Abstract
The alumina particle supported Cu component catalyst, Cu/Al2O3, was prepared by equal volume wet impregnation and evaluated through degradation of maleic acid with catalytic wet peroxide oxidation (CWPO) in this work. It was found that the Cu/Al2O3 catalyst had an excellent CWPO performance. A total organic carbon (TOC) removal rate of 98% was reached under the optimized catalytic reaction conditions. Moreover, the effect of inorganic ions on CWPO was comprehensively evaluated using different acid and alkali. The alkali had an optimum effect on TOC removal rates, while acid had an inhibitory effect. A catalytic oxidation mechanism was proposed to illustrate the CWPO process of Cu/Al2O3 catalyst. The catalytic test results as well as the proposed mechanism can provide an insight into the influence mechanism of inorganic ions on CWPO.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The efficient treatment of organic wastewater has developed to be an important task in environmental research. The main reason is the rapid development of modern industry, which has led to serious water shortage and pollution, affecting the survival and development for humankind 1 [1].
Several methods have been developed so far for organic pollutants treatment, such as advanced oxidation processes (AOPs). In AOPs, highly reactive oxidant species (such as ·OH) can be produced to degrade organic pollutants by decomposing oxidants (such as H2O2, O3 and O2). Wet peroxide oxidation (WPO) has drawn great attention due to its mild conditions and non-toxic, where H2O2 is the oxidant. The degradation rate can be accelerated by using catalysts in this process, known as catalytic wet peroxide oxidation (CWPO). Heterogeneous CWPO take the advantage of easier catalyst recycling and broader range of applications, compared to homogeneous CWPO. Nguyen et al. investigated Fe–Cu composite/H2O2 system for the degradation of hazardous organics [2]. Saleh and Taufik demonstrated Fe3O4/ZnO/graphene composites was prepared to catalytic oxidation dyes [3]. Supported metal catalysts, as a kind of heterogeneous catalyst, exhibit better catalytic performance due to its high-activity metals and carriers which provide a huge specific surface area. Wang et al. prepared an efficient Fenton system where α-Fe2O3/g-C3N4 was used as heterogeneous catalyst for pollutant degradation [4]. Lyu et al. reported mesoporous Cu/γ-Al2O3 was prepared by an evaporation-induced self-assembly process for the degradation and mineralization of aromatic pollutants [5]. Gosu et al. demonstrated the synthesized catalyst (copper-loaded activated alumina, Cu/AA) has phenomenal advantages in the simple separation and high removal efficiency in CWPO of catechol [6]. Hachemaoui et al. reported different metals (Cu, Cr, Fe and Zn) was loaded on mesoporous MCM-41 for CWPO of Acetaminophen [7].
Aromatic compounds are the most important pollutant in industrial wastewater. The aromatic rings will be cleaved by oxidants, resulting in the formation of short-chain carboxylic acids [8]. The residual short-chain carboxylic acids in the solution will leads to total organic carbon (TOC) value still remains unchanged after a long reaction time. These acids are often found in degradation progress of contaminants, while they are not attracted great attention due to its biodegradable and less toxic. Maleic acid (MA) is considered to be the main intermediate produced during the AOPs of aromatic compounds. Therefore, the degradation mechanism of MA may be helpful to explain the degradation process of aromatic compounds [9].
Inorganic ions have a significant impact on the performance of AOPs which is similar to other experimental factors, such as reaction temperature [10]. Choi et al. investigated the effects of anions on isopropylalcohol degradation using Fe/Al catalysts [11]. Liu et al. investigated the effect of Cl−, NO3−, and PO43− on sulfamethoxazole removal efficiency [12]. Kili et al. reported the presence of Cl−, SO42−and NO3− could directly interact with the organic substrate to improve its degradation rate, or led to a strong decrease in removal rate due to their lower oxidative compared to ·OH [13]. However, most of the current studies are investigated the effect of anions on the reaction rate [13, 14]. The effects of different inorganic cations existed in aqueous solutions on the degradation of organic pollutants also need to be investigated. Liu et al. investigated the effect of cations (Mg2+, Cu2+) on the oxidative degradation of Amoxicillin (AMX) catalyzed by nanoscale zero-valent iron (nZVI) [15]. The findings in the literatures reflect that the ions play a vital role in catalytic performance. In most cases, inorganic ions affect the reaction rates. In addition, the effect of inorganic ions on the catalytic activity should be considered. Therefore, it is necessary to investigate the influence of the different cations and anions on performance and composition of catalysts, as well as its consequence for the CWPO performance, accordingly.
