Abstract
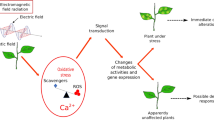
The present study investigated the impact of 1800-MHz electromagnetic field radiations (EMF-r), widely used in mobile communication, on the growth and activity of starch-, sucrose-, and phosphate-hydrolyzing enzymes in Zea mays seedlings. We exposed Z. mays to modulated continuous wave homogenous EMF-r at specific absorption rate (SAR) of 1.69±0.0 × 10−1 W kg−1 for ½, 1, 2, and 4 h. The analysis of seedlings after 7 days revealed that short-term exposure did not induce any significant change, while longer exposure of 4 h caused significant growth and biochemical alterations. There was a reduction in the root and coleoptile length with more pronounced effect on coleoptile growth (23 % reduction on 4-h exposure). The contents of photosynthetic pigments and total carbohydrates declined by 13 and 18 %, respectively, in 4-h exposure treatments compared to unexposed control. The activity of starch-hydrolyzing enzymes—α- and β-amylases—increased by ∼92 and 94 %, respectively, at an exposure duration of 4 h, over that in the control. In response to 4-h exposure treatment, the activity of sucrolytic enzymes—acid invertases and alkaline invertases—was increased by 88 and 266 %, whereas the specific activities of phosphohydrolytic enzymes (acid phosphatases and alkaline phosphatases) showed initial increase up to ≤2 h duration and then declined at >2 h exposure duration. The study concludes that EMF-r-inhibited seedling growth of Z. mays involves interference with starch and sucrose metabolism.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Non-ionizing electromagnetic field radiations (EMF-r) in the frequency range of 800–2100 MHz are the most commonly used magnetic fields for wireless communication. Today, there are 7.085 billion mobile phone users, equivalent to 96.2 % of the world population, with an average of 96.8 subscriptions per 100 individuals (ITU 2015). Mobile/cellular phones are the first EMF device with such a high frequency of use in the history of the world (Kundi 2009). The increasing exposure to mobile phone radiations and their base stations is a matter of concern because of possible adverse health effects. The available literature on the radiofrequency radiations’ exposure from cell phones is conflicting with both beneficial and harmful effects on biological systems, including birds, insects, plants (Cucurachi et al. 2013, and references therein) and humans (Kundi 2009; Khurana et al. 2010). Plants play an important role in ecosystem being primary producers; thus, it is pertinent to study the effects of EMF-r on plants. These being fixed cannot move away from the source of EMF-r and are thus continuously exposed to EMF-r. In comparison to other biological systems, relatively less is known about the ecological/biological effects of EMF-r on plants. A continuous exposure to weak magnetic field may cause discrete biotic effects at cellular, tissue, and organ level in plants (Belyavskaya 2004). Unfortunately, the exposure levels are increasing due to increased use of EMF-r emitting devices. Plants perceive and respond to radiofrequency electromagnetic radiations (Kouzmanova et al. 2009). They absorb EMF-r, convert these into weak electric currents, which damage endodermis, and consequently cause cell leakage, and make the plants more prone to soil-borne pathogens (Goldsworthy 2006). Despite the absence of neuronal network, plant neurobiologists believe that the plants are conscious and the reactions inside signaling pathways may impart biochemical basis for learning and ability to retain (Pollan 2013). Cells respond to a variety of environmental stimuli and electromagnetic energy by changing their morphology and movements. The electrical component of electromagnetic energy affects dipolar molecules such as ionic pumps, enzymes, nuclear material, and nucleotide molecules (Goodman et al. 1995). Previous studies reported that the EMF-r negatively affect seed germination, seedling growth, and development in plants (Tkalec et al. 2007; Sharma et al. 2009; Halgamuge et al. 2015). Roux et al. (2007) reported that EMF-r induces rapid molecular stress response in tomato, and the response was similar to the one found in response to cut or wound or burn. Studies have demonstrated a possible connection between negative impact of EMF-r on the growth and alterations in physiological processes, such as enzyme activities (Kouzmanova et al. 2009; Sharma et al. 2010), oxidative metabolism (Sharma et al. 2009; Tkalec et al. 2007), cell death (Sharma et al. 2010), DNA damage (Li and Chow 2001), and alterations in mitosis (Tkalec et al. 2009). However, not much is known about the interference of 1800-MHz EMF-r, widely used in mobile communication, with various metabolic pathways and the activities of enzymes catalyzing these pathways in plants. We, therefore, conducted a study to explore the effect of 1800 MHz EMF-r on the carbohydrate metabolism in Zea mays (maize). We investigated the changes in the content of reducing sugars and carbohydrates and explored the catalytic activity of various carbohydrate-metabolizing enzymes (acid and alkaline phosphatases, acid and alkaline invertases, α- and β-amylases, and starch phosphorylases) in coleoptiles of maize after ½, 1, 2 and 4 h of exposure to 1800-MHz EMF-r.
