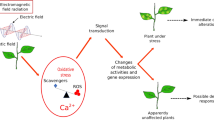
Abstract
The proliferation of wireless and other telecommunications equipment brought about by technological advances in the communication industry has substantially increased the radiofrequency radiation levels in the environment. The emphasis is, therefore, placed on investigating the potential impacts of radiofrequency radiation on biota. In this work, the impact of 2850 MHz electromagnetic field radiation (EMF-r) on early development, photosynthetic pigments, and the metabolic profile of two Brassica oleracea L. cultivars (red and green cabbage) was studied. On a daily basis for seven days, seedlings were exposed to homogeneous EMF-r for one, two, and four hours, and observations were carried out at 0-h, 1-h, and 24-h following the final dose. Irrespective of the duration of harvest, exposure to EMF-r resulted in a dose-dependent reduction in both root (from 6.3 cm to 4.0 cm in red; 6.1 cm to 3.8 cm in green) and shoot lengths (from 5.3 cm to ⁓3.1 cm in red; 5.1 cm to 3.1 cm in green), as well as a decrease in biomass (from 2.9 mg to ⁓1.1 mg in red; 2.5 to 0.9 mg in green) of the seedlings when compared to control samples. Likewise, the chlorophyll (from 6.09 to ⁓4.94 mg g−1 d.wt in red; 7.37 to 6.05 mg g−1 d.wt. in green) and carotenoid (from 1.49 to 1.19 mg g−1 d.wt. in red; 1.14 to 0.51 mg g−1 d.wt. in green) contents of both cultivars decreased significantly when compared to the control. Additionally, the contents of phenolic (28.99‒45.52 mg GAE g−1 in red; 25.49‒33.76 mg GAE g−1 in green), flavonoid (21.7‒31.8 mg QE g−1 in red; 12.1‒19.0 mg QE g−1 in green), and anthocyanin (28.8‒43.6 mg per 100 g d.wt. in red; 1.1‒2.6 mg per 100 g d.wt. in green) in both red and green cabbage increased with exposure duration. EMF-r produced oxidative stress in the exposed samples of both cabbage cultivars, as demonstrated by dose-dependent increases in the total antioxidant activity (1.33‒2.58 mM AAE in red; 1.29‒2.22 mM AAE in green), DPPH activity (12.96‒78.33% in red; 9.62‒67.73% in green), H2O2 content (20.0‒77.15 nM g−1 f.wt. in red; 14.28‒64.29 nM g−1 f.wt. in green), and MDA content (0.20‒0.61 nM g−1 f.wt. in red; 0.18‒0.51 nM g−1 f.wt. in green) compared to their control counterparts. The activity of antioxidant enzymes, i.e., superoxide dismutases (3.83‒8.10 EU mg−1 protein in red; 4.19‒7.35 EU mg−1 protein in green), catalases (1.81‒7.44 EU mg−1 protein in red; 1.04‒6.24 EU mg−1 protein in green), and guaiacol peroxidases (14.37‒47.85 EU mg−1 protein in red; 12.30‒42.79 EU mg−1 protein in green), increased significantly compared to their control counterparts. The number of polyphenols in unexposed and EMF-r exposed samples of red cabbage was significantly different. The study concludes that exposure to 2850 MHz EMF-r affects the early development of cabbage seedlings, modifies their photosynthetic pigments, alters polyphenol content, and impairs their oxidative metabolism.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The daily usage of cellular phones, Wi-Fi, and other communication devices has increased dramatically since 2000 (Kaur et al. 2021). Most of these devices' function use radiofrequency radiation (RFR), a non-ionizing form of electromagnetic field radiation (EMF-r) (Davis et al. 2023). As a result of the rapid expansion of the telecommunications industry, RFR is now continuously transmitted into the environment from a variety of sources. This has consequently increased the frequency with which the living organisms are exposed to electromagnetic fields (Kaur et al. 2021). At the end of 2022, there were over 8.4 billion mobile phone subscriptions, and it is projected to be over 9.2 billion by 2028, and of these, > 50% will be 5G subscriptions (Ericsson Mobile Subscribers Outlook Report 2021; Ericsson Mobility Report 2023). To maintain the network's functionality, base stations have increased rapidly as the number of mobile phone subscribers has increased. Surducan et al. (2020) predicted a > 30-fold rise in the concentration of RFR in the environment over the next decade due to the proliferation of base stations and wireless technology.
The EMF-r emitted by mobile phones has been categorized in Group 2B as a "Possible Human Carcinogen" (IARC 2011). To understand the biological impacts of RFRs, studies have focussed largely on animals, and the effects have been well documented in humans, rats, and insects (Vanbergen et al. 2019; Choi et al. 2020; Schuermann and Mevissen 2021). In animals, EMF-r has been shown to cause cytotoxic and genotoxic effects (Cho et al. 2014), induce changes in the cell cycle (Cleary et al. 1996), enzymatic activity (Obajuluwa et al. 2017), and gene expression (Kazemi et al. 2018). In contrast, a smaller number of studies have been conducted on plant systems. Despite a few reports highlighting the positive effects of EMF-r (Mildaziene et al. 2016, 2019), a vast majority of studies have reported that EMF-r negatively impacts the growth and development of plant species (Vian et al. 2016; Halgamuge 2017; Kaur et al. 2021). Till date, studies have investigated the negative impacts of EMF-r on seed germination, growth, and development in plants at 900 MHz (Tkalec et al. 2009; Sharma et al. 2010), 1800 MHz (Kumar et al. 2020; Stefi et al. 2018), 2100 MHz (Chandel et al. 2017, 2019b), and 2350 MHz (Chandel et al. 2019a). The EMF-r affects the metabolic pathways (in Plectranthus species at 900 MHz; Kouzmanova et al. 2009) and induces gene expression modifications (in rice at 1837.50 MHz; Kundu et al. 2021), impairs starch metabolism (at 1800 MHz in maize; Kumar et al. 2016), enhances emission of volatile compounds (in Petroselinum crispum (Mill.) Fuss, Apium graveolens L., and Anethum graveolens L.; Soran et al. 2104), and induces accumulation of stress-related mRNA transcripts (in tomato at 900 MHz; Roux et al. 2008).
Of late, the development of new cellular technologies based on higher frequencies, such as 5G, and the unrestricted expansion in smartphone usage have resulted in an unprecedented concentration of RFR in the natural environment. Keeping this in mind, we investigated the effects of 2850 MHz electromagnetic field radiation (EMF-r) on the early development, photosynthetic pigments, and metabolite composition of red and green cabbage (Brassica oleracea L.) cultivars. The 2850 MHz frequency was selected for the current study as it represents a prominent frequency within the 5G spectrum, and there is a notable scarcity of research examining its potential biological effects. Till date, only one preliminary study has reported the impact of 2850 MHz on the germination ability and early growth of chickpeas (Johal et al. 2022). The present study aims to investigate the impact of 2850 MHz EMF-r, one of the most widely used 5G frequencies, of varying exposures (0, 1, 2, and 4 h) on red and green cabbage. The observations made at 0 h, 1 h, and 24 h allow for the examination of both immediate and delayed responses, potentially revealing insights into how plants adapt to or recover from EMF-r stress over time. Cabbage, being a member of one of the most important and commonly cultivated vegetable families (Brassicaceae), offers considerable nutritional value. The two cabbage cultivars were used to investigate and identify the differential role of pigments in imparting defense mechanisms in response to EMF-r.
Materials and methods
Experimental set-up
Red and green cabbage seeds obtained from a local seed store were disinfected with NaOCl (0.1%; 7–10 min) and rinsed thoroughly with tap water and then distilled water. Pre-soaked seeds (for 10 h) were evenly spaced in 15-cm Petri dishes with Grade 1 Whatman filter paper and a thin layer of moist cotton. In all, there were four treatments: (i) Without EMF-r irradiation; (ii) EMF-r exposure for 1 h; (iii) EMF-r for 2 h; and (iv) EMF-r for 4 h. Three replicates of 15 seeds per Petri dish were maintained for each treatment. After the development of cotyledons, the Petri dishes were exposed to 2850 MHz EMF-r daily for 7 days before being placed in a chamber maintained at 22/16 (± 2) °C, 16 h light photoperiod of 240 mol m–2 s–1 and a RH of 75 ± 3%. After 7 days of EMF-r exposure, each group's seedlings were harvested at 0 h, (ii) 1 h, and (iii) 24 h, after the last treatment.
