Abstract
Current vector control strategies based on synthetic chemicals are not eco-friendly against non-target organisms; hence, alternative approaches are highly required. Commercially purchased oil of Mentha spicata (Spearmint) and Eucalyptus citriodora (Citriodora) were examined against the medical pest Cx. quinquefasciatus (Say) and their non-toxicity on the aquatic species was evaluated. Chemical screening with gas chromatography coupled with mass spectrometry (GC–MS) analysis revealed a total of 14 and 11 compounds in Citriodora and Spearmint oils, respectively, with the highest peak (%) at carvone (70.44%) and isopulegol (30.4%). The larvicidal activity on the fourth instar larvae of Cx. quinquefasciatus showed dose-dependent mortality and significance at a 100 ppm concentration 48 h post-treatment with Citriodora (76.4%, P ≤ 0.001) and Spearmint (100%, P ≤ 0.001). Additionally, the photomicrograph of the fourth instar larvae revealed significant physical abnormalities in the head and midgut tissues post-exposure to Spearmint and Citriodora oils. Moreover, the histological assay revealed severe damage in the epithelial cells and gut lumen 2 to 24 h post-treatment. The repellency percentage of adult Culex mosquitoes was prominent across both oils at 150 ppm 210 min post-exposure. Non-target toxicity on the aquatic predator showed both essential oils (Spearmint oil (17.2%) and Citriodora oil (15.2%)) are safer at the maximum treatment (200 ppm) compared to temephos (75.4% at 1 ppm). The in silico screening of phyto-compounds derived by both essential oils with BeeTox (online server) showed no contact toxicity to the honey bee Apis mellifera. Overall, the present research revealed that Spearmint and Citriodora essential oils and their active phyto-compounds were toxic to Cx. quinquefasciatus and harmless to the aquatic predator and honey bee.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mosquitoes, vectors of diseases like dengue and malaria, pose global health and economic challenges (Giunti et al. 2023). Malaria alone has caused over 247 million cases, with a mortality rate exceeding 90%, particularly affecting young children in sub-Saharan Africa (Tolle 2009; Benelli et al. 2020a; Paton et al. 2021). Moreover, climate change is expected to expand the Aedes mosquito species, elevating the risk of dengue for 4.7 billion people by 2070 (Malavige et al. 2023). Despite efforts, implementing effective mosquito control strategies, especially in the field, remains challenging (Benelli and Senthil-Nathan 2019; Benelli et al. 2020b). Diseases causing mild to severe disorders that are transmitted by Culex mosquitoes highlight the need for targeted control measures (Mazzara et al. 2023). Targeting aquatic and immobile larval mosquitoes offers a promising approach (Fillinger and Lindsay 2011). However, the widespread use of synthetic larvicides faces challenges like environmental harm and resistance (Agathokleous et al. 2023a; Benelli 2019; Haddi et al. 2023). As resistance grows, the effectiveness of commercial pesticides against filarial vectors diminishes, potentially increasing disease transmission (Hafez 2023). These identified challenges prompted us to seek an environmentally and health-centered alternative for mosquito vector control (Agathokleous et al. 2023b). Natural plant-based insecticides have emerged as a promising solution in the quest for effective alternatives (Benelli et al. 2020b). Plant-derived secondary molecules, serving as chemical defenses against herbivorous insects, exhibit properties such as feeding deterrence, insect growth influence, and toxic effect induction on mosquitoes (Dubey et al. 2010; Benelli et al. 2019). Many botanicals with insecticidal effects contain volatile oils, known as essential oils (EOs), found in plant parts like leaves, flowers, stems, and roots (Pavela 2015). These EOs, characterized by their volatility and aromatic nature, contribute to the distinctive scent and flavor of various plants (Pavela 2013, 2014).
Commercial EOs, versatile for various applications like sprays, lotions, candles, and diffusers, offer individuals the flexibility to choose the method that aligns with their preferences and needs (Osanloo et al. 2017; Benelli et al. 2018a, b). Possessing natural properties such as antibacterial, leishmanicidal, and larvicidal effects, as well as repellent activity, EOs are not only selectively effective against target organisms but also environmentally degradable and non-toxic to non-target species, making them favorable for mosquito larvae control (Osanloo et al. 2018). Existing research presents diverse studies on mosquito vector management using EOs (Khanavi et al. 2010; Vasantha-Srinivasan et al. 2017, 2018; Chellappandian et al. 2018a, b). Due to the qualitative and quantitative variations in chemical content, EOs exhibit changing toxicity against different dengue vectors (Cheng et al. 2003). The larvicidal actions of EOs are attributed to complex mixtures of volatile compounds, including terpenes, phenols, aldehydes, and esters (Benelli et al. 2018a, b). Current research emphasizes bio-rational plants from diverse regions and their insecticidal properties (Radhakrishnan et al. 2023a, b). While past literature explored the larvicidal potential of EOs, mechanistic insights into their actions on arthropod vectors remain unclear (Benelli and Senthil-Nathan 2019; Aziz et al. 2023; Kalvikkarasan et al. 2023; Vasantha-Srinivasan et al. 2024).
Mentha spicata (Spearmint), a member of the Lamiaceae family and native to Europe and Asia, is now cultivated globally, favoring temperate climates and thriving in moist habitats (Hudz et al. 2023). Known for its aromatic leaves containing EOs, including carvone, Spearmint is utilized for culinary and medicinal purposes, particularly in treating respiratory and digestive issues (de Araujo Moysés et al. 2023). Eucalyptus citriodora, part of the Eucalyptus genus in the Myrtaceae family and native to Australia, has been introduced and cultivated in various climates worldwide, including Asia, Africa, and the Americas (Amri et al. 2023). The EO derived from its leaves is particularly rich in citronellal and contributes to its lemon scent, making it valuable in the fragrance industry and as an ingredient in insect repellents (Khedhri et al. 2023). Both Spearmint and Citriodora EOs are recognized as environmentally friendly alternatives to synthetic insecticides. The importance of testing non-target toxicity against beneficial organisms such as Toxorhynchites splendens (commonly known as the mosquito fish or gambusia) is crucial in the development and use of pesticides or other control methods (Pavela et al. 2019; Vasantha-Srinivasan et al. 2023).
Overall, the objectives of the present investigation were as follows: (i) the chemical characterization of commercial Spearmint (M. spicata) and Citriodora (E. citriodora) oils using the gas chromatography-mass spectrometry (GC–MS) technique; (ii) exploring the toxic larvicidal action of EOs on the medical pest Cx. quinquefasciatus; (iii) detecting the midgut toxicity of EOs on the fourth instar larvae of the filarial vector; (iv) estimating the repellent actions of EOs on the adult Culex mosquito; and (v) In-vitro and in-silico screening of the impact of EOs on the aquatic mosquito predator and honey bee.
Materials and methods
Based on the previous research on EOs with insecticidal properties, the two vital essential bio-active oils, Citriodora (E. citriodora) and Spearmint (M. spicata), were designated. These two EOs were obtained commercially from Katyani Exports India Pvt. Ltd. (Delhi, India) and studied for their larvicidal activity and impact on the histological aspects of the midgut of the larval intestine of Cx. quinquefasciatus. The commercially purchased oils were cosmetic grade (100%), and the volatile oil was acquired through steam distillation of the herbal leaves and then further packed into sterile glass containers.
