Abstract
The historical impacts of eutrophication processes were investigated in six subtropical reservoirs (São Paulo, Brazil) using a paleolimnological approach. We questioned whether the levels of pigment indicators of algal biomass could provide information about trophic increase and whether carotenoid pigments could offer additional insights. The following proxies were employed: organic matter, total phosphorus, total nitrogen, photosynthetic pigments (by high-performance liquid chromatography), sedimentation rates, and geochronology (by 210 Pb technique). Principal component analysis indicated a gradient of eutrophication. In eutrophic reservoirs (e.g., Rio Grande and Salto Grande), levels of lutein and zeaxanthin increased over time, suggesting growth of Chlorophyta and Cyanobacteria. These pigments were significantly associated with algal biomass, reflecting their participation in phytoplankton composition. In mesotrophic reservoirs, Broa and Itupararanga, increases and significative linear correlations (r > 0.70) between pigments and nutrients are mainly linked to agricultural and urban activities. In the oligotrophic reservoir Igaratá, lower pigment and nutrient levels reflected lesser human impact and good water quality. This study underscores eutrophication's complexity across subtropical reservoirs. Photosynthetic pigments associated with specific algal groups were informative, especially when correlated with nutrient data. The trophic increase, notably in the 1990s, may have been influenced by neoliberal policies. Integrated pigment and geochemical analysis offers a more precise understanding of eutrophication changes and their ties to human factors. Such research can aid environmental monitoring and sustainable policy development.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Paleolimnological studies play a crucial role in advancing the monitoring and management of ecosystems by providing valuable insights into historical environmental processes and dynamics. Paleolimnology emerges as a tool for a more profound understanding of eutrophication phenomena. Recognized as a primary global challenge in water quality degradation (Smith and Schindler 2009), eutrophication involves the continuous and excessive influx of nutrients, especially nitrogen and phosphorus. This condition can instigate the proliferation of potentially toxic algae, resulting in biodiversity loss and an escalation of associated water treatment costs. Although numerous studies delve into eutrophication, it remains an ongoing area of scientific research due to the diverse consequences in both abiotic and biotic environments, as well as water quality degradation (Cardoso-Silva et al. 2022).
To gain a better understanding of the eutrophication process in paleolimnological studies, it is essential to incorporate a biological proxy in addition to nutrient analysis. This is because the phosphorus present in sediment may be released into the water column depending on physicochemical conditions, often failing to accurately predict the actual trophic state. Under anoxic conditions, phosphorus is typically released into the water column. In eutrophic conditions, phosphorus release from sediments can be intense even with low external loading due to intense internal loading (Cardoso-Silva et al. 2018). Photosynthetic pigments offer advantages over diatom and chrysophyte analysis, in eutrophication studies as they do not require specialized expertise for identification.
The fossil record of photopigments in the sediment represents a fraction of the phototrophic production from the water column. This sedimented biomass reflects, among other things, the pigments present in the phytoplankton community and can enhance our understanding of this community. Such pigments exist in different forms, including chlorophylls (a, b, c, and d), carotenoids (carotene and xanthophyll), and phycobilins (phycocyanin—blue color, allophycocyanin—bluish-green color, and phycoerythrin—red color) (Jeffrey et al. 1997). In terms of pigment proportions, degradation processes differ for chlorophylls and carotenoids (Makri et al. 2019). Under high oxygen conditions, chlorophylls degrade more easily, while carotenoids are more stable (Leavitt and Hodgson 2001). Conversely, when oxygen availability is low, more chlorophyll than its derivatives should be present in sediments (Yacobi et al. 1991).
Carotenoid pigments are highly reactive and capable of imparting yellow, red, or orange coloration to organisms, and can be divided into carotenes (non-oxygenated) and xanthophylls (oxygenated) (Jeffrey et al. 1997). Carotenes can include α, β, and γ carotene and lycopene. Xanthophylls, on the other hand, encompass zeaxanthin, violaxanthin, and lutein (Jeffrey et al. 1997). In addition to sediment characterization and nutrient assessment, various studies have employed photosynthetic pigments as biomarkers in natural environments ( Rowan 1989; Leavitt and Hodgson 2001; Reuss et al. 2005; Jiménez et al. 2015; Makri et al. 2019). Their taxonomic specificity for understanding anthropogenic impacts and comprehending phytoplankton abundance and composition over time distinguishes these bioindicators (Züllig 1981; Brown et al. 1984; Overmann et al. 1993; Schüller et al. 2013; Halac et al. 2020)
The analysis of photopigments has been used in the reconstruction of global primary production and the phototrophic distribution of past communities (Leavitt and Hodgson 2001; Guilizzoni et al. 2009). It has been utilized to assess overall and specific abundance of algae (Leavitt and Findlay 1994), identify specific chlorophyll degradation products (Chen et al. 2003), and indicate processes related to eutrophication (de Oliveira Soares Silva Mizael et al. 2020; Halac et al. 2020; Cardoso-Silva et al. 2022) and climate changes (Reuss et al. 2010, 2013). This method is effective because pigments are present in all autotrophic organisms and are specific to certain classes of algae (Leavitt and Findlay 1994). For instance, carotenoids such as zeaxanthin, are common pigments in Cyanobacteria, while lutein serves as an indicator for chlorophytes (Reuss et al. 2005; Wright 2005; Buchaca et al. 2019) both groups are favored during the eutrophication process. Fucoxanthins are recognized as markers for diatoms (Borghini et al. 2007; Makri et al. 2019), a group more representative of reservoirs with lower trophic levels. On the other hand, chlorophyll-a and β-carotene function as biomarkers for algal biomass, as they are present across all phytoplankton groups (Wright 2005;Reynolds 2006; Borghini et al. 2007; Buchaca et al. 2019)). Therefore, pigments can offer insights into changes in trophic conditions as well as variations in the phytoplankton community.
Studies involving pigments have been conducted for over 70 years (Leavitt and Hodgson 2001). Initially, the photopigments were predominantly identified in the sediments (Vallentyne 1957; Fogg and Belcher 1961) and employed as biochemical markers to detect the presence of former populations of phototrophic prokaryotes (Brown and Colman 1963;Leavitt and Hodgson 2001). These early studies were developed mainly in temperate regions of the Northern Hemisphere (Leavitt and Hodgson 2001). The research with photopigments has become more robust with the development of more refined techniques (Leavitt and Hodgson 2001) such as high-performance liquid chromatography (HPLC). While recent studies have been published in tropical and subtropical areas (de Oliveira Soares Silva Mizael et al. 2020; Halac et al. 2020; Cardoso-Silva et al. 2022), it is worth noting that research in these regions is still lacking.
The use of pigments addresses the shortcomings of monitoring programs that often lack regularity or have been delayed in implementation. The application of these indicators is particularly relevant in reservoirs, ecosystems susceptible to various anthropogenic impacts that hold a range of utilities for human societies, including public supply. In reservoirs, the utilization of paleolimnological techniques and pigment analysis is limited. This is attributed to the sedimentation patterns inherent in these ecosystems, which pose challenges for the precise interpretation of stratigraphic data (Shotbolt et al. 2006; Tse et al. 2015). Despite these constraints, it remains both feasible and crucial to employ these techniques in reservoirs. Doing so can provide valuable insights into ecosystem dynamics and processes, including but not limited to eutrophication (Cardoso-Silva et al. 2022).
In this study, we aimed to investigate the historical impacts related to eutrophication processes in subtropical reservoirs with varying trophic levels, utilizing geochemical variables and sedimentary photopigments as proxies. We believe that nutrients alone may not be sufficient to assess the trophic status and thus questioned whether the levels of algal biomass indicators could provide insights into trophic increase. We included the analysis of pigments associated with the main phytoplankton groups favored during eutrophication processes (Chlorophyta and Cyanobacteria). This inclusion aims to enhance the robustness of the analysis. The investigation into pigment analysis alongside nutrient assessment is driven by the need to address the limitations of monitoring programs and gain more accurate insights into trophic conditions, phytoplankton community variations, and historical impacts related to eutrophication processes. Pigments can offer valuable information that nutrients alone may not capture, providing a more comprehensive understanding of the trophic status in subtropical reservoirs.
Material and methods
Study area
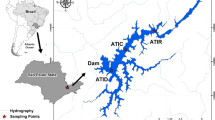
Six reservoirs from the state of São Paulo (SP-Brazil) with varying trophic levels were selected, belonging to two distinct typologies established by Cardoso-Silva et al. (2021): Broa (sampled on June 11, 2015), Barra Bonita (June 18, 2015), Salto Grande (June 25, 2015), Itupararanga (September 10, 2015), Igaratá (September 24, 2015), and Rio Grande (Billings Complex) (October 8, 2015) (Fig. 1). The Broa reservoir has undergone a process of increasing trophic levels, mainly recorded in the late 1990s (Cardoso-Silva et al. 2021). The reservoir has primarily been used for hydroelectric power generation. The Barra Bonita reservoir’s main contributing rivers (Tietê and Piracicaba rivers) are undergoing intense degradation, which is reflected in the water quality of the reservoir, currently classified as hypertrophic.