Materials and methods
Materials
Seeds of Zea mays (var. Kohinoor) were purchased locally from the seed store. Before use, these were surface disinfected with sodium hypochlorite (0.1 %, w/v) for 15 min and washed under running tap water followed by distilled water. All the chemicals and reagents used for biochemical estimations were of analytical reagent grade and procured from Sisco Research Laboratory Pvt. Ltd., India; Hi-Media, Mumbai, India; and Sigma Co., St. Louis, USA.
EMF-r exposure system
Pre-imbibed seeds (for 12 h in distilled water at 25 °C) were allowed to germinate in Petri dishes (15-cm dia) underlined with thin cotton wad and a single layer of Whatman #1 filter circle moistened with 14 ml of distilled water. In all, there were five treatment groups, each consisting of five Petri dishes with ten seeds each. No exposure was given to group-1 (control), whereas groups 2, 3, 4, and 5 were exposed to 1800 MHz of modulated continuous wave homogenous EMF-r (similar to mobile phone frequency) for ½, 1, 2, and 4 h. EMF-r was generated from radiofrequency (RF) signal generator (Agilent N9310A; Keysight Technologies, USA) supplied with 20 dBm (0.1 W) power and attached with an amplifier (Model No. ZHL-5 W-2GX+; Minicircuits, USA) and DC-regulated power supply. Exposure system was kept in an empty separate room coated with special Y shield (HSF54) RF shielding paint in order to protect from external exposure. After treatment, the experiment was laid out in a completely randomized design (CRD) in an environmentally controlled growth chamber set at day/night temperature of 30/25 (±2) °C, 76±2 % relative humidity, and a 16-h photoperiod of 240 μmol photon m−2 s−1 photon flux density.
The output power density at the plane of exposure was studied by ScanEM®-C Probe (Model No. CTK015; 3 M Technologies, USA) attached to RF power density meter (Spectran, HF-4060, range 100 MHz to 6 GHz; Aaronia AG, Germany). The average power density received at a distance of 3 cm from antenna was 332 ± 10.36 mW m−2 (1800 MHz) with a specific absorption rate (SAR) of 1.69 ± 0.0 × 10−1 W kg−1. SAR value was calculated roughly as it is quite difficult to evaluate SAR values on exposed tissues directly (Çenesiz et al. 2011). It was determined by taking the values of electrical conductivity (σ) and tissue density (ρ) for the dielectric properties of body tissue at 1800 MHz (σ = 1.70 S m−1 and ρ = 1030 kg cm−3) from IFAC (Institute of Applied Physics, Sesto Fiorentino, Italy) database (Andreuccetti et al. 1997).
Morphological parameters
After exposure to EMF-r for 7 days, the seedlings were randomly sampled from each treatment group and the seedling growth was recorded in terms of root and coleoptile length. Since the EMF-r-induced detrimental effects were more pronounced in coleoptiles (which are directly exposed to EMF-r under natural conditions), these were excised and stored at −40 °C for further biochemical studies.
Chlorophyll estimation
Total chlorophyll was extracted from coleoptiles (25 mg) in dimethyl sukphoxide (4 ml) at 60 °C for 1 h (Hiscox and Israelstem 1979). Its amount was determined spectrophotometrically as per Arnon (1949) and expressed on dry weight basis as suggested by Rani and Kohli (1991).
Estimation of carbohydrate and reducing sugar content
Carbohydrates were extracted by homogenizing 100 mg of coleoptiles with 10 ml distilled water in a pre-chilled pestle and mortar. The homogenate was centrifuged at 15,000g for 15 min at 4 °C in a cold centrifuge (Sigma Inc., USA). The supernatant was used for estimating total carbohydrate and reducing sugar content. Total carbohydrate content was estimated spectrophotometrically at 620 nm using anthrone reagent against glucose (50 μg ml−1) as a calibration standard (Loewus 1952). The reducing sugar content was determined at 520 nm against glucose as calibration standard, according to the method described by Nelson (1944).