Rearing of mosquitoes
The Cx. quinquefasciatus mosquito culture for the present study was maintained in the lab without exposure to pesticides and vectors. In addition, the cultures were maintained at specific laboratory conditions (27 ± 2 °C, relative humidity [RH], and 75–85% with a 14 h:10 h light/dark (L:D) photoperiod). Brewer’s yeast and dog biscuits in a proportion of 1:5 were provided as food to the larvae that emerged from the eggs. The pupae that emerged from the larvae were collected and placed in a plastic container with 250 mL of water. This plastic container was then kept in a 60 cm × 60 cm × 60 cm breeding cage covered with a nylon net for adult emergence. Next, Petri dishes with cotton swabs containing a 10% sucrose solution and wet raisins (dried grapes) were placed in the breeding cages for the emerging mosquitoes to eat. After 3 days of mosquito emergence, the female mosquitoes were fed with sucrose for 6 h and then delivered to a hen that was kept inside the mosquito breeding cage for blood feeding throughout the night. The fourth instar larvae were then used for conducting experiments. Our earlier research protocol was used for the insect-rearing methods (Chellappandian et al. 2018b).
Identification of essential oil components by GC–MS
The isolated EOs were dissolved using ethyl alcohol at a 1:1 ratio. Next, 2 μL of the samples were dissolved in high-performance liquid chromatography (HPLC)-grade methanol and subjected to JEOL GC mate II GC–MS (Agilent Technologies 6890N Network GC system (Mumbai, India)) equipped with a secondary electron multiplier. The column (HP5) was then fused with silica (50 m × 0.25 mm ID). The chemical characterization of the EO was adapted from the previous methodology (Vasantha-Srinivasan et al. 2018). The identification of the compounds was assessed through GC attached to a mass spectrometer. Afterward, the EO chemical structure was investigated by interpreting the GC–MS mass spectrum through the National Institute of Standard and Technology (NIST) database.
Preparation of test solutions for larvicidal activity
One gram of each test EO was transferred into a 100 mL standard volumetric flask and made up with ethanol. From this stock solution, serial volumes such as 0.25, 0.5, 0.75, 1, 2.5, 5, 7.5, and 10.0 mL of the solution were pipetted into other flasks and chlorine-free tap water was added to obtain 25, 50, 75, and 100 ppm of solution. This solution was then used for the larvicidal bioassay.
Larvicidal bioassay
The larvicidal bioassay of the EOs was conducted following the World Health Organization (2005) process with minor alterations. The fourth instar larvae were kept inside disposable plastic cups (200 mL) containing 100 mL of dechlorinated water using 25, 50, 75, and 100 ppm of EOs. During the treatment period, the larval diet was supplied in individual treatment cups, specifically if a significant mortality rate in the control was recorded. The conditions remained uninterrupted for 24 h, and the rate of mortality was logged 48 h post-treatment. The dead larvae numbers were identified at the initial experiment stages (0 and 24 h). The experiments were replicated five times, and each replication set included a control treated with an aqueous solution of dimethyl sulfoxide (DMSO (0.5%)). Larvicidal activity that displayed at least 50% mortality (lethal concentration (LC)50) and 90% mortality (LC90) within 48 h was estimated and considered for further experiments. The statistical analyses of the larvicidal assays were conducted based on the methodology of Finney (1971) Probit. In addition, the mortality (%) in the treatments was calculated with the formula adapted from Abbott’s (1925) formula (1 and 2).
Histological analysis
For the histological tests, the treated and control newly ecdysed fourth instar larvae of Cx. quinquefasciatus were separated from the laboratory culture raised with the larval diet and later incorporated with Citriodora (100 ppm) and Spearmint (75 ppm) oil. Twenty-four hours later, larval survival was observed. Afterward, the larvae were fixed in the bouins reagent post-exposure (6, 12, 24, and 48 h) for 24 h. Further experiments were conducted following the adapted protocol of Senthil-Nathan et al. (2008).
Repellent assay
The repellent activities of both EOs on the adult Culex mosquitoes treated with diverse doses (25, 50, 75, and 100 ppm) were analyzed with the improved procedure (Chellappandian et al. 2019). The whole investigation was reviewed and permitted by the institution ethical committee board (Manonmaniam Sundaranar University, Tirunelveli, India). Previously mated (5–6 days) post-emerged female gravid mosquitoes (100 total) were starved for 1 day without any blood meal in the mosquito cages (45 cm × 35 cm × 5 cm). A discernible strike with a marker pen was made on a fixed area (4 cm × 12 cm) on the forearm of every three human volunteers while the rest of the arm was protected with sleeves of paper. As a control, 0.5% DMSO was placed on one forearm of the volunteer following a similar procedure. The Cx. quinquefasciatus and Ae. aegypti mosquitoes were tested from 18.00 h to 02.00 h and 06.00 h to 14.00 h, respectively. The mosquitoes that landed on the forearm were considered and those on the hands were shaken off before they sucked the blood. The percentage of repellency was estimated using the following adapted formula (3):
where Ta represents the mosquito numbers in the control group, and Tb represents the mosquito numbers in the treatment group.
BeeTox toxicity prediction
The BeeTox is an artificial intelligence (AI) open online tool utilized to predict the acute toxicity of ligands/chemicals on honey bees (Moreira-Filho et al. 2021). In the present study, the free BeeTox online servers were used to predict the bee toxicities of the phyto-compounds of both Spearmint (11 chemicals) and Citriodora (14 chemicals) EOs identified through the GC–MS technique. Furthermore, the AI tool was designed to divide the compounds into two independent datasets according to the honey bee exposure type (contact and oral). The entire experimental protocol was adapted based on that of Moreira-Filho et al. (2021).
Non-target toxicity assay
The effects of Spearmint and Citriodora EOs on the aquatic predator and beneficial species Toxorhynchites splendens (Theobald) were determined using the previously adapted protocol (Yogarajalakshmi et al. 2020). The authentication of the aquatic predator species was given by the zoologist of Manonmaniam Sundaranar University, Tirunelveli, India. The non-target species were retained in the same ecological habitat of the aquatic mosquito larvae (dengue and filarial vector), which were isolated in separate tanks with water (47 cm diameter and 27 cm depth) at 27 ± 2 °C and 75% RH, and fed with the second instar larvae of Cx. quinquefasciatus. The aquatic predator (Tx. splendens) was exposed to different dosages (50, 100, 150, and 200 ppm) of both EOs, and the obtained results were related to the toxicity of the synthetic chemical Temephos at the 1.0 ppm dosage. In the individual treatment, a total of 20 replications were executed, and five replicates were used as a control treatment (without any chemicals). The mortality (%) was experimental 24 h post-treatment.
Statistical analyses
The mortality data of the experiment were assessed using analysis of variance (ANOVA of arcsine, logarithmic, and square root transformed percentages), and the results were expressed as a mean value of five replicates. Similarly, Tukey’s multiple range test (significance at p < 0.05) was used to analyze significant differences between the treatments using the Minitab®16 software program. The SigmaPlot version 11 of the MicroCal software was utilized for plotting the line and bar graphs. The lethal dosages necessary to develop 50 and 90% mortality (LC50 and LC90) in the larvae 24 h post-treatment were examined utilizing Probit analysis with an interval dependability (95%) using the Minitab®16 statistical software.
