Abstract
Copper is essential for various biological processes. However, excess copper has several adverse health effects. The effects of serum copper on muscle mass are poorly understood. This study aimed to investigate the association between serum copper levels and muscle mass in the US population. We utilized National Health and Nutrition Examination Survey (NHANES) data between 2011 and 2016 for analysis. Data on serum copper, muscle mass (measured using the appendicular skeletal muscle mass index (ASMI)), and covariates were extracted and analyzed. Weighted multivariate linear regression analyses and smooth curve fittings were performed to investigate the association between serum copper levels and ASMI. Subgroup analyses stratified according to age and sex were performed. In the presence of nonlinearity, threshold effect analysis was performed using a two-piecewise linear regression model. A total of 3860 participants were included in the final analysis. Serum copper levels were negatively associated with ASMI in the fully adjusted model. Furthermore, by comparing participants in the highest and lowest tertiles of serum copper levels, we found that the ASMI decreased by 0.292 kg/m2. In the sex-stratified subgroup analysis, we observed an inverse association between serum copper levels and the ASMI in both men and women. When stratified by age, the association remained significant among participants < 40 years of age, but not among those ≥ 40 years old. Smooth curve fitting revealed a nonlinear relationship between serum copper and ASMI, with an inflection point identified at 150.6 μg/dL. Our study revealed an inverse relationship between serum copper levels and muscle mass. This finding improves the current knowledge on the impact of serum copper on muscle loss and highlights the importance of serum copper homeostasis in muscle health.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sarcopenia is an age-related syndrome characterized by the loss of muscle mass, strength, and function (Genest et al. 2021). Previous studies have reported that 10% of the elderly population in the community and half of the elderly population in nursing homes are affected by sarcopenia, leading to poorer physical performance, worse quality of life, and a higher risk of adverse outcomes and mortality (Bruyère et al. 2019, Papadopoulou et al. 2020). Considering the high prevalence and serious consequences of sarcopenia, there is an urgent need to investigate its etiology and pathogenesis.
To date, the pathophysiology of sarcopenia has not been fully elucidated. Previous studies indicated that immunological dysfunction, insulin resistance, and hormonal imbalances are closely associated with sarcopenia (Cho et al. 2022, Nishikawa et al. 2021). The role of trace elements in muscle metabolism and function has recently attracted considerable attention (van Dronkelaar et al. 2018). Magnesium deficiency is thought to be associated with muscle loss and dysfunction (Yang et al. 2022). Zinc deficiency disrupts skeletal muscle proteostasis and mitochondrial function, thereby shrinking cell volume and promoting cell death (Reddy et al. 2022). A lack of iron can impair functional capacity (Neidlein et al. 2021), whereas iron overload is associated with muscle loss (Chen et al. 2022a). Copper is an essential trace element for the human body; however, it becomes toxic if its concentration exceeds a certain threshold (Tsvetkov et al. 2022). Several studies have revealed that excessive copper is associated with adverse health outcomes, such as polycystic ovarian syndrome, heart failure, and cancers (Feng et al. 2020, Huang et al. 2019, Jiang et al. 2021). Regarding the musculoskeletal system, some researchers have identified an inverse association between serum copper and handgrip strength (Luo et al. 2023), but the effect of serum copper on muscle mass remains unexplored. Therefore, we conducted this study to investigate the association between serum copper levels and muscle mass in the US population.
Materials and methods
Data source
The NHANES is a continuous program conducted by the Centers for Disease Control and Prevention (CDC). It was designed to evaluate the health and nutritional well-being of both adults and children in the USA. The NHANES comprises data from roughly 5000 participants each year and includes household interviews, physical examinations, and laboratory results. The Ethics Review Board of the National Center for Health Statistics approved all NHANES protocols, and the participants provided consent for the utilization of their data in research endeavors (Chen et al. 2022b).
