Abstract
The concentration of a range of endocrine disruptors: 17-β-estradiol, estrone, 17-α-ethinylestradiol, bisphenol-A, pentachlorophenol, triclosan, and butylbenzylphthalate, was analyzed by gas chromatography/mass spectrometry in the Wetland zone of Xochimilco, a periurban area of Mexico City, during an annual cycle. Samples were taken based on their level of use and by selecting sampling points related with activities such as agriculture, livestock, and urban, as well as their potential presence in water at the Cerro de la Estrella Wastewater Treatment Plant (WWTP) which supplies the majority of water (>90 %) to the study area. The compounds analyzed are present in a wide range of products from cosmetics to home care, pharmaceuticals, and subproducts of the food industry. The importance of identifying these compounds lies in the fact that they can disrupt the endocrine system of vertebrates, in particular reproductive gland function, affecting the development of organisms and their offspring. Pentachlorophenol, triclosan, bisphenol-A, butylbenzylphthalate, estrone, and 17-β-estradiol were detected in concentrations in nanogram-per-liter levels; 17-α-ethinylestradiol was always below the detection limit. The compounds showed a trend toward greater concentrations in the rainy season, probably due to the runoff that carries these compounds into the system.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Many of the major problems that humanity is facing in the twenty-first century are related with water quality issues. During the past five decades, along with the development of industry, there have been reports of alterations in the morphology and reproductive behavior of individuals in populations living within natural systems and in different geographical regions (Instituto Sindical de Trabajo, Ambiente y Salud (ISTAS) 2002; Agency for Toxic Substances and Diseases Registry (ATSDR) 2011). This lead to a question whose answer can be sustained in the ability to relate these alterations with interactions between living organisms and chemical compounds (Carson 1962; Schwarzenbach et al. 2010).
Experimental observations of the effects of some synthetic substances such as dichlorodiphenyltrichloroethane and dioxins in different animal species (fish, amphibians, reptiles, birds, and mammals) led in 1979 to the “Estrogens in the Environment” Conference at the US National Institute of Health and Environment, at which was confirmed that the presence of these types of substances in the environment, alters the hormonal balance of living organisms (Andrade-Ribeiro et al. 2006); thus, these were denominated endocrine disruptors (ED).
Today, one of the most relevant issues in the field of water quality is the discovery of many ED effects, such as intersex fish in UK rivers downstream of municipal wastewater discharge. This observation was attributed to the presence of estrogenic compounds in wastewater effluents (Schwarzenbach et al. 2010). Based on changes observed in different animal populations, such as the loss of the reproductive capacity of bald eagles in the northern USA or the decline of the alligator population in Lake Apopka, Florida (Carson 1962), the US Environmental Protection Agency (USEPA) in 1996 implemented a strategy for continuous monitoring of suspected compounds that can cause endocrine disruption, through the Endocrine Disruptor Screening and Testing Advisory Committee (EDSTAC 1996). The aim of EDSTAC was to develop methodologies to assess the damage of these compounds and also to emit recommendations or restrictions on their use and consumption (Patlak 1996; US Environmental Protection Agency (USEPA) 2002; Aranda 2011; Blundell 2003; Peijnenburg and Struijs 2006).
Nonetheless, there have been insufficient studies worldwide of ED in urban wastewaters and their effects on aquatic organisms (Singer et al. 2002; Servos et al. 2005; Kasprzyk-Hordem et al. 2009), to allow a clear relationship between the concentrations at which some of these pollutants are present and their toxicological effects. However, some organizations such as the USEPA, the European Union, and the Canadian Water Quality Guidelines have conducted research to provide information on the concentration ranges within which these compounds could alter hormonal processes in organisms, establishing permissible limits for the protection of natural aquatic systems. Some of the compounds tested were 4-n-nonylphenol, bisphenol-A (BPA), and butylbenzylphthalate, and it was concluded that levels must not exceed 1,000 ng/L for 4-n-nonylphenol and bisphenol-A, and 2,000 ng/L for butylbenzylphthalate (Instituto Sindical de Trabajo, Ambiente y Salud (ISTAS) 2002; Argemi et al. 2005).