EMF-r treatment system
For the generation of radiofrequency signals, a Vector Signal Generator (SMBV100A; Rohde & Schwarz, Germany) was used. The RF signal generator was fitted with an antenna and a RF power amplifier (ZFL‒2500+; Mini-circuits, USA). Petri dishes were placed 5 cm from the antenna in an EMF-r shielded chamber (75 cm × 80 cm × 75 cm). Subsequently, the Petri dishes were irradiated with 2850 MHz EMF-r. The power flux density was measured using an NBM 550 radiation field meter (Narda, Germany). At 2850 MHz EMF-r, the average power density was 632.1 ± 39.07 mW m‒2 and the specific absorption rate (SAR) was measured to be and 4.85 × 10‒1 W kg‒1. Due to the difficulty in calculating the precise value of SAR on irradiated tissues (Cenesiz et al. 2011), it was approximated using tissue density and electrical conductivity values from the database of dielectric properties of grey matter tissue at 2850 MHz (Andreuccetti et al. 1997).
Plant morphology and biomass
Red and green cabbage seedlings were harvested after one week of exposure to 2850 MHz for 1 h, 2 h, and 4 h daily. The lengths of the root and shoot were measured with a gauge ruler and expressed in centimeters (cm). After oven-drying seedlings at 60 °C for 72 h, seedling biomass was measured using a weighing scale (A&D Company Ltd., Japan; P0008; d = 0.1 mg) in milligrams (mg).
Total chlorophyll (TC) and carotenoid (Car) content
These were measured by spectrophotometry using dimethyl sulphoxide, according to Hiscox and Israelstam (1979). The TC and Car were calculated using the formulae given by Arnon (1949) and Lichtenthaler and Wellburn (1983), respectively, and presented on a dry weight basis (Rani and Kohli 1991).
Quantitation of total phenolics, flavonoids, and anthocyanins
The total phenolic content (TPC) was evaluated with the Folin-Ciocalteu reagent, according to Swain and Hillis (1959). One millilitre of extract (50 mg of plant tissue or seedlings mixed into 5 ml of distilled water and centrifuged at 15,000 g for 25 min) was dissolved in one milliliter of 50% Folin-Ciocalteu reagent. After three minutes, 1.0 ml of 20% Na2CO3 was added, and the absorbance was measured after 30 min using gallic acid as a reference at 700 nm. The TPC was expressed as mg g−1 gallic acid equivalents (GAE).
The total flavonoid concentration (TFC) was quantified using methanolic aluminium chloride (Meda et al. 2005). One millilitre of extract (10 mg plant tissue per millilitre of distilled water) was mixed with one milliliter of 2% methanolic AlCl3. After 10 min, the absorbance was read at 415 nm against quercetin as a reference. The TFC was expressed as mg g‒1 quercetin equivalents (QE).
The total anthocyanin concentration (TAC) was specified using the pH-differential method (Sutharut and Sudarat 2012). Plant tissue crushed in methanol was vortexed for 30 s and centrifuged (10,000 g for 10 min). Extracts (0.5 ml) were mixed with 0.5 ml of pH 1 (25 mM potassium chloride) and pH 4.5 (400 mM sodium acetate) buffers. The optical density was determined at two wavelengths (510 and 700 nm). The absorbance (Abs) was determined using the equation:
The concentration of anthocyanin was calculated using the equation:
449.2 g ml‒1 is the mole mass; 26,900 L mol‒1 cm‒1 is the molar extinction coefficient (ε); l is path length in cm.
Total antioxidant activity (TAA)
The TAA was determined according to Prieto et al. (1999). One milliliter of reaction solution containing 600 mM H2SO4, 0.028 M Na3PO4, and 4 mM ammonium molybdate was mixed into 100 μl of the plant extract. The samples were incubated at 95 °C for 90 min, and the optical density was read at 695 nm. TAA was expressed as mM ascorbic acid equivalents (AAE).
Free radical scavenging activity
It was determined using 1,1′-diphenyl-2-picrylhydrazyl (DPPH) as per Singh et al. (2009). Plant extract (100 μl) was mixed with 0.5 ml of 90 μM methanolic DPPH, and the volume was adjusted to 1.5 ml. A positive control, butylated hydroxytoluene, and a parallel blank (distilled water and DPPH solution) were also maintained. After incubating the samples at 25 °C in the dark for 30 min, the absorbance was measured at 515 nm.
Hydrogen peroxide (H2O2) content
H2O2 levels were determined according to the method of Velikova et al. (2000). Plant tissue (100 mg) was homogenized in 10 ml of 0.1% trichloroacetic acid (TCA) and centrifuged at 12,000 g for 15 min. The supernatant was mixed with phosphate (PO43‒) buffer (pH 7.0, 10 mM) and potassium iodide (1 M). Absorbance was measured at 390 nm, and H2O2 content was quantified using ε = 0.28 μM−1 cm−1 and expressed as nM g−1 fresh weight.
Peroxidation of lipids
It was determined in terms of the content of malondialdehyde (MDA), according to Heath and Packer (1968). Briefly, 100 mg of plant tissue was homogenized in 10 ml of 0.1% TCA and centrifuged at 12,000 g for 15 min at 4 °C. The supernatant (1 ml) was mixed with 4 ml of 0.5% thiobarbituric acid (in 20% TCA) and again centrifuged at 10,000 g for 10 min. The absorbance was read at 532 nm and 600 nm, and the MDA concentration was reported as nM g−1 fresh weight.
Antioxidant enzymes’ assay
Preparation of enzyme extract
Plant tissue (100 mg) was homogenized in 10 ml of 100 mM PO43‒ buffer (pH 7) and centrifuged for 25 min at 15,000 g. The supernatant was used for the assay of the activities of superoxide dismutases (SOD), catalases (CAT), and guaiacol peroxidases (GPX).
Superoxide dismutases
The SOD was assayed for its ability to prevent the photochemical reduction of NBT (nitroblue tetrazolium) as per Beauchamp and Fridovich (1971). To 100 µl of enzyme extract, added 900 µl of reaction mixture (50 mM PO43‒ buffer, 0.1 mM EDTA, 13 mM methionine, 13 µM riboflavin, 0.05 M sodium carbonate, and 63 µM NBT). Two sets of replicates (light and dark) were maintained, and the absorbance was read at 560 nm. The enzyme activity was expressed as enzyme unit (EU) mg−1 protein.
Catalases
The CAT activity was evaluated as per Cakmak and Marschner (1992) and calculated using ε = 2.8 mM−1 cm−1. To 0.1 ml of enzyme extract, 0.9 ml of H2O2 (10 mM) was added, and absorbance was measured at 240 nm. The activity of CAT was expressed as EU mg‒1 protein.
Guaiacol peroxidases
The GPX activity was measured according to Egley et al. (1983). One hundred microlitres of enzyme extract were added to 900 µl of reaction mixture containing 25 mM PO43‒ buffer (pH 7.0), 0.05% guaiacol, and 1 mM H2O2. A change in absorbance was recorded for one minute at 470 nm. The enzyme activity was expressed as EU mg−1 protein.
Analyses of secondary metabolites
Secondary metabolites present in two cabbage cultivars were identified using LC–MS. It was carried out using Waters micromass Q-TOF coupled to a 2795 separation module and a Unisol YVR C18 column (4.6 mm i.d., 25 cm long, thickness 5 µm). The LC–MS separation was performed using two mobile phases: A (90% methanol and 10% water) and B (40% methyl cyanide, 10% water, and 10% methanol; v/v/v). The operating conditions were: flow rate of LC solvent program = 0.8 ml min‒1, 35 ºC column temperature, and injection volume = 20 µL. The wavelength of the system was set between 190 and 800 nm to detect all the compounds. The compounds in the sample were ionized using the Electrospray Ionization (ESI) process, and a Waters 2996 photodiode was used as an array detector. For mass analysis, the desolvation flow rate, desolvation gas temperature, and source block temperature were set at 550 Lh‒1, 300 °C, and 110 ºC, respectively. The collision energy and gas flow through the cone were set to 4 eV and 30 L h−1, respectively. The capillary voltage (the voltage supplied to the electrospray capillary to stimulate ionization) was set to 3000 V, while the cone voltage (the voltage applied to the ion guide to transmit ions into the source) was set at 30 V. In the scanning mode, nitrogen and argon gases with corresponding supply pressures of 6–7 and 5–6 bar and a mass range of 60‒500 mass units were used. The data was processed using Mass Bank (https://massbank.eu/MassBank/), a spectrum database designed to identify secondary metabolites.
