Abstract
Constructing superior Z-type photocatalytic heterojunction is beneficial to effectively enlarge interface contact, improve the photo-generated carrier separation rate, and retain the high redox ability. In this work, we designed a hierarchical core–shell g-C3N4/TiO2 structure to build Z-type heterojunction via combining simple template method and pyrolysis process. A close-knit Z-type heterojunction was constructed using TiO2 as a thick core and g-C3N4 as an ultra-thin shell. The effects of lamp source, wavelength, tetracycline (TC) concentration, and photocatalyst dose on the degradation performance on TC of g-C3N4/TiO2 were inspected. 0.1TiO2/g-C3N4 photocatalyst had the best degradation rate and highest removal rate within 30 min, and its degradation rate was about 49, 23, and 5 times than pure g-C3N4, TiO2, and commercial TiO2/g-C3N4 in respect. Moreover, compared with degradation ability under Xenon lamp, LED irradiation for g-C3N4/TiO2 composites showed a remarkable selective degradation. The fast and efficient Z-type transfer pathway of 0.1 g-C3N4/TiO2 was realized by forming an optimized interface and abundant surface active sites ascribed to the combined action of thick TiO2 core and ultra-thin g-C3N4 shell. In addition, the degradation intermediates were analyzed by LC–MS and suggested pathways of degradation. The work could provide novel design concept to obtain reliable Z-type photocatalysts with hierarchical core–shell structure applied in degradation of antibiotic wastewater.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Antibiotics as a great discovery in human history has been widely used in many fields, such as medical, animal husbandry, and aquaculture (Kayal and Mandal 2022). However, the produced antibiotic wastewater causes the environmental pollution and seriously endangers the health and safety of human and other organisms. It is untoward to deal with antibiotic wastewater efficiently due to its complicated composition, biological toxicity, non-biodegradability, and high concentration. Thus, it is imperative to develop efficient and simple antibiotic treatment techniques. Among many strategies, photocatalytic technology has attracted plenty of attention due to its superiorities of good reliability, high degradation efficiency, and outstanding cyclicity. Photocatalysis, as a promising advanced oxidation process (AOP) (Dewil et al. 2017; Milh et al. 2021), can generate highly oxidizing free radicals and other strong oxidizers in the water via light irradiation, which can efficiently degrade recalcitrant pollutants into innoxious substances. It is well known that titanium oxide (TiO2) is one of the preferred photocatalysts for the commercial application, thanks to its durable physical and chemical stability, good photo-corrosion resistance, abundant natural resource, high photocatalytic efficiency, non-toxicity, and low cost (Meng et al. 2019), which shows the extensive applications, such as wastewater treatment water splitting, sterilization, and carbon dioxide reduction. Generally, there mainly exist three crystal structures of TiO2: anatase, rutile, and brookite. The anatase TiO2 commonly has the better photocatalytic activity compared with other forms of TiO2 due to the super hydrophilicity, large band gap, and high surface area. However, it still suffers several defects, such as low light utilization, low quantum efficiency, and rapid recombination between electrons (e−) and holes (h+), thus greatly limiting its photocatalytic activity. At present, many methods including morphological control (Jo and Natarajan 2015), element doping (Kong et al. 2018), dye sensitization (Hao et al. 2018), and heterojunction structure (Maziarz 2019) are widely used to improve the photocatalytic performance. In particular, the construction of direct Z-type heterojunction has been a research hotspot due to the significant advantages of this system. The system can complete the oxidation reaction on a photocatalyst with more positive valence band and reduction reaction on a photocatalyst with more negative conduction band, which can effectively accelerate the separation and migration rate of photo-generated e− and h+ and retain the high redox performance. Furthermore, it also can extend the light absorption range, reduce the unfavorable reactions, and enhance the stability of direct Z-type heterojunction (Chen et al. 2022). Hao et al. (2017) prepared rutile TiO2/graphite carbon nitride (TiO2/g-C3N4) core–shell nanorods using a saturated aqueous solution