These contaminants are able to move through the food web, and due to their chemical structure, exhibit a tendency to accumulate in tissues, mainly in adipose tissue (Guillette et al. 1994; Guillette 1995; Folmar et al. 1996, 2001; Lascombe et al. 2000; Schwarzenbach et al. 2010).
Some of reported effects in vertebrates by exposure to ED in the literature are:
-
Intersexuality, teratogenesis, and carcinogenesis (Canales et al. 2003).
-
Physiological changes generating feminization in males and masculinization in females (Crain et al. 1997; Liney et al. 2006; Oropesa 2008).
-
Synthesis and secretion of vitellogenin in male fish (Folmar et al. 1996, 2001; Hansen et al. 2003; Segner et al. 2003a, b).
-
Decreasing the thyroid function in fish and polar bears (Olea et al. 1996; Braathen et al. 2004).
-
Decreasing fertility in birds, fish, reptiles, and crustaceans (Carlsen et al. 1992; Guillette et al. 1994; Harris et al. 1997; Jobling et al. 1998; Willingham et al. 1999).
-
Generate deformities in offspring (Guillette 1995).
-
Metabolic and behavioral abnormalities (Porterfield 1994; Jacobson and Jacobson 1996; Danzo 1997; Fisher et al. 1999; Rivas et al. 2004).
-
Breast and ovarian cancer and an increased incidence of endometriosis (Colborn et al. 1993; Hunter et al. 1997; Instituto Sindical de Trabajo, Ambiente y Salud (ISTAS) 2002; Ternes et al. 1999; Argemi et al. 2005).
-
Prostate and testicular cancer, reproductive disorders characterized by a decrease in sperm count (Colborn et al. 1993; Giwercman et al. 1993; Instituto Sindical de Trabajo, Ambiente y Salud (ISTAS) 2002).
In this study, we determined the concentrations of ED in water from the Xochimilco wetland during the annual cycle 2008–2009, in the rainy and dry seasons. To our knowledge, this is the first study of ED in the Xochimilco wetland, and it is relevant due to human use of the water and the presence of aquatic endemic organisms such as the axolote (Ambystoma mexicanum).
2 Study Area
Historically, the Basin of Mexico is characterized by high biodiversity that has provided the conditions for the establishment of human settlements and the subsequent development of a traditional highly productive agricultural system known as chinampas (Jiménez-Osornio 1995). Chinampas are artificial rectangular floating agricultural plots constructed within this freshwater Xochimilco wetland, in the southern region of the basin, by means of stacking of vegetation and sediment, raising their level above that of the lake and forming a network of canals. The plots have soils rich in organic matter and a capillary irrigation system that resulted in one of the most productive agroecosystems in the world (Jiménez et al. 1995).
The chemical and biological composition of chinampas soil rendered this system extremely fertile and productive with natural irrigation and drainage (Rojas 1985). However, over the last 50 years, anthropogenic activities have stressed this ecosystem, with their transformation into urban and agricultural and livestock areas. This transformation, as well as the intensive exploitation of natural resources, has resulted in the deterioration of the system. In particular, chemical and microbiological contamination has occurred because semitreated wastewater is pumped into the canals and is also used for irrigation. The presence of harmful substances such as heavy metals, detergents, and pesticides, as well as pathogens, has been reported (Solís et al. 2006; Mazari-Hiriart and Mackay 1993, Mazari-Hiriart et al. 2008).
This study was conducted at the Xochimilco wetland, which is reminiscent of the Tenochtitlan lake system located in the Basin of Mexico. The Xochimilco wetland is located south of Mexico City between the coordinates 19°09′–19°19′ N and 98°58′–99°10′ W, with an approximate area of 36 km2 and a network of irrigation waterways that covers 207 km (Rojas 1985). The climate is temperate, ranging from 13 to 25 °C, with an average annual temperature of 16 °C and an average annual rainfall between 700 and 900 mm, which falls mainly from June to September.
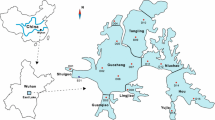
This area is also important due to the water infiltration that provides water to wells of the southern area of Mexico City. The Xochimilco wetland passes through an accelerated environmental degradation and urbanization process, transforming it into a suburban agricultural and livestock area that is irrigated with treated municipal wastewater. The location of the 19 sampling sites selected are shown in Fig. 1, and the main human activities that take place near the sampling sites are described in Table 1.
