Abstract
Catalytic wet peroxide oxidation (CWPO) has become an important deep oxidation technology for organics removal in wastewater treatments. Supported Cu-based catalysts belong to an important type of CWPO catalyst. In this paper, two Cu catalysts, namely, Cu/Al2O3-air and Cu/Al2O3-H2 were prepared and evaluated through catalytic degradation of phenol. It was found that Cu/Al2O3-H2 had an excellent catalytic performance (TOC removal rate reaching 96%) and less metal dissolution than the Cu/Al2O3-air case. Moreover, when the organic removal rate was promoted at a higher temperature, the metal dissolution amounts was decreased. Combined with hydroxyl radical quenching experiments, a catalytic oxidation mechanism was proposed to explain the above-mentioned interesting behaviors of the Cu/Al2O3-H2 catalyst for CWPO. The catalytic test results as well as the proposed mechanism can provide better guide for design and synthesis of good CWPO catalysts.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water is the basic resource for all living things on earth, however, nowadays water shortage and pollution become increasingly serious problems for humankind. The improperly treated sewage discharged from plants is one of the main reasons responsible for water pollution. Therefore, degradation of pollutants in wastewater has developed to be an important research field for reducing damage to water environment. Advanced oxidation processes (AOPs), known as a deep oxidation technology, take the advantage of oxidants that can produce highly reactive free radicals (such as ·OH, SO4·−) to oxidize organic contaminants in water. They are especially useful for the aqueous contaminants that are difficult to degrade with biologic methods. In particular, the ·OH radical does not introduce elements other than H and O into water, which fits the green chemistry idea for water treatment. For this goal, the AOP techniques using oxidants that can generate ·OH radicals, such as H2O2, O3, and molecular O2 (pure O2 or air) etc (Li et al. 2019; Fu et al. 2020; Baloyi et al. 2018) are preferred over other oxidants like S2O82−. O2 is generally used under high pressures and temperatures, and the cost of O3 is obviously higher than the other two oxidants. For example, Fu et al. (Fu et al. 2021) prepared Zn-CNTs-Cu composite for in situ generation of H2O2 to degrade 4-chlorophenol under 0.50 MPa (O2) at 150 ℃. MnCeOx catalysts were prepared and used for the catalytic wet air oxidation of phenol at 2 MPa (O2) (Geng et al. 2020).
Compared to O2, H2O2 can degrade organic substances at lower temperatures and ambient pressure. For example, Jiao et al. reported that H2O2-driven Fenton-like treatment was used for oxidation of 4-nitrophenol and Rhodamine B at the temperatures of ≤ 50 ℃ (Jiao et al. 2018).
It is undoubtedly essential to use catalysts to remove organic matters quickly and thoroughly in this progress, known as catalytic wet peroxide oxidation (CWPO). CWPO catalysts can be divided into homogeneous and heterogeneous ones in general, and the latters are more suitable for industrial applications due to their easier separation from solution. In particular, supported metal catalysts are widely applied due to high metal dispersion and therefore high catalytic activity become a research hot spot (Devlin and Harris 1984; Li et al. 2020; Wang and Tang 2021). Copper (Cu) is one of the commonly used active metals components, being lower cost compared to noble metals and higher activity compared to rare-earth metals. (Liu et al. 2022; Cui et al. 2022; Kumar et al. 2020; Jiang et al. 2022).
During the AOPs process, metal ions dissolution from supported catalysts into water can lead to second-time pollutions and thus increase the cost of post-treatment. Therefore, study on metal dissolution rules has an obvious practical significance. The dissolution of metal ions into water can be accelerated by short-chain acids, like maleic, formic and acetic acids, generated during CWPO process. And compared with the high calcination temperature (900 ℃), the low temperature (500 ℃) promotes the dissolution of Cu2+ into water (Brussino et al. 2019). Cu2+ dissolution occurs not only in CWPO, but also in other AOP processes like catalytic wet air oxidation (CWAO) (Arena et al. 2003; Santos et al. 2005). Cu2+ dissolution is often at a lower extent under neutral and alkaline conditions, while the solution acidification is inevitable because of the generation of short-chain acids during the reaction process. In addition, the dissolution decreased as the oxidation reaction temperature increases from 383 to 413 K. The reason for this trend is that acids can be oxidized quickly at higher temperatures (Zapico et al. 2017).
However, there were rare discussion on the relationship between the catalytic oxidation of organic compound and metal dissolution, especially for supported copper-based catalyst in CWPO system. More importantly, the catalytic synergetic effect between Cu and Cu+ has been proposed and verified by Shen et al. (2023). The active Cu+ sites may further enhance the catalytic performance. The findings in the literature reflect that the valence of active metal component can be important to their catalytic performance. In principle, different calcination atmosphere can lead to different valence of metal component on catalyst. Thus, it is necessary to investigate the influence of the calcination atmosphere of catalyst during catalyst preparation on the chemical status of Cu on catalyst, as well as its consequence for the CWPO performance accordingly.
In this paper, Cu/Al2O3-air and Cu/Al2O3-H2 were prepared and used for CWPO of phenol in simulated phenol solutions. It was found that Cu/Al2O3-H2 performed excellently at optimum conditions. It is interesting to find that the reaction rate was faster at higher temperatures, while the Cu2+ dissolution was lower. Based on the observed regulations, a catalytic reaction mechanism is proposed and the feasibility of this mechanism is discussed.