In this paper, alumina particle supported Cu component catalyst, denoted as Cu/Al2O3, was prepared and used for CWPO of maleic acid in simulated maleic acid solutions. It was found that the Cu/Al2O3 catalyst with a Cu loading of 5 wt% performed excellently at optimum conditions and its Cu2+ dissolution was lower. It is interesting to find that the cations could lead to a higher TOC removal rates, while anions had an inhibitory effect. Based on the observed regulations, a catalytic reaction mechanism is proposed and the feasibility of this mechanism is discussed.
Materials and methods
Materials
Alumina (Al2O3, cylinder in shape) were purchased from Nantong Jinqi Chemical Co., Ltd. Copper nitrate trihydrate (Cu(NO3)2·3H2O, AR, 99–102%), hydrogen peroxide (H2O2, ≥ 30%), maleic acid (C4H4O4, CP, 99–101%), phosphoric acid (H3PO4, AR, ≥ 85%), nitric acid (HNO3, AR, 35–68%), hydrochloric acid (HCl, AR, 36–38%), potassium hydroxide (KOH, AR, ≥ 85%), ammonium chloride (NH4Cl, AR, ≥ 99.5%), ammonium solution (NH4OH, AR, (NH3) 25–28%) were obtained from Sinopharm Group Chemical Reagent Co., Ltd. Sodium hydroxide (NaOH, AR, ≥ 96%) were obtained from Xilong Scientific Co., Ltd. Sodium diethyldithiocarbamate trihydrate (C5H10NNaS2·3H2O, AR 99%) were obtained from Shanghai McLean Biochemical Technology Co., Ltd. Deionized (DI) water was used to prepare all of the solutions in this work.
Catalyst preparation
The alumina particle supported Cu component catalyst, Cu/Al2O3, was prepared by equal volume wet impregnation. First, commercial Al2O3 (long cylinder strips in shape) was crushing and sieving to particles of 20–40 mesh as support. The 1.89 g Cu(NO3)2·3H2O was dissolved in about 7.0 mL DI water as a precursor for impregnation. Subsequently, the Cu(II) aqueous solution was added to the 9.5 g Al2O3 particles drop by drop to ensure the solution evenly distributed. Then the obtain blue solid was remained overnight and dried at 393 K for 4 h. At last, calcined at 873 K for 3 h to remove nitrate and bound water. The 5 wt% Cu/Al2O3 (5 wt% = m Cu: m Cu/Al2O3) catalysts were prepared and marked as 5% Cu/Al2O3 catalysts. According to the above steps, 1%, 2% and 10% Cu/Al2O3 catalysts was prepared by changing the dissolved Cu(NO3)2·3H2O content.
Catalytic activity evaluation
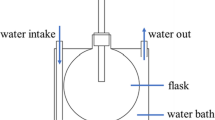
Maleic acid was used to evaluate the catalytic performance of Cu/Al2O3. The degradation reactions were performed in a 250 mL custom glass reactor with a water bath. The experimental steps were as follows. The 1 g Cu/Al2O3 catalyst was added to 200 mL maleic acid solution (500 mg/L). The mixture was stirred by magnetic stirrer the during all reactions. When the temperature was reached to the set value, the 30% H2O2 was added to the solution and the reaction was started. The aliquots were withdrawn at the regular time intervals as samples for more analysis. The used catalyst was recycled after washed by deionized water to evaluate the stability of the catalysts. The stability evaluation was the same as above typically proceeds. The influence of different ions on the reaction evaluation was similar to above typically proceeds. The difference was that 1 g Cu/Al2O3 catalyst was added to a 200 mL mixed solution of maleic acid (500 mg/L) and acid or alkali (concentration of phosphoric acid, nitric acid, hydrochloric acid, potassium hydroxide or sodium hydroxide was 0.0025 mol/L). The other steps were consistent with those typically proceeds described above.
Analytical methods for reaction solution
The TOC-L CPH (Shimadzu Production Institute, Kyoto, Japan) was used to analyze the total organic carbon (TOC) value. The TOC removal rate were calculated using Eq. 1:
Here TOCt were the TOC values at the reaction time of t min. And TOC0 were the initial value at the reaction time of 0 min.