3 Materials and Methods
3.1 Criteria for Sampling Site Selection
Selection of sampling sites was based on previous water quality information reported by Contreras (2006), Aguilar (2007), Espinosa (2008), Mazari-Hiriart et al. (2008), Zambrano et al. (2009), and Espinosa et al. (2009). Other elements taken into account were nearby urban settlements, agriculture and livestock areas, and the discharge of municipal treated water into the aquatic system. Urban areas were studied separately in two groups in order to observe the influence of the other activities taking place in the vicinity of these areas. Urban area 1 is localized nearer to Cerro de la Estrella Wastewater Treatment Plant (WWTP) effluents (La Draga and San Diego), while urban area 2 is near agriculture activities. The majority of the 19 sampling sites selected does not fulfill Mexican Water Quality Guidelines for water disposal into natural aquatic systems or treated water reuse (Diario Oficial de la Federación (DOF) 1996, 1998), which establishes the maximum permissible limits for chemical and microbiological contamination in water in Mexico.
3.2 Sample Collection
Two sampling campaigns were performed, one during the rainy (August 2008) and one during the dry season (February 2009). Triplicate samples of water (1 L) were taken in polypropylene bottles that were previously washed with distilled water, the samples were transported and stored at 4 °C and processed immediately in the laboratory.
3.3 Sample Processing
The collected samples in triplicate, as indicated by Gibson et al. (2003, 2007), were filtered in cellulose filters (pore, 0.45 μm; ∅, 1.2 mm, Whatman) adding the following surrogate standards to each of the 1-L filtrates: 50 μL of 4-n-nonylphenol, 50 μL of d16-BPA, and 25 μL d4-estrone (Supelco, IL). Then the samples were passed through extraction cartridges (Oasis HLB, MA) that were previously conditioned with 10 mL of acetone; subsequently, 5 mL of water containing 250 μL of acetic acid, and then the sample was passed through the cartridge. The cartridges were washed with 5.5 mL of the mixture of a bicarbonate buffer solution containing sodium bicarbonate (0.84 g NaHCO3 dissolved in 98 mL of high-performance liquid chromatography grade water, adjusted at (pH 10) and acetone (60:40), which was then washed with 5 mL of water prior to elution with acetone (5 mL).
3.4 Sample Preparation and Derivatization
The acetone was evaporated with nitrogen gas to a volume of approximately 500 μL; then, 1 mL of ethyl acetate and 5 mg of anhydrous sodium sulfate was added to remove residual water from the sample. The samples were transferred to glass vials (Supelco) and evaporated again to a volume of 200 μL. Internal standards were added (50 μL of d4-4-n-nonylphenol, 50 μL of d4-DEHP, and 25 μL of d3-estradiol), prior to gas chromatography (GC) analysis.
Finally, the samples were evaporated to dryness with nitrogen gas and derivatized with 15 μL of pyridine and 35 μL of BSTFA. This derivatization stops the possibility of irreversible adsorption of the analytes to be determined in the GC column through the formation of hydrogen bonding functional groups such as hydroxyl (OH−), amine (−NH3), or compounds capable of yielding a proton (H+). The samples were heated at 60 °C for 30 min, diluted with ethyl acetate (∼100 μL), and finally, 1 μL of sample was injected into a gas chromatograph (Agilent Technologies model 6890N) fitted with an HP5-MS capillary column (30 m × 0.25 mm × 0.25 μm in thickness) coupled to an Agilent Technologies 5973 series mass detector. The ions selected, retention times, and chromatogram of the compounds are depicted in Table 2 and Fig. 2.
The carrier gas was helium with a constant flow of 1.0 mL/min, inlet temperature was 250 °C, and transfer line temperature was 280 °C. Blanks were prepared by passing water samples through Oasis cartridges and subsequently treating the water collected as a blank sample.