Materials and methods
Materials
Alumina (Al2O3, cylinder in shape) were purchased from Nantong Jinqi Chemical Co., Ltd. copper nitrate trihydrate (Cu(NO3)2·3H2O, AR, 99 ~ 102%), hydrogen peroxide (H2O2, ≥ 30%), phenol (C6H5OH, AR, ≥ 99.0%), methanol (CH3OH, AR, ≥ 99.5%), isopropanol (C3H8O, AR, ≥ 99.7%), t-butanol (C4H10O, AR, ≥ 99.5%), ammonium chloride (NH4Cl, AR, ≥ 99.5%), ammonium solution (NH4OH, AR, (NH3) 25 ~ 28%) were obtained from Sinopharm Group Chemical Reagent Co., Ltd. Sodium diethyldithiocarbamate trihydrate (C5H10NNaS2 3H2O, AR 99%) were obtained from Shanghai McLean Biochemical Technology Co., Ltd. High purity hydrogen gas (99.999%) was purchased from Qingdao Henglaixiang Trading Co., Ltd. Deionized (DI) water was used to prepare all of the solutions in this work.
Catalyst preparation
The catalysts were prepared by an equal volume impregnation method. First, Al2O3 particles as catalytic support were milled into small particles in a mortar, and the 20–40 mesh particles were selected using sieves. Then the sieved Al2O3 were calcined at 500 ℃ for 4 h in a muffle furnace. A 3.775 g Cu(NO3)2·3H2O sample was dissolved in 6.75 mL DI water. Then the solution was added to 9.0 g Al2O3. Theoretical mass percentage of the Cu metal in target catalyst was 10 wt.% and the volume of solution was equal to the Al2O3 pore volume. After a 12 h wetness impregnation, the impregnated Al2O3 was put in an oven at 120 °C for 4 h for drying, and then calcined at 600 °C for 3 h in a muffle furnace. The catalyst obtained was named as Cu/Al2O3-air. Then, Cu/Al2O3-air was further treated by H2 at 600 °C for 3 h, and the obtained catalyst was named as Cu/Al2O3-H2.
Catalyst characterization
X-ray diffraction (XRD) patterns of the catalysts were obtained with a Malvern Panalytical Empyrean diffractometer and using nickel-filtered Cu Kα radiation. The patterns were recorded over 5° < 2θ < 75° using a scanning rate of 0.02°/s. The Brunauer–Emmett–Teller (BET) specific surface area was measured using Micromeritcs TriStar 3020 automatic adsorption analyzer. N2 adsorption isotherms at 77 K were used for textural studied with. The samples were degassed at 300 °C for 8 h, using liquid nitrogen as the cold trap and high-purity nitrogen as the adsorbate. X-ray photoelectron spectrum (XPS) and Auger electron spectrum (AES) were obtained by an X-ray photoelectron spectroscope (Thermo SCIENTIFIC ESCALAB250Xi) with an Al Kα radiation source.
Catalytic activity evaluation
The degradation of simulate wastewater was studied using a custom reactor as shown in Fig. 1. A round-bottomed flask with water bath was used for stable temperatures. A magnetic stirrer was used to keep the stirring speed. The solution volume was 200 mL and concentration of phenol was 280 mg/L. Before catalyst was added, the substrate solution was stirred for several minutes to reach a target temperature. Samples were taken out of the solution waiting for analysis, and taken as the initial value. After catalyst was added, there was a 10 min equilibrium period before starting the catalytic tests. As soon as 2.0 mL H2O2 was added (14.0 mol of H2O2 is required to degrade 1.0 mol of phenol stoichiometrically, and 2.0 mL 30 wt.% H2O2 is enough to degrade 200 mL of 280 mg/L phenol), the reaction started. Typically, samples were withdrawn from the solution at regular time intervals for one of the chemical oxygen demand (COD) (1 mL sample for each time measurement), total organic carbon (TOC) (5 mL sample) and UV absorption spectroscopic (2 mL sample) analysis. Typically, a 2 mL sample was withdrawn for measuring the Cu2+ concentration after 60 min of reaction.
The effects of three influencing factors including calcination atmosphere during catalytic preparation, reaction temperature in the catalytic test, and free radical trapping agents on catalytic reaction rates was studied. The effect of different calcination atmosphere, reaction temperature on the metal ion dissolution amount was also examined.
Quenching experiment
Before H2O2 was added, three quenching agents (methanol of 160 mg/L, isopropanol of 300 mg/L, and t-butanol of 375 mg/L) were mixed with three phenol oxidation systems, respectively. The concentrations of three quenching agents were controlled to be 5.0 mmol/L. Stoichometrically, the organics was sufficient to be oxidized completely by H2O2 according to Eqs. (1–4). The samples were withdrawn at different times during the reaction for determination.
Analytical methods for reaction solution
The samples were taken out from the solution during the reaction. Then the samples were diluted by DI water and analyzed through the following methods. The content of organics in water was determined by COD (HACH DR1010) and TOC (Shimadzu TOC-L CPH). The COD and TOC removal rates were calculated using Eq. (5) and Eq. (6):
where CODt and TOCt were the COD and TOC values at the reaction time of t min, respectively. And COD0 and TOC0 were the initial value at the reaction time of 0 min, respectively.
Copper ions was colored by sodium diethyldithiocarbamate under alkaline conditions, and then determined by Ultraviolet–visible (UV–Vis) absorption spectroscopy (Analytik Jena AG) at 453 nm. The copper ion concentrations were calculated through the Eq. (7):
where Abs was the absorbance of the colored solution, V was the volume of solution taken from the target sample solution, and 0.0016 was the slope of the standard curve for the relationship between Cu2+ and Abs. A series of Cu2+ solutions were prepared with different known concentration of Cu2+ (Cu mass basis) for the standard curve. The standard curve (with a zero intercept) was then obtained by plotting the absorbance (at 453 nm) of different Cu2+ solutions over their Cu2+ mass in a fix total volume of solution.