The determination of Cu(II) concentration was achieved by forming a yellow complex between sodium diethyldithiocarbamate and copper ions under alkaline conditions. The Ultraviolet–visible (UV–Vis) absorption spectroscopy (Analytik Jena AG) was used to measure the absorbance at 453 nm. The Cu(II) concentration was calculated through the Eq. 2:
Here Abs was the absorbance of the colored solution, V was the volume of solution taken from the target sample solution, and 0.0016 was the slope of the standard curve for the relationship between Cu2+ and Abs. A series of Cu2+ solutions were prepared with different known concentration of Cu2+ (Cu mass basis) for the standard curve. The standard curve (with a zero intercept) was then obtained by plotting the absorbance (at 453 nm) of different Cu2+ solutions over their Cu2+ mass in a fix total volume of solution.
Catalyst characterization
X-ray diffraction (XRD) patterns of the catalysts were obtained with a Malvern Panalytical Empyrean diffractometer and using nickel-filtered Cu Kα radiation. The patterns were recorded over 5° < 2θ < 75° using a scanning rate of 0.02°/s.
Results and discussion
Optimization of catalytic reaction conditions
To optimize the maleic acid degradation reaction conditions, the reactions with different supported amount of Cu component, reaction temperatures and H2O2 concentrations were performed.
To optimize the maleic acid degradation reaction conditions, the reactions were performed with different Cu component supported amounts, reaction temperatures and H2O2 initial concentrations. The reaction temperature was from 303 to 343 K. When the temperature was higher than 343 K, the H2O2 will efficiently decomposed to O2 and H2O [16,17,18]. The H2O2 dosage was 0.09 mol/L tentatively in this section. Stoichometrically, the maleic acid (500 mg/L) was sufficient to be oxidized completely by H2O2 (0.09 mol/L).
In Fig. 1a, the TOC removal rate of maleic acid increased with the increase of Cu component supported amounts and reaction temperature. At a lower temperature (Fig. 1a), the TOC removal rate changed slowly with different Cu component supported amounts. When the temperature was higher than 323 K, the TOC removal rate increased significantly with the increased Cu component supported amounts. At 343 K, the TOC removal rate of 5% Cu/Al2O3 catalyst reached to 96% at 30 min. It shows that the reaction temperature was more effective in improving the catalytic efficiency than the Cu component supported amounts of catalysts. Similar conclusion had also been confirmed by Qin et al. [19].
Effect of the amount of Cu component on the Al2O3 supported catalyst and reaction temperature on a TOC removal rate for the maleic acid degradation and b Cu2+ dissolution amount in aqueous solution. Reaction conditions: concentration of maleic acid = 500 mg/L, catalyst dosage = 5 g/L, H2O2 dosage = 0.09 mol/L
It is worth noting that the 5% Cu/Al2O3 catalyst with less supported Cu components also had an excellent catalytic performance which was similar to 10% Cu/Al2O3 catalyst. It indicated the formation of extra-framework copper species in the catalyst [20]. Correspondingly, these extra-framework copper species will finally prevent the reaction between catalytic active sites and reactants, such as H2O2 and maleic acid.
Accordingly, the Cu2+ dissolution amount of the solution after catalytic oxidation reaction was measured. In Fig. 1b, significant Cu2+ dissolution amount was observed in the catalytic oxidation reaction system. The reason was the involvement of the oxidation and reduction processes of Cu components during the reaction process. Interestingly, the Cu2+ dissolution amount decreased significantly as the reaction temperature increased from 303 to 343 K. The possible reasons are as follows.
The oxidation rate of maleic acid was slow at 303 K (Eq. 4). In addition, H+ may be ionized from small molecular acids which generated during the oxidation process [16]. And excessive H2O2 remained in the solution, where Eq. 5 was promoted. At 343 K, H2O2 was effectively converted into ·OH catalyzed by Cu/Al2O3 (Eq. 3) and reacted with organic compounds (Eq. 4). The competitive Eq. 5 was decelerated due to fewer reactants. Subsequently, the Cu2+ dissolution amount was decreased. The 5% Cu/Al2O3 catalyst had an excellent catalytic performance similar to 10% Cu/Al2O3 catalyst. The former one also has a lower Cu component supported amounts and Cu2+ dissolution amounts during the reaction process.
In conclusion, the TOC removal rate was proved to be strongly dependent on reaction temperature. The 5% Cu/Al2O3 catalyst had an excellent catalytic performance with a lower Cu component supported amounts and Cu2+ dissolution amounts. The TOC removal rate increased and Cu component supported amounts reduced as the reaction temperature increased. Therefore, the Cu component supported amounts and reaction temperature was confirmed as 5% and 343 K.