Enzyme extraction
To extract α- and β-amylases, 100 mg of plant tissue (coleoptiles) was homogenized in 10 ml of 0.1 M sodium PO4 3− buffer (pH 7.0) under ice-cold conditions. Acid and alkaline phosphatases were extracted in ice-cold 50 mM tris-maleate buffer (pH 7.0) containing 13 mM mercaptoethanol. Starch phosphorylases were extracted by crushing 50 mg of tissue in 5 ml of distilled water. Acid and alkaline invertases were extracted in 10 mM tris-maleate buffer (pH 7.5) containing 1 mM mercaptoethanol and 20 mM MgCl2. The homogenates were filtered through three layers of cheesecloth and centrifuged at 15,000g for 25 min at 4 °C in a cold centrifuge. The supernatants were stored at 4 °C until used for assaying the enzyme activities. An aliquot (0.5 ml) of the supernatant was used for protein estimation, using bovine serum albumin as a calibration standard (Bradford 1976). All steps of the enzyme extraction including centrifugation were done at 4 °C, and the assays were performed at 25 °C using a UV-VIS spectrophotometer (Model UV 1800; Shimadzu Corporation, Tokyo, Japan).
Starch-hydrolyzing enzymes
The activity of α-amylases was determined according to the method of Muentz (1977). The concentration of remainder starch after reacting with iodine solution was measured spectrophotometrically at 630 nm using starch (50 μg ml−1) as standard. The specific activity of β-amylases was assayed as per the method of Bernfeld (1955), later modified by Dure (1960) using starch as a substrate and dinitrosalicylic acid as a reaction inhibitor. The absorbance of yellow to orange color thus developed was measured at 560 nm against a calibration standard of maltose (50 μg ml−1). The specific activities of both α- and β-amylases were expressed as μkat mg−1 protein. The activity of starch phosphorylases was determined at 660 nm against a calibration standard of potassium dihydrogen orthophosphate (Fiske and Subbarow 1952) and expressed as μkat mg−1 protein. One katal (kat) is the amount of the enzyme required to catalyze the transformation of one mole of substrate per second.
Sucrose-metabolizing enzymes
The activities of acid and alkaline invertases were measured in terms of reducing sugars produced, according to the method given by Nelson (1944). For the estimation of acid invertases, 0.2 M sodium acetate buffer (pH 4.8) was used, whereas for alkaline invertases, 0.2 M sodium phosphate buffer (pH 8) was used. Enzymatic assay was conducted spectrophotometrically at 620 nm against a calibration standard of glucose, and the specific activity of enzyme was expressed as μkat mg−1 protein.
Phosphohydrolytic enzymes
The activities of phosphohydrolytic enzymes—acid and alkaline phosphatases—were assayed at 410 nm in terms of p-nitrophenol, according to the method given by Malik and Singh (1980). For acid phosphatases, 0.1 M acetate buffer (pH 5.0) was used, whereas for alkaline phosphatases, 0.1 M glycine-NaOH buffer (pH 10.5) was used. The activity was expressed as μkat mg−1 protein.
Statistical analyses
For each exposure treatment, five Petri dishes (each with 10 seeds) were maintained in a completely randomized design with each Petri dish serving as an independent replicate. For enzyme assays, there were five replicated (independent) tissue samples (one pooled sample from each Petri dish) as replicate The data were analyzed using one-way analysis of variance followed by the comparison of mean values using post hoc Tukey’s test at P ≤ 0.05.
Results
Effect of EMF-r on seedling growth
The effect of EMF-r on seedling length and chlorophyll content of Zea mays after 7 days of exposure has been presented in Table 1 and Fig. 1. No difference in the seedling length and the amount of photosynthetic pigment was observed at lower exposure period. However, a significant reduction in root length (17 %), coleoptile length (23 %), and chlorophyll content (13 %) was observed at 4-h exposure period (Table 1). The inhibitory effect was more pronounced on coleoptile length than on root length (Fig. 1).
Effect on carbohydrate and reducing sugars content
Total carbohydrate content increased up to 1-h exposure treatments over that in the control, whereas it declined by 14 and 18 % (significant at P ≤ 0.05) at 2 and 4 h exposure treatments, respectively (Table 2). The amount of reducing sugars showed significant increment at ≥1 h exposure period. It increased by 30, 66, and 82 % at 1, 2, and 4 h exposure treatments, respectively, compared to the control (Table 2).