3.5 Statistical Analysis
We performed principal component (PC) analysis to explore the relationship between the concentration of ED at this study’s different sampling sites. From distribution of sites and their concentrations in a scatter plot of the first two main components that explain greatest variance, it is possible to observe the difference between the sites and to explore their behavior, including all of the compounds. Because Student’s t test analysis demonstrated that there are significant differences among seasons, PC analyses were performed separately employing SPSS ver.17.0 software (SPSS, Inc., USA 2008) for each of the periods.
4 Results and Discussion
Based on European regulations and those of the USEPA, the concentrations at which the compounds are analyzed in the study area are low despite the anthropogenic activities that take place in the area. Figure 3 illustrates averages and standard deviations (SD) of the concentrations of the compounds determined during the 2008–2009 annual cycle (rainy and dry seasons) at each Xochimilco wetland site and grouped by activity as depicted in Table 1.
The present conditions in Xochimilco wetland water, despite its being heavily impacted by human activities, do not show ED concentrations that may pose a risk in this ecosystem, although it is noteworthy that these compounds are bioaccumulative. This is based on the literature, in which some compounds have been evaluated in vitro by different methods, in which toxicological effects in living organisms or cell cultures are detected in concentrations of micorgrams per liter.
However, it is important to consider that these compounds may accumulate in adipose tissue and sediment, so organisms can be exposed to higher ED concentrations. It is also important to be aware that ED in the Xochimilco wetland can affect endemic amphibian, crustacean, and fish species, such as the axolotl (A. mexicanum) and the crayfish (Cambarellus montezumae), and also can contaminate the groundwater recharge areas that provides water to one of the most populated metropolitan areas of the world.
Results of PC analyses for the dry season, that is, PC1 and PC2 (Table 3; Fig. 4), explained a cumulative variance of 86 %. PC1 (61 %) was mainly explained by two compounds: the concentration of BPA presented a positive value, and the concentration of estradiol, a negative value. The PC2 analysis (25 %) presented a positive value for the concentration of estradiol and a negative one for triclosan (TRC). The sites were 12 and 13 at PC1 positive values, indicating that the concentration of BPA in these sites explain the spatial behavior of this compound to a greater degree than the other sample sites. Moreover, sites 7 and 11 exhibited negative behavior associated with estradiol concentrations (Table 3). At this time, the grouping of sites do not reflect a relationship conferred by their spatial location, which may be a result of the quality of water being utilized for recharging Xochimilco canals, because at present the contribution of precipitation is negligible, which reduces the effect of site.
PC analysis for the rainy season (Table 3) (Fig. 5) explained 97 % of the variance in the first two components. PC1 explained 72 % of variance: this component was positively related with the concentration of butylbencylphthalate (BuBePh) concentration and negatively related with those of estradiol, pentachlorophenol (PCP), TRC, and estrone. PC2 analysis was principally related with the concentration of BPA and this relationship was positive. The PC1 and PC2 figures grouped the sites, suggesting that the site’s features and their spatial location exert an influence on the behavior of the compounds. This behavior was consistent in the majority of sites, except at site 4, which showed high concentrations of BPA (140.33 ng/μL), BuBePh (17.38 ng/μL), and estradiol (1.72 ng/μL) and low concentrations of the remaining compounds. The sites located in the urban area 1 (s10, s11, s12, and s13) form a group with high concentrations of PCP (2.49–3.89 ng/μL), TRC (52.74–71.93 ng/μL), and BPA (29.35–8.42 ng/μL) in contrast with the group consisting of sites 14–17 localized within urban area 2, which have lower concentrations of these same compounds, as observed in Fig. 3. Although both areas are urban, their supply and location is different because urban area 1 is near the agricultural area and the high concentration of TRC, BPA, and PCP in the rainy season may be due to runoff, while in urban area 2, the values of TRC, BPA, and PCP are lower, a fact that can reflect the effect of the La Draga and San Diego effluents, both directly from the Cerro de la Estrella WWTP.