Locations of reservoirs (São Paulo, Brazil) and the sampling sites in dam areas, except for the Igaratá reservoir, where a point near the Dom Pedro highway was sampled (modified from Cardoso-Silva et al. 2021)
The Salto Grande and Itupararanga reservoirs are affected by intensive agricultural activities along their shores, in addition to receiving sewage inputs from urban areas (Buzelli and da Cunha-Santino 2013; Frascareli et al. 2015; Tundisi et al. 2015). Salto Grande has been predominantly classified as eutrophic, and Itupararanga as mesotrophic. Currently, the Itupararanga reservoir is a significant source of public water supply. The Igaratá and Rio Grande reservoirs also serve as sources of public water supply. Among these reservoirs, the Igaratá reservoir exhibits a lesser degree of degradation compared to the others, currently holding an oligotrophic classification (Frascareli et al. 2018).
The Rio Grande reservoir, situated within the Billings Complex, is categorized as an “urban reservoir” (Alves da Silva et al. 2005) due to its location. It is consistently subjected to socio-environmental impacts stemming from disordered and irregular occupation, and the discharge of untreated effluents, which exacerbates the process of eutrophication in the region. In recent decades, this reservoir has seen repeated applications of copper sulfate and hydrogen peroxide in attempts to manage phytoplankton growth (Mariani and Pompêo 2008; Biamont-Rojas et al. 2023). Detailed information regarding the sampled depths and general morphometric characteristics of each reservoir can be found in Table 1.
Sampling
As described by (Cardoso-Silva et al. 2021), for each reservoir, three sediment cores were collected in the deepest reservoir area, generally the dam area. The samples were collected from the deepest areas of the reservoirs. In each collection, samples representing the first centimeters of water at the water-sediment interface were chosen. This ensured that the surface layer of sediment was captured. The length of the sediment column was maximized to preferably represent the operational period of the reservoirs. Considering that lakes and reservoirs have relatively low sedimentation rates, collectors of up to 50 cm are sufficient for geochronology using Pb-210 (Cazotti et al. 2006). All analyses and sampling were conducted to prevent pigment degradation. To achieve this, samples were shielded from light, oxygen, and high temperatures, following recommendations from the literature (Leavitt and Hodgson 2001).
The cores were shielded from light and kept in refrigerated boxes until sample processing in the laboratory. Each core was divided into 2-cm intervals, and the resulting samples were securely stored in sterilized plastic containers safeguarded from exposure to light. The sectioning of the samples was conducted under an inert atmosphere with a flow of gaseous nitrogen, aiming to minimize contact with oxygen, which acts as a catalyst in the degradation of phytoplankton pigments (Leavitt and Hodgson 2001). Among these cores, one was designated for determining organic matter (OM), particle size, and nutrient content, while another was allocated for geochronological analysis, and the third was earmarked for pigment analysis. To prevent pigment degradation, the samples designated for pigment analysis were processed under conditions that minimized exposure to light and stored in a freezer at – 20 °C until extraction. Sequential numbering was assigned to the core slices from top to bottom and the depths of the cores ranged from 20 to 36 cm.
Geochronology and sedimentation rate
Sediment geochronology and sedimentation rate were previously published by (Cardoso-Silva et al. 2021) and were performed as follows: after sediments were dried and ground, portions were dispatched to the Radioisotope Service of the Research, Technology, and Innovation Centre at the University of Seville (Spain) for geochronological analysis. The samples were securely stored in containers for a minimum of 20 days, sealed to prevent air entry, allowing gaseous 222Rn to achieve secular equilibrium within the 238U decay series. Concentrations of 210Pb were estimated using alpha spectrometry (Alpha Analyst, Canberra). Analytical reagents of high purity were procured from Merck (HNO3, HF, and HCl) and Panreac (ascorbic and boric acids). Deionized water with a resistivity of 18.0 MΩ cm was sourced from a Millipore system.
The digestion procedure was based on the modified US EPA 3050 method (US EPA United States Environmental Protection Agency 1996) by (Laissaoui et al. 2013). The resulting spectra were analyzed using Genie 2000 software, incorporating decay corrections to compute the activities of 210Po and 209Po. Successful alpha particle spectrometry yielded high chemical yields (> 50%). The quantification limit was determined by measuring several blank samples over a 3-day background count period. Vertical profiles of 210Pb and 226Ra were employed within a constant rate of supply (CRS) model to (Appleby and Oldfield 1978) establish an age-depth model and derive sedimentation rates (SRs) for each core. This model is widely accepted for such purposes, offering a consistent mathematical framework to model the dilution and concentration of unsupported 210Pb in aquatic systems, which are subject to shifts in sedimentation rates. The SR was expressed as centimeters per year (cm/y). Reservoir core parameters for CRS age model dating are detailed in Cardoso-Silva et al. (2021). More details in geochronology are present in supplementary material (SM 1).
Organic matter and nutrients
The OM content was determined by the loss on ignition (LOI) method, which involves the combustion of the OM in an oven at 550 °C (Meguro 2000). The determination of total phosphorus content (TP) in the sediment followed the method by Andersen (1976). The assessment of total nitrogen levels (TN) was conducted through Kjeldahl digestion (APHA-American Public Health Association 2002), and absorbances were read on a Femto UV/VIS Cirrus 80 spectrophotometer. Data from OM, TP, and TN were also previously presented in Cardoso-Silva et al. (2021).
Photosynthetic pigments
The quantification of pigments was carried out using high-performance liquid chromatography (HPLC), employing a high-performance liquid chromatograph (UHPLC focused Dionex Ultimate 3000) with an automatic sampler, UV and Fluorescence detectors; C18 chromatographic column (Acclaim 120, inner diameter 5 μm, 4.6 × 250 mm); Ultrasonic Sonicator (Eco-Sonics Ultrasonic).
The photosynthetic pigments were analyzed as described by (Airs et al. 2001; Squier et al. 2002). About 1 g of fresh sediment was sonicated for 10 min at a power of 25 W with 10 mL of acetone. After that, the samples were centrifuged for 5 min at 2000 rpm. The supernatant was filtered at 45 μm and stored in 1.5-mL vials, kept refrigerated at 4 °C for HPLC analysis. The entire procedure was conducted in low-light conditions, with just enough illumination for the analysis, at a temperature of 17 °C, with the sample manipulation taking place under ice bath conditions. All containers used for storing and processing the samples were encased in aluminum foil. During analysis, the samples were transported in sealed bags with ice to maintain low temperatures.
For the HPLC analyses, a C18 column was used with a gradient of 1 mL/min and an injection of 100 μL. The chromatographic conditions were adjusted to obtain a symmetric peak and a shorter run time. The parameters were set according to the proposal by Chen et al. (2001): Phase A (80:20 methanol: 0.5 M ammonium acetate pH 7.2), Phase B (90:10 acetonitrile: water), and Phase C (ethyl acetate), with a total analysis time of 20 min at room temperature. All solvents used were ultrapure. The standard pigments chlorophyll (Chl-a), fucoxanthin (Fuc), beta-carotene (Bet), lutein (Lut), and zeaxanthin (Zea) used were from Sigma-Aldrich.
As the analyzed reservoirs undergo varying impacts from the eutrophication process. The groups of cyanobacteria and chlorophytes are particularly favored during this process. Studies conducted in the mentioned reservoirs (Nishimura et al. 2008; Vicentin et al. 2018; Rodrigues et al. 2019; Pompêo and Moschini-Carlos 2022) indicate that cyanobacteria constitute a significant portion of the phytoplankton biomass. In light of this and considering the availability of standards for the calibration curve provided by the Brazilian distributor Sigma-Aldrich, we chose to use the pigments lutein and zeaxanthin as representatives of trophic increases. These were selected in conjunction with indicators of algal biomass, chlorophyll-a, and beta-carotene.
Data analysis
The obtained data of nutrient and pigment contents were normalized using the Shapiro-Wilk test in the PAST software, and values with p > 0.05 were considered normal. They were analyzed using descriptive statistical techniques and multivariate statistical methods. Pigment values equal to zero were replaced with half of the minimum value for each variable (Romero-Viana et al. 2009). Principal component analysis (PCA) was performed based on a correlation matrix, aiming to summarize the set of environmental variables and produce orthogonal axes that express a portion of the variability within the original variables (Legendre and Legendre 1998). Data analyses were conducted using the PAST 3.0 computational software (Hammer et al. 2001).
Results
Eutrophic reservoirs
Data for sedimentation was previously reported by (Cardoso-Silva et al. 2021) Figs. 2a, b presents the vertical profiles obtained for the unsupported 210Pb geochronological analyses and SRs, respectively. In the Salto Grande and Barra Bonita reservoirs, the highest SRs were observed in the uppermost layers of the cores, corresponding to the 2010s, when the local environmental agency recorded increases in eutrophication (CETESB 2016).