Study population
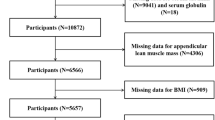
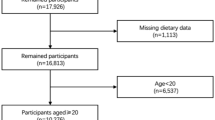
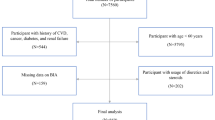
In this study, we analyzed data obtained from three 2-year cycles of NHANES (2011–2016). The eligible participants had complete data on serum copper levels, appendicular skeletal muscle mass (ASM, kg), and standing height. We excluded participants who were pregnant (n = 238), had cancer (n = 1448), or lacked data on serum copper levels (n = 21,355), ASM (n = 2527), and standing height (n = 474). (Figure 1 illustrates the screening processes.) Data on serum copper level, appendicular lean muscle mass, standing height, and covariates were extracted and analyzed for each participant.
Study variables
Serum copper
The independent variable, serum copper (μg/dL), was measured by inductively coupled plasma dynamic reaction cell mass spectrometry (ICP-DRC-MS).
Muscle mass
Dual-energy X-ray absorptiometry (DXA) scans (Hologic, Inc., Bedford, Massachusetts) provided bone and soft tissue measurements of the entire body, including the head, trunk, arms, and legs. ASM was defined as the sum of the lean muscle mass of both arms and legs (Chen et al. 2021b). ASMI (kg/m2) was calculated by dividing the ASM by the height square (ASM/ht2) (Kim et al. 2016).
Covariates
Based on previous literature, the following variables were selected as covariates (Bruno et al. 2022, Chen et al. 2022a, Chen et al. 2022b, Prokopidis &Witard 2022). The demographic variables included age, sex (man or woman), race (Hispanic, non-Hispanic White, non-Hispanic Black, and other races), education level (< high school, high school, > high school), and the family income to poverty ratio (FIPR). Based on the analytic guideline of NHANES, the FIPR was categorized as follows: low, <1.3; middle, 1.3–3.5; and high, >3.5 (Johnson et al. 2013). Participants who smoked fewer than 100 cigarettes in their lifetime were considered as never smokers. Participants who had smoked more than 100 cigarettes but had not smoked at the time of the survey were defined as former smokers. Those who had smoked 100 cigarettes in their lifetime and smoked cigarettes at the time of the survey were categorized as current smokers (Liu et al. 2019). Vigorous recreational activities were defined as active or inactive by the questionnaire “do you do any high-intensity sports, fitness, or recreational activities that cause large increases in breathing or heart rate like running or basketball for at least 10 min continuously in a typical week?” (Wu et al. 2023). Body mass index (BMI, kg/m2) was calculated using body measurements. White blood cell counts (WBC, 1000 cells/μL) and hemoglobin levels (g/dL) were measured using the Coulter® DxH 800 analyzer. Glycohemoglobin levels were measured using a G7/G8 Glycohemoglobin analyzer. Insulin levels were measured using a TOSOH AIA-900 Chemistry Analyzer. Fasting glucose levels were measured using a Roche/Hitachi Cobas C 501 Chemistry Analyzer. Serum albumin (g/dL), globulin (g/dL), total protein (g/dL), aspartate aminotransferase (AST) (IU/L), uric acid (mg/dL), and creatinine levels (mg/dL) were measured using a Beckman UniCel® DxC800 Synchron. High-density lipoprotein (HDL)-cholesterol (mg/dL), low-density lipoprotein (LDL)-cholesterol (mg/dL), total cholesterol (mg/dL), and triglyceride (mg/dL) levels were measured using Roche/Hitachi Modular P Chemistry Analyzer. Vitamin D levels were measured using high-performance liquid chromatography-tandem mass spectrometry (Bruno et al. 2022, Chen et al. 2022b). The details of the laboratory procedures, data processing, quality control, and analytic notes are available on the NHANES website (http://wwwn.cdc.gov/nchs/nhanes/) (Johnson et al. 2013).