Five sites located within the agricultural zone contain low concentrations of PCP (0–1.13 ng/μL), BPA (7.78–12.63 ng/μL), estrone (1.02–1.35 ng/μL), and estradiol (0.11–0.15 ng/μL). Furthermore, sites 7 and 9, while not spatially near, share the use of agricultural land; these sites have average remaining concentrations with regard to sites for the compounds estradiol (0.24–0.86 ng/μL), BPA (4.27–18.32 ng/μL), and TRC (29.45–33.81 ng/μL). Finally, sites 1–3 and 8 are grouped by filing mean concentrations of TRC (26.41–31.71 ng/μL) and BPA (15.20–33.17 ng/μL), low concentrations of estrone (1.67–2.29 ng/μL) with the exception of site 8 (10.38 ng/μL), and high concentrations of estradiol (0.98–1.68 ng/μL); these sites comprise land use for livestock except for site 8, whose land use is agricultural.
Estradiol was found at highest concentrations during the rainy season, primarily in areas where animal raising activities are carried out, as well as urban farming, which may be due to the use of this hormone by the livestock sector to accelerate development and reproduction in animals.
5 Conclusions
Water pollution requires an effective set of policies, technologies, and scientific advances in very different scales to establish a regulatory effort on the global scale, mainly with new contaminants such as ED. Human activities in proximity to groundwater systems must be evaluated based on local characteristics such as the soil type, topography, irrigation, and drainage systems that ensure optimal use of water as well as the implementation of policies and methodologies for reducing the pollution effect.
Based on the statistical analysis, the effect of season and/or activities in different areas regarding the concentration of certain compounds shows that current conditions in Xochimilco wetland water, despite its being impacted by human activities, reflected in high nutrient concentrations and microbiological counts (Mazari-Hiriart et al. 2008) do not exhibit concentrations of ED that may pose a risk in this ecosystem. Nonetheless, this study provides information on the contaminant release in urban-rural infringement setting.
It is also important to be aware that the Xochimilco wetland represents a habitat for several endemic amphibian, crustacean, and fish species such as the axolotl (A. mexicanum) and the crayfish (C. montezumae), and that these share this Wetland’s environment within Mexico City, one of the world’s most populated metropolitan areas. The main environmental problems in terms of endemic species confronted include the following: water quality alteration due to several types of compounds, as well as the introduction of exotic species such as tilapia (Orochromis aureus) and carp (Cyprinus carpio) (Zambrano et al. 2001). ED may simply comprise one additional factor that adds stress to these species that are already under considerable pressure from various other factors. The Xochimilco wetland possesses historical, cultural and environmental values; the wetland represents the agricultural site where vegetables were produced for the Mexica indigenous culture, at present is a Ramsar site (Ramsar, Iran, 1971 Convention on Wetlands of International Importance) (Pérez Mujica 2012), in addition to the importance of being a groundwater supply for the southern area of Mexico City.
References
Agency for Toxic Substances and Diseases Registry (ATSDR). Web site: (http://www.atsdr.cdc.gov/). Consulted 2011.
Aguilar, J. (2007). Detección de factores de virulencia de Escherichia coli, Shigella y Salmonella en agua superficial y subterránea de Xochimilco. Tesis de Maestría en Ciencias Biológicas. Instituto de Ecología, UNAM. México.
Andrade-Ribeiro, A., Pacheco-Ferreira, A., Nóbrega da Cunha, C., & Mendes-Kling, A. (2006). Disruptores endocrinos: potencial problema para la salud pública y medio ambiente. Revista de Biomedicina, 17, 146–150.
Aranda, M. Página Web Disruptores Endocrinos. (Web site: (http://disruptor.ugr.es/). Consulted 2011.
Argemi, F., Cianni, N., & Porta, A. (2005). Disrupción endocrina: perspectivas ambientales y salud pública. Acta Bioquim Clin Latinoamer, 39(3), 291–300.
Blundell, T. (2003). Chemicals in Products Safeguarding the Environment and Humans. Twenty fourth Health Report (Vol. 5827, p. 307). London, UK: Royal Commission on Environmental Pollution.
Braathen, M., Derocher, A., Wigg, O., Sormo, E., Lie, E., Skaare, C., & Jenssen, B. (2004). Relationships between bisphenol-A and thyroid hormones in female and male polar bears (Ursus maritimus). Environmental Health Perspectives, 112(8), 826–833.
Canales, A., de Celis, R., Salgado, H., & Feria, A. (2003). Xentoestrógenos: función y efectos. Rev Digital Cien Tecnol e-GNOSIS 01: 1-11. (www.e-gnosis.udg.mx). México: Universidad de Guadalajara.