UV–Vis was also used to judge the changes of characteristic peaks of phenol (271 nm) and intermediates.
Results and discussion
Characterization of basic physiochemial properties
XRD
The XRD pattern of catalysts and catalytic support are shown in Fig. 2. The diffraction peaks of Al2O3 at 2θ of 33.3, 35.7, 66.8° corresponded well to (107), (114) and (1114) lattice planes of aluminum oxide-Al2O3 (PDF-#51–0769) (Zapico et al. 2017). The diffraction peaks of Al2O3 were weakened after the Cu component was loaded, while position of Al2O3 diffraction peaks were not changed. The Al2O3 peaks of Cu/Al2O3-air and Cu/Al2O3-H2 remained unchanged. The diffraction peaks of Cu/Al2O3-air at 2θ of 35.4, 35.5 and 38.7°, corresponded respectively to (002), (11–1) and (111) lattice planes of tenorite-CuO (PDF-#48–1548) (Xin et al. 2021). The diffraction peaks of Cu/Al2O3-H2 at 2θ of 43.3, 50.4 and 74.1°, corresponded to (111), (200) and (220) lattice planes of copper-Cu (PDF-#04–0836). In addition, diffraction peaks of CuAlO2 (PDF-#40–1037) existed in both Cu/Al2O3-air and Cu/Al2O3-H2 samples. In contrast, to that the peaks assigning to CuO were not observed in Cu/Al2O3-H2. This difference indicated that the interaction of Cu and Al in CuAlO2 was stronger than Cu and O in CuO, and Cu(I) was difficult to be further reduced. Therefore, the CuAlO2 peak was still observed after calcination under H2 atmosphere. The results indicated that the Cu components existed as CuO and CuAlO2 in Cu/Al2O3-air catalyst, while existed as Cu and CuAlO2 in Cu/Al2O3-H2 catalyst.
XPS
The XPS was then used to further investigate the chemical status of Cu in Cu/Al2O3-H2 and Cu/Al2O3- air (Fig. 3). In Fig. 3a, two peaks located at around ~ 933 and ~ 953 eV and strong satellite peaks can be clearly seen from the high-resolution Cu 2p XPS spectrum. The peak at ~ 953 eV can be assigned to Cu. Satellite peaks still existed after the H2 calcination, which indicated the possibility of Cu(II). The two catalysts had similar peaks, but there was a slight difference. The binding energy of Cu 2p1/2 shifted from ~ 965.5 (in Cu/Al2O3-air) to ~ 966.7 eV (in Cu/Al2O3-H2) and Cu 2p3/2 shifted from ∼934.6 (in Cu/Al2O3-air) to ∼933.3 eV (in Cu/Al2O3-H2) (Larson 1974; Huang et al. 2019). It indicated that Cu(II) partially transformed to Cu(I) and Cu(0) after calcination in H2. The AES spectrum (Fig. 3b) shows the peak of ~ 915.8 eV was shift to ~ 914.5 and ~ 917.2 eV after calcined in H2 (Shen et al. 2023). The peak of Cu/Al2O3-air was assigned to Cu (II) and two peaks of Cu/Al2O3-H2 can be corresponded to Cu(I) and Cu(0), respectively. This indicated that the Cu(I) and Cu(0) existed in Cu/Al2O3-H2. Combining the results of high-resolution Cu 2p and AES spectra, the Cu component existed mainly as Cu(II) in Cu/Al2O3-air, and existed as Cu(I), Cu(0) and Cu(II) in Cu/Al2O3-H2. The results of XPS were consistent with the results of XRD and further indicated the existence of Cu with different valence, which is beneficial for studying the relationship between the chemical status of catalyst and its catalytic performance.
N2 adsorption–desorption isotherm
The 77K-N2 adsorption–desorption isotherms are shown in Fig. 4. Both of these N2 adsorption–desorption isotherms belonged to an IUPAC type IV isotherm with a H1 hysteresis loop (Yang et al. 2021). This indicated that the catalysts were mesoporous and had uniform pore size distributions. The BET surface area, pore volume and pore width of Al2O3, Cu/Al2O3-air and Cu/Al2O3-H2 obtained from the N2 adsorption–desorption isotherms are listed in Table 1. It can be noticed that the BET surface area and pore volume of catalyst was decreased (from 163 to 142 m2/g for Cu/Al2O3-air for BET area for instance), while the pore width increased (from 10.7 to 13.7 nm for Cu/Al2O3-air for instance). The changes in pore volume and pore size suggested that a part of pores were occupied by Cu component, and these occupied pores were mainly smaller pores. The BET surface area was further decreased from 142 to 131 m2/g for Cu/Al2O3-H2. However, the pore width was almost unchanged (13.7 nm). This phenomenon suggested that the pores were further filled due to the clustered Cu(0), and Cu(0) was obtained from reduction of Cu(II) by H2 in the "XRD" Section.