In order to determine the optimum concentration of H2O2 required in the reaction, the reaction with different initial H2O2 concentrations were carried out. The result was shown in Fig. 2a. The TOC removal rate increased as the concentration of H2O2 increased. The TOC removal rate of the reactions with 0.09, 0.13 and 0.18 mol/L H2O2 was much higher than that of 0.04 mol/L H2O2. The TOC removal rate of 0.09 mol/L and 0.13 mol/L H2O2 was significantly different at beginning of the reaction (0–20 min). After 20 min, the TOC removal rate was similar. The results show that the amount of ·OH produced by 0.09 mol/L H2O2 was sufficient to degrade 500 mg/L of maleic acid.
When concentration of H2O2 was higher than 0.13 mol/L, the TOC removal rate remains unchanged due to the well-known ·OH scavenging effect (Eqs. 6–8) [11, 21].
These reactions are not conducive to the reaction of organic molecules with ·OH, resulting in a proximate TOC removal rate. Although other free radicals (e.g., ·OOH) are generated, their oxidation potential was much lower than that of ·OH, resulting in the reaction was inhibited [11].
According to the results of Cu2+ dissolution amount (Fig. 2b), 0.04 mol/L H2O2 had the lowest TOC removal rate and highest Cu2+ dissolution amount, which was different from 0.09, 0.13 and 0.18 mol/L H2O2. The 0.09 mol/L H2O2 had the lowest Cu2+dissolution amount. The addition of excess H2O2 had little effect on increasing in TOC removal rate. And the Cu2+dissolution amount cannot be significantly reduced. Therefore, 0.09 mol/L H2O2 was adopted in the subsequent experiments.
The influence of different ions on the reaction
Different acid and alkali (phosphoric acid, nitric acid, hydrochloric acid, potassium hydroxide, sodium hydroxide) were added to investigate the influence of various ions (PO43−, NO3−, Cl−, K+, Na+) on the degradation of maleic acid and the results are shown in Fig. 3. The reaction was promoted in the presence of alkali whereas inhibited in the presence of acid. The reaction was the fastest after added potassium hydroxide. The TOC removal rate of the reaction at 2 min was 3.9 times higher than that of phosphoric acid, which having the lowest TOC removal rate. The similar results were also observed by Choi et al. [11]. The inhibitory effect of phosphoric acid was most obvious on the reaction, followed by nitric acid and hydrochloric acid.
The effects of various anions on the a TOC removal rate and b Cu2+ dissolution amounts in the catalytic wet peroxide oxidation of maleic acid. Reaction conditions: concentration of maleic acid = 500 mg/L, catalyst (5% Cu/Al2O3) dosage = 5 g/L, H2O2 dosage = 0.09 mol/L, reaction temperature = 343 K, and concentration of phosphoric acid, nitric acid, hydrochloric acid, potassium hydroxide, sodium hydroxide = 0.0025 mol/L
Inorganic ions have different impact on the catalytic performance in degrading maleic acid [10]. The reaction with alkali might be promoted via the following two ways. At first, the maleic acid aqueous solution was acidic. The dissolution of Cu components will be promoted in acidic solution at the presence of H2O2 (See Sect. 3.1). The solution could be neutralized by the alkali, making the Cu components supported on the catalyst more stable. Because copper catalysts exhibit poor catalytic performance under acidic conditions. Secondly, cation could participate the catalytic reaction to promote the generation of ·OH in the reaction. Ni et al. reported that adding alkaline earth metals to catalyst could improve their catalytic oxidation performance [22].
The reaction with acid might be inhibited via the following two ways. First, these anions of acid might be oxidized in the presence of ·OH. For example, The Cl− could react with ·OH to produce ClOH·− (Eq. 9). The ClOH·− can be further transformed to Cl· under acid conditions (Eq. 10) [23]. The reduction potential of Cl· (2.4 V) was lower than ·OH (2.8 V). The ·OH can react with PO43− to produce PO4·2− (Eq. 11), The ·OH can react with NO3− to produce NO3· (Eq. 12). These radicals, such as Cl· and PO4·2−, have lower reaction rate with organic compounds. Thus, the reaction with acids usually presented inhibition phenomenon compared to ·OH.