Statistical analysis
The data are presented as mean ± SE of three replicates. The significant differences between EMF-r treatments and observation groups were analyzed using one-way ANOVA and Tukey’s test at P ≤ 0.05. A t-test was employed to assess the significant differences between red and green cabbage at P ≤ 0.05.
Results
Effect of 2850 MHz on early growth and biomass
EMF-r exposure affected the early growth (root and shoot length, and biomass) of both red and green cabbage seedlings in a dose-dependent manner. In the control (non-irradiated group), the root length, shoot length, and biomass were measured to be 6.1 cm, 5.3 cm, and 2.7 mg in red cabbage, and 5.9 cm, 4.9 cm, and 2.2 mg in green cabbage, respectively (Table S1, supplementary material). In samples harvested immediately after the EMF-r treatment (0 h), root growth declined significantly by 19 and 34% in red and 22 and 35% in green cabbage at 2- and 4-h of EMF-r treatment, respectively, over control. Shoot length also decreased significantly by ~ 18 and 33% in red cabbage and ~ 22 and 36% in green cabbage upon EMF-r exposure for 2- and 4-h. The biomass of seedlings declined over the control by ~ 7, 37, and 55% (significant at P ≤ 0.05) in red cabbage and ~ 13, 40, and 59% in green cabbage upon 1-, 2-, and 4-h EMF-r exposure (Fig. 1).
Variations in root length, shoot length and biomass of red and green cabbage upon exposure to 2850 MHz EMF-r for 0, 1, 2 and 4 h daily for 7 days. Data presented as mean ± SE of three biological replicates. Different alphabets (upper case for red cabbage and lower case for green cabbage) represent significant difference among treatment groups at P ≤ 0.05 applying post hoc Tukey’s test. * represents significant difference between the two cabbage cultivars at = P ≤ 0.05 using student t-test
In control samples harvested after 1 h, the root length, shoot length, and biomass were found to be 6.1 cm, 5.2 cm, and 2.8 mg in red cabbage, and 6.0 cm, 4.8 cm, and 2.3 mg in green cabbage, respectively (Table S1, supplementary material). However, the root length exhibited a decrease of ~ 19–34% in red cabbage and ~ 21–35% in green cabbage upon treatment for 2 and 4 h, respectively. Shoot length declined significantly by ~ 19 to 40% in red cabbage and ~ 18 to 32% in green cabbage upon 2 h and 4 h of EMF-r treatment. The biomass of seedlings showed a reduction of ~ 10, 46, and 60% in red cabbage and ~ 13, 39, and 56% in green cabbage upon 1, 2, and 4 h of treatment, respectively. Similarly, after 24 h, the root length, shoot length, and biomass of the control group were found to be 6.3 cm, 5.2 cm, and 2.9 mg in red cabbage, and 6.1 cm, 5.1 cm, and 2.5 mg in green cabbage, respectively (Table S1, supplementary material). In contrast, root length (at 24 h after exposure) declined by ~ 19 and 31% in red cabbage and ~ 22 and 36% in green cabbage upon EMF-r treatment for 2- and 4-h, respectively. Upon 2 and 4 h of EMF-r exposure, a significant reduction (15 and 35% in red cabbage and ~ 17 and 33% in green cabbage, respectively) in shoot length was found over control. However, the biomass of seedlings showed a decrease of ~ 10, 44, and 55% in red cabbage and ~ 12, 40, and 56% in green cabbage upon 1, 2, and 4 h of treatment, respectively (Fig. 1). In general, the three harvested groups (0, 1, and 24 h) showed a decreasing trend of reduction in early growth and biomass of both red and green cabbage seedlings (but not significant). Moreover, there were no significant differences in the root and shoot lengths of both red and green cabbages subjected to EMF-r exposure (except in samples harvested after 24 h of 4-h treatment). A significant difference between the two groups in terms of biomass was observed only in samples that were harvested after the 1-h treatment.
Effect of 2850 MHz on the photosynthetic pigment profile
The chlorophyll and carotenoid contents declined with the increase in EMF-r exposure time in both cultivars. In the control samples, chlorophyll content was found to be 5.95, 6.00, and 6.08 mg g−1 in red cabbage and 7.37, 7.17, and 7.31 mg g−1 in green cabbage at 0, 1, and 24 h, respectively. However, carotenoid content of control samples was 1.43, 1.44, and 1.49 mg g−1 in red cabbage and 1.11, 1.14, and 1.13 mg g−1 in green cabbage, respectively (Table S1, Supplementary material). The chlorophyll content declined over the control by ~ 6 and 16% in red cabbage and ~ 8 and 12% in green cabbage when measured immediately (0 h) after exposure for 2 and 4 h, respectively (Fig. 2). Likewise, carotenoid content decreased significantly by 7 to 17% in red cabbage and ~ 13 to 54% in green cabbage upon exposure for 2 and 4 h, respectively, over the control group. Similarly, at 2 h and 4 h of exposure, samples harvested 1 h after treatment showed a decrease in chlorophyll content by 13–18% and 5–11% in red and green cabbage, respectively. A reduction in carotenoid content upon 2 and 4 h of EMF-r exposure was found to be ~ 9 and 13% in red cabbage and ~ 24 and 48% in green cabbage, respectively, over control. Likewise, in samples harvested 24 h after treatment, the decrease in chlorophyll content was ~ 5 and 15% (red cabbage) and ~ 8 and 17% (green cabbage) after 2 and 4 h of treatment, respectively. The carotenoid content upon 2 and 4 h of EMF-r exposure was reduced by ~ 5 and 13% in red cabbage and ~ 21 and 32% in green cabbage, respectively, compared to the control samples. Further, green cabbage showed higher chlorophyll content, whereas red cabbage showed higher carotenoid content, and the difference in chlorophyll and carotenoid content between the two cultivars, i.e., red, and green cabbage, was statistically significant (P ≤ 0.05) at 0, 1, 2, and 4 h exposures in all three observation groups (Fig. 2).
Total chlorophyll and carotenoid content of red and green cabbage upon exposure to 2850 MHz EMF-r for 0, 1, 2, and 4 h daily for 7 days. Data presented as mean ± SE of three biological replicates. For details of the different letters used in this figure, kindly refer to the caption of Fig. 1
Effect of 2850 MHz on phenols, flavonoids, and anthocyanin content
In both red and green cabbage, TPC, TFC, and TAC were higher in EMF-treated samples compared to controls. The phenolic, flavonoid, and anthocyanin content of red and green cabbage was found to be 34.82, 25.68 mg GAE g−1, 22.40 and 13.60 mg QE g−1 and 32.30, 1.20 mg per 100 g d.wt., respectively, in the control samples (Table S2, supplementary material). The phenolic content of EMF-r exposed samples harvested immediately (0 h) increased by 8, 20, and 26% in red cabbage and by 6, 17, and 26% in green cabbage after 1, 2, and 4 h, respectively, when compared to the unexposed group (Fig. 3). Likewise, flavonoid content was also increased by ~ 12, 26, and 45% in red and ~ 5, 25, and 39% in green cabbage upon EMF-r exposure of 1, 2, and 4 h, respectively. Compared to the control, EMF-r exposed samples exhibited more anthocyanin content. Upon 1, 2, and 4 h of irradiation, an increase of ~ 6, 23, and 34% and ~ 33, 91, and 116% was observed in red and green cultivars, respectively. Analysis of phenol, flavonoid, and anthocyanin content was also done after EMF-r exposure for 1 and 24 h. In the control samples harvested at 1 h, the phenolic content in red and green cabbage was determined to be 29.39 and 25.91 mg GAE g−1, respectively. The flavonoid content was measured to be 21.20 mg QE g−1 in red cabbage and 13.70 mg QE g−1 in green cabbage. Additionally, the anthocyanin content was found to be 31.20 and 1.20 mg per 100 g d.wt. in red and green cabbage, respectively (Table S2, Supplementary material). In samples harvested 1-h after EMF-r exposure, the phenolic content increased by ~ 18, 31, and 51% in red and ~ 7, 21, and 30% in green cabbage upon 1, 2, and 4 h of EMF-r exposure. The flavonoid content after 1, 2, and 4 h EMF-r exposure increased by ~ 16, 36, and 50% in red and ~ 5, 17, and 32% in green cabbage, respectively. An increase of ~ 7, 20, and 30% and ~ 63, 98, and 118% was observed in the anthocyanin content of red and green cabbage upon EMF-r exposure for 1, 2, and 4 h, respectively. Similarly, in samples harvested 24 h after treatment, the phenolic, flavonoid, and anthocyanin content of red and green cabbage was found to be 28.99 and 25.49 mg GAE g−1, 21.70 and 12.00 mg QE g−1, and 28.80 and 1.20 mg per 100 g d.wt., respectively, in the control samples (Table S2, Supplementary material). The phenolic content increased in the range of 16 to 25% in the red cultivar and 8 to 23% in the green cultivar upon EMF-r exposure of 1 to 4 h, compared to the control. Over the unexposed samples, upon 1–4 h of EMF-r exposure, the flavonoid content increased by 6 to 38% in red cabbage and 12 to 47% in green cabbage, respectively. Anthocyanin content was enhanced by ~ 21, 34, and 47% in red cabbage and ~ 41, 75, and 108% in green cabbage, respectively, upon 1, 2, and 4 h of EMF-r exposure compared to the control (Fig. 3).