Influence factors for the catalytic performance in phenol oxidation by H2O2 in water
Reaction temperature
The performance of different catalysts was evaluated by phenol oxidation reaction with H2O2. To determine the catalytic activity at an optimal reaction temperature, the COD removal rates were calculated and the UV absorption spectrums of the solution were measured during the reaction process. The COD removal rate of phenol gradually increased with the increase of reaction temperature before reaching 70 ℃ (Fig. 5). Unexpectedly, the initial reaction rate at 80 ℃ was faster than 70 ℃, while the COD removal rate at 80 ℃ was lower at the reaction rate of 60 min. The reason can be accounted to the case that high temperature promoted the catalytic degradation reaction on one hand, and self-decomposition rate of H2O2 to H2O and O2 was also accelerated on the other (Luo et al. 2020; Xia et al. 2011; Liang et al. 2019). Therefore, the optimal reaction temperature was 70 ℃, since at this condition the catalytic reaction rate and self-decomposition of H2O2 were comprehensively considered.
The UV absorption spectrums of solution during reaction are shown in Fig. 6a (40 ℃) and 5b (70 ℃). As expected, the characteristic peak of phenol at 270 nm decreased slightly at 40 ℃. There was a UV absorption band in the wavelength range of 190 and 260 nm, indicating that a large number of degradation intermediates still existed in the solution, such as hydroquinone, resorcinol, p-benzoquinone, and catechol (Huang et al. 2021a; Taran et al. 2018). The characteristic peak of phenol decreased significantly at 70 ℃. However, the COD removal rate finally stabilized around 93% at 70 ℃, which was due to the residual organic small molecules that were difficult to degrade, such as formic acid, acetic acid and oxalic acid (Guo et al. 2021).
Calcination atmosphere during catalyst preparation
In order to study the effect of calcination atmosphere on catalyst activity, Cu/Al2O3-air and Cu/Al2O3-H2 were used for catalytic oxidation reactions for comparison. The COD removal rates during reactions were measured and compared. The result can be seen in Fig. 7.
Compared with the case of no catalyst being used (-None in Fig. 7a), the reaction was promoted by two catalysts effectively (Fig. 7a). The COD removal rate was nearly not observable at 40 ℃ when no catalyst was used. There was no visible change in color during the reaction process. In the presence of catalyst, the solution turned brown and gradually faded as the reaction proceed, which suggested that p-benzoquinone was generated and discussed in the "Reaction temperature" Section. It was difficult to oxidize phenol at 40 ℃. The COD removal rate of Cu/Al2O3-H2 (~ 82%) at 1 h was significantly higher than that of Cu/Al2O3-air catalyst (~ 52%) at 1 h.
In Fig. 7b, it can be seen that the COD removal rate (~ 22%) was higher than 40 ℃ at 70 ℃ after 1 h reaction even without using catalyst. This may be due to self-decomposition of H2O2 at 70 ℃, generating reactive oxidant species. At 70 ℃, Al2O3 replaced the catalyst as comparison, and the catalytic test results for the pure support were presented in Supporting Information (Figure S1). The results show that there was no significant change in COD removal compared to the case without using catalyst after reaction. The COD removal rate was significantly improved and the color of the reaction solution changed rapidly after a certain catalyst was added. As expected, the catalytic performance of Cu/Al2O3-H2 was better than Cu/Al2O3-air in terms of COD removal rate. In principle, the COD removal may also be contributed from the adsorption of organic compounds by the catalyst during the catalytic reaction. Therefore, the adsorption experiment of Al2O3, Cu/Al2O3-air and Cu/Al2O3-H2 without hydrogen peroxide was performed to investigate the adsorption effect (in Figure S2). It was found that the adsorption effect of the catalysts was not significant in the reaction from Figure S2.
The Cu component in Cu/Al2O3-air were existed in form of high valent oxides, which was an inactive state for CWPO. After calcined by H2, high valent Cu oxides were converted into zero or low valent oxides (Cu(I)) (see "XRD" and "XPS" Section). For example, Cu elements in CuAlO2 and Cu were existed in the form of Cu(0) and Cu(I) according to the XRD results. These states were conducive to the redox reactions, and could have an excellent catalytic activity accordingly. H2O2 would react with Cu(0) and Cu(I) to generate ·OH, and react with Cu(I) and Cu(II) to generate ·OH (Huang et al. 2021b). Therefore, the catalytic performance of Cu/Al2O3-H2 was better than Cu/Al2O3-air under the same conditions. However, the COD removal rate did not reach to 100%, which was due to the formation of some refractory small organic molecules. These molecules existed at a low concentration that were difficult to degrade.
Reusability is important for a catalyst. The reusability performance of the two Cu catalysts was tested through five consecutive experiments. The results are shown in Figure S3. From the results shown in Figure S3, Cu/Al2O3-H2 showed an excellent catalytic performance after 4 cycles of recycling without a significant decrease in COD removal rate. Compared to Cu/Al2O3-air, the recyclability performance of Cu/Al2O3-H2 was better. One possible reason was that the interaction between the active metal and the carrier was stronger after calcination in H2, resulting in less Cu2+ dissolution amounts (vide infra).
Effects of reaction temperature and calcination atmosphere on metal dissolution
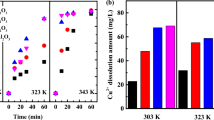
In order to investigate the factors affecting the Cu2+ dissolution amount in solution, the metal dissolution amounts (in terms of Cu2+ concentration) of catalysts in DI water, H2O2 aqueous solution, phenol aqueous solution, and the solution after catalytic oxidation were measured at different temperatures (Fig. 8). The dissolution amounts were under 5 mg/L in DI water, H2O2 aqueous solution and phenol aqueous solution. There are some differences in copper dissolution due to wear caused by stirring. In other words, DI water, H2O2 and phenol had a weak effect on promoting the Cu2+ dissolution in the Cu/Al2O3-air system.