route, which presented higher photocatalytic degradation performance of Rhodamine B in contrast with pure TiO2 and pure g-C3N4 under visible-light on account of the superb separation efficiency of photo-generated carriers by a Z-scheme heterojunction. Zuo et al. (2021) fabricated a Z-scheme TiO2/ZnIn2S4 nanoflower heterojunction from ultrathin TiO2 nanosheets integrated into ZnIn2S4, showing an enhanced photocatalytic H2 and O2 evolution activities and wonderful stability without sacrificial agent due to the higher redox potentials and prohibition of carrier recombination in system. Yu et al. (2013) synthesized a direct g-C3N4/TiO2 Z-type photocatalyst via an easy calcination method using P25 TiO2 (30 nm) and urea as the raw materials. The intimate heterojunctions were composed of P25 nanoparticles enfolded by ultrathin g‐C3N4 sheets, which could enable the fast transport of carriers at the interface to obtain the improved decomposition performance of formaldehyde (HCHO). Therefore, it was essential to optimize the direct Z-scheme migration route via constructing suitable nano-heterojunction structure. As a non-metallic photocatalyst,g-C3N4 could be applied in the degradation, hydrogen production, and reduction of carbon dioxide for superiorities of high specific surface area, stable properties, and low cost (Pan et al. 2022, 2023;). It was reported that Z-type g-C3N4/TiO2 heterojunctions for advanced oxidation were developed via numbers of methods such as wetness impregnation, defect-assisted modification, saturated aqueous solution method, and solvothermal method. However, there were few reports on using template method to construct a hierarchical core–shell structure to establish Z-type g-C3N4/TiO2 heterojunction. Furthermore, although Z-type g-C3N4/TiO2 presented extended photocatalytic activity and stability via different methods, it was still a great challenging to construct optimal Z-type g-C3N4/TiO2 heterojunction to obtain the reliable interface for rapid divorce and transfer of carriers (Rehman et al. 2022).
Herein, we designed a hierarchical core–shell g-C3N4/TiO2 structure via combining of a simple template method and pyrolysis process. The tight Z-scheme heterojunction was built from TiO2 as a thick core and g-C3N4 as an ultrathin shell, which could vastly shorten the transfer distance and enhanced the movement and division efficiency of photogenerated carriers. The effects at different conditions (such as light source, wavelength band, dose, and TiO2 amount) on the photocatalytic degradation performances of antibiotic were measured in detailed. The composite showed the selective efficient degradation ability under LED light irradiation. The g-C3N4/TiO2 sample presented enhanced degradation ability of antibiotic due to their coupling effect from special interface and Z-scheme pathway in contrast to pure g-C3N4 and TiO2.
Experimental part
Preparation of porous TiO2 nanomaterials
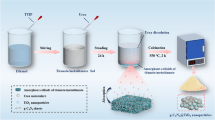
First, 0.12 g of silica (SiO2) nanopowders were dissolved in 80 ml of ethanol; then, 0.5 ml ammonium hydroxide was added and the blend was mixed evenly by stirring for 60 min at indoor temperature. Then, 1.2 ml of tetrabutyl titanate (TBT) was put into solution, and the reaction was stirred continuously at 50 °C for 16 h. The reaction was washed with deionized water and absolute ethanol, and dried in vacuo at 60 °C for 10 h. Subsequently, the products were kept at 550 °C in air for 2 h, and the heating rate was 5 °C/min to prepare SiO2@TiO2 composites. The composites were treated with 5% hydrogen fluoride solution to remove the SiO2 template. Ultimately, the porous anatase TiO2 nanomaterials were prepared by cleaning with deionized water and absolute ethanol. Then, the products were dried under vacuum at 60 °C for 10 h.
Preparation of direct Z-type g-C3N4/TiO2 composites
The flow chart for preparing direct Z-type TiO2/g-C3N4 composites was shown in Fig. S1. First, porous TiO2 (0.05 g, 0.1 g, and 0.15 g) and thiourea (2 g) were added into ethanol and blended for 1 h. Subsequently, the obtained product was then dried and kept at 550 °C for 2 h, and temperature rate was 5 °C/min. After cooling, the xTiO2/g-C3N4 composites were obtained, which were recorded as 0.05TiO2/g-C3N4, 0.1TiO2/g-C3N4 and 0.15TiO2/g-C3N4, respectively. Pure g-C3N4 was prepared by holding thiourea at 550 °C for 2 h at a temperature rate of 5 °C/min. For comparison, the commercial TiO2 (0.1 g) was also used for preparing commercial TiO2/g-C3N4 at the same experimental conditions.