Statistical analysis
Statistical analyses were performed using R 3.4.3 (https://www.r-project.org/) and EmpowerStats 2.0 (http://www.empowerstats.com), with P < 0.05 considered statistically significant. All estimates were calculated using the NHANES sample weights. Continuous variables were compared using a weighted linear regression model, and categorical variables were compared using a weighted chi-square test. Weighted multivariable linear regression analyses were conducted to explore the association between serum copper levels and the ASMI. Subgroup analyses stratified according to age and sex were performed. Generalized additive models and smooth curve fittings were used to detect the nonlinear relationships. In the presence of nonlinearity, a two-piecewise linear regression model was used to analyze the threshold effect.
Results
A total of 3860 participants were included in the final analysis. There were 1965 men with a mean age of 32.992 years and 1895 women with a mean age of 32.714 years. Men were more likely than women to have higher levels of hemoglobin, AST, creatinine, uric acid, albumin, total protein, glycohemoglobin, fasting glucose, LDL, triglyceride, ASM, and ASMI and lower levels of serum copper, WBC, globulin, HDL, and vitamin D (Table 1).
Association between serum copper and ASMI
Table 2 presents three sets of regression models used in this study: unadjusted model (model 1), partially adjusted model (model 2), and fully adjusted model (model 3). We observed negative associations between serum copper and ASMI in both model 1 (β = −0.006, 95% confidence interval [CI] −0.008, −0.004, P < 0.00001) and model 3 (β = −0.003, 95% CI −0.004, −0.001, P = 0.0088), but not in model 2 (β = 0.003, 95% CI 0.002, 0.005, P = 0.00006). Furthermore, by comparing participants in the highest vs. the lowest tertile of serum copper, we found that ASMI decreased by 0.292 kg/m2, P trend < 0.001 (model 3).
When stratified by sex, negative associations were observed between serum copper and ASMI in both men (model 3: β = −0.004, 95% CI −0.007, −0.000, P = 0.03940) and women (model 3: β = −0.002, 95% CI −0.004, −0.001, P = 0.01041) (Table 3).
When stratified by age, a negative association was observed between serum copper and ASMI in those aged < 40 years (model 3: β = −0.003, 95% CI −0.005, −0.001, P = 0.00445), but not in those aged 40 ≥ years (model 3: β = −0.002, 95% CI −0.006, 0.001, P = 0.14760) (Table 4).
Threshold effect analysis
Smooth curve fitting revealed a nonlinear relationship between serum copper and ASMI, with an inflection point identified at 150.6 μg/dL. When serum copper was < 150.6 μg/dL, a one-unit increase in serum copper was associated with 0.005 units reduction in ASMI (Fig. 2 and Table 5). Stratified by age and sex, nonlinear relationships were detected among women, those who aged < 40 years, and those who aged ≥ 40 years, with the inflection points identified at 134.2 μg/dL, 167.4 μg/dL, and 139.1 μg/dL, respectively (Fig. 3 and Table 6).
Discussion
Muscle health plays an important role in maintaining function and independence in older ages (Lee et al. 2022). As co-factors of metalloenzymes, minerals involved in various biological processes are indispensable for survival. A growing body of evidence suggests that essential and non-essential trace elements are correlated with body composition and metabolic syndrome (Ngu et al. 2022). Magnesium is positively correlated with muscle mass and function (Welch et al. 2017). Calcium, potassium, and sodium are essential metals for healthy and normal muscle function (van Dronkelaar et al. 2018). Iron is a double-edged sword. Literature reports that iron deficiency leads to physical dysfunction, whereas excessive iron deposition is toxic (Chen et al. 2022a, Ho et al. 2022). Copper is also an essential micronutrient, and alterations in its serum levels can potentially affect human health. Our results revealed that serum copper levels were negatively associated with the ASMI, especially in men and women aged < 40 years.