Total phenolic, flavonoid, and anthocyanin content of red and green cabbage upon exposure to 2850 MHz EMF-r for 0, 1, 2, and 4 h daily for 7 days. Data presented as mean ± SE of three biological replicates. For details of the different letters used in this figure, kindly refer to the caption of Fig. 1
In general, the phenols, flavonoids, and anthocyanins were more abundant in red than green cabbage at 1–4 h of EMF-r exposure. These differences were statistically significant (P ≤ 0.05) between red and green cabbage in all three groups (0, 1, and 24 h after EMF-r treatment). Furthermore, a statistically significant variation in phenolic content was observed among the three harvesting groups or the control, 1 h, 2 h, and 4 h treatment groups.
Effect of 2850 MHz on the total antioxidant activity and radical scavenging activity
In both red and green cabbage, EMF-r exposed samples had significantly higher total antioxidant activity and radical scavenging activity than control samples. The antioxidant activity in the control samples was found to be 1.35, 1.37, and 1.33 mM AAE in red cabbage and 1.31, 1.29, and 1.32 mM AAE in green cabbage at 0, 1, and 24 h, respectively. However, DPPH radical scavenging activity of control samples was 17.98, 16.24, and 12.96% in red cabbage and 10.39, 10.44, and 9.62% in green cabbage at 0, 1, and 24 h, respectively (Table S2, Supplmentary material). The samples harvested immediately after EMF-r treatment for 1-, 2-, and 4-h showed a significant increase in antioxidant activity (80–252%) and radical scavenging activity (39–335%) in red cabbage and 120–332% and 63–551% in green cabbage, respectively (Fig. 4). In samples harvested 1 h after the exposure, the antioxidant activity increased by ~ 56‒197% in red cabbage and ~ 120‒332% in green cabbage upon 1, 2- and 4 h of EMF-r exposure. The radical scavenging activity upon exposure for 1, 2, and 4 h increased by ~ 51‒350% in red and ~ 70‒520% in green cabbage, respectively. Similarly, 24 h after treatment, red cabbage seedlings exhibited increased antioxidant (~ 90‒265%) and radical scavenging (~ 87‒464%) activities, while ~ 75‒282%, and ~ 74‒560% increases were observed in green cabbage upon EMF-r exposure for 1–4 h. A significant difference in the antioxidant activity of red and green cabbage was observed at 2 h EMF-r exposure treatment in the samples harvested at 0 h. The radical scavenging activity in red cabbage was significantly different from that in the green cabbage at 2 h of EMF-r exposure treatment across all observation groups (0, 1, and 24 h) and at 4 h EMF-r exposure treatment in the samples harvested immediately after treatment, i.e., 0 h observation group. However, no changes were observed among the three harvesting groups for 0, 1, 2, and 4 h of EMF-r exposure.
Total antioxidant activity, free radical scavenging activity, and H2O2 and MDA content of red and green cabbage upon exposure to 2850 MHz EMF-r for 0, 1, 2, and 4 h daily for 7 days. Data presented as mean ± SE of three biological replicates. For details of the different letters used in this figure, kindly refer to the caption of Fig. 1
Effect of 2850 MHz on H2O2 and MDA contents
The H2O2 and MDA content increased significantly in EMF-r exposed samples compared to the control. In control, H2O2 content was found to be 21.42, 22.38, and 20.00 nM g−1 f.wt. in red cabbage, and 15.71, 14.76, and 14.28 nM g−1 f.wt. in green cabbage at 0, 1, and 24 h, respectively. However, the MDA content of the control samples was 0.24, 0.23, and 0.20 nM g−1 f.wt. in red cabbage and 0.21, 0.19, and 0.18 nM g−1 f.wt. in green cabbage at 0, 1, and 24 h, respectively (Table S3, Supplementary material). Upon 1, 2, and 4 h of irradiation, an increase of ~ 42‒260% and ~ 39‒165% in H2O2 content and ~ 45‒309% and ~ 33‒142% in MDA content was observed in red and green cultivars, respectively. in samples harvested immediately after EMF-r treatment (Fig. 4). An increase of ~ 31‒206% was observed in the H2O2 content in red cabbage and ~ 45‒319% in green cabbage upon EMF-r exposure for 1-, 2-, and 4-h, respectively. However, the MDA content was increased by ~ 21‒152% in red and ~ 36‒163% in green cabbage upon EMF-r exposure for 1, 2, and 4 h in test samples harvested 1 h after the exposure.
Similarly, in the case of samples harvested 24 h after treatment, H2O2 content showed an increase of ~ 35‒235% and ~ 46‒296%, and MDA content increased by ~ 50‒145% and ~ 44‒161% in red and green cabbage, respectively, upon 1-, 2-, and 4-h of treatment over the control (Fig. 4). However, no significant differences were noticed among the three observation groups (samples harvested 0, 1, and 24 h after treatment). These alterations were insignificant in both red and green cabbage at 0, 1, and 4 h of EMF-r exposure.
Effect of 2850 MHz on the antioxidative enzyme activities
The SOD, CAT, and GPX activities were significantly higher in 2850 MHz exposed samples compared to control ones in both cabbage cultivars. In the control samples harvested at 0 h, the SOD, CAT, and GPX activities were found to be 3.94, 2.00, and 15.17 EU mg−1 protein in red cabbage and 4.19, 1.19, and 14.55 EU mg−1 protein in green cabbage, respectively (Table S3, Supplementary material). Compared to control, in test samples harvested immediately after EMF-r treatment (0 h), the activities of SOD and CAT increased in the range of ~ 27‒106%, ~ 43‒270% in red cabbage and ~ 19‒78%, ~ 77‒425% in green cabbage, respectively, upon 1–4 h of exposure (Fig. 5). Similarly, GPX activity increased significantly in samples exposed to EMF-r compared to controls. An increase of ~ 40‒213% and ~ 25‒189% was observed in GPX activity in red and green cultivars, respectively.
SOD, CAT and GPX activity of red and green cabbage upon exposure to 2850 MHz EMF-r for 0, 1, 2, and 4 h daily for 7 days. Data presented as mean ± SE of three biological replicates. For details of the different letters used in this figure, kindly refer to the caption of Fig. 1
Enzymatic activities were also observed at 1 and 24 h after EMF-r exposure. In the control samples harvested at 1 h, the SOD, CAT, and GPX activities were found to be 4.10, 1.97, and 14.94 EU mg−1 protein in red cabbage and 4.24, 1.12, and 12.30 EU mg−1 protein in green cabbage, respectively (Table S3, Supplementary material). In seedlings harvested 1 h after the treatment (exposure for 1 to 4 h), the SOD activity was increased by 17‒92% and 11‒66% in red and green cabbage, respectively. Exposure to EMF-r for 1 to 4 h increased the activity of CAT by 63‒261% (P ≤ 0.05) and 107‒440% (P ≤ 0.05) in red and green cabbage, respectively. Likewise, an increase in GPX activity by 28‒235% in red cabbage and by 41‒225% in green cabbage was observed upon EMF-r exposure for 1, 2, and 4 h.