Phosphorus levels showed variations in the distribution pattern except in the Rio Grande reservoir. Total nitrogen (TN) and organic matter (OM) levels exhibited an increasing trend in both remote and more recent periods (Fig 3). Pigment levels, in general, showed the highest records in the most recent layers of the sediment profile, particularly zeaxanthin and lutein (Fig 3). Fucoxanthin levels were below the limit of detection (LD) in the Rio Grande reservoir. In the Rio Grande reservoir, lutein and zeaxanthin levels tended to increase from around 1950, with the highest records at the surface, measuring 399.04 and 292.35 μg/g OM, respectively (Fig. 3). Chlorophyll-a and β-carotene exhibited a concentration peak at a depth of 18 to 20 cm, with 249.41 and 145.06 μg/g OM, respectively, in the year 1956, shortly after the reservoir’s construction (Fig 3). The pigment fucoxanthin was not detected (LD: 0.014 μg/mL). In the Rio Grande reservoir, there were no variations in phosphorus levels; however, increases in TN and OM were observed in the 1940–1960s (20.0–18.0 cm) before the establishment of the monitoring program in the area.
Concentrations of sedimentary photopigments in sediment cores of the Reservoirs Barra Bonita and Rio Grande (São Paulo- Brazil). Chl-a: chlorophyll-a; Lut: lutein; Zea: zeaxanthin; Fuc: fucoxanthin; Bet: ẞ-carotene; OM: organic matter; TP: total phosphorus; TN: total nitrogen; and OM: organic matter
In the Barra Bonita reservoir, chlorophyll-a recorded its highest value (283.00 μg/g OM) in 1988 (Fig 3). β-Carotene exhibited the lowest levels in the upper sediment layers, corresponding to the years 2008 to 2015, with an average of 359.81 μg/g OM. For fucoxanthin, between the period of 1975 to 2011, the highest record was in 2015 (76.07 μg/g OM). Lutein and zeaxanthin presented variations along the sediment profile. In Barra Bonita, phosphorus and nitrogen levels also showed variations with both increases and decreases throughout the core (Fig 3). The reservoir’s early operational phase (approximately 1963 to 1980) was marked by increases in TN and a decrease in TP. The pattern was reversed during the 1990s, with increases in TP and a decrease in TN. Increases were again recorded for TN and OM at 4 cm (~ 2011).
In Salto Grande, the highest pigment levels were recorded in the more recent layers from the 2000s onwards: 4 to 6 cm (~ 2011) for fucoxanthin (79.29 μg/g OM); 6 to 8 cm (~ 2007) for lutein (619.47 μg/g OM) and zeaxanthin (521.14 μg/g OM) and 8 to 10 cm (~ 2002) for chlorophyll-a (229.05 μg/g OM) (Fig. 4). The Salto Grande reservoir exhibited increases in TP and OM from 12.0- to 18.0-cm depth (1954–1986). The highest OM percentage occurred in the mid-1980s (approximately 1986), while TN concentrations began to decrease in the 1950s (14.0–18.0 cm, 1954–1978) (Fig 4).
Mesotrophic reservoirs
Increases in SR (Fig 2b) were observed mainly in the Broa reservoir and can be attributed to eutrophication which was corroborated by a significant correlation between SR and total nitrogen (r = 0.67, p < 0.10) (Cardoso-Silva et al. 2021); SR and Chl-a (r = 0.60, p < 0.10); and SR and Lut (r = 0.62). Phosphorus levels did not show significant changes over time in the Broa and Itupararanga reservoirs (Fig. 5). On the other hand, TN and OM concentrations exhibited more pronounced variations in the sediment profile (Fig. 5). Across all reservoirs, the levels of pigments Chl-a, fucoxanthin, and zeaxanthin showed varying degrees of increase over time, suggesting an increase in trophic status in these environments (Fig. 5). The highest levels were mainly recorded from the 1990s and 2000s. However, for fucoxanthin and β-carotene, the increase towards the top of the core is not always evident. In the Broa reservoir, higher concentrations of chlorophyll-a and zeaxanthin were observed at a depth of 4 to 6 cm (~ 2010), measuring 161.70 and 220.85 μg/g OM, respectively. For lutein, the highest concentrations were recorded both in the layer of 4 to 6 cm and in the year of reservoir construction, 1936. In this case, the increase in chlorophytes during the early years of reservoir operation is expected, as there is an increase in trophic status in the initial years of these ecosystems. The pigment fucoxanthin showed values above the LQ (0.046 μg/mL) only from the year 2004. β-Carotene levels did not indicate significant changes over time. Increases in TN and organic matter concentrations were observed, especially from the 1990s (14.0 cm, 1992).
In Itupararanga, increases in the concentrations of pigments chlorophyll-a, lutein, and zeaxanthin were recorded in the more recent layers from 6 to 8 cm (~ 1995) (Fig. 5). For chlorophyll-a, the increase was gradual up to the surface (~ 2015, 108.41 μg/g OM) (Fig. 5). The pigment fucoxanthin showed an increase only from the year 2003. β-carotene levels remained below the LD (0.014 μg/mL) throughout the sediment profile. In the Itupararanga reservoir, TN concentrations tended to increase in the surface layers between the years 2012 and 2015 (3.73 mg/g), while OM levels decreased towards the base-top direction (Fig. 5).
Oligotrophic reservoir
Profiles for the Igaratá sediment core revealed episodes of dilution of unsupported 210Pb, which could be directly related to increases in the SR. TP and TN levels showed changes over time, however, there is no clear pattern of increase associated with an escalation in the eutrophication process. After the year of Igaratá reservoir construction (1969), the pigments lutein and zeaxanthin experienced a decrease in concentration, reaching 18 μg/g OM (4 to 6 cm depth ~ 2009) (Fig. 6). The pigment chlorophyll-a exhibited higher concentrations only at the top of the sediment profile (~ 2015), measuring 16.84 μg/g OM. The pigments fucoxanthin and β-carotene were not detected.
Integrated analysis of the reservoirs
Reservoirs classified as more eutrophic typically display elevated levels of total pigment mass, such as Rio Grande (774.04 ± 239.30 μg/g OM) and Salto Grande (774.04 ± 239.30 μg/g OM). Those categorized as mesotrophic and oligotrophic exhibited lower total pigment mass levels, as observed in Igaratá (132.24 ± 63.83 μg/g OM) (Table 2). In terms of the analyzed pigments, the coefficient of variation surpassed 30% for most reservoirs, except for Broa and Igaratá (for Chl-a), with figures even reaching 223% for fucoxanthin in Barra Bonita. This variability is likely attributed to significant data differences between the upper and lower sections of the sediment core and could be linked to potential shifts in phytoplankton communities as well as the degradation process of these compounds. In terms of carotenoids, lutein content was found to be the highest in all reservoirs, followed by zeaxanthin and fucoxanthin (Figs. 3, 4, 5, and 6). Despite the elevated levels of lutein in all tested reservoirs, the paired t-test did not reveal significant differences between these pigments in Salto Grande, Itupararanga, Igaratá, and Rio Grande reservoirs (p > 0.01).
Through a PCA, it can be observed that the greatest variability in the data was explained by the first two axes (59.91%) (Fig. 7). The main components influencing the arrangement along axis 1 were lutein (0.879), zeaxanthin (0.810), TN (0.745), and Chl-a (0.665), while along axis 2, TP (− 0.662) and OM (0.635) had the most influence (Fig. 7 and Table 3). The PCA highlights the formation of a degradation gradient, with the eutrophic reservoirs Barra Bonita and Rio Grande influenced by Chl-a and TP levels, and the Salto Grande reservoir primarily influenced by zeaxanthin and lutein levels. The Broa reservoir was impacted by OM and fucoxanthin levels. The mesotrophic reservoir Itupararanga and oligotrophic reservoir Igaratá were considered to have lesser impact concerning variables associated with the eutrophication process.
Discussion
Eutrophic reservoirs
In both the Rio Grande and Salto Grande reservoirs, the levels of lutein and zeaxanthin increased over time, suggesting an increase in the Chlorophyta and Cyanobacteria groups (Leavitt and Hodgson 2001; Jiménez et al. 2015). Significant Pearson correlations between algal biomass indicators support the significant involvement of these groups in the composition of the phytoplankton community (RG: ß-carotene and Lut: r = 0.53 and ß-carotene and Zea: r = 0.55; SG: Chl-a and Zea: r = 0.50; BB: Chl-a and Lut: r = 0.56 and Chl-a and Zea: r = 0.61). Such algal groups are commonly associated with the growth of the eutrophication process. The increase in TN and OM levels throughout the sediment profile, especially in the surface layers, may be linked to a greater influx of nutrients originating from anthropogenic activities in the watershed of these reservoirs.