Copper is significantly involved in various physiological and pathological conditions by regulating various biological processes, including mitochondrial oxidation, phosphorylation, iron mobilization, blood coagulation, antioxidant defense, and neuropeptide maturation (Liu & Miao 2022, Xiao et al. 2022). A complex regulatory mechanism maintains copper homeostasis and prevents copper deficiency or excess (Kim et al. 2010). Copper deficiency can cause leukopenia, myeloneuropathy, cardiac hypertrophy, and heart failure (Gaier et al. 2012, Liu & Miao 2022). However, copper overload leads to the generation of free radicals, which induces oxidative stress. Previous studies have reported that excessive copper levels can lead to progressive liver injury, diabetes mellitus, metabolic syndrome, Alzheimer’s disease, and death (Chen et al. 2021a, Gaier et al. 2012, Lu et al. 2021). Some scholars have speculated that there might be a connection between increased serum copper levels and decreased muscle mass and function with age (Cruz-Jentoft &Sayer 2019, Malavolta et al. 2015). In a cross-sectional study, Wu et al. found that higher serum concentrations of copper were associated with an increased fat percentage in the limbs (Wu et al. 2023). Consistent with this, another study revealed that children with the highest serum levels of copper had an increased risks of obesity compared with those with the lowest levels (Fan et al. 2017). Furthermore, a meta-analysis reported that compared with controls, the serum copper concentration was higher in obese children (Gu et al. 2020). In a cohort study, Gaier et al. found that participants with higher copper levels displayed slower walking speeds and a longer time on the get-up-and-go test (Gaier et al. 2012). Furthermore, Lee et al. reported that hair copper concentrations were significantly higher in participants with low muscle mass than in those with normal muscle mass, even after adjusting for covariates (Lee et al. 2022). First, we observed a negative association between serum copper levels and muscle mass in the general population.
Two decades ago, researchers found that serum copper concentration increased with age (Madarić et al. 1994). It has been proposed that this change may be driven by age-related increases in oxidative stress, chronic inflammation, and hormonal changes (Malavolta et al. 2015, Milanino et al. 1993). Recently, some scholars have suggested that PM2.5 may be another reason for elevated copper levels. PM2.5 particles can adsorb and bind with heavy metals such as copper. When these particles are inhaled into the respiratory tract, copper can diffuse through the alveolar walls into the blood circulation and accumulated in the liver. When copper storage exceeds the liver’s detoxification capacity of the liver, the liver releases copper, leading to an increase in serum copper concentration.(Julian F.B. Mercer 2001; López-Alonso et al. 2006). Animal experiments have shown that serum copper levels increase with exposure to PM2.5. (Li et al. 2015). A cross-sectional study in China found that serum copper and ceruloplasmin levels were higher in workers with silicosis than those without silicosis, and these levels were positively correlated with the exposure time (Beshir et al. 2015). Other components of PM2.5, such as smoke, may also affect the serum copper levels. Women who smoked had higher serum copper concentrations than nonsmokers. (Jasińska-Starczewska et al. 2017). In addition to PM2.5, other factors such as excessive copper intake and metabolic disorders may also cause elevated serum copper concentrations (N C O’Neill and M S Tanner 1989; Julian F.B. Mercer 2001). One study has shown that long-term exposure to PM2.5 is significantly associated with an increase in the prevalence of sarcopenia (Zhang et al. 2023). In low- and middle-income countries, each 10-μg/m3 increase in average outdoor PM2.5 concentration is associated with a 0.70-kg decrease in grip strength (Lin et al. 2019). Chen et al. found that long-term exposure to PM2.5 was associated with reduced skeletal muscle mass and increased body fat mass in older people (Chen et al. 2019). Although the link between excessive copper intake and various disorders has gradually been recognized, the underlying mechanisms have not been sufficiently delineated. Some studies have revealed that elevated serum copper levels are associated with the dysregulation of sex hormones. Copper has a xenoestrogenic effect on the estradiol feedback pathway, which is assumed to form a negative feedback loop in the hypothalamus-pituitary-gonadal axis and reduces testosterone and estradiol release (Roychoudhury et al. 2016). Testosterone and estradiol are essential for maintaining skeletal muscle mass and function. They can stimulate satellite cell proliferation and limit inflammatory stress damage (Sipilä et al. 2013). Additionally, a meta-analysis confirmed that the serum copper levels were positively associated with inflammatory markers, suggesting that copper may modulate inflammation (Wang et al. 2023). Chronic low-grade inflammation has been confirmed to be an important reason of muscle loss (Chen et al. 2022b). Furthermore, elevated levels of serum copper levels can trigger oxidative stress by promoting reactive oxygen species (ROS) production and decreasing glutathione levels (Jomova &Valko 2011). Excessive ROS may damage macromolecules such as lipids, proteins, and nucleic acids, thereby causing cuproptosis (Fig. 4) (Tsvetkov et al. 2022). Therefore, it is necessary to focus on the prevention, monitoring, and management of copper overload.