Second, the catalyst performance was affected by acid. The cycle of Cu(I)/Cu(II) was the key for the catalytic stability. During the process, Cu(II) can form the complexation with anions, such as phosphate ions. Thus, the amount of Cu(II) in the solution was reduced, which affected the cycle of Cu(I)/Cu(II), further affecting the catalytic performance. Compared to phosphoric acid, the removal rate of reaction with nitric acid was higher. The phosphorus with lower electronegativity in phosphoric acid is more prefer to form complexes compared to nitrogen in nitric acid. Thus, it is more difficult to form copper sulfate complexes than that of copper nitrate complexes, which can lead to a lower TOC removal rate [24]. It is worth noting that the addition of phosphoric acid did not cause a large amount of Cu2+ dissolution, which was not similar to hydrochloric acid and nitric acid. This phenomenon indicated that phosphoric acid mainly reduced the reaction rate through the first inhibitory effect type. During the reaction process, the PO43− tended to react with ·OH to form PO4·2−, rather than formed complexes with Cu2+ to reduce the reaction rate. In addition, the anions ionized by short chain acids, such as maleic acid and its degradation intermediates, may also have a similar complex effect on copper ions. In conclusion, the reaction will be promoted in the presence of alkali whereas inhibited in the presence of acid.
The reusability performance of catalysts
Stability and reusability of catalysts are important evaluation indicators for industrial applications. Stability testing was performed through six cycles of maleic acid degradation with the same catalyst under the same conditions. The results are shown in Fig. 4. The TOC removal rates of the reaction catalyzed by 5% Cu/Al2O3 reached to 90% until 4th cycle, and less than 80% at 5th cycle. The Cu2+ dissolution amounts in six cycles were lower than 30 mg/L (Fig. 4b). The catalyst also exhibited good deposition properties and could be completely deposited within a short time after stopping the agitation. Therefore, this catalyst has good practical application prospects [25].
The XRD pattern of fresh and used catalysts are shown in Fig. 5. The diffraction peaks of Al2O3 at 2θ of 33.3, 35.7 and 66.8° corresponded well to (107), (114) and (1114) lattice planes of aluminum oxide-Al2O3 (PDF-#51-0769). The diffraction peaks of Al2O3 were weakened after the Cu component was loaded, while position of Al2O3 diffraction peaks were not changed. The Al2O3 peaks of used Cu/Al2O3 catalyst remained unchanged. The diffraction peaks of Cu/Al2O3 catalyst at 2θ of 35.4, 35.5 and 38.7°, corresponded respectively to (002), (11-1) and (111) lattice planes of tenorite-CuO (PDF-#48-1548) [26, 27]. In addition, diffraction peaks of CuO and CuAlO2 (PDF-#40-1037) existed in Cu/Al2O3 catalyst samples. And the peaks of CuO were disappeared in the pattern of 1st and 6th used catalysts samples. This difference indicated that the interaction of Cu and Al in CuAlO2 was stronger than Cu and O in CuO. The results indicated that the catalyst structure was changed slightly during the reaction process. The crystal structure of the carrier remained unchanged, and the peaks of CuO was disappeared, which may be due to the loss during the reaction process and the possible transformation into other components such as CuAlO2.
Proposed reaction mechanism
In above sections, the effects of reaction temperature, Cu component supported amounts and H2O2 initial concentration on catalytic reaction rates and dissolution of the Cu component were studied (section “Optimization of catalytic reaction conditions”), and the experiments with different ions were performed (section “The influence of different ions on the reaction”). Some interesting clews have been concluded in the catalytic reaction results. Especially, the reaction was promoted in the presence of alkali whereas the reaction was inhibited in the presence of acid. To better explain the process of maleic acid degradation catalyzed by Cu/Al2O3 at present of different ions, the catalytic reaction mechanism was proposed, discussed and tested. The proposed reaction mechanism is summarized as follows.
The Cu component, maleic acid, H2O2 and different ions are involved in the proposed catalytic reaction mechanism. At first, the possible reactions between the cycle of Cu(II)/Cu(I) and H2O2 were discussed. H2O2, as an oxidant, can be converted into ·OOH and ·OH, respectively catalyzed by Cu(II) and Cu(I) [28, 29], and the valence conversion of Cu component (Cu(II)/Cu(I)) was completed in this process. Therefore, two possible reactions (Eqs. 13, 14) are included in the proposed mechanism.
Then the ·OH reacted with maleic acid to generate CO2 and H2O (Eq. 4). However, the Eq. 4 was not completed only in one step. The maleic acid was converted into short-chain acids firstly (Eq. 15) [30]. Then, the short-chain acids were further oxidized to CO2 and H2O. The H+ ionized from short-chain acids that have not been degraded will contribute to form acidic solutions (Eq. 16).