Material characterization
The XRD diffraction pattern of the sample was determined by D8 ADVANCE diffractometer (Cu Kα diffraction source) test result (test range was 2θ = 10 ~ 80°, scanning speed was 5° min−1). The Fourier transform infrared (FTIR) spectra of each sample were measured with Nicolet Nexus 470 infrared spectrometer (test range was 400–4000 cm−1, with KBr as substrate). Scanning electron microscope (SEM, JSM-7800F) and transmission electron microscope(TEM, FEI Talos 200S) were used to analyze the morphology characteristics and element distribution of each sample. X-ray photoelectron spectroscopy (XPS) of each sample was obtained by X-ray photoelectron spectrometer (Thermo Scientific K-Alpha). Ultraviolet–visible (UV–Vis) reflectance spectra of all samples were obtained by spectrophotometer (UV2600) (test wavelength range was 300–400 nm, and high-purity BaSO4 was used as reference). The Steady-state PL spectra of the samples were tested by QuantaMaster™-40 with an excitation wavelength of 365 nm.
Photocatalytic degradation experiment
The photocatalytic activity of all specimens were estimated by photocatalytic degradation of TC. First, 50 ml TC water solution was diffused with10 mg photocatalyst. After dark reaction in 30 min, the equilibrium of adsorption–desorption was reached; then, experimental light source was turned on. An 80 W LED lamp (λ = 365 nm, 420 nm, 850 nm) and a 300-W Xe lamp (λ = 200–400 nm) were selected as the experimental light sources, and the distance between the lamp cap and the reaction vessel was about 10 cm. The reaction beaker was placed on the magnetic agitator to ensure that the reaction solution was in a stable state of continuous agitation, and then, the agitator and light source were turned on to conduct the photocatalytic degradation experiment. By gathering a certain amount of reaction solution at different times (0, 1, 3, 5, 15, 30 min) and filtered by 0.22 μm filter head to determine TC concentration (measured at 300–400 nm by ultraviolet spectrophotometer).
Photoeletric chemical test
The CHI 660E electrochemical workstation (three electrode system) was used for photochemical tests. The preparation process of the working electrode was as followed: 5 mg samples were added into a 5-ml centrifuge tube, then 40 μL membrane solution, 250 μL glycol, and 250 μL anhydrous ethanol were added, and then, ultrasonic was performed for 30 min to gain a uniform suspension. Then, the suspension (80 μL) was evenly covered on FTO conductive glass with a pipette gun, and was placed at 80 °C for 6 h until dry. The electrolyte was Na2SO4 aqueous solution (0.5 mol/L). The counter electrode was platinum electrode, and reference electrode was Ag/AgCl. The photocurrent, electrochemical impedance, and Schottky characteristics of the materials were tested by using this workstation.
Results and discussion
XRD and FTIR analysis
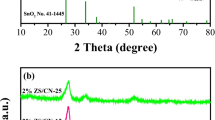
Figure 1a shows the XRD patterns of TiO2, g-C3N4, and xTiO2/g-C3N4 samples. And there existed two characteristic diffraction peaks in pure g-C3N4, located at 13.1° and 28.1°° corresponding to (100) crystal plane of in-plane structural stacking in g-C3N4 and the (002) crystal plane caused by interlayer stacking of conjugated aromatic hydrocarbons (JCPDS No.87–1526) (Li et al. 2017). There were obvious diffraction peaks at 25.5°, 37.6°, 48.1°, 53.9°, 55.0°, 62.7°, and 68.8°, corresponding to crystal planes of (101), (004), (200), (105), (211), (204), and (116) in TiO2 samples, respectively, indicating that only anatase phase TiO2 existed (JCPDS No.21–1272) (Li et al. 2022). For xTiO2/g-C3N4 sample, there existed obvious diffraction peaks of TiO2. However, it is difficult to observe the characteristic peak of g-C3N4 in xTiO2/g-C3N4 owing to poor content of g-C3N4 obtained from thiourea after pyrolysis. Furthermore, with raising TiO2 amount, the peak intensity and position of xTiO2/g-C3N4 composites had no evident change, indicating that the addition of TiO2 had little influence on the phase composition.