In samples harvested at 24 h after EMF-r exposure, the SOD, CAT, and GPX activities of control samples were found to be 3.83, 1.81, and 14.37 EU mg−1 protein in red cabbage and 4.42, 1.04, and 13.57 EU mg−1 protein in green cabbage, respectively (Table S3, supplementary material). The SOD and CAT levels were up-regulated in samples harvested after 24 h of treatment by ~ 15‒105% and ~ 50‒244% in red cabbage and 7‒56% and ~ 107‒443% in green cabbage, respectively, upon 1–4 h of EMF-r exposure. Compared to control, GPX levels increased by ~ 21‒214% in red cabbage and ~ 22‒159% in green cabbage, respectively. Higher levels of SOD enzymes were observed in the 1 h observation group for red cabbage over green cabbage. Furthermore, the levels of the CAT enzyme showed a statistically significant increase (P ≤ 0.05) in red cabbage compared to green cabbage. This difference was significant in the control across all observation groups (0, 1, and 24 h) (Fig. 5).
Effect of 2850 MHz on the secondary metabolites of red and green cabbage
The EMF-r irradiated (for 4 h) and control (non-irradiated) red and green cultivars were harvested immediately after exposure (0 h), and analyzed for the presence of secondary metabolites. In general, control samples showed more secondary metabolites compared to EMF-r exposed ones. The LC–MS analysis revealed 348 constituents putatively identified in the control and EMF-r exposed samples of red and green cabbage. These belonged to three major groups: polyphenols, alkaloids, and terpenoids. Of these, polyphenols were the major components. These were significantly lower in EMF-r exposed red cabbage samples (55% vs. 63% in control samples). Polyphenols were followed by terpenoids, which constituted 30 and 25% in control and EMF-r exposed red cabbage, respectively. In contrast, alkaloids were more abundant in EMF-r exposed (20%) samples compared to control (7%) samples (Fig. 6 a, b). However, in green cabbage, these secondary metabolites were nearly the same in both control and EMF-r exposed samples (Fig. 6 c, d).
Discussion
The current study revealed the detrimental effects of mobile phone radiation at 2850 MHz on the early development, physiological and biochemical attributes, antioxidant activity, and secondary metabolites of two cabbage cultivars, red and green. It is well known that both secondary metabolites and pigments play a significant role in plant defense under various abiotic stresses, including EMF-r (Stefi et al. 2018). Two cultivars of cabbage, i.e., red, and green, were chosen to investigate if there was any variation in the responses of different coloured cultivars to the cell phone EMF-r. The present study is the first to examine such distinct responses between two cultivars of a plant differing in pigment content. In response to EMF-r (1–4 h), early growth (root length, shoot length, and biomass) was reduced in a dose-dependent manner in both cultivars over the control. The impact of 1 and 4 h of EMF-r treatment was more pronounced on the root length and dry weight of green cabbage compared to red cabbage. In both cultivars, samples exposed to EMF-r for 4 h showed the greatest growth reduction. This indicates that the effects of EMF-r on plant growth and morphology are influenced by the intensity and duration of exposure in a dose-dependent manner. Such types of growth inhibitory effects in response to cellphone radiation in the frequency range of 900 MHz, 1805–1850 MHz, and 1 GHz have also been demonstrated by Sharma et al. (2009), Chen and Chen (2014), and Racuciu et al. (2015), respectively. The reduction in plant height could be due to lignification and cell wall thickening or secondary metabolite accumulation due to activation of the Shikmik acid pathway under EMF-r exposure (Stefi et al. 2018). Vishki et al. (2012) opined that peroxidases might also be responsible, as these degrade indole-3-acetic acid (IAA), a growth regulator, thereby resulting in a decrease in cell wall extensibility and ultimately root and shoot length under EMF-r stress conditions.
Photosynthetic pigments–chlorophyll, and carotenoids– play a vital role in plant growth and development. The variation in their concentration indicates the health of a plant, especially under abiotic stress (Wang et al. 2020). In response to EMF-r exposure (for 0, 1, 2, and 4 h), a decrease in total chlorophyll and carotenoids content was observed in both red and green cabbage. In fact, EMF-r of diverse frequencies alter photosynthetic activity in many plants (Sandu et al. 2005; Stefi et al. 2017). Chloroplasts are one of the key targets of reactive oxygen species (ROS). This could be attributed to ultrastructural alterations in chloroplasts (Ahmad et al. 2020), which may be due to ROS overproduction that damages the lipid membrane of the chloroplast, thereby lowering photosynthetic efficiency. This ultimately leads to lower primary productivity and biomass in EMF-r exposed samples (Stefi et al. 2017). ROS inhibits photosystem I and II activity, along with alterations in the cytochrome b6f complex and ATP synthase gene expression levels in plants (Varshikar and Tan 2017), thereby resulting in a significant decrease in chlorophyll and carotenoid content (Xi et al. 2005; Tkalec et al. 2007; Chandel et al. 2017; Tang et al. 2018). The pigment content was significantly different in red and green cabbage irrespective of EMF-r exposure duration and sample harvest time (0, 1, and 24 h). The reason for the higher chlorophyll content in green cabbage is due to its characteristic green color and chlorophyll’s ability to absorb light in the blue and red parts of the electromagnetic spectrum while reflecting green light, thus indicating high chlorophyll content in the plant (Paciulli et al. 2017). However, in red cabbage, the presence of anthocyanins enhances the contrast with carotenoid colors (red, orange, and yellow), thereby making the carotenoids more prominent (Martínez-Zamora et al. 2021).
Plants develop complex mechanisms that defend against the detrimental effects of a wide range of abiotic stresses. Among these, phenolics are the key antioxidants to quench ROS (Siddhuraju 2007). Cebulak et al. (2017) opined that abiotic stress such as UV-C, microwave, and ultrasonication increased the production of phenolic compounds in chokeberry (Aronia melanocarpa [Michx.] Elliott). Various abiotic stresses are well known to activate the phenylpropanoid biosynthetic pathway, resulting in the accumulation of numerous phenolic compounds (Yaqoob et al. 2022). These polyphenolic compounds, because of their redox properties, neutralize free radicals and quench singlet and doublet oxygen (Siddhuraju 2007). In the present study, it was observed that, in response to EMF-r, the phenolic and flavonoid content increased in both cabbage cultivars. Compared to green cabbage, red cabbage showed a significantly higher phenolic and flavonoid content. It agrees with a previous study reporting an increased content of phenols and flavonoids upon field radiation exposure in chokeberries (Cebulak et al. 2017). Furthermore, it is worth noting that the phenolic content exhibited significant differences in all the post-treatment observation groups (0, 1, and 24 h). This variation could be attributed to the induction of oxidative stress, which may lead to the utilization of phenolic compounds and consequently an overall decline in phenolic levels. These observations parallel the findings of Rabelo et al. (2020), who reported a decrease in phenolic compounds in Malpighia emarginata DC after 7 days of UV-C irradiation, suggesting that changes in phenolic compounds in response to EMF-r exposure may evolve over time.
Many genes involved in flavonoid biosynthesis are stimulated under stressful conditions (Shomali et al. 2022). The production of anthocyanin pigments via the shikimic acid pathway is greatly enhanced under environmental stresses, such as visible and UV-B radiation, freezing temperatures, and water stress (Gao et al. 2021; Shomali et al. 2022). In the present study, anthocyanin content was enhanced with the increase in EMF-r exposure duration. Red cabbage showed a significantly higher anthocyanin content than green cabbage, in which only traces of the pigment were found. These findings corroborate those of Cebulak et al. (2017), who reported a significant increase in the anthocyanin pigment content in chokeberry fruits upon exposure to UV-C radiation.
In the present study, the contents of H2O2 and MDA (a measure of lipid peroxidation), ROS scavenging, and TAA increased in both cultivars after EMF-r irradiation. These observations agree with previous studies reporting greater generation of these oxidative stress markers (H2O2 and MDA) under EMF-r exposure (Chandel et al. 2017; Sharma et al. 2009; Tkalec et al. 2007). There has been a direct correlation between stress tolerance and a rise in antioxidant activity and free radical scavenging capability in plants (Sharma et al. 2012). The rise in antioxidant and radical scavenging activity in the 2 and 4 h EMF-r exposure treatments in red cabbage may be attributed to its higher anthocyanin content. Anthocyanins are known for their strong antioxidant properties (Ashfaq et al. 2020), which further contributes to the increased antioxidant activity observed in red cabbage over green cabbage (Ashfaq et al. 2020). In a plant system, CAT and SOD are the key enzymes metabolizing H2O2 and superoxide anion radicals and function as the primary defense against ROS. The superoxide ions are dismutated by SOD into molecular oxygen and H2O2, whereas H2O2 is decomposed by CAT into H2O and O2 (del Río et al. 2018). In the current study, cabbage plants grown under EMF-r stress showed increased activities of SOD and CAT, implying enhanced expression of SOD and CAT–encoding genes in the EMF-r exposed samples (Mosa et al. 2018). Such findings are in accordance with Bułdak et al. (2012) and Sharma et al. (2009), who observed higher levels of SOD and CAT in response to EMF-r. A significant enhancement was noticed in the activity of GPX upon EMF-r exposure. The results are in corroboration with those of Sharma et al. (2010), who demonstrated increased levels of peroxidase and other antioxidant enzymes in Vigna radiata (L.) R.Wilczek seedlings irradiated with cell phone radiation (at 900 MHz) for 0.5, 1, 2, and 4 h duration. The significant difference in the enzymatic activity of CAT between red and green cabbage in control samples across all the observation groups (0, 1, and 24 h) could be attributed to various genetic, physiological, and environmental factors.