Carlsen, E., Giwercman, A., Keiding, N., & Skakkebaek, N. (1992). Evidence for decreasing quality of semen during the past 50 years. British Medical Journal, 305, 609–613.
Carson, R. (1962). The Silent Spring. Spain: Grijalba. 344.
Contreras, V. (2006). Distribución potencial del Ambystoma mexicanum en los canales de la zona chinampera de Xochimilco. Tesis de licenciatura (Biología). México: Facultad de Ciencias, UNAM.
Colborn, T., Vom-Saal, F., & Soto, A. (1993). Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environmental Health Perspectives, 101, 378–384.
Crain, A., Guillette, L., Rooney, A., & Pickford, D. (1997). Alterations in steroidogenesis in alligators (Alligator mississipiensis) exposed naturally and experimentally to enviromental contaminants. Environmental Health Perspectives, 105, 528–533.
Danzo, B. (1997). Environmental xenobiotics may disrupt normal endocrine function by interfering with the binding of physiological ligands to steroid receptors and binding proteins. Environmental Health Perspectives, 105, 294–301.
Diario Oficial de la Federación (DOF). (1996). Límites máximos permisibles para contaminantes básicos.
Diario Oficial de la Federación (DOF) 1998. Límites máximos permisibles de contaminación en aguas residuales tratadas para su reuso. Endocrine Disruptor Screening and Testing Advisory Committee (EDSTAC). Final Report 1996. EPA/743/R-96/00.
Endocrine Disruptor Screening and Testing Advisory Committee (EDSTAC) (1996). Final report, EPA/743/R-96/00
Espinosa, A. C. (2008). Presencia de virus entéricos en agua: efecto de la calidad del agua sobre su estabilidad e infectividad. Tesis de Doctorado en Ciencias Biomédicas. México: Instituto de Ecología, UNAM.
Espinosa, A.C., Árias, C.F., Sánchez-Colón, S., Mazari-Hiriart, M. (2009). Comparative study of enteric viruses, coliphages and indicator bacteria for evaluating water quality in a tropical high-altitude system. Environ Health 8(49):10.1186/069X.
Fisher, J., Turner, K., Brown, D., & Sharpe, R. (1999). Effect of neonatal exposure to estrogenic compounds on development of the rat through puberty to adulthood. Environmental Health Perspectives, 107, 397–405.
Folmar, L., Denslow, N., Kroll, K., Enblom, J., Metcalfe, J., & Guillette, L. (1996). Comparative estrogenicity of estradiol in an in vitro male fish (Cyprinodon variegatus), vitellogenin bioassay. Aquatic Toxicology, 49, 77–88.
Folmar, L., Denslow, N., Rao, V., Chow, M., Crain, D., Louis, J., & Gillette, L. (2001). Vitellogenin induction and reduced serum testosterone concentrations in fetal male carp (Cyprinus carpio) captured near a major metropolitan sewage treatment plant. Environmental Health Perspectives, 104, 1096–1101.
Gibson, R., Wang, M., Padgett, E., & Beck, A. (2003). Analysis of 4-n-nonylphenols, phthalates and polychlorinated bisphenyls in soils and biosolids. Chemosphere, 61, 1336–1344.
Gibson, R., Becerril-Bravo, E., Silva-Castro, V., & Jiménez, B. (2007). Determination of acidic pharmaceuticals and potential endocrine disrupting compounds in wastewaters and spring waters by selective elution and analysis by gas chromatography-mass spectrometry. Journal of Chromatography, 43, 31–39.
Giwercman, A., Carlsen, E., Keiding, N., et al. (1993). Evidence for increasing incidence of abnormalities of the human testis: A review. Environmental Health Perspectives Supplements, 101(2), 65–71.
Guillette, L., Gross, T., Masson, G., Matter, J., Percival, H., & Woodward, A. (1994). Developmental abnormalities of the gonad and abnormal sex hormone concentrations in juvenile alligators from contaminated lakes in Florida, USA. Environmental Health Perspectives, 102, 680–688.
Guillette, L. (1995). Endocrine disrupting enviromental contaminants and developmental abnormalities in embryos. Human and Ecological Risk Assessment, 1, 25–36.