Results
Identification of chemical components in the essential oils
The EO is screened through GC–MS to identify the major components responsible for the insecticidal activity of the Cx. quinquefasciatus larvae. In the Spearmint oil, the GC–MS analysis of its components revealed about 11 components (Table 1). Out of these, the major components were estragole (22.7%) and carvone (70.44%), and the minor components were β-pinene (3.41%) and α-pinene (1.43%). The chromatogram is presented in (Fig. 1A). The GC–MS analysis of Citriodora oil revealed 14 identified compounds (Table 2 and Fig. 1B). The chief ingredients of Eucalyptus citriodora oil were citronellal (9.34%), citronellol (17.39%), isopulegol (30.44%), and 2,6-Octadiene 2,6, dimethyl. The minor components identified were x-pinene (1.35%), eucalyptol (1.75%), caryophyllene (4.59%), 4-bromo-n-butyl (3.36%), and 2-methylhexacosane (2.88%).
Larvicidal activity
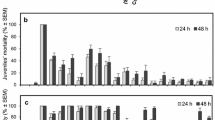
The larvicidal actions of Citriodora EO 24 h post-treatment displayed reliant mortality activity on the fourth instar larvae of Cx. quinquefasciatus. The mortality percentage was significant at the maximum dosage (100 ppm) with 76.4% (F4,20 = 41.31, P ≤ 0.001) 24 h post-treatment, whereas 25 ppm caused 30.2% mortality (Fig. 2A). Similarly, at 48 h post-treatment with Citriodora (100 ppm), a prominent mortality rate of 83.5% (F4,20 = 18.54, P ≤ 0.001) was seen, though it was not significant with the other treatment dosages of 75 ppm (73.1%, F4,20 = 13.76, P ≤ 0.001) and 50 ppm (67.9%, F4,20 = 19.99, P ≤ 0.001), respectively (Fig. 2B). Correspondingly, the larvicidal action of Spearmint EO displayed a significant mortality rate across 24 and 48 h of treatment against the fourth instar larvae. The maximum dosage (100 ppm) only produced an 18.77% (F4,20 = 19.55, P ≤ 0.001) mortality rate 24 h post-treatment (Fig. 2C). Despite 100 ppm Spearmint oil causing 100% (F4,20 = 17.88, P ≤ 0.001) mortality 48 h post-treatment, it was significant with the other treatment dosages of 50 ppm (45.21%, F4,20 = 19.33, P ≤ 0.001) and 25 ppm (39.11%, F4,20 = 15.61, P ≤ 0.001), respectively (Fig. 2D). The LC50 values of Spearmint oil against the fourth instar larvae of the filarial vector were seen at 51.00 ppm (24 h) and 32.99 ppm (48 h), while the LC90 values were recorded at 146.5 ppm (24 h) and 133.64 ppm (48 h). Correspondingly, the Citriodora oil delivered LC50 values at 45.36 ppm (24 h) and 29.15 ppm (48 h), and LC90 values at 214.5 ppm (24 h) and 196.3 ppm (48 h) (Table 3). Notably, all EOs tested delivered an LC50 value > 500 ppm 48 h post-treatment and were considered as effective larvicides. The confidence intervals (95%) of the larvae mortality percentage were also determined. Overall, the results showed that the Citriodora and Spearmint oils were effective if their confidence interval did not overlap and were significantly different.
Mortality of different essential oils against IV instar Cx. quinquefasciatus. A Citriodora essential oil treatment post 24 h. B Citriodora essential oil treatment post 48 h. C Spearmint essential oil treatment post 24 h. D Spearmint essential oil treatment post 48 h. Means (± SEM) followed by the same letters above bars indicate no significant difference (P ≤ 0.05) according to a Tukey’s test
Photomicrography analysis
The fourth instar larvae of the filarial vector showed significant abnormalities in their midgut and head position post-exposure (24 h) to Citriodora and Spearmint oils (Fig. 3). Moreover, the midgut epithelial layer, gut lumen, and anal segments were severely damaged by the Citriodora oil treatment (Fig. 3B) as compared to that of the control (Fig. 3A). Similarly, the head, anal, and midgut segments collapsed following the Spearmint oil treatment (Fig. 3C), whereas the control appeared normal with a clear head, midgut tissues, and anal segment layers.
Histological studies
The mid-gut of the arthropod larvae was subdivided into two different regions, each including one characteristic cell type. Depending on their stage of development, the clear cells displayed different degrees of apical swelling into the gut lumen, reducing intercellular contacts with the neighboring cells and displaying nuclei and brush border degeneration, as shown in the Cx. quinquefasciatus control (Fig. 4H). Dark cells showed normal intercellular contacts along the whole lateral plasma membranes, normal nuclei, a well-developed brush border, and a normal adhesive basal lamina, as observed in the control sections of Cx. quinquefasciatus.
Lateral section (LS) of mid gut of fourth instar of Cx. quinquefasciatus larvae treated with Citriodora oil (CO). A Four hours after treatment. B Eight hours after treatment. C Sixteen hours after treatment. D Twenty-four hours after treatment. E Larvae treated with Spearmint oil 4 h after treatment. F Spearmint oil 8 h after treatment. G Spearmint oil 16 h after treatment. H Spearmint oil 24 h of treatment. I Untreated control. PM, peritrophic membrane; GL, gut lumen; EL, epithelial layer
Four hours post-treatment
When compared with the control group (Fig. 4I), the Citriodora-treated larvae did not show much change. In the Spearmint-treated larvae, even after the 4-h treatment, there were signs of gut wall and epithelial cell degeneration and non-distinct larval segments when compared with the control. In addition, the peritrophic membrane also started to undergo degeneration (Fig. 4E and F).
Eight hours post-treatment
The analysis of the anterior mid-gut showed a progression of swollen clear cells, vacuoles, and degenerated nuclei in both oil treatments, with the effect being more pronounced in the Citriodora-treated larvae (Fig. 4B). In the posterior mid-gut, disruption of the junctional complexes among the dark cells progressed apically, together with their cytoplasmic and nuclear lysis, local detachment from the basal lamina, and degeneration of microvilli. In the Spearmint-treated larvae, the epithelial cells were collapsed. Moreover, the epithelial cells showed signs of degeneration when compared to those of the control (Fig. 4B and I).
Sixteen hours post-treatment
Treatment with either EO was followed by severe degeneration of the larval gut cells. Additionally, there was complete degeneration of the peritrophic membrane and epithelial cells in the Citriodora EO treatment (Fig. 4A–C). The epithelial cells showed signs of degeneration in the Citriodora EO-treated larvae though the peritrophic membrane was visible (Fig. 4C).
Twenty-four hours post-treatment
Maximum epithelial cells are vacuolated and degenerated. Gut-histological examination results revealed significant toxicity with EOs among the two diverse parts of the mid-gut epithelium based on the exposure period. The key cyto-pathological changes were outsized vacuoles of dissimilar sizes with damaged membranes at the epithelial cell’s apical sides, wide cellular damage to the peritrophic membrane and gut lumen, and disruption of the brush border cells. Additionally, larval segmentation was completely lost, indicating heavy necrosis, and the nucleus showed complete dissolution.
Repellent activity
The repellency percentage was significant against both the Spearmint and Citriodora oil treatments, up to the maximum protection time of 210 min. The Spearmint oil treatment delivered a maximum protection percentage (98.4%, F4,20 = 27.33, P ≤ 0.001) up to the maximum protection period of 210 min (Fig. 5A). In addition, the Citriodora EO treatment delivered a significant repellent percentage (97.3%, F4,20 = 18.93, P ≤ 0.001) until the maximum protection time of 210 min (Fig. 5B).