Plants produce secondary metabolites as defense compounds to minimize oxidative stress–induced damage (Erb and Kliebenstein 2020). Most of the metabolites found in the present study belonged to the polyphenol family, followed by terpenoids and alkaloids. Increased accumulation of secondary metabolites and induction of plant defense mechanisms has been reported under various abiotic stresses, including extremely low-frequency EMF-r (Šamec et al. 2021). Under long-term exposure to radio waves (1800 MHz up to 96–144 h), the phenolic and flavonoid contents increased up to 24 h; however, there was a decline in secondary metabolites upon prolonged exposure (Upadhyaya et al. 2021, 2022). In our study, the number of polyphenolic compounds in red cabbage decreased upon exposure to EMF-r compared to their control counterparts, while there were no significant variations in polyphenolic compounds in green cabbage. A possible reason for the decrease in metabolic compounds could be due to the down-regulation of the PAL (phenylalanine ammonia-lyase) gene (Singh et al. 2012). Furthermore, red cabbage contains more polyphenolic compounds than green cabbage, which could explain why there is less damage caused by EMF-r exposure in red cabbage.
The extent to which plants recover after being exposed to EMF-r was also investigated in the present study. The plants were allowed to recover under controlled conditions for 1 and 24 h in the absence of EMF-r. However, the three observation groups did not vary significantly, thereby implying that changes persisted over time after exposure to EMF-r. These observations are supported by the findings of Chandel et al. (2019b), who reported that exposure of the Allium cepa L. plant to 2100 MHz incited cytotoxic and genotoxic damage in root meristem cells, but no significant difference was found in the samples harvested 24 h after treatment. In general, exposure to EMF-r stimulates systemic responses within plant tissue that affect whole plant metabolism. Within the plant system, radiation changes the electric polarity of the tissues, thereby affecting biomolecules and charged ions. These ultimately alter the ionic movement, leading to the propagation of improper signals responsible for morphological, metabolic, biochemical, and physiological adjustments in plants (Kaur et al. 2021).
Conclusions
The study concludes that the 2850 MHz electromagnetic field radiation exerts a detrimental effect on plants by interfering with their functioning at various stages of growth and development, pigment content, and antioxidant responses. The effects were dose-dependent, and the maximum damage was caused at higher exposure durations, implying that the biological effects are dependent on the exposure time. Furthermore, EMF-r exposure treatment reduced growth, biomass, and photosynthetic pigment content while increasing levels of phenols, flavonoids, anthocyanins, and overall antioxidant activity compared to control. The effects of EMF-r were more pronounced in green cabbage than in red cabbage. The red cabbage contained greater amounts of carotenoids, phenols, flavonoids, and anthocyanins. These compounds play a significant role in shielding against oxidative stress, potentially enabling red cabbage to better manage the stress induced by EMF-r. The findings obtained from the present study emphasize the need to better understand the mechanism of EMF-r as an abiotic stress at the molecular level using genomics and proteomics approaches.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Ahmad R, Ali S, Abid M, Rizwan M, Ali B, Tanveer A, Ahmad I, Azam M, Ghani MA (2020) Glycinebetaine alleviates the chromium toxicity in Brassica oleracea L. by suppressing oxidative stress and modulating the plant morphology and photosynthetic attributes. Environ Sci Pollut Res 27:1101–1111. https://doi.org/10.1007/s11356-019-06761-z
Andreuccetti D, Fossi R, Petrucci C (1997) An Internet resource for the calculation of the dielectric properties of body tissues in the frequency range 10 Hz–100 GHz, IFACCNR, Florence (Italy). Available online at http://niremf.ifac.cnr.it/tissprop/. Accessed 17 Aug 2021.
Arnon DI (1949) Copper enzymes in isolated chloroplasts Polyphenoloxidase in Beta vulgaris. Plant Physiol 24:1–15. https://doi.org/10.1104/pp.24.1.1
Ashfaq F, Butt MS, Bilal A, Tehseen S, Suleria HA (2020) Comparative assessment of free radical scavenging ability of green and red cabbage based on their antioxidant vitamins and phytochemical constituents. Curr Bioact Compd 16:1231–1241. https://doi.org/10.2174/1573407216666200127130014
Beauchamp C, Fridovich I (1971) Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–286. https://doi.org/10.1016/0003-2697(71)90370-8
Bułdak RJ, Polaniak R, Bułdak Ł, Żwirska-Korczala K, Skonieczna M, Monsiol A, Kukla M, Dulawa-Budlak A, Birkner E (2012) Short-term exposure to 50 Hz ELF-EMF alters the cisplatin-induced oxidative response in AT478 murine squamous cell carcinoma cells. Bioelectromagnetics 33:641–651. https://doi.org/10.1002/bem.21732
Cakmak I, Marschner H (1992) Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol 98:1222–1227. https://doi.org/10.1104/pp.98.4.1222
Cebulak T, Oszmiański J, Kapusta I, Lachowicz S (2017) Effect of UV-C radiation, ultra-sonication electromagnetic field and microwaves on changes in polyphenolic compounds in chokeberry (Aronia melanocarpa). Molecules 22:1161. https://doi.org/10.3390/molecules22071161
Çenesiz M, Atakişi O, Akar A, Önbilgin G, Ormancı N (2011) Effects of 900 and 1800 MHz electromagnetic field application on electrocardiogram, nitric oxide, total antioxidant capacity, total oxidant capacity, total protein, albumin and globulin levels in guinea pigs. Kafkas Univ Vet Fak Derg 17:357–362
Chandel S, Kaur S, Issa M, Singh HP, Batish DR, Kohli RK (2019a) Exposure to mobile phone radiations at 2350 MHz incites cyto-and genotoxic effects in root meristems of Allium cepa. J Environ Health Sci Eng 17:97–104. https://doi.org/10.1007/s40201-018-00330-1
Chandel S, Kaur S, Issa M, Singh HP, Batish DR, Kohli RK (2019b) Appraisal of immediate and late effects of mobile phone radiations at 2100 MHz on mitotic activity and DNA integrity in root meristems of Allium cepa. Protoplasma 256:1399–1407. https://doi.org/10.1007/s00709-019-01386-y
Chandel S, Kaur S, Singh HP, Batish DR, Kohli RK (2017) Exposure to 2100 MHz electromagnetic field radiations induces reactive oxygen species generation in Allium cepa roots. J Microsc Ultrastruct 5:225–229. https://doi.org/10.1016/j.jmau.2017.09.001
Chen H-Y, Chen C (2014) Effects of mobile phone radiation on germination and early growth of different bean species. Pol J Environ Stud 23:1949–1958. https://doi.org/10.15244/pjoes/24254
Cho S, Lee Y, Lee S, Choi YJ, Chung HW (2014) Enhanced cytotoxic and genotoxic effects of gadolinium following ELF-EMF irradiation in human lymphocytes. Drug Chem Toxicol 37:440–447. https://doi.org/10.3109/01480545.2013.879662
Choi YJ, Moskowitz JM, Myung SK, Lee YR, Hong YC (2020) Cellular phone use and risk of tumors: systematic review and meta-analysis. Int J Environ Res Public Health 17:8079. https://doi.org/10.3390/ijerph17218079
Cleary SF, Cao G, Liu LM (1996) Effects of isothermal 2.45 GHz microwave radiation on the mammalian cell cycle: comparison with effects of isothermal 27 MHz radiofrequency radiation exposure. Bioelectrochem Bioenerg 39:167–173. https://doi.org/10.1016/0302-4598(95)05037-X
Davis D, Birnbaum L, Ben-Ishai P, Taylor H, Sears M, Butler T Scarato T (2023) Wireless technologies, non-ionizing electromagnetic fields and children: Identifying and reducing health risks. Curr Probl Pediatr Adolesc Health Care 53:101374. https://doi.org/10.1016/j.cppeds.2023.101374
del Río LA, Corpas FJ, López-Huertas E, Palma JM (2018) Plant superoxide dismutases: Function under abiotic stress conditions. In: Gupta D, Palma J, Corpas F (eds) Antioxidants and antioxidant enzymes in higher plants. Springer, Cham. https://doi.org/10.1007/978-3-319-75088-0_1
Egley GH, Paul RN, Vaughn KC, Duke SO (1983) Role of peroxidase in the development of water-impermeable seed coats in Sida spinosa L. Planta 157:224–232. https://doi.org/10.1007/BF00405186
Erb M, Kliebenstein DJ (2020) Plant secondary metabolites as defenses, regulators, and primary metabolites: The blurred functional trichotomy. Plant Physiol 184(1):39–52. https://doi.org/10.1104/pp.20.00433
Ericsson Mobile Subscribers Outlook Report (2021) 5G on the road to mass market. https://www.ericsson.com/en/reports-and-papers/mobility-report/dataforecasts/mobile-subscriptions-outlook. Accessed 30 March 2023.