Hansen, H., Hock, B., Marx, A., Sherry, J., & Bilitewski, B. (2003). Endocrine disrupting on environmental sources. Environmental Research, 202, 4–7.
Harris, C., Henttu, P., Parker, M., & Sumpter, J. (1997). The estrogenic activity of phthalate esters in vitro. Environmental Health Perspectives, 105, 802–811.
Hunter, D., Hankinson, S., Laden, F., Colditz, G., Manson, J., Willett, W., Speizer, F., & Wolf, M. (1997). Plasma organochlorine levels and the risk of breast cancer. New England Journal of Medicine, 337(18), 1253–1258.
Instituto Sindical de Trabajo, Ambiente y Salud (ISTAS). (2002). Curso de Introducción a los Disruptores Endocrinos: 1-34. España: Barcelona.
Jacobson, J., & Jacobson, S. (1996). Intellectual impairment in children exposed to polychlorinated biphenyls in utero. The New England Journal of Medicine, 335(11), 783–789.
Jiménez-Osornio, J. J. (1995). Componentes esenciales de la tecnología chinampera. In R. T. Rojas (Ed.), Presente, Pasado y Futuro de las Chinampas (pp. 77–80). México, D.F., México: Centro de Investigaciones y Estudios Superiores en Antropología Social. Patronato del Parque Ecológico de Xochimilco.
Jiménez, J. J., Rojas-Rabiela, T., del Amo, S., & Gómez-Pompa, A. (1995). Conclusiones y recomendaciones del taller. In T. Rojas Rabiela (Ed.), Presente, Pasado y Futuro de las Chinampas (pp. 19–43). México, D.F., México : Centro de Investigaciones y Estudios Superiores en Antropología Social. Patronato del Parque Ecológico de Xochimilco.
Jobling, S., Nolan, M., Tyler, C., Brighty, G., & Sumpter, J. (1998). Widespread sexual disruption in wild fish. Environmental Sciences, 32, 2498–2506.
Kasprzyk-Hordem, B., Dinsdale, R. M., & Guwy, A. J. (2009). The removal of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs during wastewater treatment and its impact on the quality of receiving waters. Water Research, 43(2), 363–380.
Lascombe, I., Beffa, D., Rüeg, U., Tarradellas, J., & Wahli, W. (2000). Estrogenic activity assessment of environmental chemicals using in vitro assays: identification of two new estrogenic compounds. Environmental Health Perspectives, 108, 621–629.
Liney, K., Hagger, J., Tyler, C., Depledge, M., & Jobling, S. (2006). Health effects in fish of long-term exposure to effluents from wastewater treatment works. Environmental Health Perspectives, 114, 81–87.
Mazari-Hiriart, M., & Mackay, D. (1993). Potential for groundwater contamination in Mexico City. Environmental Science and Technology, 27(5), 794–802.
Mazari-Hiriart, M., Ponce de León, S., López-Vidal, Y., Islas-Macías, P., Amieva-Fernández, R., & Quiñones-Falconi, G. (2008). Microbiological implications of periurban agriculture and water reuse in Mexico City. PLoS One, 3, 1–8. http://www.plosone.org.
Olea, N., Pulgar, P., Pérez, F., Olean-Serrano, A., Rivas, A., Novillo-Fertell, V., Pedraza, A. M., & Sonnenschein, C. (1996). Estrogenicity of resin-based composites and sealants used in dentistry. Environmental Health Perspectives, 104, 298–305.
Oropesa, A. (2008). Disruptores endocrinos en el medio ambiente: caso del 17-α-etinilestradiol. Observatorio Medioambiental, 11, 63–76.
Patlak, M. (1996). A testing deadline for endocrine disrupters. Environmental Science and Technology, 30, 540–544.
Peijnenburg, W., & Struijs, J. (2006). Occurrence of phthalate esters in the environment in The Netherlands. Ecotoxicology and Environmental Safety, 63, 204–215.
Pérez Mujica, L. H. (2012). Problemática ambiental de Xochimilco. Tesis de licenciatura (Biología). México: Facultad de Ciencias, UNAM.