Metal dissolution of catalysts in DI water, H2O2 aqueous solution, phenol aqueous solution and reaction solution at different temperature: (a) 40 ℃ Cu/Al2O3-air, (b) 40 ℃ Cu/Al2O3-H2, (c) 70 ℃ Cu/Al2O3-air and (d) 70 ℃ Cu/Al2O3-H2. Reaction conditions: concentration of phenol = 280 mg/L, catalysts dosage = 5 g/L, H2O2 dosage = 0.09 mol/L
An obvious ions dissolution was observed for the catalytic oxidation reaction system, partly because the process of oxidation and reduction of the Cu component was involved in the reaction solution. Interestingly, the Cu2+ amount decreased significantly as the reaction temperature increased from 40 ℃ to 70 ℃. There are two competitive reactions of H2O2 during the phenol oxidation (Eqs. (1) and (8)).
The oxidation rate of phenol was slow at 40 ℃ (Eq. (1)). In addition, H+ may be ionized from small molecular acids generated during the oxidation process (Guo et al. 2021). And excessive H2O2 remained in the solution, where Eq. (8) was promoted. While Eq. (1) was faster at 70 ℃, H2O2 was utilized effectively to convert into ·OH (discussed in the "Quenching experiments of reactive oxidant species" Section) and reacted with organic compounds. The competitive Eq. (8) decelerated due to fewer reactants. Correspondingly, the Cu2+ dissolution amount was decreased. Compared with Cu/Al2O3-air, Cu/Al2O3-H2 has a slightly reduced Cu2+ dissolution amount, and at the same time had a better catalytic performance (in the "Calcination atmosphere during catalyst preparation" Section). The Cu component existed as Cu(0) and Cu(I) in Cu/Al2O3-H2. It reflected that the interaction between metal and carrier in Cu/Al2O3-H2 was stronger, leading to a lower metal dissolution than Cu/Al2O3-air.
Based on the results mentioned above, DI water, H2O2 and phenol alone could not promote ion dissolution. Pretreatment with H2 could improve the catalytic performance and reduce the dissolution of metal ions slightly. Meanwhile, it was also found that the catalytic reaction rate was accelerated and metal dissolution was reduced at a higher temperature. This phenomenon is undoubtedly beneficial to reducing metal ion pollution and saving post-treatment costs.
Quenching experiments of reactive oxidant species
Alcohols are usually applied as hydroxyl radical scavengers, such as methanol, isopropanol and t-butanol (Schneider et al. 2020; Huang et al. 2021b; Yang et al. 2021; Manos et al. 2022). Quenching experiments were performed to confirm that the catalytic oxidation of phenol by H2O2 over copper based catalysts involved a free radical oxidation mechanism. Furthermore, the reaction mechanism in the "Proposed reaction mechanism" Section was proposed based on the free radical oxidation mechanism. The UV–Vis absorption spectrums during the CWPO reactions with alcohols are obtained in Fig. 9.
The UV–Vis absorption spectrum confirmed that the absorption peaks (at 270 nm) declined evidently after methanol, isopropanol or t-butanol was added (Fig. 9a-d). These indicated that the reaction was inhibited to some extent. Since hydroxyl radical partially reacted with scavengers, and their reaction with phenol was slower in rate.
The inhibitory effect of methanol and t-butanol was similar. The position of the 10 min curve (Fig. 9d) was similar to the position of the 30 min ~ 60 min curve (Fig. 9a and c). Isopropanol had the optimum inhibitory effect. The substances with higher electron density tend to be preferentially attacked by free radicals (Lin et al. 2023). The electronegativity of O2− in an organic compound follows the order of t-butanol > isopropanol > methanol. Unexpectedly, isopropanol had a better inhibitory effect eventually. This might be due to its smaller steric hindrance. The inhibitory effect of t-butanol was similar to that of methanol due to steric hindrance.
When the reaction was inhibited, the CWPO solution was observed to be brown. It was just similar to the case of the reaction occurred at 40 ℃ (see "Calcination atmosphere during catalyst preparation" Section). A new absorption peak appeared (~ 246 nm), indicating the formation of p-benzoquinone or its analogues during phenol degradation (Kuboyama et al. 1974). This peak decreased slowly as the reaction continued. In addition, isopropanol had the optimum inhibitory effect which made the transition process occurred more slowly. The results indicated that the production of hydroxyl radical was promoted by catalysts during the reaction. This process can be inhibited after adding radical scavengers.
Proposed reaction mechanism
In above sections, the effects of reaction temperature and calcination atmosphere in the catalyst preparation process on catalytic reaction rates and dissolution of the Cu component were studied in the "Influence factors for the catalytic performance in phenol oxidation by H2O2 in water" and "Effects of reaction temperature and calcination atmosphere on metal dissolution" Sections, and the free radical quenching experiments were performed in the "Quenching experiments of reactive oxidant species" Section. Some interesting clews can be seen in the catalytic reaction results. Especially, the Cu dissolution amount in the reaction was much more than the non-reaction system, and when the reaction temperature was higher, the dissolution amount was lower. To better explain the process of catalytic reaction of Cu-catalyzed phenol oxidation as well as the Cu2+ dissolution behavior, the catalytic mechanism of Cu/Al2O3-H2 was further proposed, discussed and tested. The proposed reaction mechanism is summarized in Fig. 10.