Limitations
This study has some limitations. Firstly, due to the cross-sectional nature of this study, the causal relationship between serum copper levels and muscle loss could not be evaluated. Secondly, due to the lack of data on muscle strength and function, the associations between serum copper and these parameters could not be explored. Thirdly, DXA scans were performed only in 8–59-year-old participants in this study; therefore, studies targeting elderly populations, who are more susceptible to muscle loss, are still needed.
Conclusion
The present study demonstrated an inverse relationship between serum copper and muscle mass. This finding helps us understand the impact of serum copper on muscle loss and highlights the importance of serum copper homeostasis in muscle health. Further studies are required to investigate these underlying mechanisms.
Data availability
All data were extracted from the NHANES database (http://www.cdc.gov/nchs/nhanes/). Data are available from the corresponding author on a reasonable request.
Abbreviations
- NHANES:
-
National Health and Nutrition Examination Survey
- CDC:
-
Centers for Disease Control and Prevention
- DXA:
-
dual-energy X-ray absorptiometry
- ASM:
-
appendicular skeletal muscle mass
- ASMI:
-
appendicular skeletal muscle mass index
- FIPR:
-
family income to poverty ratio
- BMI:
-
body mass index
- WBC:
-
white blood cell counts
- AST:
-
aspartate aminotransferase
References
Bruno RR, Rosa FC, Nahas PC, de Branco FMS, de Oliveira EP (2022) Serum α-carotene, but not other antioxidants, is positively associated with muscle strength in older adults: NHANES 2001-2002. Antioxidants (Basel) 11:2386
Bruyère O, Beaudart C, Ethgen O, Reginster JY, Locquet M (2019) The health economics burden of sarcopenia: a systematic review. Maturitas 119:61–69
Chen C, Zhou Q, Yang R, Wu Z, Yuan H, Zhang N, Zhi M, Zhang Y, Ni X, Wang Z, Gao D, Zhu X, Cai J, Yang Z, Sun L (2021a) Copper exposure association with prevalence of non-alcoholic fatty liver disease and insulin resistance among US adults (NHANES 2011-2014). Ecotoxicol Environ Saf 218:112295
Chen L, Shivappa N, Dong X, Ming J, Zhao Q, Xu H, Liang P, Cheng M, Liu J, Sun P, Ban B (2021b) Association between appendicular skeletal muscle index and leukocyte telomere length in adults: a study from National Health and Nutrition Examination Survey (NHANES) 1999-2002. Clin Nutr 40:3470–3478
Chen Z, Chen J, Song C, Sun J, Liu W (2022a) Association between serum iron status and muscle mass in adults: results from NHANES 2015-2018. Front Nutr 9:941093
Chen Z, Song C, Yao Z, Sun J, Liu W (2022b) Associations between albumin, globulin, albumin to globulin ratio and muscle mass in adults: results from the national health and nutrition examination survey 2011-2014. BMC Geriatr 22:383
Cho MR, Lee S, Song SK (2022) A review of sarcopenia pathophysiology, diagnosis, treatment and future direction. J Korean Med Sci 37:e146
Cruz-Jentoft AJ, Sayer AA (2019): Sarcopenia. Lancet 393, 2636-2646
Fan Y, Zhang C, Bu J (2017) Relationship between selected serum metallic elements and obesity in children and adolescent in the U.S. Nutrients 9:104
Feng Y, Zeng JW, Ma Q, Zhang S, Tang J, Feng JF (2020) Serum copper and zinc levels in breast cancer: a meta-analysis. J Trace Elem Med Biol 62:126629
Gaier ED, Kleppinger A, Ralle M, Mains RE, Kenny AM, Eipper BA (2012) High serum Cu and Cu/Zn ratios correlate with impairments in bone density, physical performance and overall health in a population of elderly men with frailty characteristics. Exp Gerontol 47:491–496