Non-target toxicity
BeeTox (in silico) screening of EOs
The in silico toxicological screening of the major metabolites of Spearmint and Citriodora EOs showed that all compounds were non-toxic against the honey bee Apis mellifera under acute contact toxicity. Despite this, all compounds (except isomenthol, eucalyptol, 3-hydroxypropanenitrile, 5-(1-bromo-1-methyl-ethyl)-2-methyl-cyclohexanone, and bicyclononadiene diepoxide) induced acute oral toxicity against the bees with a maximum LD50 from caryophyllene oxide (194.8894 μg/bee), isomenthone (100.7472 μg/bee), and paramethasone (95.2246 μg/bee), respectively (Table 4).
Aquatic predator (in vitro) toxicity screening
The aquatic mosquito predator (Tx. splendens) toxicity test showed that the maximum treatment dosage of temephos (1 ppm) caused the highest morality rate in the aquatic predator (75.4%) and it is significant on other dosages of EOs and the control (F4,20 = 14.73, P ≤ 0.001). The highest treatment dosage (200 ppm) of the EOs, however, caused minimal toxicity in the mosquito predator (Spearmint oil (17.2%) and Citriodora oil (15.2%)), respectively) (Fig. 6).
Discussion
Botanical compounds displayed higher ovicidal, larvicidal, and repellent actions towards the initial or adult phases of the arthropod vectors, disturbing respiratory, endocrine, water balance, and nervous systems (Benelli and Cornara 2021; Şengül Demirak and Canpolat 2022). Alkaloids, aromatic chemicals, and EOs derived from herbs are frequently utilized for botanical-based natural repellents (Pavela and Benelli 2016). Traditionally, EOs have been competently utilized against diverse medically challenging insects and crop pests across nations (Sedaghat et al. 2011; Sánchez-Gómez et al. 2022). Additionally, they are possible replacements for synthetic chemicals generally utilized on mosquitoes. EOs are intricate natural blends of phyto-compounds that contain volatile molecules, which are usually terpenes and sesquiterpenes (hydrocarbons), phenylpropenes, and oxygenated hydrocarbons, in different ranges (Moemenbellah-Fard et al. 2020; Noorpisheh Ghadimi et al. 2020; Osanloo et al. 2020). With the ever-growing interest in the use of EOs as an alternative for successful vector control, the present study is aimed at understanding and drawing a meaningful comparison between the impact of different EOs on mosquito larvicidal action, their toxicity, and their effect on the histological profile of the hindgut of Cx. quinquefasciatus larvae. Based on the LC50 values of the 24-h and 48-h treatments, of the different oils studied, Spearmint oil showed an LC50 value of 51.38 ppm during the 24-h exposure while that of Citriodora was 45.36 ppm. Additionally, in the 24-h treatment, Citriodora showed mortality rates of 68% and 74% at 50 and 100 ppm, respectively, while Spearmint oil showed the least mortality (16%) at a 100 ppm concentration.
To select an efficient EO, two criteria should be met: (i) the EOs need to deliver significant mortality rates across the standard larvicidal examinations (WHO 2009) to attain a lethal concentration (LC50) ≤ 100 ppm, and (ii) phyto-chemicals and their peak area percentages should be screened (Pavela 2015). Based on these criteria, all EOs tested showed good larvicidal potential with a LC50 less than 100 ppm. Comparable studies have been conducted by Chaiphongpachara et al. (2020) in screening seven marketable herbal oils (East Indian lemongrass, cassia, bay, cinnamon, holy basil, ginger, and sweet basil) for their larvicidal actions on the Ae. aegypti dengue larvae.
The results showed that cinnamon oil induced significant larvicidal actions with an LC50 (0.03 ppm) and LC90 (0.04 ppm). The mortality increased with the concentration, which was also observed in our study where the mortality was dose-dependent. When the treated larvae were visible for 48 h in our study, the EOs tested showed low LC50 values (less than 50 ppm), indicating progressive toxicity throughout exposure. Interestingly, when the LC90 values were compared, Spearmint and Citriodora oils showed LC90 values of 68.68 and 50.61 ppm, respectively, in a 24-h exposure period, indicating highly active EOs against mosquito larvae (Pavela 2009). The LC90 value is also considered important, which most researchers have failed to recognize even though it indicates the dosage that delivered the determined decline towards the ensuing generations of mosquitoes. When we compare the LC90 values between the two EOs, though the 24-h LC50 values are quite close for the Citriodora (45.36) and Spearmint (51 ppm) oils, the LC90 values are remarkably different (146.51 ppm for Spearmint oil and 214.5 ppm for Citriodora oil). This indicates that Spearmint oil has a greater potential as a larvicide. It is well-proven that larvicidal efficacy, a minimal LC50 value that does not always require minimal concentration, is adequate to induce mortality. Likewise, this is true in our case for all EOs, which is in agreement with other studies where the estimated LC50 and LC90 values for EOs derived from Thymus vulgare and Satureja hortensis for the Culex larvae were not the same (de Morais et al. 2007). In addition, Rattan (2010) revealed that the LC50 (36 ppm) and LC90 (47 ppm) dosages were determined for Ae. aegypti larvae post-treatment with Piper permucronatum-derived volatile oils. Also, the LC50 and LC90 values of the EO derived from Cinnamomum osmophloeum were 36 ppm and 79 ppm, respectively, when used against the mosquito vector (Cheng et al. 2004). Similarly, the dosages required to attain larval mortality in the EO can be determined by diverse aspects including the selection of the larval instar, cuticle penetration ability of the selected compounds, and ambient temperature, along with the mechanistic actions (Tripathi et al. 2003, 2009; Pavela et al. 2009; Rattan 2010). These underlying factors could explain the difference in mortality of the larvae.
The bioactivity of the EOs towards the arthropod mosquito larvae is related to their chemical composition. In our study, the major constituents of the selected EOs were estragole and carvone for Spearmint oil and citronellal for Citriodora oil. Carvone is reported to possess larvicidal action, which is similar to the Spearmint oil action in our study. These chemicals have also been reported to have insecticidal properties (Santos et al. 2011; Govindarajan et al. 2012; Bullangpoti et al. 2018). The Eucalyptus species, which belongs to the Myrtaceae family, is a prevalent harvesting plant in South Asian countries including India, South Korea, and Vietnam. Amid the different Eucalyptus species, Eucalyptus citriodora-derived EOs are enriched with a higher percentage of the bio-active molecule citronellal. Similarly, our phyto-chemical screening of E. citriodora oil also revealed a higher peak area percentage in citronellal (7.47%), citronellol (4.11%), dl-isopulegol (40.42%), and isopulegol (12.14%). Recent studies by Kweka et al. (2016) reported the mosquito larvicidal activity of carvone. Earlier studies by Rahuman et al. (2008) and Nasir et al. (2015) have reported the mosquito larvicidal activity of ( −)-isopulegol and other monoterpenes against An. gambiae. Additionally, the mosquito larvicidal potential of β-sitosterol was illustrated by Ryan and Byrne (1988). The two monoterpenes, carvone and ( −)-isopulegol, are among the chemical constituents identified by GC–MS analysis in Spearmint and Citriodora oils and they have contributed towards the larvicidal activity of these EOs. While the insecticidal activity is attributable to the chemical constituents of the EO tested, in most cases, as EOs are complex mixtures of several compounds, the mechanism that causes this activity against immature larvae is often difficult to identify because the biological effects are due to the individual components acting as synergic mixtures of these components (Maggi and Benelli 2018). Previous screenings in the literature mostly concluded that CVOs were more active against the insect pests as compared to their isolated phyto-compounds. Phenylpropanoids and monoterpene hydrocarbons, recognized as the significant compound classes, induced higher larval mortality. In our study, carvone and estragole, which are present in Spearmint oil, and citronellal and isopulegol, present in Citriodora oil, showed good larvicidal activity with an LC50 less than or equal to 50 ppm, thus confirming the constituents responsible for larvicidal performance. It is hypothesized that (Enan 2001; Moola et al. 2023) the lipophilicity of the components plays an important role in larvicidal action, which enhances the passage through the cuticle of the insect, thereby generating toxicity as evidenced by the greater activity of these EOs in our present study. When mosquitoes are exposed to EOs or their constituents, the compounds can disrupt their normal nervous system function. This disruption can lead to hyperactivity, causing the affected insects to exhibit abnormal and erratic behaviors (Patrick et al. 2006).