The Cu2+ dissolution behavior is illustrated in the maleic acid CWPO reaction system. When the reaction temperature and concentration of H2O2 was higher, the TOC removal was higher. At the same time, the Cu2+ dissolution was lower (Fig. 1). This trend was explainable with the aid of Eqs. 4, 15, and 16 as follows. When maleic acid in the reaction solution was fully mineralized Eq. 4, the contribution of Eqs. 15–16 was relatively less. Thus, the concentration of the short-chain acids was less, and their electroionized forms was also less. The maleic acid and its degradation intermediates are important species in maintaining the system acidity, and the acidic conditions are the main reason for the large Cu2+ dissolution amount. Therefore, when organics was more completed degraded, the Cu2+ dissolution amount was lower.
Notably, the reaction rate will be changed when alkali and acids are added to the system. The reaction will be promoted in the presence of alkali whereas inhibited in the presence of acid (section “The influence of different ions on the reaction”). The reaction with alkali can be promoted in two ways. At first, the maleic acid solution could be neutralized by the alkali. The leaching amount of copper will be reduced compared to the original system, which making the Cu components supported on the catalyst more stable. Secondly, the cation may participate the catalytic reaction to promote the generation of ·OH in the reaction. The reaction with acid can be inhibited in two ways. First, these anions of acid might be oxidized in the presence of ·OH. These obtained radicals, such as Cl· and PO4·2−, have lower reaction rate with organic compounds than ·OH. Second, during the process, Cu(II) can form the complexation with anions, such as phosphate ions. Thus, the amount of Cu(II) in the solution will be reduced, which affected the cycle of Cu(I)/Cu(II), further affecting the catalytic performance.
In addition, H2O2 may not be fully converted into ·OH and accompanied with self-decomposition (Eq. 17). This process will be accelerated at higher temperature. The reactive oxidant species may be consumed by themselves (Eqs. 6–8), and the organic matters may not be oxidized.
To better support the reaction mechanism proposed above, the TOC removal rates, Cu2+ dissolution and pH values during the reaction are collected in Table 1.
Due to the dissociation of H+ by water and the acidity of the maleic acid solution (pH 2.5), a small amount of Cu2+ was dissolved in the solution. As soon as the H2O2 added, the reaction began. The short-chain acids were formed from maleic acid, which can be ionized to H+, and then oxidized to CO2 and H2O. The pH value of the solution increased slightly during this process. The final pH value of the solution was remained weakly acidic because of the dissolution of refractory short-chain acids and CO2. The Cu component participated in the redox reaction and complete valence state transitions during the reaction process. Simultaneously, the acidic solution formed from dissociation of acids, leading to large metal dissolution amount.
Conclusion
In this work, different Cu/Al2O3 catalysts were prepared for the CWPO reaction of maleic acid. The catalytic reaction conditions were optimized and the effect of different inorganic ions on catalytic performance were investigated. The primary findings are summarized as follows.
-
(i)
The 5% Cu/Al2O3 catalyst showed an excellent catalytic performance for maleic acid degradation under optimized reaction conditions. The TOC removal rates reached to 98%. At the same time, 5% Cu/Al2O3 catalyst maintained a lower Cu2+ dissolution amount during reaction.
-
(ii)
The effect of reaction temperature on the TOC removal rate was optimum, followed by Cu component supported amounts and H2O2 concentrations. Acid lead to a higher TOC removal rates, while alkali had an inhibitory effect on the catalytic degradation reaction.
-
(iii)
A catalytic reaction mechanism was proposed mainly for explanation of the effect of different inorganic ions on catalytic performance. The proposed mechanism is consistent with experimental findings.
Data availability
The raw data presented in this study are available from the first author if request.