Genest F, Rak D, Bätz E, Ott K, Seefried L (2021) Sarcopenia and malnutrition screening in female osteoporosis patients-a cross-sectional study. J Clin Med 10
Gu K, Li X, Xiang W, Jiang X (2020) The relationship between serum copper and overweight/obesity: a meta-analysis. Biol Trace Elem Res 194:336–347
Ho V, Lee CT, Merchant RA (2022) The “Iron Tale”- iron indices and handgrip strength in community-dwelling adults. Aging Clin Exp Res 34:3025–3032
Huang L, Shen R, Huang L, Yu J, Rong H (2019) Association between serum copper and heart failure: a meta-analysis. Asia Pac J Clin Nutr 28:761–769
Jiang Q, Zhang F, Han L, Zhu B, Liu X (2021) Serum copper level and polycystic ovarian syndrome: a meta-analysis. Gynecol Obstet Invest 86:239–246
Johnson CL, Paulose-Ram R, Ogden CL, Carroll MD, Kruszon-Moran D, Dohrmann SM, Curtin LR (2013) National health and nutrition examination survey: analytic guidelines, 1999-2010. Vital Health Stat 2:1–24
Jomova K, Valko M (2011) Advances in metal-induced oxidative stress and human disease. Toxicology 283:65–87
Kim BE, Turski ML, Nose Y, Casad M, Rockman HA, Thiele DJ (2010) Cardiac copper deficiency activates a systemic signaling mechanism that communicates with the copper acquisition and storage organs. Cell Metab 11:353–363
Kim KM, Jang HC, Lim S (2016) Differences among skeletal muscle mass indices derived from height-, weight-, and body mass index-adjusted models in assessing sarcopenia. Korean J Intern Med 31:643–650
Lee YA, Kim HN, Song SW (2022) Associations between hair mineral concentrations and skeletal muscle mass in Korean adults. J Nutr Health Aging 26:515–520
Liu B, Sun Y, Xu G, Snetselaar LG, Ludewig G, Wallace RB, Bao W (2019) Association between body iron status and leukocyte telomere length, a biomarker of biological aging, in a nationally representative sample of US adults. J Acad Nutr Diet 119:617–625
Liu Y, Miao J (2022) An emerging role of defective copper metabolism in heart disease. Nutrients 14
Lu CW, Lee YC, Kuo CS, Chiang CH, Chang HH, Huang KC (2021) Association of serum levels of zinc, copper, and iron with risk of metabolic syndrome. Nutrients 13
Luo L, Xu J, Jiang R, Yao B, Di J (2023) Association between serum copper, zinc and their ratio and handgrip strength among adults: a study from National Health and Nutrition Examination Survey (NHANES) 2011-2014. Environ Sci Pollut Res Int 30:29100–29109
Madarić A, Ginter E, Kadrabová J (1994) Serum copper, zinc and copper/zinc ratio in males: influence of aging. Physiol Res 43:107–111
Malavolta M, Piacenza F, Basso A, Giacconi R, Costarelli L, Mocchegiani E (2015) Serum copper to zinc ratio: relationship with aging and health status. Mech Ageing Dev 151:93–100
Milanino R, Marrella M, Gasperini R, Pasqualicchio M, Velo G (1993) Copper and zinc body levels in inflammation: an overview of the data obtained from animal and human studies. Agents Actions 39:195–209
Neidlein S, Wirth R, Pourhassan M (2021) Iron deficiency, fatigue and muscle strength and function in older hospitalized patients. Eur J Clin Nutr 75:456–463
Ngu YJ, Skalny AV, Tinkov AA, Tsai CS, Chang CC, Chuang YK, Nikolenko VN, Zotkin DA, Chiu CF, Chang JS (2022) Association between essential and non-essential metals, body composition, and metabolic syndrome in adults. Biol Trace Elem Res 200:4903–4915