Histopathological observations on the larvae treated with the EOs tested showed greater damage to mid-gut cells that was progressive over different exposure periods (4-, 8-, 16-, and 24-h treatments) compared to no damage in the control. In all EOs tested, the damage to the peritrophic membrane, epithelial membrane, and foregut, midgut, and hind-gut basement membrane regions was well-illustrated. The structural breakdown resulted in gut lumen leakage and led to major functional malformations. These were mainly due to the volatile constituents present in the EOs such as carvone in Spearmint, and citronellal and dl-isopulegol in Citriodora. Moreover, our results are similar to those of previous reports (Thanigaivel et al. 2018; Chellappandian et al. 2019; Karthi et al. 2020). The degenerative effect of the midgut cells in all Spearmint- and Citriodora-treated larvae seems to agree with the study of intense degenerative reactions in the anterior, posterior, and thorax midgut regions of Ae. aegypti larvae caused by targeting ion transporting cells in the gastric caeca of the thorax region and the posterior and anterior midgut of the epithelial cells where osmoregulation connected machineries including H + V-ATPase are significantly expressed in the dengue mosquito larvae (Volkman and Peters 1989). Overall, the essential components (mainly terpenes and sesquiterpenes) act against larvae in modulating the detoxification enzyme coupled with a cytotoxic effect in the immature stages, bringing about greater susceptibility to Spearmint and Citriodora EOs.
By examining various biological endpoints, including different species and life stages, non-target toxicity screening provides a more comprehensive understanding of the potential risks associated with a chemical (Vasantha-Srinivasan et al. 2016; Ponsankar et al. 2016). Non-target toxicity screening helps assess the impact of chemicals on ecosystems, including aquatic and terrestrial environments. This is crucial for protecting biodiversity and maintaining the health of natural ecosystems (Pisa et al. 2015; Stenrod et al. 2016). Our present screening of Spearmint and Citriodora EOs against the non-target mosquito predator had less impact or harmless actions as compared to that of the commercial chemical. This information can be used for understanding the non-target effects of botanical oils and allows for the development of appropriate risk mitigation measures to minimize environmental impact. Moreover, in silico toxicity predictions enable the early identification of potentially hazardous compounds, allowing researchers and regulators to prioritize chemicals for further investigation or regulatory scrutiny (Zulkifli et al. 2023). The present in silico predictions of the major metabolites of Spearmint and Citriodora using the BeeTox server offer a valuable and efficient approach to assessing the potential non-toxic impact of phyto-chemicals, thus contributing to advancements in safety assessment, regulatory compliance, and ethical considerations in scientific research and product development.
Conclusions
The two EOs studied for their larvicidal effects indicated that Spearmint oil is more toxic to larval stages due to its larvicidal action as well as its considerable mortality rate when the larvae are exposed for longer periods (48 h). In search of alternative strategies for mosquito larval control, the use of plant-based EOs like Spearmint and Citriodora oils, along with integrated mosquito management strategies, will provide a sustainable solution. Moreover, the EOs delivered minimal or less toxicity against the aquatic predator Tx. splendens (in vitro) and honey bees (in silico). Moving forward, exploring the mechanisms of action of these EOs and their individual components can provide valuable insights. Furthermore, investigating the toxicity of individual chemicals within the EOs and their impact on key detoxifying enzymes in dengue larvae can deepen our understanding of the intricate interactions involved in mosquito control. This avenue of research holds significant promise for the development of targeted and efficient mosquito control strategies. Overall, the study highlights the potential use of natural commercial oils as effective larvicidal agents and emphasizes the importance of integrating plant-based EOs into mosquito management practices.
Data availability
All data are available within the manuscript. Also, all materials used in this research were cited in this work.
References
Abbott WS (1925) A method of computing the effectiveness of an insecticide. J Econ Entomol 18(2):265–267
Agathokleous E, Blande JD, Calabrese EJ, Guedes RNC, Benelli G (2023a) Stimulation of insect vectors of pathogens by sublethal environmental contaminants: a hidden threat to human and environmental health? Environ Pollut 336:122422
Agathokleous E, Blande JD, Masui N, Calabrese EJ, Zhang J, Sicard P, Guedes RN, Benelli G (2023b) Sublethal chemical stimulation of arthropod parasitoids and parasites of agricultural and environmental importance. Environ Res 237:116876
Amri I, Khammassi M, Ben Ayed R, Khedhri S, Mansour MB, Kochti O, Pieracci Y, Flamini G, Mabrouk Y, Gargouri S, Hanana M (2023) Essential oils and biological activities of Eucalyptus falcata, E. sideroxylon and E. citriodora growing in Tunisia. Plants 12(4):816
Aziz AT, Panneerselvam C, Edward-Sam E (2023) Toxicity of plants as insecticides against human pathogenic mosquito vectors of Saudi Arabian strains-a review. Entomol Res 53:323–332
Benelli G (2019) Managing mosquitoes and ticks in a rapidly changing world–facts and trends. Saudi J Biol Sci 26(5):921–929
Benelli G, Cornara D (2021) Arthropod vectors and vector-borne pathogens: know your enemy for not succumbing the battle. Entomol Gen 41(5):415–418
Benelli G, Senthil-Nathan S (2019) Together in the fight against arthropod-borne diseases: a one health perspective. Int J Environ Res Public Health 16(23):4876
Benelli G, Pavela R, Petrelli R, Cappellacci L, Canale A, Senthil-Nathan S, Maggi F (2018a) Not just popular spices! Essential oils from Cuminum cyminum and Pimpinella anisum are toxic to insect pests and vectors without affecting non-target invertebrates. Ind Crops Prod 124:236–243
Benelli G, Pavela R, Giordani C, Casettari L, Curzi G, Cappellacci L, Petrelli R, Maggi F (2018b) Acute and sub-lethal toxicity of eight essential oils of commercial interest against the filariasis mosquito Culex quinquefasciatus and the housefly Musca domestica. Ind Crops Prod 112:668–680
Benelli G, Pavela R, Zorzetto C, Sánchez-Mateo CC, Santini G, Canale A, Maggi F (2019) Insecticidal activity of the essential oil from Schizogyne sericea (Asteraceae) on four insect pests and two non-target species. Entomol Gen 39(1):1–9