References
Yang Y, Liu M, You X, Li Y, Lin H, Chen JP (2024) A novel bimetallic Fe-Cu-CNT catalyst for effective catalytic wet peroxide oxidation: reaction optimization and mechanism investigation. Chem Eng J 479:147320. https://doi.org/10.1016/j.cej.2023.147320
Nguyen TB, Dong C-D, Huang CP, Chen C-W, Hsieh S-L, Hsieh S (2020) Fe-Cu bimetallic catalyst for the degradation of hazardous organic chemicals exemplified by methylene blue in Fenton-like reaction. J Environ Chem Eng 8:104139. https://doi.org/10.1016/j.jece.2020.104139
Saleh R, Taufik A (2019) Degradation of methylene blue and congo-red dyes using Fenton, photo-Fenton, sono-Fenton, and sonophoto-Fenton methods in the presence of iron(II,III) oxide/zinc oxide/graphene (Fe3O4/ZnO/graphene) composites. Sep Purif Technol 210:563–573. https://doi.org/10.1016/j.seppur.2018.08.030
Wang Y, Song H, Chen J, Chai S, Shi L, Chen C, Wang Y, He C (2020) A novel solar photo-Fenton system with self-synthesizing H2O2: enhanced photo-induced catalytic performances and mechanism insights. Appl Surf Sci 512:145650. https://doi.org/10.1016/j.apsusc.2020.145650
Lyu L, Zhang L, Wang Q, Nie Y, Hu C (2015) Enhanced Fenton catalytic efficiency of gamma-Cu-Al2O3 by sigma-Cu2+-ligand complexes from aromatic pollutant degradation. Environ Sci Technol 49:8639–8647. https://doi.org/10.1021/acs.est.5b00445
Gosu V, Dhakar A, Zhang TC, Surampalli RY, Subbaramaiah V (2023) Using innovative copper-loaded activated alumina (Cu/AA) as the catalyst for catalytic wet peroxidation (CWPO) of catechol. Environ Sci Pollut Res 30:40576–40587. https://doi.org/10.1007/s11356-022-24930-5
Hachemaoui M, Molina CB, Belver C, Bedia J, Mokhtar A, Hamacha R, Boukoussa B (2021) Metal-loaded mesoporous MCM-41 for the catalytic wet peroxide oxidation (CWPO) of acetaminophen. Catalysts 11:219. https://doi.org/10.3390/catal11020219
Kaale D (2013) Performance of activated carbons in the catalytic wet peroxide oxidation (CWPO) of maleic acid. J Eng Technol 5:189–199. https://doi.org/10.5897/JETR09.061
Huang Y, Sheng B, Wang Z, Liu Q, Yuan R, Xiao D, Liu J (2018) Deciphering the degradation/chlorination mechanisms of maleic acid in the Fe(II)/peroxymonosulfate process: an often overlooked effect of chloride. Water Res 145:453–463. https://doi.org/10.1016/j.watres.2018.08.055
Wang J, Wang S (2021) Effect of inorganic anions on the performance of advanced oxidation processes for degradation of organic contaminants. Chem Eng J 411:128392. https://doi.org/10.1016/j.cej.2020.128392
Choi J, Jeong J-H, Chung J (2013) Degradation of acetone and isopropylalcohol in electronic wastewater using Fe- and Al-immobilized catalysts. Chem Eng J 218:260–266. https://doi.org/10.1016/j.cej.2012.11.004
Liu Y, Guo J, Chen Y, Tan N, Wang J (2020) High-efficient generation of H2O2 by aluminum-graphite composite through selective oxygen reduction for degradation of organic contaminants. Environ Sci Technol 54:14085–14095. https://doi.org/10.1021/acs.est.0c05974
Kilic MY, Abdelraheem WH, He X, Kestioglu K, Dionysiou DD (2019) Photochemical treatment of tyrosol, a model phenolic compound present in olive mill wastewater, by hydroxyl and sulfate radical-based advanced oxidation processes (AOPs). J Hazard Mater 367:734–742. https://doi.org/10.1016/j.jhazmat.2018.06.062
Wang S, Wang J (2018) Radiation-induced degradation of sulfamethoxazole in the presence of various inorganic anions. Chem Eng J 351:688–696. https://doi.org/10.1016/j.cej.2018.06.137
Liu Y, Zha S, Rajarathnam D, Chen Z (2017) Divalent cations impacting on Fenton-like oxidation of amoxicillin using nZVI as a heterogeneous catalyst. Sep Purif Technol 188:548–552. https://doi.org/10.1016/j.seppur.2017.07.061
Luo X, Hu H, Pan Z, Pei F, Qian H, Miao K, Guo S, Wang W, Feng G (2020) Efficient and stable catalysis of hollow Cu9S5 nanospheres in the Fenton-like degradation of organic dyes. J Hazard Mater 396:122735. https://doi.org/10.1016/j.jhazmat.2020.122735
Liang H, Xiao K, Wei L, Yang B, Yu G, Deng S, Duan H, Zhu C, Li J, Zhang J (2019) Decomplexation removal of Ni(II)-citrate complexes through heterogeneous Fenton-like process using novel CuO-CeO2-CoOx composite nanocatalyst. J Hazard Mater 374:167–176. https://doi.org/10.1016/j.jhazmat.2019.04.031