Effect on starch-hydrolyzing enzymes
EMF-r exposure (for ≥1 h) significantly increased the activities of α- and β-amylases by 24–92 and 28–94 %, respectively, over the control, during 1 to 4 h of exposure (Table 3). On the contrary, the activity of starch phosphorylases declined upon EMF-r exposure. It declined by 36, 53, and 74 % in response to 1, 2, and 4 h exposure treatments (Table 3).
Effect on sucrolytic and phosphohydrolytic enzymes
The activities of sucrolytic and phosphohydrolytic enzymes increased significantly on exposure to EMF-r for ≥1 h (Table 4). The activities of acid and alkaline invertases increased by 16–88 and 82–266 %, respectively, on 1 to 4 h of exposure to EMF-r over the control (Table 4). The specific activity of acid phosphatases increased by 14 and 23 % at 1- and 2-h exposure treatment, respectively (Table 4). However, it declined by 14 % in response to 4-h exposure treatment (Table 4). The activity of alkaline phosphatases increased over the control by 21 % at 1-h exposure treatment and then declined by 10 and 28 % at 2- and 4-h exposure treatments, respectively (Table 4).
Discussion
The present study demonstrated that EMF-r significantly decline the root and coleoptile length of Z. mays, particularly at higher exposure period of 2 and 4 h, thereby impeding seedling growth. Such a reduction in seedling length on exposure to EMF-r is corroborated by similar findings made earlier with cell phone radiations (Sharma et al. 2009; Singh et al. 2012), radiofrequency EMF-r (Tkalec et al. 2009; Akbal et al. 2012), and magnetic fields (Peñuelas et al. 2004). On the contrary, pre-treatment with magnetic fields has been found to alleviate the growth inhibition induced by abiotic stresses (Flórez et al. 2007; Hajnorouzi et al. 2011). In general, the response(s) of the plant systems to EMF-r are substantial and inconsistent; it varies with exposure type, strength and duration (intensity and frequency), oxidative state, and the genotype (Shabrangi and Majd 2009). The reduction in the photosynthetic pigments on EMF-r exposure suggests interference of EMF-r with the photosynthetic machinery, as reported earlier by Hirano et al. (1998) and Sandu et al. (2005).
In the present study, there was an initial rise in the amount of total carbohydrates at short duration of exposure (≤1 h); however, at longer duration of exposure (≥2 h), a significant decrease was observed. To the best of our knowledge, no such report highlighting such a trend in carbohydrate content on EMF-r exposure is available. Notwithstanding, studies have reported an increase in total soluble sugar and carbohydrate content on exposure to 945-MHz radiations in maize (Khalafallah and Sallam 2009) and to 50 Hz (at 6 kV/m field strength) electric field in wheat (Hanafy et al. 2006). In contrast, exposure to cell phone EMF-r (power density = 8.55 μW cm−2) caused a decline in carbohydrate content in roots of Vigna radiata, 1 week after exposure (Sharma et al. 2010). Carbohydrate content in the plants varies widely due to the interaction of plant with environment (Rosa et al. 2004). To avoid unfavorable abiotic conditions, plants accumulate different metabolites; and different carbohydrates accumulate depending on the type of stress (Ranwala and Miller 1998). However, at higher stress levels, there occurs a decline in carbohydrate content (Ranwala and Miller 1998), as observed in our study.
The decrease in carbohydrate content might be due to the increased activities of starch-hydrolyzing enzymes or decrease in the photosynthetic pigments as no new carbohydrates are synthesized due to impaired photosynthesis (as observed in the present study). Starch formed during photosynthesis is the interim reserve form of fixed carbon, whereas sucrose is the most suitable source of carbon and energy. In plant cells, starch is hydrolyzed by α- and β-amylases forming glucose and maltose (Niittyla et al. 2004), either independently or cumulatively, which are further used in glycolytic pathway or in the synthesis of sucrose (Stanley et al. 2005).
We observed a significant increase in the activities of α- and β-amylases on increasing the duration of EMF-r exposure. It is in accordance with an earlier study of Sharma et al. (2010) who also observed an increase in the activity of amylases in V. radiata roots on EMF-r exposure. The increased activity of amylases under EMF-r exposure results in an increase in the amount of sugars, an important source of energy for plants to keep growing under abiotic stress conditions (Kaplan and Guy 2004; Mahajan et al. 2013).