The reactants in the proposed catalytic reaction mechanism involves the Cu component, phenol, and H2O2, as shown in Fig. 10. At first, the possible reactions between the Cu component and H2O2 and their consequences were discussed. The reaction began as soon as H2O2 was added into the reaction system. H2O2 can be converted into ·OH and ·OOH, respectively catalyzed by Cu(0) and Cu(I) (Huang et al. 2021b; Covinich et al. 2016), and the Cu component on the catalyst completed valence conversion in this process. The ·OOH might contribute to the overall catalytic oxidation reactions which had not been confirmed, and was considered as product of the valence transition of the catalyst in this work. Therefore, in the proposed mechanism in Fig. 10, four possible reactions (Eqs. (9–12)) are included.
In the above reactions, Cu(0) and Cu(I) reacted with H2O2 to generate Cu(II). This process can occur in the solution or on the surface of the catalyst. However, when catalysts and H2O2 existed alone in the solution at 40 ℃ and 70 ℃ (see in Fig. 8), there was no significant Cu2+ dissolution. This phenomenon implicated that the Cu(II) species generated in this process existed on the surface of the catalyst rather than in the aqueous solution when the solution was non-acidic. However, situations may be different if the solution is acidic, that is, H2O2 could lead to a side reaction of Cu2+ dissolution under acidic conditions (Eq. 8), and this reaction was a competitive reaction of Eq. 1 (discussed in the "Effects of reaction temperature and calcination atmosphere on metal dissolution" Section).
Secondly, when the solution system only contained catalysts and phenol, there was rather lower Cu2+ dissolution amount (see in Fig. 8). This is due to the absence of H2O2, and thus no more Cu2+ can be generated through the reactions (Eqs. 9–10).
Thirdly, based on the above two points of discussion, the Cu2+ dissolution behavior is explainable when both of phenol and H2O2 existed in the CWPO reaction system. The ·OH reacted with phenol to generate CO2 and H2O (Eq. 13). The existences of ·OH species was supported by the quenching experiments in the "Quenching experiments of reactive oxidant species" Section, indicating the contribution of ·OH to the catalytic reaction.
However, the above process was not completed only in one step. The phenol was oxidized to converted into short-chain acids firstly (Eq. 14) (Guo et al. 2021; Taran et al. 2018). Then, short-chain acids were further oxidized to CO2 and H2O eventually (Eq. 15).
When the reaction temperature was higher, the COD removal was higher (Figs. 5 and 7) and the Cu2+ dissolution was lower (Fig. 8). This trend was explainable with the aid of Eqs. (13–15) as follows. When phenol in water was more completely mineralized (Eq. 13), the contribution of Eq. 14 and Eq. 15 was less. Thus, the concentration of the short-chain acids including their electroionized forms was less. Since the short-chain acids were important species for maintaining the system acidity during the CWPO reaction, and the acidic conditions were the main reason for the Cu2+ dissolution, therefore, the Cu2+ dissolution amount was lower when phenol was more completed degraded.
In addition, part of H2O2 may not completely converted into ·OH, accompanied with self-decomposition (Eq. 16). This process can be accelerated at high temperatures. The reactive oxidant species, like ·OH and ·OOH, may not completely interact with organic matters, and may also be consumed by themselves (Eq. 17).
To better support the reaction mechanism proposed above as well as in Fig. 10, the TOC removal rates, Cu2+ dissolution and pH values during the reaction are collected in Table 2. Due to the dissociation of H+ by water and the weak acidity of the phenol solution (pH = 6.68), a small amount of Cu2+ was dissolved in the solution. After the catalyst reached adsorption saturation, the solution become alkaline slightly. As soon as the reaction began, short-chain acids were formed from phenol, which can produce H+ by ionization, and then oxidized to CO2 and H2O. During this process, the pH value decreased firstly and then increased slightly. Due to the dissolution of CO2 and refractory short-chain acids solution with a low concentration, the final pH value of the solution was remained weakly acidic. During the reaction process, the participation of Cu component in the redox reaction and the acidic solution due to the dissociation of H+, leading to large metal dissolution amount.
Conclusion
In this work, the Cu/Al2O3-air and Cu/Al2O3-H2 catalysts were prepared for the CWPO reaction of phenol. The catalytic performance and metal dissolution behavior of these catalysts were investigated. The primary findings are summarized as follows.
-
(i)
The Cu/Al2O3-H2 showed an excellent catalytic performance for phenol degradation under optimized reaction conditions. The COD and TOC removal rates reached to 89% and 96%, respectively. At the same time, Cu/Al2O3-H2 maintained a lower Cu2+ dissolution amount during reaction compared to the Cu/Al2O3-air case.
-
(ii)
More interestingly, the faster the reaction rate was, the less Cu2+ dissolution was observed. In particular, the catalytic reaction rate at 70 ℃ was higher than 40 ℃, and at the same time the Cu2+ leaching amount was lower.
-
(iii)
Combined with the quenching experiments, a catalytic reaction mechanism was proposed mainly for explanation of the relationship between CWPO reaction rate and dissolution amount of Cu2+. The proposed mechanism is consistent with experimental findings.
Data availability
The raw data presented in this study are available from the first author if request.