Figure 1b shows the chemical compositions of the samples and FTIR spectra were used for analysis. For single TiO2 and xTiO2/g-C3N4 samples, the absorption peak at 400–800 cm−1 was caused by the stretching vibration of Ti–O-Ti. And 1600 cm−1 and 3350 cm−1 were absorption peaks caused by the adsorption of H2O and surface O–H vibration (Monga and Basu 2019). The sharp absorption peak of pure g-C3N4 at 808 cm−1 was mainly due to out-of-plane bending vibration of triazine unit. The forceful and broad absorption band between 1200 and 1700 cm−1 was mainly attributed to the stretching vibration in C-N and C = N of typical S-triazine ring. The broad absorption peaks in the range of 3450–3150 cm−1 were assigned to the tensile vibration of N–H or O–H (Jiang et al. 2018a, b). Compared with single g-C3N4, recognize typical characteristic peak of g-C3N4 in xTiO2/g-C3N4 was tough, mainly thanks to low percentage of g-C3N4 in composite. Besides, xTiO2/g-C3N4 retained the characteristic absorption peak of TiO2. With raising TiO2 content, the intensity of the stretching vibration peak of Ti–O-Ti was significantly enhanced. However, the position of Ti–O-Ti peak was not changed, suggesting that the addition of g-C3N4 could be incapable of changing the chemical structure of the composites and could effectively build a heterojunction, which was conductive to the rapid transfer of carriers at the interface.
XPS analysis
To explore electronic chemical states of TiO2, g-C3N4, and xTiO2/g-C3N4, XPS analysis was conducted. Figure S2 presents the full XPS spectra of the samples that elements of C, N, O, and Ti were in composite simultaneously. Figure 1c is a high-resolution C 1 s spectrum with two peaks located at 284.8 eV and 288.3 eV, corresponding to sp2 hybridized C in N–C = N and sp2 C–C bond, respectively (Jiang et al. 2018a, b). The 286.4 eV was the peak which was corresponded to sp2 C of aromatic ring on heptazine unit. In Fig. 1d, the N 1 s spectrum of pure g-C3N4 had three peaks at 398.6 eV, 400.1 eV, and 401.2 eV in consistent with the -NH2 functional group, N-(C)3, and sp2 hybrid N(C-N = C), respectively. The high-resolution spectrum of O 1 s was presented in Fig. 1f. And peaks in pure TiO2 at 529.2 eV and 531.2 eV were related to Ti–O bond and O–H bond adsorbed on the surface, respectively (Qiu et al. 2018). The Ti 2p spectrum of pure TiO2 in Fig. 1e presented a binding energy of 456.9 eV in accordance with the split peak Ti 2p3/2 and the other binding energy at 463.6 eV corresponded to the split peak of Ti 2p1/2. It could be found that compared with pure g-C3N4, these binding energies of 0.1TiO2/g-C3N4 had an apparent negative shift. Conversely, the binding energy at different positions for 0.1TiO2/g-C3N4 appeared an evident positive shift than that of TiO2, implying the viable electron move path from TiO2 to g-C3N4.
SEM analysis
Figure 2 presents the figures of SEM and element mapping of xTiO2/g-C3N4 so as to investigate the micromorphology, element composition, and distribution of the composites. Figure 2a–b show the SEM of 0.05TiO2/g-C3N4 sample. It could be seen from the figure that the composite material was mainly composed of nanospheres and nanoparticles. Many nanoparticles and pores were found on the surface of the nanosphere in the corresponding enlarged image, indicating that the sample had a good hierarchical and porous structure and the surface of the hierarchical structure was flat. Pure TiO2 appeared as relatively smooth nanospheres in Fig. S3. Once TiO2 was covered with g-C3N4 nanoparticles, the active sites on the surface of composite increased, which promoted the rapid transfer of photogenerated charge carriers. With increasing TiO2 amount, no apparent changes of the morphology for 0.1TiO2/g-C3N4 were observed from the SEM images in both low and high magnification (Fig. 2c and d). With further raising TiO2 amount, the particle sizes of 0.15TiO2/g-C3N4 (Fig. 2e and f) increased significantly due to the accumulation of higher TiO2 content, implying that the TiO2 amount had a great impact on the microstructure of the composites. In addition, it could be seen that C, N, O, and Ti elements coexisted from EDS element mapping of 0.1TiO2/g-C3N4, and their distribution was uniform. And no Si element could be found, illustrating the completely etching of SiO2 as the porous template. It was summed that g-C3N4 could cover on the exterior of TiO2 to achieve a close heterojunction, which could enhance the separation and migration rates of carriers.
TEM analysis
Figure 2h shows the TEM images of 0.1 g-C3N4/TiO2. It could be noted that the nanospheres were consist of many nanoparticles with uniform sizes, leading to the formation of obvious pores, which was in keeping with results of SEM. The corresponding TEM image proved nanoparticles from hierarchical structure with sizes of 10 ~ 20 nm were well distributed further which was presented in Fig. 2i. The corresponding HRTEM image in Fig. 2j further testified that the lattice distance of the sample was about 0.357 nm, which was in great accordance with the (101) crystal plane of anatase TiO2. Furthermore, the TiO2 surface was covered with ultrathin g-C3N4 sheets, suggesting a thick core-thin shell structure of the composite to form suitable heterojunctions, which was conducive to improve the carrier separation rate to obtain high photocatalytic efficiency.