Benelli G, Pavela R, Rakotosaona R, Nzekoue FK, Canale A, Nicoletti M, Maggi F (2020a) Insecticidal and mosquito repellent efficacy of the essential oils from stem bark and wood of Hazomalania voyronii. J Ethnopharmacol 248:112333
Benelli G, Petrelli R, Canale A (2020b) Arthropod-borne disease control at a glance: what’s new on drug development? Molecules 25(21):5175
Bullangpoti V, Mujchariyakul W, Laksanavilat N, Junhirun P (2018) Acute toxicity of essential oil compounds (thy-mol and 1, 8-cineole) to insectivorous guppy, Poecilia reticulata Peters, 1859. Agri Nat Res 52(2):190–194
Chaiphongpachara T, Laojun S, Wassanasompong W (2020) Screening seven commercial essential herb oils for larvicidal activity against the mosquito Aedes aegypti (Linnaeus), a vector of the dengue virus. J Appl Pharm Sci 10(7):043–050
Chellappandian M, Thanigaivel A, Vasantha-Srinivasan P, Edwin ES, Ponsankar A, Selin-Rani S, Kalaivani K, Senthil-Nathan S, Benelli G (2018a) Toxicological effects of Sphaeranthus indicus Linn. (Asteraceae) leaf essential oil against human disease vectors, Culex quinquefasciatus Say and Aedes aegypti Linn., and impacts on a beneficial mosquito predator. Environ Sci Pollut Res 25:10294–10306
Chellappandian M, Vasantha-Srinivasan P, Senthil-Nathan S, Karthi S, Thanigaivel A, Ponsankar A, Kalaivani K, Hunter WB (2018b) Botanical essential oils and uses as mosquitocides and repellents against dengue. Environ Intern 113:214–230
Chellappandian M, Senthil-Nathan S, Vasantha-Srinivasan P, Karthi S, Thanigaivel A, Kalaivani K, Sivanesh H, Stanley-Raja V, Chanthini KM, Shyam-Sundar N (2019) Target and non-target botanical pesticides effect of Trichodesma indicum (Linn) R. Br. and their chemical derivatives against the dengue vector, Aedes aegypti L. Environ Sci Pollut Res 26:16303–16315
Cheng SS, Chang HT, Chang ST, Tsai KH, Chen WJ (2003) Bioactivity of selected plant essential oils against the yellow fever mosquito Aedes aegypti larvae. Biores Technol 89(1):99–102
Cheng SS, Liu JY, Tsai KH, Chen WJ, Chang ST (2004) Chemical composition and mosquito larvicidal activity of essential oils from leaves of different Cinnamomum osmophloeum provenances. J Agri Food Chem 52(14):4395–4400
de Araujo Moysés D, dos Santos Martins HP, Ribeiro MS, da Rocha Galucio NC, de Souza RR, dos Santos Correa RM, de Arimateia Rodrigues do Rego J, Dolabela MF, Vale VV (2023) Mentha sp. essential oil and its applicability in Brazil. In: Essential oils: extraction methods and applications, vol 14, pp 125–155
de Morais SM, Facundo VA, Bertini LM, Cavalcanti ESB, dos Anjos Júnior JF, Ferreira SA, de Brito ES, de Souza Neto MA (2007) Chemical composition and larvicidal activity of essential oils from Piper species. Biochem Syst Ecol 35(10):670–675
Dubey N, Shukla R, Kumar A, Singh P, Prakash B (2010) Global scenario on the application of natural products in integrated pest management programmes. In: Natural products in plant pest management, pp 1–20. https://doi.org/10.1079/9781845936716.0001
Enan E (2001) Insecticidal activity of essential oils: octopaminergic sites of action. Comp Biochem Physiol c: Toxicol Pharmacol 130:325–337
Fillinger U, Lindsay SW (2011) Larval source management for malaria control in Africa: myths and reality. Malaria J 10(1):1–10
Finney DJ (1971) Statistical logic in the monitoring of reactions to therapeutic drugs. Method Info Med 10(04):237–245
Giunti G, Becker N, Benelli G (2023) Invasive mosquito vectors in Europe: from bioecology to surveillance and management. Acta Trop 239:106832
Govindarajan M, Sivakumar R, Rajeswari M, Yogalakshmi K (2012) Chemical composition and larvicidal activity of essential oil from Mentha spicata (Linn.) against three mosquito species. Parasitology Res 110:2023–2032
Haddi K, Nauen R, Benelli G, Guedes RNC (2023) Global perspectives on insecticide resistance in agriculture and public health. Entomol Gen 43:495–500
Hafez AM (2023) First comprehensive report of the resistance of Culex quinquefasciatus Say (Diptera: Culicidae) to commonly used insecticides in Riyadh, Saudi Arabia. Heliyon 9(1):e12709
Hudz N, Kobylinska L, Pokajewicz K, Horčinová Sedláčková V, Fedin R, Voloshyn M, Myskiv I, Brindza J, Wieczorek PP, Lipok J (2023) Mentha piperita: essential oil and extracts, their biological activities, and perspectives on the development of new medicinal and cosmetic products. Molecules 28(21):7444
Karthi S, Vasantha-Srinivasan P, Ganesan R, Ramasamy V, Senthil-Nathan S, Khater HF, Radhakrishnan N, Amala K, Kim TJ, El-Sheikh MA, Krutmuang P (2020) Target activity of Isaria tenuipes (Hypocreales: Clavicipitaceae) fungal strains against dengue vector Aedes aegypti (Linn.) and its non-target activity against aquatic predators. J Fungi 29:196
Kalvikkarasan K, Muthusamy J, Prabhakaran VS, Pandiyan R, Narayanaswamy R, Muthu K, Sengodan K, Raja G, Sengottayan SN, Selvaraj GK, Rajakrishnan R (2023) In-vitro and in-silico analysis of methanolic crude extracts of Mountain knotgrass Aerva lanta (L.) against two lepidopteran pests and non-target species. Toxin Rev 21:1–16
Khanavi M, Norouzi M, Tabatabaee H, Noudeh AS, Safavi SB, Shafiee A (2010) Chemical compositions and antiviral effects of the essential oil of Zataria multiflora Boiss. and Origaunum majorana L. J Med Plants 9:128–137
Khedhri S, Marwa K, Boukhris Bouhachem S, Pieracci Y, Flamini G, Gargouri S, Amri I Hamrouni L (2023) Tunisian Eucalyptus essential oils: exploring their potential for biological applications. Plant Biosyst- Int J Deal Aspects Plant Biol 1–26. https://doi.org/10.1080/11263504.2023.2287531
Kweka EJ, Lima TC, Marciale CM, de Sousa DP (2016) Larvicidal efficacy of monoterpenes against the larvae of Anopheles gambiae. Asian Pac J Trop Biomed 6(4):290–294
Maggi F, Benelli G (2018) Essential oils from aromatic and medicinal plants as effective weapons against mosquito vectors of public health importance. In: Benelli G, Mehlhorn H (eds) Mosquito-borne diseases. Parasitology research monographs, vol 10. Springer, Cham, pp 69–129. https://doi.org/10.1007/978-3-319-94075-5_6
Malavige GN, Sjö P, Singh K, Piedagnel JM, Mowbray C, Estani S, Lim SCL, Siquierra AM, Ogg GS, Fraisse L, Ribeiro I (2023) Facing the escalating burden of dengue: challenges and perspectives. PLOS Glob Public Health 3(12):e0002598
Mazzara E, Spinozzi E, Maggi F, Petrelli R, Fiorini D, Scortichini S, Perinelli DR, Bonacucina G, Ricciardi R, Pavela R, Benelli G (2023) Hemp (Cannabis sativa cv. Kompolti) essential oil and its nanoemulsion: prospects for insecticide development and impact on non-target microcrustaceans. Ind Crops Prod 203:117161