The Rio Grande reservoir is highly impacted by the discharge of organic effluents (Mariani and Pompêo 2008; Silva et al. 2014; Cardoso-Silva et al. 2021; Biamont-Rojas et al. 2023) and has been considered eutrophic since the 1950s (Wengrat et al. 2019; Cardoso-Silva et al. 2021). Despite the application of copper sulfate from the 1970s onwards (Biamont-Rojas et al. 2023) to control algal blooms, the reservoir exhibited the highest algal biomass and the highest pigment concentrations, indicating the high primary productivity of the system. The reservoir sediments are characterized by their anoxic nature (Mariani and Pompêo 2008), which promotes the release of phosphorus into the water column, thereby maintaining high trophic conditions. To achieve recovery and restoration, in addition to controlling nutrient sources, there is a need to address the phosphorus feedback present in the environment. Positive experiences in the restoration of eutrophic reservoirs can be found in (Barçante et al. 2020; Lan et al. 2021)
In Salto Grande, the eutrophication process was intensified primarily from the 1970s onwards (Martins et al. 2011; Cardoso-Silva et al. 2021) due to intensive agricultural activity and urbanization (Fonseca and Matias 2015) in the region. Additionally, the Salto Grande reservoir exhibits spatial complexity due to the influence of a large upstream reservoir (Chavantes) and the input of the Rio Pardo, a secondary tributary, which introduces high nutrient loads (Gomes et al. 2012). Between the years 2007 and 2011, higher levels of lutein and zeaxanthin were in line with higher sedimentation rates of 0.65 cm/year, as also observed in other studies: Sanger 1988; Chen et al. 2005; Romero-Viana et al. 2009. Sedimentation rates have been increasing, indicating a rise in erosive processes (Cardoso-Silva et al. 2021) that transport nutrients into the water body, promoting increased productivity, as suggested by the obtained data.
The Barra Bonita sediment exhibited increases in pigments, TN, and OM from the start of the reservoir’s operation until the early 1980s. These changes have been attributed to various factors as reported by Cardoso-Silva et al. (2021): 1) mineralization of OM present in the recently inundated reservoir zone and 2) population growth leading to elevated effluent discharges within the reservoir’s watershed (David et al. 2016). It should also be considered that in the 1970s, exotic tilapia species were intentionally introduced to boost fishing and economic activity in the region, which might have contributed to the increase in pigment levels. The introduction of exotic species into an ecosystem can intensify predation and competitive exclusion, potentially leading to an initial increase in organic matter and primary productivity. The decline in nutrient levels since the 1980s is likely connected to improvements in sanitation in 1979 and 1981 (Leme-Pompeu and Mucare 1983). Subsequent increases in TN and OM were observed at 4.0 cm (2011) (Fig. 3), possibly due to greater discharges of industrial and domestic effluents (Buzelli and da Cunha-Santino 2013; David et al. 2016) along with the application of fertilizers in agricultural areas (Buzelli and da Cunha-Santino 2013). A significant correlation was found between SR and total phosphorus (r = 0.59, p < 0.10) for the Barra Bonita reservoir, corroborating the influence of eutrophication on SR (Sanger 1988).
Despite SG, RG, and BB reservoirs being recognized as eutrophic for decades, the isolated analysis of P and Chl-a does not reliably predict the trophic increase in these environments. Degradation of Chl-a generally occurs rapidly, so it is poorly preserved in sediment (Sanger 1988), especially in tropical regions, where higher temperatures and faster metabolism can hinder pigment preservation (Buchaca et al. 2019). Additionally, the high concentrations of zeaxanthin observed mainly in eutrophic reservoirs may contribute to Chl-a degradation. Zeaxanthin is efficient in quenching triplet chlorophyll, a phytoplanktonic species that generally has lower light sensitivity due to higher zeaxanthin content (Schubert and García-Mendoza 2008; Betterle et al. 2010). Upon deposition in sediments, zeaxanthin could facilitate the degradation of chlorophyll-a. In light of this, it was observed that in all reservoirs, except for Barra Bonita, the zeaxanthin pigment was present in higher concentrations than chlorophyll-a. This suggests that zeaxanthin might have contributed to the reduction of chlorophyll-a within the sediment profile, in conjunction with other factors such as oxidative processes.
Specific pigments derived from populations of planktonic algae, such as chlorophytes (lutein) and cyanobacteria (zeaxanthin), usually follow similar patterns (Romero-Viana et al. 2009), which was also observed in all reservoirs analyzed in the present study through significant Pearson linear correlations: RG- Lut and Zea: r = 0.99; SG-Lut and Zea: r = 0.81; BB-Lut and Zea: r = 0.95. It is worth noting that lutein levels can also come from the decomposition of aquatic macrophyte biomass (Martins et al. 2011), especially in Salto Grande (Minhoni et al. 2017) and Barra Bonita reservoirs (Minhoni et al. 2017), which have experienced multiple episodes of these organisms’ proliferation.
Zeaxanthin pigments are chemically stable (Itoh et al. 2003), while fucoxanthin usually degrades faster than Chl-a (Periotto and Tundisi 2013; Campregher and Martins 2017), so its absence does not necessarily mean that diatoms are not present in the water column. Due to their long-standing recognition as eutrophic reservoirs, there is a tendency for biotic homogenization and reduced abundance of diatoms. Currently, a significant portion of the evaluated reservoirs shows dominance of the cyanobacteria phytoplankton group (unpublished data).
Mesotrophic reservoirs
The Broa reservoir underwent a historical milestone in the 1970s with the construction of hydroelectric complexes and development in its surroundings (Periotto and Tundisi 2013; Campregher and Martins 2017), resulting in an increase in nutrient load that has led to a shift in trophic status from oligotrophic to mesotrophic (Tundisi and Matsumura-Tundisi 2013; Moraes et al. 2023). The construction of residences and farms in the watershed of this reservoir along with the absence of sewage treatment (Tundisi et al. 2015), and intensified agricultural activities (Silva 2015) starting from the 1990s (Cardoso-Silva et al. 2021) justify the observed increases in Chl-a and zeaxanthin levels, organic matter, and TN between the years ~ 1992 and 2015. A factor that leads us to infer the increase in trophic status in this study is the significant Pearson correlations between TP and OM (r = 0.74), TN and Chl-a (r = 0.77), OM and Fucoxanthin (r = 0.67), and zeaxanthin and lutein (r = 0.75).
The leaching of nutrients, especially from non-point sources such as agriculture activities in the watershed, increases the nutrient input to the reservoir (da Anjinho et al. 2021), has led to an increase in the proliferation of potentially toxic cyanobacteria (e.g., Raphidiopsis raciborskii, previously named as Cylindropermopsis raciborskii (Tundisi et al. 2015), represented in this study by the zeaxanthin pigment. Sedimentation rates corroborate the degradation increase in the region due to land use and occupation, particularly in the year 1992 when the rate was 0.92 cm/year. The presence of lutein in the region is associated not only with the presence of Chlorophytes but also with the occurrence of aquatic macrophytes, which occupy up to 10% of the reservoir area (Periotto and Tundisi 2013). The sediment in the reservoir can be consistently classified as polluted, as it consistently exceeds the nitrogen concentration limit set by current local regulations (4.8 mg N/g) (Cardoso-Silva et al. 2021). The trophic state could be higher if the reservoir’s retention time (< 25 days) was not low (Tundisi et al. 2015).
The Itupararanga reservoir also showed an increasing trend in TP and Chl-a levels. The reservoir has been classified as mesotrophic since the 1970s (Cardoso-Silva et al. 2021), and the intense agricultural activities in its vicinity (Abreu and Tonello 2015; Frascareli et al. 2015) are the main factors responsible for the results observed in this research. In the mid-1990s, the Itupararanga reservoir exhibited the highest sedimentation rate (2.9 cm/year), and the following year had some of the highest levels of lutein (408.97 μg/g OM) and zeaxanthin (629.05 μg/g OM) pigments. These observations may be associated with a decrease in reservoir flow in 1993, which reduced dissolved oxygen levels and a significant fish mortality event (Manfredini et al. 2015). Fish mortality increases autochthonous organic matter and intensifies oxygen depletion due to bacterial activity in organic matter decomposition. High sedimentation rates and low oxygen levels can be related to the preservation of photosynthetic pigments (Sanger 1988; Romero-Viana et al. 2009). The Itupararanga reservoir showed a significant correlation between chlorophyll-a and lutein levels (r = 0.77), as well as lutein and zeaxanthin levels (r = 0.96), suggesting that algal biomass is influenced by the Chlorophytes and Cyanobacteria groups. Analysis of monitoring data provided by the local environmental agency shows a notable increase in Chl-a concentrations since 2002. Previous studies (Cunha and Calijuri 2011; Beghelli et al. 2016) have documented the prevalence of Cyanobacteria in the Itupararanga reservoir, likely attributed to elevated water temperatures, especially during the summer months, and substantial ammonium and nitrate concentrations within the reservoir reveals (Gasparini Fernandes Cunha et al. 2017). Recent years have also witnessed substantial populations of Raphidiopsis raciborskii (Casali et al. 2017) and the detection of saxitoxins in the reservoir (dos Santos et al. 2022). Therefore, Ituparanga has exhibited a long-standing historical trend of eutrophication, underscoring the imperative need to mitigate nutrient loads within the watershed.