References
Arena F, Giovenco R, Torre T, Venuto A, Parmaliana A (2003) Activity and resistance to leaching of Cu-based catalysts in the wet oxidation of phenol. Appl Catal B 45(1):51–62. https://doi.org/10.1016/s0926-3373(03)00163-2
Baloyi J, Ntho T, Moma J (2018) Synthesis of highly active and stable Al/Zr pillared clay as catalyst for catalytic wet oxidation of phenol. J Porous Mater 26(2):583–597. https://doi.org/10.1007/s10934-018-0667-3
Brussino P, Gross MS, Banús ED, Ulla MA (2019) CuO/TiO2-ZrO2 wire-mesh catalysts for phenol wet oxidation: Substrate effect on the copper leaching. Chem Eng Process 146:107686. https://doi.org/10.1016/j.cep.2019.107686
Cui KP, He YY, Xu KJ, Zhang Y, Chen CB, Xu ZJ, Chen X (2022) Degradation of tetracycline hydrochloride by Cu-Doped MIL-101(Fe) loaded diatomite heterogeneous fenton catalyst. Nanomaterials (Basel) 12(5). https://doi.org/10.3390/nano12050811
Covinich LG, Massa P, Fenoglio RJ, Area MC (2016) Oxidation of hazardous compounds by heterogeneous catalysis based on Cu/Al2O3 system in Fenton-type reactions. Crit Rev Env Sci Tech 46(23–24):1745–1781. https://doi.org/10.1080/10643389.2016.1267449
Devlin HR, Harris IJ (1984) Mechanism of the oxidation of aqueous phenol with dissolved oxygen. Ind Eng Chem Fundam 23:5. https://doi.org/10.1021/i100016a002
Fu P, Wang L, Li G, Hou Z, Ma Y (2020) Homogenous catalytic ozonation of aniline aerofloat collector by coexisted transition metallic ions in flotation wastewaters. J Environ Chem Eng 8(3). https://doi.org/10.1016/j.jece.2020.103714
Fu T, Gong X, Guo J, Yang Z, Liu Y (2021) Zn-CNTs-Cu catalytic in-situ generation of H2O2 for efficient catalytic wet peroxide oxidation of high-concentration 4-chlorophenol. J Hazard Mater 401:123392. https://doi.org/10.1016/j.jhazmat.2020.123392
Geng L, Chen B, Yang J, Shui C, Ye S, Fu J, Chen B (2020) Synergistic effect between Mn and Ce for active and stable catalytic wet air oxidation of phenol over MnCeOx. Appl Catal A: Gen 604. https://doi.org/10.1016/j.apcata.2020.117774
Guo PC, Qiu HB, Yang CW, Zhang X, Shao XY, Lai YL, Sheng GP (2021) Highly efficient removal and detoxification of phenolic compounds using persulfate activated by MnOx@OMC: Synergistic mechanism and kinetic analysis. J Hazard Mater 402:123846. https://doi.org/10.1016/j.jhazmat.2020.123846
Huang C, Wen J, Sun Y, Zhang M, Bao Y, Zhang Y, Liang L, Fu M, Wu J, Ye D, Chen L (2019) CO2 hydrogenation to methanol over Cu/ZnO plate model catalyst: Effects of reducing gas induced Cu nanoparticle morphology. Chem Eng J 374:221–230. https://doi.org/10.1016/j.cej.2019.05.123
Huang H, Zhang H, Yan Y (2021a) Preparation of novel catalyst-free Fe3C nanocrystals encapsulated NCNT structured catalyst for continuous catalytic wet peroxide oxidation of phenol. J Hazard Mater 407:124371. https://doi.org/10.1016/j.jhazmat.2020.124371
Huang Z, Shen M, Liu J, Ye J, Asefa T (2021b) Facile synthesis of an effective g-C3N4-based catalyst for advanced oxidation processes and degradation of organic compounds. J Mater Chem A 9(26):14841–14850. https://doi.org/10.1039/d1ta01325d
Jiang S, Zhao Z, Cui K, Tang Y, Du X, He B, Xiong W (2022) Catalytic wet peroxide oxidation of phenolic wastewater on novel Cu/Mn-UiO-66@Al2O3 ceramic tube membrane catalysts. Chem Eng J 430:132787. https://doi.org/10.1016/j.cej.2021.132787
Jiao Y, Wan C, Bao W, Gao H, Liang D, Li J (2018) Facile hydrothermal synthesis of Fe(3)O(4)@cellulose aerogel nanocomposite and its application in Fenton-like degradation of Rhodamine B. Carbohydr Polym 189:371–378. https://doi.org/10.1016/j.carbpol.2018.02.028
Kuboyama A, Matsuzaki S, Takagi H, Arano H (1974) Studies of the π-π* Absorption bands of p-Quinones and o-Benzoquinone. B Chem Soc JPN 47(7):3. https://doi.org/10.1246/bcsj.47.1604
Kumar A, Prasad B, Garg KK (2020) Efficiently degradation of acrylonitrile from aqueous solution by La0.5Ce0.5MO3 (M = Fe, Cu and Co) perovskite-like catalyst: optimization and reaction pathways. J Water Process Eng 36. https://doi.org/10.1016/j.jwpe.2020.101314
Larson PE (1974) X-ray induced photoelectron and Auger spectra of Cu, CuO, Cu2O and Cu2S thin films. J Electron Spectrosc 4:213–218
Li J, Pham AN, Dai R, Wang Z, Waite TD (2020) Recent advances in Cu-Fenton systems for the treatment of industrial wastewaters: role of Cu complexes and Cu composites. J Hazard Mater 392:122261. https://doi.org/10.1016/j.jhazmat.2020.122261
Li M, Li W, Bolton JR, Blatchley ER 3rd, Qiang Z (2019) Organic pollutant degradation in water by the vacuum-ultraviolet/ultraviolet/H2O2 process: inhibition and enhancement roles of H2O2. Environ Sci Technol 53(2):912–918. https://doi.org/10.1021/acs.est.8b05831