Photocatalytic degradation performances
The antibiotic tetracycline (TC) was used as the degradation pollutant, and the initial concentration of its solution was 60 mg/L during the whole degradation processes. Figure S4 shows the photocatalytic performances of 0.1TiO2/g-C3N4 under different degradation conditions. First, the pollutant concentration basically had no change under dark adsorption condition after 30 min, indicating that the adsorption reached the equilibrium. Subsequently, TC was degraded using irradiation of light at different wavelengths which was presented in Fig. S4(a), (d), and (g). It was found that the sample had the highest degradation rate of TC in 30 min under 365 nm attributed to the super light absorption ability under this wavelength, which could produce more photogenic charge carriers (Xu et al. 2020; Perumal et al. 2023). Meanwhile, it was easy to see that the degradation curves of 0.1TiO2/g-C3N4 under 365@420 nm and 365 nm were similar, showing that the addition of visible light hardly enhanced the degradation effect of the samples. Besides, it was worth noting that 0.1TiO2/g-C3N4 processed a moderate photocatalytic degradation rate under 200–400 nm, revealing that the sample had a good photocatalytic ability under ultraviolet B and C (UVB and UVC) due to the satisfying light absorption capacity. Also, the sample showed no photocatalytic effect under the near-infrared light of 850 nm attributed to the negligible light absorption level at this wavelength. The photocatalytic degradation kinetic curves of 0.1TiO2/g-C3N4 sample were fitted by the following formula:
C (mg/L) was the density of TC in solution in that moment, C0 (mg/L) was the initial concentration of TC, and K (min−1) was the rate constant of reaction (Liu et al. 2019; Yu et al. 2020). The obtained information was completely conformed to the quasi first-order kinetic equation after linear fitting. The K values at various wavelengths (200 ~ 400 nm, 365 nm, 420 nm, 850 nm, and 365@420 nm) were 0.030, 0.049, 0.014, 0.000421, and 0.042, respectively, further inferring the efficient degradation of the sample at ultraviolet and visible regions.
Figure S4(c) shows the degradation efficiency of 0.1TiO2/g-C3N4 photocatalyst (10 mg) at different concentrations of TC under 365 nm. The results showed the degradation ability gradually declined with growing TC concentration. The degradation kinetic curves of 0.1TiO2/g-C3N4 were accord with the first-order kinetic equation which were presented in Fig. S4(f). The corresponding K values of TC in Fig. S4(i) at different concentrations were 0.076, 0.074, 0.054, and 0.049, respectively, showing a decreasing degradation rate with raising TC concentration. Generally, under the same degradation conditions, the catalyst had a fixed number of active groups and electron–hole pairs. When TC concentration was low, the active free radicals of the sample were sufficient to degrade pollutant effectively. However, when TC concentration reached a higher concentration, the sample showed insufficient number of active free radicals leading to the weakness of photocatalysis and obvious decline of degradation rate. Figure S4(b, e, h) show the degradation performances of 0.1TiO2/g-C3N4 using various photocatalyst doses under 365 nm. Under the same test conditions, the degradation efficiency was positively correlated with photocatalyst amount. With increasing photocatalyst doses, the degradation efficiency was significantly enhanced. The pollutant could be completely removed after 30 min when adding a highest dose (15 mg), corresponding to a maximum degradation rate constant (0.076) and the best degradation ability because the high dose of photocatalyst provided more surface active sites and photo-generated carriers, which could effectively improve the photocatalytic degradation efficiency (Zhang et al. 2022).
Figure 3a shows the degradation performance of different samples under 365 nm using 10 mg photocatalyst. Among these samples, the degradation ability of prepared xTiO2/g-C3N4 composites by template method was superior to that of commercial TiO2/g-C3N4 due to more active sites for photogenic carrier transfer. Moreover, it could be found that after the dark adsorption reached the equilibrium, the value of C/C0 for 0.1TiO2/g-C3N4 showed a more significant decrease with the extension of time compared with other samples, showing its highest degradation performance. From the fitted degradation kinetics curves (Fig. 3c), the degradation capability of xTiO2/g-C3N4 was superior to single g-C3N4. Besides, degradation efficiency was first enhanced and then weakened sharply with raising TiO2 amount. The increase of particle size of the composite and the decrease of the surface active sites after adding excessive TiO2 were not favoring to rapid migration of photogenerated carriers. Figure 3e shows the degradation kinetic rates of different samples. It could be clearly inferred that the 0.1TiO2/g-C3N4 composite presented the fastest degradation rate (K = 0.049), which were about 49, 23, 4, 9, and 5 times to single g-C3N4, TiO2, 0.05TiO2/g-C3N4, 0.15TiO2/g-C3N4, and commercial TiO2/g-C3N4, respectively. It was confirmed that the appropriate heterojunction interface and good hierarchical structure of 0.1TiO2/g-C3N4 were conducive to the efficient separation and transfer of carries, which was compatible with the SEM analysis results (Hunge et al. 2021; Tang et al. 2021).