A suite of integrated practices can be employed to mitigate nonpoint source pollution in these regions, as suggested by the 4 Rs framework proposed by Xue et al. (2020):
-
1)
Source reduction: mitigating excessive fertilizer application in agricultural fields is essential. Precision in applying recommended dosages, coupled with the adoption of agroforestry practices incorporating trees and shrubs to enhance nutrient absorption and diminish water runoff, represents a viable approach. Furthermore, optimizing irrigation systems for efficiency is crucial in minimizing water runoff and nutrient loss.
-
2)
Process retention: the interception and filtration of nutrients/pollutants within the agricultural landscape before their ingress into lakes or rivers constitute a critical strategy. Employing physical barriers such as retention basins can effectively capture sediments and nutrients, preventing their discharge into watercourses.
-
3)
Nutrient reuse: the recycling and reuse of filtered nutrients present an opportunity for sustainable nutrient management.
-
4)
Ecological restoration: undertaking ecological measures to restore balance in the ecosystem is imperative. For instance, ecological restoration efforts in the Broa reservoir could involve reforesting its shores, which have experienced a considerable loss of typical cerrado vegetation cover (Pompêo and Moschini-Carlos 2022), and preserving riparian vegetation along water bodies within the basin.
Moreover, providing financial incentives to farmers embracing sustainable practices, such as subsidies for the implementation of nutrient management technologies, is crucial. Additionally, managing nonpoint sources that export nutrients into water bodies (Strokal et al., 2020), coupled with ongoing monitoring and enforcement, represents essential daily practices for curtailing nutrient sources.
Oligotrophic reservoir
The Igaratá reservoir exhibited the lowest pigment levels, indicating the lowest degree of impact compared to the other assessed reservoirs. Decreases in TN may be associated with denitrification processes releasing nitrogen in gaseous form into the water column (Hou et al. 2014; Cardoso-Silva et al. 2018, 2021). Significant correlations between Chl-a and lutein (r = 0.77) and zeaxanthin (r = 0.75) suggest the involvement of these groups in algal biomass. The drought that occurred in the reservoir between 1998 and 2003 may have favored the increase in pigments, as they become more stable when water levels decrease (Louda et al. 1998). Drought periods can impact various aspects of ecosystem dynamics, including primary productivity (Mosley 2015; Rocha Junior et al. 2018). In certain instances, drought conditions may contribute to sediment resuspension ((Rosen and Van Metre 2010) due to higher temperatures and increased wind action. Consequently, nutrients in the sediment can be released into the water column, promoting phytoplankton growth. During drought conditions, the concentration of nutrients in the water may also rise due to reduced dilution from inflowing water (van Vliet and Zwolsman 2008). This can lead to higher nutrient levels in the remaining water, potentially fostering phytoplankton growth. The increased nutrient concentrations during and after droughts can heighten the risk of eutrophication when normal water flow resumes (Rocha Junior et al. 2018). Upon the return of inflow, the accumulated nutrients may be swiftly transported into the water body, promoting algal blooms and other adverse ecological effects.
The absence of fucoxanthin and ß-carotene pigments throughout the sediment profile in the Igaratá reservoir may be linked to the historically low anthropogenic activity in these environments and the good water quality, as indicated by results published by (Frascareli et al. 2018). To maintain the oligotrophic conditions of the reservoir, it is essential to regulate land use and occupations along the hydrographic basin. This requires efficient public policies and rigorous monitoring measures to prevent irregular occupations. Additionally, it is crucial to preserve or reforest riparian forests to reduce nutrient input. Water quality monitoring should be conducted regularly and frequently, with an expansion of the observation point network. This way, it will be possible to identify early signs of degradation, allowing for the immediate implementation of necessary measures. It is also imperative to expand the sewage collection and treatment infrastructure in accordance with the population growth of the region. These measures should be particularly considered during the current era of climate change, where drought episodes become more frequent. As discussed, dry periods can lead to increased nutrient availability, promoting eutrophication and the subsequent bloom of cyanobacteria.
Integrated analysis
The analysis of the phytoplankton community and water quality data in the studied reservoirs, conducted during the same period as the sediment profile collection (Rodrigues et al. 2019), reinforces the information obtained from the sediment. The authors identified a trophic gradient like that found in the present study as follows: Barra Bonita, Rio Grande, Salto Grande, Broa, Itupararanga, and Igaratá reservoirs. Through specific richness, the authors observed a predominance of green algae. Meanwhile, the dominant group in terms of abundance was cyanobacteria, especially in the eutrophic reservoirs. In the current study, a significant portion of the biomass is attributed to the Chlorophyta group. In the Salto Grande, Itupararanga, Igaratá, and Rio Grande reservoirs, the distinctions between pigments indicative of Chlorophyta and Cyanobacteria are not statistically significant. This underscores the significance of the Chlorophyta and Cyanobacteria groups in shaping the biomass composition within these reservoirs.
Rodrigues et al. (2019) recorded the lowest values of diversity in the most eutrophic reservoirs: Barra Bonita, Salto Grande, Broa, and Itupararanga reservoirs (Rodrigues et al. 2019). Several cyanobacteria blooms have already been reported in these eutrophic reservoirs: Barra Bonita (Araújo et al. 2023), Salto Grande (Pamplona-Silva et al. 2018), Broa (Tundisi et al. 2015), and Itupararanga (De Souza Beghelli et al. 2016). The Rio Grande reservoir frequently undergoes applications of algaecides such as hydrogen peroxide and copper sulfate (Mariani and Pompêo 2008; Frascareli et al. 2018), which may have led to a classification of the phytoplankton community that does not accurately reflect its trophic status (Silva et al. 2014). The classification of the ecological potential of the reservoirs, evaluated through the evenness index, characterized the more eutrophic reservoirs by the worst classifications, while the less eutrophic reservoirs had better classifications (Rodrigues et al. 2019). The presented data reveal a gradient of degradation in the reservoirs that persists to the present day. It is imperative to enhance public policies and investments aimed at the recovery of the assessed reservoirs.
Conclusion and recommendations
We observed that isolated analysis of nutrient levels may not accurately predict increases in trophic conditions. The combined analysis of characteristic carotenoid pigments from the Chlorophyta and Cyanobacteria groups and chlorophyll-a enhances the analysis. In terms of carotenoids, lutein content, a Chlorophyta biomarker, was found to be the highest in all reservoirs, followed by zeaxanthin Cyanobacteria biomarker. Despite the elevated levels of lutein, in the Salto Grande, Itupararanga, Igaratá, and Rio Grande reservoirs, the differences in pigments representing Chlorophyta and Cyanobacteria are not statistically noteworthy. Significant correlations between the Chlorophyta and Cyanobacteria carotenoids and algal biomass also suggest the relevance of these groups in the composition of the phytoplankton community. Increases in pigments occurred in conjunction with neoliberal policies implemented during the 1990s. The increases were more evident in the Salto Grande, Broa, and Ituparanga reservoirs. In the Rio Grande, the most impacted of the reservoirs, the applications of algaecides justify significant variations over time in the distribution pattern of phytoplankton pigments. In the Barra Bonita reservoirs, the increase in trophic conditions was more intense in the 1980s and tended to decrease subsequently, showing the effectiveness of sanitation improvements for that period. However, the improvement in trophy did not persist over time and nowadays the Barra Bonita is still classified as eutrophic. As for Igaratá, which is the least impacted among the reservoirs, the increase in trophic conditions through pigment and nutrient analysis is not evident. Organizing the reservoirs by trophic level, we obtain the following classification: RG > SG > BB > Br > It > Ig. The nutrient load, which is accountable for recurrent algae blooms and the proliferation of macrophytes in certain reservoirs, must be curtailed.
It is crucial to implement strategies for mitigating both point and non-point sources of pollution. While the management and identification of point sources are more feasible, they persist as a primary cause of water quality degradation in various regions of Brazil including most of the evaluated reservoirs. The control of irregular occupations in watershed areas, such as in the Rio Grande, which contribute to nutrient input through the discharge of untreated effluents, requires enhancement. Current investments in monitoring land use and occupancy in these areas, as well as in effluent treatment, remain inadequate. There is a pressing need for political commitment and substantial investments in the sanitation sector, particularly in sewage collection and treatment.
To address non-point sources, particularly in reducing fertilizer use, nutrient retention, and reuse, economic incentives, awareness programs, and training are necessary. Farmers need to be empowered to adopt sustainable practices. The adoption of effective policies is vital to encourage the use of more precise techniques in agriculture. Additionally, ecological restoration efforts demand collaboration between the public and private sectors, along with increased awareness of the importance of such initiatives. Monitoring and controlling non-point sources of nutrients require significant resources and a robust regulatory framework. The successful implementation of these practices depends on close cooperation between governmental bodies, researchers, and the agricultural community. Synergy among these stakeholders is essential for achieving sustainable goals in environmental management and water resource preservation.