Liang H, Xiao K, Wei L, Yang B, Yu G, Deng S, Zhang J (2019) Decomplexation removal of Ni(II)-citrate complexes through heterogeneous Fenton-like process using novel CuO-CeO2-CoOx composite nanocatalyst. J Hazard Mater 374:167–176. https://doi.org/10.1016/j.jhazmat.2019.04.031
Lin Y, Chen J, Li H, Chen L, Yuan B, Shi C, Xie Y (2023) Synergistic and efficient degradation of acid red 73 by UV/O3/PDS: Kinetic studies, free radical contributions and degradation pathways. Environ Res 216:114449. https://doi.org/10.1016/j.envres.2022.114449
Liu X, Wang X, Yang W, Yuan F, Wang B, Peng Q (2022) Impregnating biochar with Fe and Cu by bioleaching for fabricating catalyst to activate H2O2. Appl Microbiol Biotechnol 106(5–6):2249–2262. https://doi.org/10.1007/s00253-022-11853-x
Luo X, Hu H, Pan Z, Pei F, Qian H, Miao K, ... Feng G (2020) Efficient and stable catalysis of hollow Cu9S5 nanospheres in the Fenton-like degradation of organic dyes. J Hazard Mater 396:122735. https://doi.org/10.1016/j.jhazmat.2020.122735
Manos D, Papadopoulou F, Margellou A, Petrakis D, Konstantinou I (2022) Heterogeneous activation of persulfate by LaMO3 (M=Co, Fe, Cu, Mn, Ni) perovskite catalysts for the degradation of organic compounds. Catalysts 12(2):187. https://doi.org/10.3390/catal12020187
Santos A, Yustos P, Quintanilla A, Ruiz G, Garcia-Ochoa F (2005) Study of the copper leaching in the wet oxidation of phenol with CuO-based catalysts: causes and effects. Appl Catal B 61(3–4):323–333. https://doi.org/10.1016/j.apcatb.2005.06.006
Schneider JT, Firak DS, Ribeiro RR, Peralta-Zamora P (2020) Use of scavenger agents in heterogeneous photocatalysis: truths, half-truths, and misinterpretations. Phys Chem Chem Phys 22(27):15723–15733. https://doi.org/10.1039/d0cp02411b
Shen Z, Yan J, Wang M, Xing L, Huang B, Zhou H, Li W, Chen L, Shi J (2023) Cu/Cu+ synergetic effect in Cu2O/Cu/CF electrocatalysts for efficient nitrate reduction to ammonia. ACS Sustain Chem Eng 11:9433–9441. https://doi.org/10.1021/acssuschemeng.3c01514
Taran OP, Zagoruiko AN, Yashnik SA, Ayusheev AB, Pestunov AV, Prosvirin IP, ... Parmon VN (2018) Wet peroxide oxidation of phenol over carbon/zeolite catalysts. Kinetics and diffusion study in batch and flow reactors. J Environ Chem Eng 6(2):2551–2560. https://doi.org/10.1016/j.jece.2018.03.017
Wang J, Tang J (2021) Fe-based Fenton-like catalysts for water treatment: Preparation, characterization and modification. Chemosphere 276:130177. https://doi.org/10.1016/j.chemosphere.2021.130177
Xia M, Long M, Yang Y, Chen C, Cai W, Zhou B (2011) A highly active bimetallic oxides catalyst supported on Al-containing MCM-41 for Fenton oxidation of phenol solution. Appl Catal B 110:118–125. https://doi.org/10.1016/j.apcatb.2011.08.033
Xin S, Liu G, Ma X, Gong J, Ma B, Yan Q, Xin Y (2021) High efficiency heterogeneous Fenton-like catalyst biochar modified CuFeO2 for the degradation of tetracycline: Economical synthesis, catalytic performance and mechanism. Appl Catal B 280:119386. https://doi.org/10.1016/j.apcatb.2020.119386
Yang L, Ren X, Zhang Y, Chen Z, Wan J (2021) One-step synthesis of a heterogeneous catalyst: Cu+-decorated triazine-based g-C3N4 nanosheet formation and catalytic mechanism. J Environ Chem Eng 9(4):105558. https://doi.org/10.1016/j.jece.2021.105558
Zapico RR, Marín P, Díez FV, Ordóñez S (2017) Assessment of phenol wet oxidation on CuO/γ-Al2O3 catalysts: Competition between heterogeneous and leached-copper homogeneous reaction paths. J Environ Chem Eng 5(3):2570–2578. https://doi.org/10.1016/j.jece.2017.04.050
Funding
Support from the National Natural Science Foundation of China (21576291), the Fundamental Research Funds for the Central Universities (23CX03007A), and the Natural Science Foundation of Rizhao City (RZ2021ZR40) is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
Wumin Zhang: catalyst preparation, characterization, reaction test, writing. Huimin Zhuang: catalytic test, reaction setup construction, discussion. Yu Guo: catalyst preparation, characterization, paper revision. Hua Chi: catalyst preparation, characterization, reaction test. Qiuyue Ding: reaction setup construction. Lu Wang: catalyst preparation, reaction test. Yanyan Xi: catalyst characterization, discussion. Xufeng Lin: idea development, research funding provision, writing and organization.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent to publish
Not applicable.
Competing interests
There are no conflicts to declare.
Additional information
Responsible Editor: Ricardo A. Torres-Palma
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, W., Zhuang, H., Guo, Y. et al. Wet peroxide oxidation process catalyzed by Cu/Al2O3: phenol degradation and Cu2+ dissolution behavior. Environ Sci Pollut Res 31, 26916–26927 (2024). https://doi.org/10.1007/s11356-024-32781-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-32781-5