Ericsson Mobility Report update: Global 5G subscriptions top one billion (2023). https://www.ericsson.com/en/news/2023/2/emr-february-2023-update. Accessed 29 March 2023
Gao HN, Jiang H, Cui JY, You CX, Li YY (2021) The effects of hormones and environmental factors on anthocyanin biosynthesis in apple. Plant Sci 312:111024. https://doi.org/10.1016/j.plantsci.2021.111024
Halgamuge MN (2017) Weak radiofrequency radiation exposure from mobile phone radiation on plants. Electromagn Biol Medic 36(2):213–235. https://doi.org/10.1080/15368378.2016.1220389
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplast. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198. https://doi.org/10.1016/0003-9861(68)90654-1
Hiscox JD, Israelstam GF (1979) A method for the extraction of chlorophyll from leaf tissue without maceration. Can J Bot 57:1332–1334. https://doi.org/10.1139/b79-163
IARC, International Agency for Research on Cancer (2011) IARC classifies radiofrequency electromagnetic fields as possibly carcinogenic to humans (Press Release No:208, 31 May) https://www.iarc.who.int/wp-content/uploads/2018/07/pr208_E.pdf. Accessed 15 June 2021.
Johal N, Batish D, Pal A, Chandel S, Pal M (2022) Investigating the effects of 2850 MHz electromagnetic field radiations on the growth, germination and antioxidative defense system of chickpea (Cicer arietinum L.) seedlings. Russ J Plant Physiol 69:136. https://doi.org/10.1134/S1021443722060310
Kaur S, Vian A, Chandel S, Singh HP, Batish DR, Kohli RK (2021) Sensitivity of plants to high frequency electromagnetic radiation: Cellular mechanisms and morphological changes. Rev Environ Sci Biotechnol 20:55–74. https://doi.org/10.1007/s11157-020-09563-9
Kazemi M, Sahraei H, Aliyari H, Tekieh E, Saberi M, Tavacoli H, Meftahi GH, Ghanaati H, Salehi M, Hajnasrollah M (2018) Effects of the extremely low frequency electromagnetic fields on NMDA-receptor gene expression and visual working memory in male Rhesus macaques. Basic Clin Neurosci 9:167–176. https://doi.org/10.29252/NIRP.BCN.9.3.167
Kouzmanova M, Dimitrova M, Dragolova D, Atanasova G, Atanasov N (2009) Alterations in enzyme activities in leaves after exposure of Plectranthus sp. plants to 900 MHz electromagnetic field. Biotechnol Biotechnol Equip 23:611–615. https://doi.org/10.1080/13102818.2009.10818499
Kumar A, Kaur S, Chandel S, Singh HP, Batish DR, Kohli RK (2020) Comparative cyto-and genotoxicity of 900 MHz and 1800 MHz electromagnetic field radiations in root meristems of Allium cepa. Ecotoxicol Environ Saf 188:109786. https://doi.org/10.1016/j.ecoenv.2019.109786
Kumar A, Singh HP, Batish DR, Kaur S, Kohli RK (2016) EMF radiations (1800 MHz)-inhibited early seedling growth of maize (Zea mays) involves alterations in starch and sucrose metabolism. Protoplasma 253:1043–1049. https://doi.org/10.1007/s00709-015-0863-9
Kundu A, Vangaru S, Bhattacharyya S, Mallick AI, Gupta B (2021) Electromagnetic irradiation evokes physiological and molecular alterations in rice. Bioelectromagnetics 42:173–185. https://doi.org/10.1002/bem.22319
Lichtenthaler HK, Wellburn AR (1983) Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem Soc Trans 11:591–592. https://doi.org/10.1042/bst0110591
Martínez-Zamora L, Castillejo N, Artés-Hernández F (2021) UV-B radiation as abiotic elicitor to enhance phytochemicals and development of red cabbage sprouts. Horticulturae 7:567. https://doi.org/10.3390/horticulturae7120567
Meda A, Lamien CE, Romito M, Millogo J, Nacoulma OG (2005) Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem 91:571–577. https://doi.org/10.1016/j.foodchem.2004.10.006
Mildažienė V, Aleknavičiūtė V, Žūkienė R, Paužaitė G, Naučienė Z, Filatova I, Lyushkevich V, Haimi P, Tamošiūnė I, Baniulis D (2019) Treatment of common sunflower (Helianthus annus L.) seeds with radio-frequency electromagnetic field and cold plasma induces changes in seed phytohormone balance, seedling development and leaf protein expression. Sci Rep 9(1): https://doi.org/10.1038/s41598-019-42893-56437
Mildaziene V, Pauzaite G, Malakauskiene A, Zukiene R, Nauciene Z, Filatova I, Azharonok V, Lyushkevich V (2016) Response of perennial woody plants to seed treatment by electromagnetic field and low-temperature plasma. Bioelectromagnetics 37(8):536–548. https://doi.org/10.1002/bem.22003
Mosa KA, El-Naggar M, Ramamoorthy K, Alawadhi H, Elnaggar A, Wartanian S, Ibrahim E, Hani H (2018) Copper nanoparticles induced genotoxicty, oxidative stress, and changes in superoxide dismutase (SOD) gene expression in cucumber (Cucumis sativus) plants. Front Plant Sci 9:872. https://doi.org/10.3389/fpls.2018.00872
Obajuluwa AE, Akinyemi AJ, Afolabi OB, Adekoya K, Sanya JO, Ishola AO (2017) Exposure to radio-frequency electromagnetic waves alters acetylcholinesterase gene expression, exploratory and motor coordination-linked behaviour in male rats. Toxicol Rep 4:530–534. https://doi.org/10.1016/j.toxrep.2017.09.007
Paciulli M, Palermo M, Chiavaro E, Pellegrini N (2017) Chlorophylls and colour changes in cooked vegetables. In: Yahia EM (eds) Fruit and Vegetable Phytochemicals: Chemistry and Human Health, 2nd edn. Wiley, United States, pp 703–719. https://doi.org/10.1002/9781119158042.ch31
Prieto P, Pineda M, Aguilar M (1999) Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem 269:337–341. https://doi.org/10.1006/abio.1999.4019
Rabelo MC, Bang WY, Nair V, Alves RE, Jacobo-Velázquez DA, Sreedharan S, de Miranda MRA, Cisneros-Zevallos L (2020) UVC light modulates vitamin C and phenolic biosynthesis in acerola fruit: Role of increased mitochondria activity and ROS production. Sci Rep 10:21972. https://doi.org/10.1038/s41598-020-78948-1
Racuciu M, Iftode C, Miclaus S (2015) Inhibitory effects of low thermal radiofrequency radiation on physiological parameters of Zea mays seedlings growth. Rom J Phys 60:603–612
Vishki RF, Majd A, Nejadsattari T, Arbabian S (2012) Study of effects of extremely low frequency electromagnetic radiation on biochemical changes in Satureja bachtiarica L. Int J Sci Technol Res 1:77–82
Rani D, Kohli RK (1991) Fresh matter is not an appropriate relation unit for chlorophyll content: Experience from experiments on effects of herbicide and allelopathic substance. Photosynthetica 25:655–658
Roux D, Vian A, Girard S, Bonnet P, Paladian F, Davies E, Ledoigt G (2008) High frequency (900 MHz) low amplitude (5 V m− 1) electromagnetic field: a genuine environmental stimulus that affects transcription, translation, calcium and energy charge in tomato. Planta 227:883–891. https://doi.org/10.1007/s00425-007-0664-2
Šamec D, Karalija E, Šola I, Vujčić Bok V, Salopek-Sondi B (2021) The role of polyphenols in abiotic stress response: The influence of molecular structure. Plants 10:118. https://doi.org/10.3390/plants10010118
Sandu DD, Goiceanu C, Ispas A, Creanga I, Miclaus S, Creanga DE (2005) A preliminary study on ultra high frequency electromagnetic fields effect on black locust chlorophylls. Acta Biol Hung 56:109–117. https://doi.org/10.1556/abiol.56.2005.1-2.11