Porterfield, S. (1994). Vulnerability of developing brain to thyroid abnormalities: environmental insults to the thyroid system. Environmental Health Perspectives, 102, 125–130.
Rojas, R. (1985). La cosecha del agua en la Cuenca de México y la pesca en el medio lacustre y chinampera de San Luis Tlaxialtemalco. México, D.F., México: Centro de Investigaciones y Estudios Superiores en Antropología Social. Patronato del Parque Ecológico de Xochimilco.
Rivas, A., Granada, A., Jiménez, M., & Olea, N. (2004). Exposición humana a disruptores endocrinos. Ecosistemas Revista científica y técnica de ecología y medio ambiente de la Asociación Española de Ecología Terrestre (AEET), 13(3), 7–12.
Schwarzenbach, R. P., Egli, T., Hofstetter, T. B., Von-Gunten, U., & Wehrli, B. (2010). Global water pollution and human health. Annual Review of Environment and Resources, 35, 109–136.
Segner, H., Navas, J., Schäfers, C., & Wenzel, A. (2003a). Potencies of estrogenic compounds in in vitro screening assays and in life cycle tests with zebrafish in vivo. Ecotoxicology and Environmental Safety, 54, 315–322.
Segner, H., Carrol, K., Fenske, M., Janssen, C., Maack, G., Pascoe, D., Schäfers, C., Vandenbergh, G., Watts, M., & Wenzel, A. (2003b). Identification of endocrine disrupting effects in aquatic vertebrates and invertebrates. Report from the European IDEA project. Ecotoxicology and Environmental Safety, 54, 302–314.
Servos, M., Bennie, D., Burnison, B., Jurkovik, A., McInnis, R., Neheli, T., Schnell, A., Seto, P., Smyth, S., & Ternes, T. (2005). Distribution of estrogens 17-β-estradiol and estrone in Canadian Municipal Wastewater Treatment Plants. Science of the Total Environment, 336, 155–170.
Singer, H., Müller, S., Tixier, C., & Pillonel, L. (2002). Triclosan occurrence and fate of a widely used biocide in the aquatic environment: field measurements in wastewater treatment plants, surface waters and lake sediments. Environmental Science and Technology, 36, 1998–2004.
Solís, C., Sandoval, J., Pérez-Vega, H., & Mazari-Hiriart, M. (2006). Irrigation water quality in southern Mexico City based on bacterial and heavy metal analyses. Nuclear Instruments and Methods in Physics Research, 249, 592–595.
Ternes, T., Stumpf, M., Müller, J., Heberer, K., Wilken, R., & Servos, M. (1999). Behavior and occurrence of estrogens in municipal sewage treatment plants. Institute of Investigations in Germany, Canada and Brazil. Science of the Total Environment, 225, 81–90.
US Environmental Protection Agency (USEPA) (2002). Endocrine Disruptor Screening Program Report to Congress. (http://www.epa.gov/).
Willingham, E., Rhen, T., Sakata, J., & Crews, D. (1999). Embryonic treatment with xenobiotics disrupts steroid hormone profiles in hatchling red-eared slider turtles (Trachemys scripta elegans). Environmental Health Perspectives, 108, 329–332.
Zambrano, L., Contreras, V., Mazari-Hiriart, M., & Zarco-Arista, A. E. (2009). Spatial heterogeneity of water quality in highly degraded tropical freshwater ecosystem. Environmental Management, 43, 249–263.
Zambrano, L., Scheffer, M., & Martínez-Ramos, M. (2001). Catastrophic response of lakes to response to benthivorous fish introduction. Oikos, 94, 344–350.
Acknowledgments
We acknowledge the support provided by Roberto Altamirano, a Xochimilco fisherman, during field work, the Water Reuse Group coordinated by Dr. Blanca Jiménez at the Instituto de Ingeniería, UNAM, for analytical work with respect to analysis of compounds, and Alma Delia de los Ríos for her help with the graphic material presented.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Díaz-Torres, E., Gibson, R., González-Farías, F. et al. Endocrine Disruptors in the Xochimilco Wetland, Mexico City. Water Air Soil Pollut 224, 1586 (2013). https://doi.org/10.1007/s11270-013-1586-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-013-1586-1