Xia M, Long M, Yang Y, Chen C, Cai W, Zhou B (2011) A highly active bimetallic oxides catalyst supported on Al-containing MCM-41 for Fenton oxidation of phenol solution. Appl Catal B 110:118–125. https://doi.org/10.1016/j.apcatb.2011.08.033
Qin X, Wang Z, Guo C, Guo R, Lv Y, Li M (2022) Fulvic acid degradation in Fenton-like system with bimetallic magnetic carbon aerogel Cu-Fe@CS as catalyst: response surface optimization, kinetic and mechanism. J Environ Manag 306:114500. https://doi.org/10.1016/j.jenvman.2022.114500
Li L, Hu C, Zhang L, Yu G, Lyu L, Li F, Jiang N (2019) Framework Cu-doped boron nitride nanobelts with enhanced internal electric field for effective Fenton-like removal of organic pollutants. J Mater Chem A 7:6946–6956. https://doi.org/10.1039/C9TA00255C
Huang Z, Shen M, Liu J, Ye J, Asefa T (2021) Facile synthesis of an effective g-C3N4-based catalyst for advanced oxidation processes and degradation of organic compounds. J Mater Chem A 9:14841–14850. https://doi.org/10.1039/d1ta01325d
Ni C, Hou J, Li L, Li Y, Wang M, Yin H, Tan W (2020) The remarkable effect of alkali earth metal ion on the catalytic activity of OMS-2 for benzene oxidation. Chemosphere 250:126211. https://doi.org/10.1016/j.chemosphere.2020.126211
Yang Y, Pignatello JJ, Ma J, Mitch WA (2014) Comparison of halide impacts on the efficiency of contaminant degradation by sulfate and hydroxyl radical-based advanced oxidation processes (AOPs). Environ Sci Technol 48:2344–2351. https://doi.org/10.1021/es404118q
Gurtekin E, Celik A, Aydin E (2022) Degradation and mineralization of tetracycline and oxytetracycline by Fenton process: effect of inorganic anions. Desalin Water Treat 261:299–307. https://doi.org/10.5004/dwt.2022.28508
Yang L, Ren X, Zhang Y, Chen Z, Wan J (2021) One-step synthesis of a heterogeneous catalyst: Cu+-decorated triazine-based g-C3N4 nanosheet formation and catalytic mechanism. J Environ Chem Eng 9:105558. https://doi.org/10.1016/j.jece.2021.105558
Brussino P, Gross MS, Ulla MA, Banús ED (2023) Copper and iron-based monolithic catalysts for phenol catalytic wet peroxide oxidation (CWPO): support and iron effects on the catalytic performance. J Environ Chem Eng 11:110858. https://doi.org/10.1016/j.jece.2023.110858
Xin S, Liu G, Ma X, Gong J, Ma B, Yan Q, Chen Q, Ma D, Zhang G, Gao M, Xin Y (2021) High efficiency heterogeneous Fenton-like catalyst biochar modified CuFeO2 for the degradation of tetracycline: economical synthesis, catalytic performance and mechanism. Appl Catal B 280:119386. https://doi.org/10.1016/j.apcatb.2020.119386
Ma D, Yi H, Lai C, Liu X, Huo X, An Z, Li L, Fu Y, Li B, Zhang M, Qin L, Liu S, Yang L (2021) Critical review of advanced oxidation processes in organic wastewater treatment. Chemosphere 275:130104. https://doi.org/10.1016/j.chemosphere.2021.130104
Parvulescu VI, Epron F, Garcia H, Granger P (2022) Recent progress and prospects in catalytic water treatment. Chem Rev 122:2981–3121. https://doi.org/10.1021/acs.chemrev.1c00527
Taran OP, Zagoruiko AN, Yashnik SA, Ayusheev AB, Pestunov AV, Prosvirin IP, Prihod’ko RV, Goncharuk VV, Parmon VN (2018) Wet peroxide oxidation of phenol over carbon/zeolite catalysts. Kinetics and diffusion study in batch and flow reactors. J Environ Chem Eng 6:2551–2560. https://doi.org/10.1016/j.jece.2018.03.017
Funding
Support from the National Natural Science Foundation of China (21576291), and the Natural Science Foundation of Rizhao City (RZ2021ZR40) is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
Wumin Zhang: catalyst preparation, characterization, reaction test, writing. Yu Guo: catalyst preparation, characterization, reaction test, writing. Qiuyue Ding: reaction setup construction. Junxiao Jin: catalyst preparation, reaction test. Yanyan Xi catalyst characterization, discussion. Xufeng Lin: idea development, research funding provision, writing and organization.
Corresponding author
Ethics declarations
Competing interests
There are no competing interests to declare.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, W., Guo, Y., Ding, Q. et al. The degradation of maleic acid with wet peroxide oxidation catalyzed by Al2O3-supported Cu catalyst: effect of inorganic ions. Reac Kinet Mech Cat (2024). https://doi.org/10.1007/s11144-024-02662-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11144-024-02662-6