In addition to measures designed to mitigate the influx of nutrients into the assessed reservoirs, the recovery process, despite presenting numerous challenges, can find inspiration from global instances, including examples within Brazil, such as the case of Lake Paranoá in Brasília-DF (Brazil) (Angelini et al. 2008). Current palliative measures, which involve the application of algaecides based on copper sulfate (Cardoso-Silva et al. 2021), may potentially provide a temporary reduction in trophic levels; however, they are controversial due to the potential contamination of sediments with copper. Other physical, chemical, and biological techniques can be implemented either individually or in combination, and their application should be assessed on a case-by-case basis. Examples include reducing the residence time of water, mechanically removing macrophytes, and employing practices such as flushing (reservoir gate opening).
Regardless of the techniques applied, the effectiveness in restoration and recovery is directly linked to implementing efficient measures in water resource management, often requiring substantial investments from the public sector. A noteworthy example of encouragement for recovery can be found in the European water resource management system, the Water Framework Directive (WFD). Embracing an ecological approach, this model has propelled advancements in the management and enhancement of water quality (Cardoso-Silva et al. 2013). While challenges persist, it is imperative to draw inspiration from successful initiatives, such as the aforementioned one, to guide more efficient remediation strategies. Commitment to implementing public policies and appropriate investments is essential to confront and overcome the obstacles associated with remediating pollution sources, thereby contributing to the preservation and revitalization of water resources.
References
Abreu MC, Tonello KC (2015) Estimativa do balanço hídrico climatológico da bacia hidrográfica do rio Sorocaba–São Paulo Estimate of the water balance of the Sorocaba river watershed–SP. Ambiência 11. https://doi.org/10.5935/ambiencia.2015.03.01
Airs RL, Atkinson JE, Keely BJ (2001) Development and application of a high resolution liquid chromatographic method for the analysis of complex pigment distributions. J Chromatogr A 917. https://doi.org/10.1016/S0021-9673(01)00663-X
Alves da Silva MEP, de Castro PMG, Maruyama LS, de Paiva P (2005) Levantamento Da Pesca E Perfil Socioeconômico Dos Pescadores Artesanais Profissionais No Reservatório Billings. Boletim do Instituto de PescaInstituto da Pesca 35:531–543
Andersen JM (1976) An ignition method for determination of total phosphorus in lake sediments. Water Res 10. https://doi.org/10.1016/0043-1354(76)90175-5
Angelini R, Bini LM, Starling FLRM (2008) Efeitos de diferentes intervenções no processo de eutrofização do Lago Paranoá (Brasília - DF). Oecologia brasiliensis 12. https://doi.org/10.4257/oeco.2008.1203.15
APHA- American Public Health Association (2002) Standard methods for the examination of water and wastewater. American Public Health Association, Washington
Appleby PG, Oldfield F (1978) The calculation of lead-210 dates assuming a constant rate of supply of unsupported 210Pb to the sediment. Catena (Amst) 5. https://doi.org/10.1016/S0341-8162(78)80002-2
Araújo BM, Negri RG, Moraes Ananias PH et al (2023) Remotely sensed-based analysis about climatic and landscape change effects on phytoplankton bloom in Barra Bonita Reservoir (São Paulo State, Brazil). J Appl Remote Sens 17. https://doi.org/10.1117/1.jrs.17.014509
Barçante B, Nascimento NO, Silva TFG et al (2020) Cyanobacteria dynamics and phytoplankton species richness as a measure of waterbody recovery: response to phosphorus removal treatment in a tropical eutrophic reservoir. Ecol Indic 117. https://doi.org/10.1016/j.ecolind.2020.106702
Beghelli FG, Pompêo ML, Páscoli M et al (2016) Can a one-sampling campaign produce robust results for water quality monitoring? A case of study in Itupararanga reservoir, SP, Brazil. Acta Limnol Bras 28. https://doi.org/10.1590/s2179-975x3115
Betterle N, Ballottari M, Hienerwadel R et al (2010) Dynamics of zeaxanthin binding to the photosystem II monomeric antenna protein Lhcb6 (CP24) and modulation of its photoprotection properties. Arch Biochem Biophys 504. https://doi.org/10.1016/j.abb.2010.05.016
Biamont-Rojas IE, Cardoso-Silva S, Bitencourt MD et al (2023) Ecotoxicology and geostatistical techniques employed in subtropical reservoirs sediments after decades of copper sulfate application. Environ Geochem Health 45. https://doi.org/10.1007/s10653-022-01362-1
Borghini F, Colacevich A, Bargagli R (2007) Water geochemistry and sedimentary pigments in northern Victoria Land lakes, Antarctica. Polar Biol 30. https://doi.org/10.1007/s00300-007-0274-2
Brown S, Colman B (1963) Oscillaxanthin in lake sediments. Limnol Oceanogr 8. https://doi.org/10.4319/lo.1963.8.3.0352
Brown SR, McIntosh HJ, Smol JP (1984) Recent paleolimnology of a meromictic lake: fossil pigments of photosynthetic bacteria. SIL Proceedings 1922-2010:22. https://doi.org/10.1080/03680770.1983.11897499
Buchaca T, Kosten S, Lacerot G et al (2019) Pigments in surface sediments of South American shallow lakes as an integrative proxy for primary producers and their drivers. Freshw Biol 64. https://doi.org/10.1111/fwb.13317
Buzelli GM, da Cunha-Santino MB (2013) Diagnosis and analysis of water quality and trophic state of Barra Bonita reservoir. SP. Revista Ambiente e Agua 8. https://doi.org/10.4136/ambi-agua.930
Campregher R, Martins RC (2017) O “Modelo Broa” e a produção de conhecimento científico sobre o meio ambiente. Desenvolvimento e Meio Ambiente 40. https://doi.org/10.5380/dma.v40i0.49243
Cardoso-Silva S, Ferreira PADL, Figueira RCL et al (2018) Factors that control the spatial and temporal distributions of phosphorus, nitrogen, and carbon in the sediments of a tropical reservoir. Environ Sci Pollut Res 25:31776–31789
Cardoso-Silva S, Ferreira T, Pompêo MLM (2013) Diretiva quadro d’água: Uma revisão crítica e a possibilidade de aplicação ao brazil. Ambiente e Sociedade 16. https://doi.org/10.1590/S1414-753X2013000100004
Cardoso-Silva S, Mizael JO, Frascareli D et al (2021) Paleolimnological evidence of environmental changes in seven subtropical reservoirs based on metals, nutrients, and sedimentation rates. Catena (Amst) 206. https://doi.org/10.1016/j.catena.2021.105432
Cardoso-Silva S, Mizael JOSS, Frascareli D et al (2022) Geochemistry and sedimentary photopigments as proxies to reconstruct past environmental changes in a subtropical reservoir. Environ Sci Pollut Res 29. https://doi.org/10.1007/s11356-022-18518-2
Casali SP, Dos Santos ACA, De Falco PB, Do Carmo Calijuri M (2017) Influence of environmental variables on saxitoxin yields by Cylindrospermopsis raciborskii in a mesotrophic subtropical reservoir. J Water Health 15. https://doi.org/10.2166/wh.2017.266
Cazotti RI, Gomes ACF, Nascimento M, Mozeto A (2006) Geocronologia isotópica (210 Pb e 226Ra) de sedimentos límnicos: determinação de velocidades e taxas de sedimentação, e de idades. In: Mozeto A, Umbuzeiro G, Wilson J (eds) Métodos de coleta, análises físico-químicas e ensaios biológicos e ecotoxicológicos de sedimentos de água doce, 1st edn. Cubo, São Carlos, pp 37–57