Invertases regulate the supply of sucrose to different cellular processes by hydrolyzing sucrose into hexose monomers (glucose and fructose), whenever needed, and play an important role in modulating environmental stresses (Roitsch and Gonzalez 2004). Hexoses are involved in maintaining osmotic pressure, defense response, and cell differentiation and development (Sturm and Tang 1999). The decreased activity of invertase(s) suggests interference with the carbon partitioning, growth regulation, and development (Ranwala and Miller 1998). We observed a dose-dependent increase in the activity of invertases under EMF-r exposure, thereby limiting the availability of sucrose in the cells. These findings are in agreement with those of Canli et al. (2011) and Taskin et al. (2013), who demonstrated increased production of invertases in Rhodotorula glutinis and Aspergillus niger OZ-3 on exposure to extremely low frequency magnetic fields.
Phosphohydrolytic enzymes (acid and alkaline phosphatases) regulate the inorganic phosphorus (Pi) supply to different parts of growing plants to upkeep the speed of growth and rate of physiological reactions in plant cells (Duff et al. 1994). In the present study, the activity of acid and alkaline phosphatases was increased at lower exposure duration, whereas a decline was observed at higher exposure period. The increased activity of phosphatases observed at short duration of exposure might be required to regulate the supply of Pi because of increased respiration and increased demand of sucrose in the growing seedling on exposure to EMF-r exposure and thus keep the plant growing (Agoreyo 2010). However, the decrease in phosphatase activity at higher exposure results in lesser availability of Pi and hence the reduced growth (Olmos and Hellin 1997).
Conclusions
In summary, our results suggest that 1800-MHz EMF-r interferes with carbohydrate metabolism. EMF-r exposure induces alterations in the contents of carbohydrates and reducing sugars and the activities of starch metabolizing enzymes: amylases, phosphatases, phosphorylases, and invertases. The study suggests that EMF-r-induced perturbations in carbohydrate metabolism are one of the factors resulting in growth inhibition on EMF-r exposure. The present study bears significance in view of the rapidly increasing EMF-r in the communication range in the natural environment and their possible interference with the physiological and biochemical processes in the plants. The study implies that proper risk assessment of EMF-r should be undertaken to develop management strategies for reducing EMF-r pollution in the natural environment.
References
Agoreyo BO (2010) Acid phosphatase and alkaline phosphatase activities in ripening fruit of Musa paradisiaca L. Plant Omics J 3:66–69
Akbal A, Kiran Y, Sahin A, Turgut-Balik D, Balik HH (2012) Effects of electromagnetic waves emitted by mobile phones on germination, root growth, and root tip cell mitotic division of Lens culinaris medik. Pol J Environ Stud 21(1):23–29
Andreuccetti D, Fossi R, Petrucci C (1997) An internet resource for the calculation of the dielectric properties of body tissues in the frequency range 10 Hz–100 GHz. http://niremfifaccnrit/tissprop/Florence, (Italy): IFAC-CNR; Based on data published by C Gabriel et al. in 1996
Arnon DI (1949) Copper enzymes in isolated chloroplasts: polyphenol oxidase in Beta vulgaris. Plant Physiol 24:1–15
Belyavskaya NA (2004) Biological effects due to weak magnetic field on plants. Adv Space Res 34:1566–1574
Bernfeld P (1955) Amylases, α and β. Methods Enzymol 1:149–158
Bradford MM (1976) Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Canli O, Erdal S, Taskin M, Kurbanoglu EB (2011) Effects of extremely low magnetic field on the production of invertase by Rhodotorula glutinis. Toxicol Ind Health 27:35–39
Çenesiz M, Atakişi O, Akar A, Önbilgin G, Ormancı N (2011) Effects of 900 and 1800 MHz electromagnetic field application on electrocardiogram, nitric oxide, total antioxidant capacity, total oxidant capacity, total protein, albumin and globulin levels in guinea pigs. J Fac Vet Med Univ Kafkas 17(3):357–362