Moemenbellah-Fard MD, Abdollahi A, Ghanbariasad A, Osanloo M (2020) Antibacterial and leishmanicidal activities of Syzygium aromaticum essential oil versus its major ingredient. Eugenol Flav Frag J 35(5):534–540
Moola AK, Ayyadurai T, Balasubramani S, Vignesh R, Mohan PK, Sathish S, Diana RKB (2023) Chemical com-position and larvicidal activity against Aedes aegypti larvae from Hyptis suaveolens (L.) Poit essential oil. J Nat Pest Res 3:100018
Moreira-Filho JT, Braga RC, Lemos JM, Alves VM, Borba JV, Costa WS, Kleinstreuer N, Muratov EN, Andrade CH, Neves BJ (2021) BeeTox AI: an artificial intelligence-based web app to assess acute toxicity of chemicals to honey bees. Artif Intel Life Sci 1:100013
Nasir S, Batool M, Hussain SM, Nasir I, Hafeez F, Debboun M (2015) Bioactivity of oils from medicinal plants against immature stages of dengue mosquito Aedes aegypti (Diptera: Culicidae). Inter J Agri Biol 17(4):843–847
Noorpisheh Ghadimi S, Abedini MR, Sarkari B, Savardashtaki A, Mikaeili F (2020) Neobalantidium coli: first molecular identification from the Eurasian wild boar, Susscrofa in Bushehr province, southwestern Iran. Vet Med Sci 6(1):142–146
Osanloo M, Amani A, Sereshti H, Abai MR, Esmaeili F, Sedaghat MM (2017) Preparation and optimization nanoemulsion of Tarragon (Artemisia dracunculus) essential oil as effective herbal larvicide against Anopheles stephensi. Ind Crops Prod 109:214–219
Osanloo M, Sereshti H, Sedaghat MM, Amani A (2018) Nano-emulsion of Dill essential oil as a green and potent larvicide against Anopheles stephensi. Environ Sci Pollut Res 25:6466–6473
Osanloo M, Arish J, Sereshti H (2020) Developed methods for the preparation of electro spun nanofibers containing plant-derived oil or essential oil: a systematic review. Polym Bull 77:6085–6104
Paton RS, Kamau A, Akech S, Agweyu A, Ogero M, Mwandawiro C, Mturi N, Mohammed S, Mpimbaza A, Kariuki S, Otieno NA (2021) Malaria infection and severe disease risks in Africa. Science 373(6557):926–931
Patrick ML, Aimanova K, Sanders HR, Gill SS (2006) P-type Na+/K+-ATPase and V-type H+-ATPase expression patterns in the osmoregulatory organs of larval and adult mosquito Aedes aegypti. J Exp Biol 209:4638–4651
Pavela R (2009) Larvicidal property of essential oils against Culex quinquefasciatus Say (Diptera: Culicidae). Ind Crops Prod 30(2):311–315
Pavela R (2013) Efficacy of naphthoquinones as insecticides against the house fly, Musca domestica L. Ind Crops Prod 43:745–750
Pavela R (2014) Insecticidal properties of Pimpinella anisum essential oils against the Culex quinquefasciatus and the non-target organism Daphnia magna. J Asia-Pacif Entomol 17:287–293
Pavela R (2015) Essential oils for the development of eco-friendly mosquito larvicides: a review. Ind Crops Prod 76:174–187
Pavela R, Benelli G (2016) Ethnobotanical knowledge on botanical repellents employed in the African region against mosquito vectors–a review. Exper Parasitol 167:103–108
Pavela R, Vrchotová N, Tříska J (2009) Mosquitocidal activities of thyme oils (Thymus vulgaris L.) against Culex quinquefasciatus (Diptera: Culicidae). Parasitol Res 105:1365–1370
Pavela R, Benelli G, Petrelli R, Cappellacci L, Lupidi G, Sut S, Dall’Acqua S, Maggi F (2019) Exploring the insecticidal potential of boldo (Peumus boldus) essential oil: toxicity to pests and vectors and non-target impact on the microcrustacean Daphnia magna. Molecules 24(5):879
Pisa LW, Amaral-Rogers V, Belzunces LP, Bonmatin JM, Downs CA, Goulson D, Kreutzweiser DP, Krupke C, Liess M, McField M, Morrissey CA (2015) Effects of neonicotinoids and fipronil on non-target invertebrates. Environ Sci Pollut Res 22:68–102
Ponsankar A, Vasantha-Srinivasan P, Senthil-Nathan S, Thanigaivel A et al (2016) Target and non-target toxicity of botanical insecticide derived from Couroupita guianensis L. flower against generalist herbivore, Spodoptera litura Fab. and an earthworm, Eisenia foetida Savigny. Ecotoxicol Environ Saf 133:260–270
Radhakrishnan N, Karthi S, Raghuraman P, Ganesan R, Srinivasan K, Edwin ES, Ganesh-Kumar S, Mohd Esa N, Senthil-Nathan S, Vasantha-Srinivasan P, Krutmuangh P (2023a) Chemical screening and mosquitocidal activity of essential oil derived from Mikania scandens (L.) Willd. against Anopheles gambiae Giles and their non-toxicity on mosquito predators. All Life 16(1):2169959
Radhakrishnan N, Vasantha-Srinivasan P, Wadaan MA, Baabbad A, Vinayagam R, Kang SG (2023b) STITCH, physicochemical, ADMET, and in silico analysis of selected mikania constituents as anti-inflammatory agents. Processes 11:1722
Rahuman AA, Gopalakrishnan G, Venkatesan P, Geetha K (2008) Isolation and identification of mosquito larvicidal com-pound from Abutilon indicum (Linn.) sweet. Parasitol Res 102:981–988
Rattan RS (2010) Mechanism of action of insecticidal secondary metabolites of plant origin. Crop Prot 29(9):913–920
Ryan MF, Byrne O (1988) Plant-insect coevolution and inhibition of acetylcholinesterase. J Chem Ecol 14:1965–1975
Sánchez-Gómez S, Pagán R, Pavela R, Mazzara E, Spinozzi E, Marinelli O, Zeppa L, Morshedloo MR, Maggi F, Canale A, Benelli G (2022) Lethal and sublethal effects of essential oil-loaded zein nanocapsules on a zoonotic disease vector mosquito, and their non-target impact. Ind Crops Prod 176:114413
Santos SR, Melo MA, Cardoso AV, Santos R, de Sousa DP, Cavalcanti SC (2011) Structure–activity relationships of larvicidal monoterpenes and derivatives against Aedes aegypti Linn. Chemosphere 84(1):150–153
Sedaghat MM, Dehkordi AS, Abai MR, Khanavi M, Mohtarami F, Abadi YS, Rafi F, Vatandoost HASSAN (2011) Larvicidal activity of essential oils of Apiaceae plants against malaria vector. Anopheles Stephensi Iranian J Arthropod-Borne Diseases 5(2):51
Şengül Demirak MŞ, Canpolat E (2022) Plant-based bio-insecticides for mosquito control: impact on insecticide resistance and disease transmission. Insects 13(2):162
Senthil-Nathan S, Choi MY, Seo HY, Paik CH, Kalaivani K, Kim JD (2008) Effect of azadirachtin on acetylcholinesterase (AChE) activity and histology of the brown planthopper Nilaparvata lugens (Stål). Ecotoxicol Environ Saf 70(2):244–250
Stenrod M, Almvik M, Eklo OM, Gimsing AL, Holten R, Künnis-Beres K, Larsbo M, Putlies L, Siimes K, Turka I, Uusi-Kämppä J (2016) Pesticide regulatory risk assessment, monitoring, and fate studies in the northern zone: recommendations from a Nordic-Baltic workshop. Environ Sci Pollut Res 23:15779–15788
Thanigaivel A, Vasantha-Srinivasan P, Edwin ES, Ponsankar A, Selin-Rani S, Chellappandian M, Kalaivani K, Senthil-Nathan S, Benelli G (2018) Development of an eco-friendly mosquitocidal agent from Alangium salvifolium against the dengue vector Aedes aegypti and its biosafety on the aquatic predator. Environ Sci Pollut Res 25:10340–10352