CETESB (2016) Relatório de qualidade das águas interiores do Estado de São Paulo 2015. Cetesb, São Paulo
Chen N, Bianchi TS, Bland JM (2003) Implications for the role of pre- versus post-depositional transformation of chlorophyll-a in the Lower Mississippi River and Louisiana shelf. Mar Chem 81. https://doi.org/10.1016/S0304-4203(02)00138-X
Chen N, Bianchi TS, McKee BA (2005) Early diagenesis of chloropigment biomarkers in the lower Mississippi River and Louisiana shelf: Implications for carbon cycling in a river-dominated margin. Mar Chem 93. https://doi.org/10.1016/j.marchem.2004.08.005
Chen N, Bianchi TS, McKee BA, Bland JM (2001) Historical trends of hypoxia on the Louisiana shelf: application of pigments as biomarkers. Org Geochem 32. https://doi.org/10.1016/S0146-6380(00)00194-7
Cunha DGF, Calijuri MDC (2011) Variação sazonal dos grupos funcionais fitoplanctônicos em braços de um reservatório tropical de usos múltiplos no estado de são paulo (Brasil). Acta Bot Bras 25. https://doi.org/10.1590/S0102-33062011000400009
da Anjinho PS, MAGA B, Costa CW, Mauad FF (2021) Environmental fragility analysis in reservoir drainage basin land use planning: a Brazilian basin case study. Land Use Policy 100. https://doi.org/10.1016/j.landusepol.2020.104946
David GS, Castro Campanha PMG, de Maruyama LS, Carvalho ED (2016) Artes de pesca artesanal nos reservatórios de Barra Bonita e Bariri: monitoramento pesqueiro na Bacia do Médio Rio Tietê. Bol Inst Pesca 42. https://doi.org/10.20950/1678-2305.2016v42n1p29
De Souza Beghelli FG, Frascareli D, Pompêo MLM, Moschini-Carlos V (2016) Trophic state evolution over 15 years in a tropical reservoir with low nitrogen concentrations and cyanobacteria predominance. Water Air Soil Pollut 227. https://doi.org/10.1007/s11270-016-2795-1
dos Santos ML, Dörr F, Dörr FA et al (2022) Permanent occurrence of Raphidiopsis raciborskii and cyanotoxins in a subtropical reservoir polluted by domestic effluents (Itupararanga reservoir, São Paulo, Brazil). Environ Sci Pollut Res 29. https://doi.org/10.1007/s11356-021-16994-6
Fogg GE, Belcher JH (1961) Pigments from the bottom deposits of an English Lake. New Phytologist 60. https://doi.org/10.1111/j.1469-8137.1961.tb06246.x
Fonseca MF, Matias LF (2015) ANÁLISE DO USO DA TERRA E DO COMPONENTE CLINOGRÁFICO POR MEIO DE GEOPROCESSAMENTO: O ENTORNO DO RESERVATÓRIO DE SALTO GRANDE - SP. Boletim de Geografia 32. https://doi.org/10.4025/bolgeogr.v32i3.20888
Frascareli D, Beghelli FGDS, Da Silva SC, Carlos VM (2015) Heterogeneidade espacial e temporal de variáveis limnológicas no reservatório de Itupararanga associadas com o uso do solo na Bacia do Alto Sorocaba-SP. Revista Ambiente Água 10. https://doi.org/10.4136/ambi-agua.1715
Frascareli D, Cardoso-Silva S, de Oliveira Soares-Silva Mizael J et al (2018) Spatial distribution, bioavailability, and toxicity of metals in surface sediments of tropical reservoirs, Brazil. Environ Monit Assess 190. https://doi.org/10.1007/s10661-018-6515-8
Gasparini Fernandes Cunha D, de Melo F, Lima V, Menegante Néri A et al (2017) Uptake rates of ammonium and nitrate by phytoplankton communities in two eutrophic tropical reservoirs. Int Rev Hydrobiol 102. https://doi.org/10.1002/iroh.201701900
Gomes MN, Perbiche-Neves G, Naliato DA (2012) Limnology of two contrasting hydroelectric reservoirs (storage and run-of-river) in Southeast Brazil. In: Samadi-Boroujeni, H (ed) Hydropower - practice and application, 1st edn. In Tech, London, pp 167 -184
Guilizzoni P, Massaferro J, Lami A et al (2009) Palaeolimnology of Lake Hess (Patagonia, Argentina): multi-proxy analyses of short sediment cores. Hydrobiologia 631. https://doi.org/10.1007/s10750-009-9818-5
Halac S, Mengo L, Guerra L et al (2020) Paleolimnological reconstruction of the centennial eutrophication processes in a sub-tropical South American reservoir. J South Am Earth Sci 103. https://doi.org/10.1016/j.jsames.2020.102707
Hammer Ø, Harper DAT, Ryan PD (2001) Past: Paleontological Statistics Software Package for education and data analysis. Palaeontol Electron 4:1–9
Hou D, He J, Lü C et al (2014) Spatial variations and distributions of phosphorus and nitrogen in bottom sediments from a typical north-temperate lake, China. Environ Earth Sci 71. https://doi.org/10.1007/s12665-013-2683-6
Itoh N, Tani Y, Nagatani T, Soma M (2003) Phototrophic activity and redox condition in Lake Hamana, Japan, indicated by sedimentary photosynthetic pigments and molybdenum over the last ∼250 years. J Paleolimnol 29. https://doi.org/10.1023/A:1024407210928
Jeffrey SW, Mantoura RFC, Wright SW (1997) Phytoplankton pigments in oceanography: guidelines to modern methods. UNESCO, Paris
Jiménez L, Romero-Viana L, Conde-Porcuna JM, Martínez CP (2015) Sedimentary photosynthetic pigments as indicators of climate and watershed perturbations in an alpine lake in southern Spain. Limnetica 34. https://doi.org/10.23818/limn.34.33
Laissaoui A, Mas JL, Hurtado S et al (2013) Radionuclide activities and metal concentrations in sediments of the Sebou Estuary, NW Morocco, following a flooding event. Environ Monit Assess 185. https://doi.org/10.1007/s10661-012-2922-4
Lan C, Ji Y, Wang J, Yang H (2021) Water quality restoration of a drinking water outlet area in a eutrophic reservoir using hypolimnetic oxygenation in Southwest China. Pol J Environ Stud 30. https://doi.org/10.15244/pjoes/124776
Leavitt PR, Findlay DL (1994) Comparison of fossil pigments with 20 years of phytoplankton data from eutrophic Lake 227, Experimental Lakes Area, Ontario. Can J Fish Aquat Sci 51. https://doi.org/10.1139/f94-232
Leavitt PR, Hodgson DA (2001AD) Sedimentary pigments. In: Smol JP, Birks HJB, Last WM (eds) Tracking environmental change using lake sediments. Volume 3: Terrestrial, algal, and siliceous indicators, 1st Kluwer Academic Publishers, Dordrecht, pp 295–325
Legendre P, Legendre L (1998) Numerical ecology. Elsevier Science, Amsterdam
Leme-Pompeu S, Mucare W (1983) A hundred years of Barra Bonita History. <https://barrabonita.sp.gov.br/?page=livro-100-anos-de-historia>
Louda JW, Li J, Liu L, et al (1998) Chlorophyll-a degradation during cellular senescence and death. In: Org Geochem 29. https://doi.org/10.1016/S0146-6380(98)00186-7
Makri S, Lami A, Lods-Crozet B, Loizeau JL (2019) Reconstruction of trophic state shifts over the past 90 years in a eutrophicated lake in western Switzerland, inferred from the sedimentary record of photosynthetic pigments. J Paleolimnol 61. https://doi.org/10.1007/s10933-018-0049-5
Manfredini FN, Guandique MEG, Rosa AH (2015) A história ambiental de Sorocaba. Sorocaba: Unesp – Câmpus Experimental de Sorocaba. Unesp- <http://www.sorocaba.unesp.br/Home/Eventos191/historia-ambiental-editora-ebook.pdf>, Sorocaba
Mariani CF, Pompêo MLM (2008) Potentially bioavailable metals in sediment from a tropical polymictic environment-Rio Grande Reservoir, Brazil. J Soils Sediments 8. https://doi.org/10.1007/s11368-008-0018-0
Martins D, Marchi SR, Costa NV et al (2011) Levantamento de plantas aquáticas no reservatório de salto grande. Americana-SP. Planta Daninha 29. https://doi.org/10.1590/S0100-83582011000100025
Meguro M (2000) Metodos em Ecologia. Universidade de São Paulo, São Paulo.