Cucurachi S, Tamis WLM, Vijver MG, Peijnenburg WJGM, Bolte JFB, de Snoo GR (2013) A review of the ecological effects of radiofrequency electromagnetic fields (RF-EMF). Environ Int 51:116–140
Duff SMG, Sarath G, Plaxton WC (1994) The role of acid phosphatase in plant phosphorus metabolism. Physiol Plant 90:791–800
Dure LS (1960) Site of origin and extent of activity of amylases in maize germination. Plant Physiol 35:925–934
Fiske CH, Subbarow Y (1952) The colorimetric determination of phosphorus. J Biol Chem 56:375
Flórez M, Carbonell MV, Martínez E (2007) Exposure of maize seeds to stationary magnetic fields: effects on germination and early growth. Environ Exp Bot 59:68–75
Goldsworthy A (2006) Effects of electrical and electromagnetic fields on plants and related topics. In: Volkov AG (ed) Plant electrophysiology—theory and methods. Springer, Berlin Heidelberg, pp 247–267
Goodman EM, Greenebaum B, Marron MT (1995) Effects of electromagnetic fields on molecules and cells. Int Rev Cytol 158:279–338
Hajnorouzi A, Vaezzadeh M, Ghanati F, Jamnezhad H, Nahidian B (2011) Growth promotion and a decrease of oxidative stress in maize seedlings by a combination of geomagnetic and weak electromagnetic fields. J Plant Physiol 168:1123–1128
Halgamuge M, See K, Eberhardt J (2015) Reduced growth of soybean seedlings after exposure to weak microwave radiation from GSM 900 mobile phone and base station. Bioelectromagnetics 36:87–95
Hanafy MS, Mohamed HA, Elham A, El-Hady EAA (2006) Effect of low frequency electric field on growth characteristics and protein molecular structure of wheat plant. Rom J Biophys 16:253–271
Hirano MH, Ohta A, Abe K (1998) Magnetic field effects on photosynthesis and growth of the cyanobacterium Spirulina platensis. J Ferment Bioeng 86:313–316
Hiscox JD, Israelstem GF (1979) A method for the extraction of chlorophyll from leaf tissue without maceration. Can J Bot 57:1332–1334
Kaplan F, Guy CL (2004) β-amylase induction and the protective role of maltose during temperature shock. Plant Physiol 135:1674–1684
Khalafallah AA, Sallam SM (2009) Response of maize seedlings to microwaves at 945 MHz. Rom J Biophys 19:49–62
Khurana VG, Hardell L, Everaert J, Bortkiewicz A, Carlberg M, Ahonen M (2010) Epidemiological evidence for a health risk from mobile phone base stations. Int J Occup Environ Health 16(3):263–267
Kouzmanova M, Dimitrova M, Dragolova D, Atanasova G, Atanasov N (2009) Alterations in enzyme activities in leaves after exposure of Plectranthus sp. plants to 900 MHz electromagnetic field. Biotechnol Biotechnol Equip 23(2):611–615
Kundi M (2009) The controversy about a possible relationship between mobile phone use and cancer. Environ Health Perspect 117:317–324
Li SH, Chow KC (2001) Magnetic field exposure induces DNA degradation. Biochem Biophys Res Commun 280(5):1385–1388
Loewus FA (1952) Improvement in anthrone method for determination of carbohydrates. Anal Chem 24:219
Mahajan P, Singh HP, Batish DR, Kohli RK (2013) Cr(VI) imposed toxicity in maize seedlings assessed in terms of disruption in carbohydrate metabolism. Biol Trace Elem Res 156:316–322
Malik CP, Singh MB (1980) Plant enzymology and histo-enzymology. Kalyani Publishers, New Delhi, India
Muentz K (1977) The function of the pod for protein storage in seeds of Vicia faba L. 5. Isoenzymes of α-amylase during pod development of field beans. Phytochemistry 16:1491–1494
Nelson N (1944) A photometric adaptation of the Somogyi method for the determination of glucose. J Biol Chem 153:375–380
Niittyla T, Messerli G, Trevisan M, Chen J, Smith AM, Zeeman SC (2004) A previously unknown maltose transporter essential for starch degradation in leaves. Science 303:87–89
Olmos E, Hellin E (1997) Cytochemical localization of ATPase plasma membrane and acid phosphatase by cerium based in a salt-adapted cell line of Pisum sativum. J Exp Bot 48:1529–1535
Peñuelas J, Llusiá J, Martínez B, Fontcuberta J (2004) Diamagnetic susceptibility and root growth responses to magnetic fields in Lens culinaris, Glycine soja, and Triticum aestivum. Electromagn Biol Med 23:97–112