Nishikawa H, Fukunishi S, Asai A, Yokohama K, Nishiguchi S, Higuchi K (2021) Pathophysiology and mechanisms of primary sarcopenia (review). Int J Mol Med 48
Papadopoulou SK, Tsintavis P, Potsaki P, Papandreou D (2020) Differences in the prevalence of sarcopenia in community-dwelling, nursing home and hospitalized individuals. A systematic review and meta-analysis. J Nutr Health Aging 24:83–90
Prokopidis K, Witard OC (2022) Understanding the role of smoking and chronic excess alcohol consumption on reduced caloric intake and the development of sarcopenia. Nutr Res Rev 35:197–206
Reddy SS, Addi UR, Pullakhandam R, Reddy GB (2022) Dietary zinc deficiency disrupts skeletal muscle proteostasis and mitochondrial biology in rats. Nutrition 98:111625
Roychoudhury S, Nath S, Massanyi P, Stawarz R, Kacaniova M, Kolesarova A (2016) Copper-induced changes in reproductive functions: in vivo and in vitro effects. Physiol Res 65:11–22
Sipilä S, Narici M, Kjaer M, Pöllänen E, Atkinson RA, Hansen M, Kovanen V (2013) Sex hormones and skeletal muscle weakness. Biogerontology 14:231–245
Tsvetkov P, Coy S, Petrova B, Dreishpoon M, Verma A, Abdusamad M, Rossen J, Joesch-Cohen L, Humeidi R, Spangler RD, Eaton JK, Frenkel E, Kocak M, Corsello SM, Lutsenko S, Kanarek N, Santagata S, Golub TR (2022) Copper induces cell death by targeting lipoylated TCA cycle proteins. Science 375:1254–1261
van Dronkelaar C, van Velzen A, Abdelrazek M, van der Steen A, Weijs PJM, Tieland M (2018) Minerals and sarcopenia; the role of calcium, iron, magnesium, phosphorus, potassium, selenium, sodium, and zinc on muscle mass, muscle strength, and physical performance in older adults: a systematic review. J Am Med Dir Assoc 19:6–11.e3
Wang H, Zhang R, Shen J, Jin Y, Chang C, Hong M, Guo S, He D (2023) Circulating level of blood iron and copper associated with inflammation and disease activity of rheumatoid arthritis. Biol Trace Elem Res 201:90–97
Welch AA, Skinner J, Hickson M (2017) Dietary magnesium may be protective for aging of bone and skeletal muscle in middle and younger older age men and women: cross-sectional findings from the UK Biobank cohort. Nutrients 9:1189
Wu H, Li Q, Zhang K, Zhao J (2023) The association between serum copper and obesity and all-cause mortality: the NHANES 2011-2016. Environ Sci Pollut Res Int 30:31395–31407
Xiao L, Yang C, Gu W, Liu R, Chen D (2022) Associations between serum copper, zinc, selenium level and sex hormones among 6-19 years old children and adolescents in NHANES 2013-2016. Front Endocrinol (Lausanne) 13:924338
Yang SW, Chen YY, Chen WL (2022) Association between oral intake magnesium and sarcopenia: a cross-sectional study. BMC Geriatr 22:816
Author information
Authors and Affiliations
Contributions
ZC wrote the manuscript. ZC, DH, DW, CS, and JS collected and analyzed the data. WL designed this study and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All NHANES protocols were approved by the Ethics Review Board of the National Center for Health Statistics, and informed consent was obtained from each participant. We confirmed that all methods were performed in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Lotfi Aleya
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, ., Hu, D., Wu, D. et al. Association between serum copper levels and muscle mass: results from NHANES 2011–2016. Environ Sci Pollut Res 31, 6847–6856 (2024). https://doi.org/10.1007/s11356-023-31599-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-31599-x