Minhoni RTDA, Pinheiro MPMA, Filgueiras R, Zimback CRL (2017) Sensoriamento remoto aplicado ao monitoramento de macrófitas aquáticas no reservatório de barra, Bonita, SP. IRRIGA 22:. https://doi.org/10.15809/irriga.2017v22n2p330-342
Mizael JD, Cardoso-Silva S, Frascareli D et al (2020) Ecosystem history of a tropical reservoir revealed by metals, nutrients and photosynthetic pigments preserved in sediments. Catena (Amst) 184. https://doi.org/10.1016/j.catena.2019.104242
Moraes MD, Rodrigues RD, Podduturi R et al (2023) Prediction of cyanotoxin episodes in freshwater: a case study on microcystin and saxitoxin in the Lobo Reservoir, São Paulo State, Brazil. Environments - MDPI 10. https://doi.org/10.3390/environments10080143
Mosley LM (2015) Drought impacts on the water quality of freshwater systems; review and integration. Earth Sci Rev 140. https://doi.org/10.1016/j.earscirev.2014.11.010
Nishimura PY, Moschini-Carlos V, Pompêo MLM et al (2008) Phytoplankton primary productivity in Rio Grande and Taquacetuba branches (Billings Reservoir, Sao Paulo, Brazil). SIL Proceedings 1922-2010:30. https://doi.org/10.1080/03680770.2008.11902081
Overmann J, Sandmann G, Hall KJ, Northcote TG (1993) Fossil carotenoids and paleolimnology of meromictic Mahoney Lake, British Columbia, Canada. Aquat Sci 55. https://doi.org/10.1007/BF00877257
Pamplona-Silva MT, Gonçalves LC, Marin-Morales MA (2018) Genetic toxicity of water contaminated by microcystins collected during a cyanobacteria bloom. Ecotoxicol Environ Saf 166. https://doi.org/10.1016/j.ecoenv.2018.09.090
Periotto NA, Tundisi JG (2013) Ecosystem Services of UHE Carlos Botelho (Lobo/Broa): a new approach for management and planning of dams multiple-uses. Braz J Biol 73. https://doi.org/10.1590/s1519-69842013000300003
Pompêo M, Moschini-Carlos V (2022) Assessment of water quality degradation in the Carlos Botelho Reservoir (Broa) (Itirapina, São Paulo State, Brazil), using Sentinel 2 satellite images. Engenharia Sanitaria e Ambiental 27. https://doi.org/10.1590/S1413-415220210002
Reuss N, Conley DJ, Bianchi TS (2005) Preservation conditions and the use of sediment pigments as a tool for recent ecological reconstruction in four Northern European estuaries. Mar Chem 95. https://doi.org/10.1016/j.marchem.2004.10.002
Reuss NS, Anderson NJ, Fritz SC, Simpson GL (2013) Responses of microbial phototrophs to late-Holocene environmental forcing of lakes in south-west Greenland. Freshw Biol 58. https://doi.org/10.1111/fwb.12073
Reuss NS, Hammarlund D, Rundgren M et al (2010) Lake ecosystem responses to Holocene climate change at the subarctic tree-line in Northern Sweden. Ecosystems 13. https://doi.org/10.1007/s10021-010-9326-5
Reynolds CS (2006) The ecology of Phytoplankton. Cambridge University, Cambridge
Rocha Junior CA, Costa MR, Menezes RF et al (2018) Water volume reduction increases eutrophication risk in tropical semi-arid reservoirs. Acta Limnol Bras 30. https://doi.org/10.1590/s2179-975x2117
Rodrigues EHC, Vicentin AM, Machado LDS et al (2019) Phytoplankton, trophic state and ecological potential in reservoirs in the State of São Paulo, Brazil. Revista Ambiente e Agua 14. https://doi.org/10.4136/ambi-agua.2428
Romero-Viana L, Keely BJ, Camacho A et al (2009) Photoautotrophic community changes in Lagunillo del Tejo (Spain) in response to lake level fluctuation: two centuries of sedimentary pigment records. Org Geochem 40. https://doi.org/10.1016/j.orggeochem.2008.11.010
Rosen MR, Van Metre PC (2010) Assessment of multiple sources of anthropogenic and natural chemical inputs to a morphologically complex basin, Lake Mead, USA. Palaeogeogr Palaeoclimatol Palaeoecol 294. https://doi.org/10.1016/j.palaeo.2009.03.017
Rowan KS (1989) Photosynthetic pigments of algae. Cambridge University, Cambridge
Sanger JE (1988) Fossil pigments in paleoecology and paleolimnology. Palaeogeogr Palaeoclimatol Palaeoecol 62. https://doi.org/10.1016/0031-0182(88)90061-2
Schubert N, García-Mendoza E (2008) Photo inhibition in red algal species with different carotenoid profiles. J Phycol 44. https://doi.org/10.1111/j.1529-8817.2008.00590.x
Schüller SE, Allison MA, Bianchi TS et al (2013) Historical variability in past phytoplankton abundance and composition in Doubtful Sound, New Zealand. Cont Shelf Res 69. https://doi.org/10.1016/j.csr.2013.09.021
Shotbolt L, Hutchinson SM, Thomas AD (2006) Sediment stratigraphy and heavy metal fluxes to reservoirs in the southern Pennine uplands, UK. J Paleolimnol 35. https://doi.org/10.1007/s10933-005-1594-2
Silva RT (2015) Águas e saneamento na macro metrópole paulista: o desafio da integração de escopos. Rev Iberoamerican Urbanism 12:137–157
Silva SC, Nishimura PY, Padial PR et al (2014) Compartimentalização e qualidade da água : o caso da Represa Billings. Bioikos 28. https://periodicos.puc-campinas.edu.br/bioikos/issue/view/293 Acessed 15 January 2024
Smith VH, Schindler DW (2009) Eutrophication science: where do we go from here? Trends Ecol Evol 24. https://doi.org/10.1016/j.tree.2008.11.009
Strokal M, Kahil T, Wada Y, Albiac J, Bai Z, Ermolieva T, Langan S, Ma L, Oenema O, Wagner F, Zhu X, Kroeze C (2020) Cost-effective management of coastal eutrophication: A case study for the Yangtze river basin. Resour. Conserv. Recycl. 154. https://doi.org/10.1016/j.resconrec.2019.104635
Squier AH, Hodgson DA, Keely BJ (2002) Sedimentary pigments as markers for environmental change in an Antarctic lake. Org Geochem 33. https://doi.org/10.1016/S0146-6380(02)00177-8
Tse TJ, Doig LE, Leavitt PR et al (2015) Long-term spatial trends in sedimentary algal pigments in a narrow river-valley reservoir, Lake Diefenbaker, Canada. J Great Lakes Res 41. https://doi.org/10.1016/j.jglr.2015.08.002
Tundisi JG, Matsumura-Tundisi T (2013) The ecology of UHE Carlos Botelho (Lobo-Broa Reservoir) and its watershed, São Paulo, Brazil. Freshw Rev 6. https://doi.org/10.1608/frj-6.2.727
Tundisi JG, Matsumura-Tundisi T, Tundisi JEM et al (2015) A bloom of cyanobacteria (Cylindrospermopsis raciborskii) in UHE Carlos Botelho (Lobo/Broa) reservoir: a consequence of global change? Braz J Biol 75.https://doi.org/10.1590/1519-6984.24914
US EPA United States Environmental Protection Agency (1996) Method 3050B. Acid digestion of sediments, sludges and soil. . Revision 2. December. https://www.epa.gov/sites/default/files/2015-06/documents/epa-3050b.pdf. Accessed 26 January 2015
Vallentyne JR (1957) Carotenoids in a 20,000-year-old sediment from Searles Lake, California. Arch Biochem Biophys 70. https://doi.org/10.1016/0003-9861(57)90076-0
van Vliet MTH, Zwolsman JJG (2008) Impact of summer droughts on the water quality of the Meuse river. J Hydrol (Amst) 353. https://doi.org/10.1016/j.jhydrol.2008.01.001
Vicentin AM, Rodrigues EHC, Moschini-Carlos V, Pompêo MLM (2018) Is it possible to evaluate the ecological status of a reservoir using the phytoplankton community? Acta Limnol Bras 30. https://doi.org/10.1590/s2179-975X13717
Wengrat S, Bennion H, de Ferreira PAL et al (2019) Assessing the degree of ecological change and baselines for reservoirs: challenges and implications for management. J Paleolimnol 62. https://doi.org/10.1007/s10933-019-00090-4
Wright SW (2005) Analysis of phytoplankton populations using pigment markers. Workshop on pigment analysis of Antarctic microorganisms. University of Malaya, June 20-July 1
Xue L, Hou P, Zhang Z et al (2020) Application of systematic strategy for agricultural non-point source pollution control in Yangtze River basin, China. Agric Ecosyst Environ 304. https://doi.org/10.1016/j.agee.2020.107148
Yacobi YZ, RFC M, Llewellyn CA (1991) The distribution of chlorophylls, carotenoids and their breakdown products in Lake Kinneret (Israel) sediments. Freshw Biol 26. https://doi.org/10.1111/j.1365-2427.1991.tb00503.x
Züllig H (1981) On the use of carotenoid stratigraphy in lake sediments for detecting past developments of phytoplankton. Limnol Oceanogr 26. https://doi.org/10.4319/lo.1981.26.5.0970
Acknowledgements
The authors are grateful to the UNESP Post-Doctoral Program (PROPe 13/2022), the Department of Environmental Engineering (ICTS- UNESP); the Ecology Department at the Biosciences Institute of the University of Sao Paulo, and the Chemistry Department at the Oceanographic Institute of the University of Sao Paulo. The authors thank two anonymous reviewers for their constructive comments which have improved the quality of our manuscript.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Funding
Financial support for this work was provided by FAPESP (Fundacao de Amparo a Pesquisa do Estado de Sao Paulo, grants 2014/22581–8, 2016/17266–1, 2016/15397–1, and 2019/10845–4), CAPES (Coordenacao de Aperfeicoamento de Pessoal de Nivel Superior, PNPD and DAAD grant 88887.165060/2018–00), and the UNESP Post-Doctoral Program (PROPe 13/2022).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design as follows: Sheila Cardoso-Silva: conceptualization, writing original draft, formal analysis, investigation, and editing; Juliana Oliveira Soares Silva Mizael: conceptualization, writing original draft, formal analysis, and investigation; Paulo Alves de Lima Ferreira: formal analysis and writing review; Daniele Frascareli: formal analysis, investigation, and writing review. Rubens Cesar Lopes Figueira: formal analysis and writing review. Eduardo Vicente: formal analysis and writing review. Marcelo Pompeo: supervision, funding acquisition, project administration, and writing review. Viviane Moschini-Carlos: supervision, funding acquisition, project administration, and writing review. All authors commented, read, and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Thomas Hein
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 49 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cardoso-Silva, ., Mizael, J.S.S., Frascareli, D. et al. Past environmental changes: using sedimentary photosynthetic pigments to enhance subtropical reservoir management. Environ Sci Pollut Res 31, 22994–23010 (2024). https://doi.org/10.1007/s11356-024-32574-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-32574-w