Schuermann D, Mevissen M (2021) Manmade electromagnetic fields and oxidative stress-biological effects and consequences for health. Int J Mol Sci 22(7):3772. https://doi.org/10.3390/ijms22073772
Sharma P, Jha AB, Dubey RS, Pessarakli M (2012) Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot 2012:217037. https://doi.org/10.1155/2012/217037
Sharma VP, Singh HP, Batish DR, Kohli RK (2010) Cell phone radiations affect early growth of Vigna radiata (mung bean) through biochemical alterations. Z Naturforsch C 65:66–72. https://doi.org/10.1515/znc-2010-1-212
Sharma VP, Singh HP, Kohli RK, Batish DR (2009) Mobile phone radiation inhibits Vigna radiata (mung bean) root growth by inducing oxidative stress. Sci Total Environ 407:5543–5547. https://doi.org/10.1016/j.scitotenv.2009.07.006
Shomali A, Das S, Arif N, Sarraf M, Zahra N, Yadav V, Aliniaeifard S, Chauhan DK, Hasanuzzaman M (2022) Diverse physiological roles of flavonoids in plant environmental stress responses and tolerance. Plants 11(22):3158. https://doi.org/10.3390/plants11223158
Siddhuraju P (2007) Antioxidant activity of polyphenolic compounds extracted from defatted raw and dry heated Tamarindus indica seed coat. LWT Food Sci Technol 40:982–990. https://doi.org/10.1016/j.lwt.2006.07.010
Singh HP, Mittal S, Kaur S, Batish DR, Kohli RK (2009) Characterization and antioxidant activity of essential oils from fresh and decaying leaves of Eucalyptus tereticornis. J Agric Food Chem 57:6962–6966. https://doi.org/10.1021/jf9012407
Singh HP, Sharma VP, Batish DR, Kohli RK (2012) Cell phone electromagnetic field radiations affect rhizogenesis through impairment of biochemical processes. Environ Monit Assess 184:1813–1821. https://doi.org/10.1007/s10661-011-2080-0
Soran ML, Stan M, Niinemets Ü, Copolovici L (2014) Influence of microwave frequency electromagnetic radiation on terpene emission and content in aromatic plants. J Plant Physiol 171:1436–1443. https://doi.org/10.1016/j.jplph.2014.06.013
Stefi AL, Margaritis LH, Christodoulakis NS (2017) The aftermath of long-term exposure to non-ionizing radiation on laboratory cultivated pine plants (Pinus halepensis M.). Flora 234:173–186. https://doi.org/10.1016/j.flora.2017.07.016
Stefi AL, Vassilacopoulou D, Margaritis LH, Christodoulakis NS (2018) Oxidative stress and an animal neurotransmitter synthesizing enzyme in the leaves of wild growing myrtle after exposure to GSM radiation. Flora 243:67–76. https://doi.org/10.1016/j.flora.2018.04.006
Surducan V, Surducan E, Neamtu C, Mot AC, Ciorîță A (2020) Effects of long-term exposure to low-power 915 MHz unmodulated radiation on Phaseolus vulgaris L. Bioelectromagnetics 41:200–212. https://doi.org/10.1002/bem.22253
Sutharut J, Sudarat J (2012) Total anthocyanin content and antioxidant activity of germinated colored rice. Int Food Res J 19:215–221
Swain T, Hillis WE (1959) The phenolic constituents of Prunus domestica. I.—The quantitative analysis of phenolic constituents. J Sci Food Agric 10:63–68. https://doi.org/10.1002/jsfa.2740100110
Tang C, Yang C, Yu H, Tian S, Huang X, Wang W, Cai P (2018) Electromagnetic radiation disturbed the photosynthesis of Microcystis aeruginosa at the proteomics level. Sci Rep 8:479. https://doi.org/10.1038/s41598-017-18953-z
Tkalec M, Malarić K, Pavlica M, Pevalek-Kozlina B, Vidaković-Cifrek Ž (2009) Effects of radiofrequency electromagnetic fields on seed germination and root meristematic cells of Allium cepa L. Mutat Res Genet Toxicol Environ Mutagen 672(2):76–81. https://doi.org/10.1016/j.mrgentox.2008.09.022
Tkalec M, Malarić K, Pevalek-Kozlina B (2007) Exposure to radiofrequency radiation induces oxidative stress in duckweed Lemna minor L. Sci Total Environ 388:78–89. https://doi.org/10.1016/j.scitotenv.2007.07.052
Upadhyaya C, Patel I, Upadhyaya T, Desai A, Patel U, Pandya K (2021) Investigation of mobile communication radio frequency exposure on the medicinal property of Jasminum grandiflorum L. In 5th International Conference on Computing Methodologies and Communication (ICCMC), Erode, India, 2021, pp 212–218. https://doi.org/10.1109/ICCMC51019.2021.9418324
Upadhyaya C, Upadhyaya T, Patel I (2022) Attributes of non-ionizing radiation of 1800 MHz frequency on plant health and antioxidant content of Tomato (Solanum Lycopersicum) plants. J Radiat Res Appl Sci 15:54–68. https://doi.org/10.1016/j.jrras.2022.02.001
Vanbergen AJ, Potts SG, Vian A, Malkemper EP, Young J, Tscheulin T (2019) Risk to pollinators from anthropogenic electro-magnetic radiation (EMR): Evidence and knowledge gaps. Sci Total Environ 695:133833. https://doi.org/10.1016/j.scitotenv.2019.133833
Varshikar D, Tan FC (2017) Salt and drought stress affects electron transport chain genes in rice. Intl J Adv Appl Sci 4:106–110. https://doi.org/10.21833/ijaas.2017.02.018
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain treated bean plants. Plant Sci 151:59–66. https://doi.org/10.1016/S0168-9452(99)00197-1
Vian A, Davies E, Gendraud M, Bonnet P (2016) Plant responses to high frequency electromagnetic fields. Biomed Res Int 2016:1830262. https://doi.org/10.1155/2016/1830262
Wang Z, Chen J, Fan Y, Cheng Y, Wu X, Zhang J, Wang B, Wang X, Yong T, Liu W, Liu J, Du J, Yang W, Yang F (2020) Evaluating photosynthetic pigment contents of maize using UVE-PLS based on continuous wavelet transform. Comput Electron Agric 169:105160. https://doi.org/10.1016/j.compag.2019.105160
Xi G, li Y, Cao Y, Song, Q (2005) The difference of chlorphyll fluorescence dynamics process and the system of photosynthetic pigment in leaf of spinach and tobacco under the action of low level microwave electromagnetic field. Acta Photonica Sin 34:1023-1027 (In Chinese with English Summary)
Yaqoob U, Jan N, Raman PV, Siddique KHM, John R (2022) Crosstalk between brassinosteroid signaling, ROS signaling and phenylpropanoid pathway during abiotic stress in plants: Does it exist? Plant Stress 4:100075. https://doi.org/10.1016/j.stress.2022.100075
Acknowledgements
APH is thankful to the Council of Scientific and Industrial Research (CSIR), New Delhi, India, for research fellowship.
Funding
None.
Author information
Authors and Affiliations
Contributions
SK and DRB: Project leader, supervision of the work; research design; AV: Conceptualization; Manuscript preparation and editing; APH: Establishment and execution of experiment, data collection, and manuscript preparation; HPS: Data analysis, Manuscript preparation and editing; RKK: Research design and manuscript editing. All authors reviewed the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Responsible Editor: Gangrong Shi
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Handa, A.P., Vian, A., Singh, H.P. et al. Effect of 2850 MHz electromagnetic field radiation on the early growth, antioxidant activity, and secondary metabolite profile of red and green cabbage (Brassica oleracea L.). Environ Sci Pollut Res 31, 7465–7480 (2024). https://doi.org/10.1007/s11356-023-31434-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-31434-3