Pollan M (2013) The intelligent plant. The New Yorker. Available online at URL: http://www.newyorker.com/magazine/2013/12/23/the-intelligent-plant
Rani D, Kohli RK (1991) Fresh matter is not an appropriate relation unit for chlorophyll content: experience from experiments on effects of herbicide and allelopathic substance. Photosynthetica 25:655–667
Ranwala AP, Miller WB (1998) Sucrose cleaving enzymes and carbohydrate pools in Lilium longiflorum floral organs. Physiol Plant 103:541–550
Roitsch T, Gonzalez MC (2004) Function and regulation of plant invertases: sweet sensations. Trends Plant Sci 9:606–613
Rosa M, Hilal M, González JA, Prado FE (2004) Changes in soluble carbohydrates and related enzymes induced by low temperature during early developmental stages of quinoa (Chenopodium quinoa) seedlings. J Plant Physiol 161:683–689
Roux D, Vian A, Girard S, Bonnet P, Paladian F, Davies E, Ledoigt G (2007) High frequency (900 MHz) low amplitude (5 V m−1) electromagnetic field: a genuine environmental stimulus that affects transcription, translation, calcium and energy charge in tomato. Planta 227:883–891
Sandu DD, Goiceanu IC, Ispas A, Creanga I, Miclaus S, Creanga DE (2005) A preliminary study on ultra high frequency electromagnetic fields effect on black locust chlorophylls. Acta Biol Hung 56:109–117
Shabrangi A, Majd A (2009) Comparing effects of electromagnetic fields (60 Hz) on seed germination and seedling development in monocotyledons and dicotyledons. PIERS Proceedings, Moscow, Russia
Sharma VP, Singh HP, Kohli RK, Batish DR (2009) Mobile phone radiation inhibits Vigna radiata (mung bean) root growth by inducing oxidative stress. Sci Total Environ 407(21):5543–5547
Sharma VP, Singh HP, Batish DR, Kohli RK (2010) Cell phone radiations affect early growth of Vigna radiata (mung bean) through biochemical alterations. Z Naturforsh C 65(1–2):66–72
Singh HP, Sharma VP, Batish DR, Kohli RK (2012) Cell phone electromagnetic field radiations affect rhizogenesis through impairment of biochemical processes. Environ Monit Assess 184:1813–1821
Stanley D, Farnden KJF, MacRae EA (2005) Plant α-amylases: functions and roles in carbohydrate metabolism. Biologia (Bratislava) 16:65–71
Sturm A, Tang GQ (1999) The sucrose-cleaving enzymes of plants are crucial for development, growth and carbon partitioning. Trends Plant Sci 4:401–407
Taskin M, Esim N, Genisel M, Ortucu S, Hasenekoglu I, Canli O, Erdal S (2013) Enhancement of invertase production by Aspergillus niger OZ-3 using low-intensity static magnetic fields. Prep Biochem Biotechnol 43:177–188
ITU (International Telecommunication Union) (2015) ITU World Telecommunication/ICT Indicators database, 2014. Available online at URL: http://www.itu.int/en/ITU-D/Statistics/Documents/statistics/2015/ITU_Key_2005-2015_ICT_data.xlsPages/publications/wtidaspx. Accessed on May 24, 2015
Tkalec M, Malarić K, Pevalek-Kozlina B (2007) Exposure to radiofrequency radiation induces oxidative stress in duckweed Lemna minor L. Sci Total Environ 388:78–89
Tkalec M, Malarić K, Pavlica M, Pevalek-Kozlina B, Vidaković-Cifreka Z (2009) Effects of radiofrequency electromagnetic fields on seed germination and root meristematic cells of Allium cepa L. Mutat Res 672:76–81
Acknowledgments
The authors acknowledge the Ministry of Environment and Forests (India) and Department of Science and Technology (India) for financial assistance. Arvind Kumar is thankful to the University Grants Commission for providing research fellowship under UGC-BSR scheme.
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Peter Nick
Rights and permissions
About this article
Cite this article
Kumar, A., Singh, H.P., Batish, D.R. et al. EMF radiations (1800 MHz)-inhibited early seedling growth of maize (Zea mays) involves alterations in starch and sucrose metabolism. Protoplasma 253, 1043–1049 (2016). https://doi.org/10.1007/s00709-015-0863-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-015-0863-9