Tolle MA (2009) Mosquito-borne diseases. Curr Prob Pediatr Adolesc Health Care 39:97–140
Tripathi AK, Prajapati V, Kumar S (2003) Bioactivities of l-carvone, d-carvone, and dihydrocarvone toward three stored product beetles. J Econom Entomol 96(5):1594–1601
Tripathi AK, Upadhyay S, Bhuiyan M, Bhattacharya PR (2009) A review on prospects of essential oils as biopesticide in in-sectpest management. J Pharmacogn Phytother 1:52–63
Vasantha-Srinivasan P, Senthil-Nathan S, Thanigaivel A, Edwin ES, Ponsankar A, Selin-Rani S, Pradeepa V, Sakthi-Bhagavathy M, Kalaivani K, Hunter WB, Duraipandiyan V (2016) Developmental response of Spodoptera litura Fab. to treatments of crude volatile oil from Piper betle L. and evaluation of toxicity to earthworm, Eudrilus eugeniae Kinb. Chemosphere 155:336–347
Vasantha-Srinivasan P, Senthil-Nathan S, Ponsankar A, Thanigaivel A, Edwin ES, Selin-Rani S, Chellappandian M, Pradeepa V, Lija-Escaline J, Kalaivani K, Hunter WB (2017) Comparative analysis of mosquito (Diptera: Culicidae: Aedes aegypti Liston) responses to the insecticide Temephos and plant derived essential oil derived from Piper betle L. Ecotoxicol Environ Saf 139:439–446
Vasantha-Srinivasan P, Chellappandian M, Senthil-Nathan S, Ponsankar A, Thanigaivel A, Karthi S, Edwin ES, Selin-Rani S, Kalaivani K, Maggi F, Benelli G (2018) A novel herbal product based on Piper betle and Sphaeranthus indicus essential oils: toxicity, repellent activity and impact on detoxifying enzymes GST and CYP450 of Aedes aegypti Liston (Diptera: Culicidae). J Asia-Pac Entomol 21(4):1466–1472
Vasantha-Srinivasan P, Shanmuga-Priya S, Han YS, Radhakrishnan N, Karthi S, Elsadek MF, Mustafa AEZM, Senthil-Nathan S (2023) Phyto-chemical screening, insecticidal potential and detoxifying enzyme inhibition of Ficus auriculata (Lour.) extracts, against the mosquito vector and non-target aquatic predator. Biocatal Agric Biotechnol 53:102864
Vasantha-Srinivasan P, Unni PKS, Karthi S, Ganesan R, Senthil-Nathan S, Chellappandian M, Radhakrishnan N, Rajagopal R, Patcharin K (2024) Bio-efficacy of chloroform crude extracts of chick weed Ageratum conyzoides (Linn.) against the tobacco cutworm Spodoptera litura (Linn.) and their non-toxicity against the beneficial earthworm. J King Saud Univ-Sci 36(1):102930
Volkman A, Peters W (1989) Investigations on the midgut caeca of mosquito larvae. II. Functional aspects. Tissue Cell 21:253–261
WHO (2009) Guidelines for efficacy testing of mosquito repellents for human skin. WHO/HTM/NTD/WHOPES/2009.4. Control of neglected tropical diseases. World Health Organization, Geneva
World Health Organization (2005) Guidelines for laboratory and field testing of mosquito larvicides. World Health Organization
Yogarajalakshmi P, Poonguzhali TN, Ganesan R, Karthi S, Senthil-Nathan S et al (2020) Toxicological screening of marine red algae Champia parvula (C. Agardh) against the dengue mosquito vector Aedes aegypti (Linn.) and its non-toxicity against three beneficial aquatic predators. Aquat Toxicol 222:105474
Zulkifli MH, Abdullah ZL, Yusof NISM, Fauzi FM (2023) In silico toxicity studies of traditional Chinese herbal medicine: a mini review. Curr Opin Struct Biol 80:102588
Acknowledgements
We are grateful to Prof. Dr. K. G. Sivaramakrishnan for his encouragement and support for conducting this investigation. We would also like to thank Ms. Chandini, research scholar, Division of Bio-pesticides and Environmental Toxicology, Sri Paramakalyani Centre for Excellence in Environmental Sciences, Manonmaniam Sundaranar University, Alwarkurichi, Tirunelveli, Tamil Nadu, for her support on data and statistical analyses in Minitab. The authors wish to acknowledge the support received from the “Regional Innovation Strategy (RIS)” through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (MOE) (2021RIS-002), and Department of Science and Technology (DST-FIST), India under the FIST program (SR/FIST/LS-1/2019/522).The authors extend their appreciation to the Researchers supporting project number (RSP2024R414) King Saud University, Riyadh, Saudi Arabia.
Funding
This research was supported by the “Regional Innovation Strategy (RIS)” through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (MOE) (2021RIS-002) and the Department of Science and Technology (DST-FIST), India, under the FIST program (SR/FIST/LS-1/2019/522). Also, this research was funded by King Saud University, Riyadh, Saudi Arabia Project number (RSP2024R414).
Author information
Authors and Affiliations
Contributions
Pavana Sivadasan Unni: conceptualization of the study, investigation, interpretation of results, validation, writing-original draft, supervision. Pandiyan Kirupaanntha-Rajan: conceptualization of the study, investigation, interpretation of results, validation, writing-original draft, supervision. Prabhakaran Vasantha-Srinivasan: conceptualization of the study, development of methodology, investigation, interpretation of results, validation, writing-review, supervision, project administration, resources. Sridhar Srinivasan: conceptualization of the study, investigation, interpretation of results, writing-review, supervision. Yeon Soo Han: conceptualization of the study, development of methodology, investigation, interpretation of results, writing-review, project administration, funding acquisition. Sengodan Karthi: conceptualization of the study, development of methodology, investigation, interpretation of results, validation, writing-review. Narayanaswamy Radhakrishnan: conceptualization of the study, development of methodology, investigation, interpretation of results, validation, writing-review, resources. Ki Beom Park: conceptualization of the study, investigation, interpretation of results, validation, writing-review, resources. Rajakrishnan Rajagopal: conceptualization of the study, development of methodology, investigation, interpretation of results, validation, writing-review, resources. Sengottayan Senthil-Nathan: conceptualization of the study, development of methodology, investigation, interpretation of results, writing-review, project administration.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All listed authors have approved the manuscript before submission, including the names and order of authors.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Giovanni Benelli
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Unni, P.S., Kirupaanntha-Rajan, P., Vasantha-Srinivasan, P. et al. Chemical composition and toxicity of commercial Mentha spicata and Eucalyptus citriodora essential oils on Culex quinquefasciatus and non-target insects. Environ Sci Pollut Res 31, 21610–21631 (2024). https://doi.org/10.1007/s11356-024-32249-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-32249-6