The degradation performance of different samples (10 mg) under the irradiation of 200–400 nm was seen in Fig. 3b, d, f. It was interesting to note that the prepared xTiO2/g-C3N4 composites showed similarities in degradation performance and the K values kept in the range of 0.021 to 0.030, suggesting that the additive amount of TiO2 had lesser effect on the photocatalytic performance of these composites using Xenon irradiation under ultraviolet light. Unlike Xenon irradiation at 200–400 nm, the ability of photocatalysis existed considerable difference in xTiO2/g-C3N4 composites and K values remained in 0.006 to 0.049, implying that LED irradiation under 365 nm had remarkable selective degradation for various composites. It also could be seen that 0.1TiO2/g-C3N4 composite among all samples had the best photocatalytic efficiency under both two light irradiations in respect due to the suitable hierarchical structure and coated heterojunction. And the removal rate (R) and K value of TC was lower at 200–400 nm (R = 83%, K = 0.030) than at 365 nm (R = 95%, K = 0.049) because the sample produced fewer photogenerated carriers under 200–400 nm, which resulted a weaker degradation performance.
Photocatalytic degradation mechanism
UV − vis diffuse reflection spectroscopy was used to test the absorbance and forbidden band width (Eg) of samples in Fig. 4. Figure 4a shows the absorption edges of single TiO2 and g-C3N4 were about 370 nm and 467 nm, respectively (Zong et al. 2019). The positions of absorption edge for xTiO2/g-C3N4 and 0.15TiO2/g-C3N4 were essentially consistent with pure TiO2, indicating that TiO2 amount had little influence on the absorption edge of the composites. And 0.1TiO2/g-C3N4 displayed higher absorbance under ultraviolet and visible regions in contrast with pure TiO2 due to the construction of appropriate core–shell structure, leading an effective enhancement in degradation property under 365 nm and 420 nm. Figure 4b presents the calculated curves which indicated the Eg of single TiO2, g-C3N4 were 3.17 and 2.38 eV, respectively. It was found that Eg of xTiO2/g-C3N4 was superior to single g-C3N4, showing stronger redox ability.
The Mott Schottky (M-S) curves obtained from various frequencies were presented in Fig. 4c, d. It was clearly seen that the slopes of samples of M-S curves of at different frequencies were positive, revealing the materials had n-type semiconductor characteristic. The conduction band potential (ECB) of g-C3N4 and TiO2 could be gained from M-S curves. The intercept with the x-axis of M-S curves was the flat-band potential (Efb (Ag/AgCl)) of TiO2 and g-C3N4, which were − 0.58 V and − 0.93 V, respectively. In conformity with the formula Efb (NHE) = Efb (Ag/AgCl) + Eθ (Ag/AgCl) + 0.059 pH (pH = 6.8, Eθ (Ag/AgCl) = 0.197 eV), the conduction band potential (ECB) was generally 0.3 V lower than that of EfB (NHE). Thus, the ECB values of TiO2, g-C3N4 were − 0.28 and − 0.63 V (vs NHE). From the above result, the band gaps (Eg) of single TiO2, g-C3N4 were 2.91 and 2.38 eV, respectively. The valence band potentials (EVB) were corresponded to 2.63 and 1.72 V (vs NHE) by combining with EVB = ECB + Eg.
The recombination and migration process of carriers for different samples were measured by PL spectroscopy in Fig. 5a. The PL intensity of single g-C3N4 was the best attribute to the rapid recombination of carriers. On contrary, the porous TiO2 had awfully low intensity, inferring a fast separation and migration rate of carriers. Also, it could be observed that xTiO2/g-C3N4 had extremely low PL strength due to their ideal heterostructures. The result clarified that the core–shell structure of the composites could effectively promote the carrier transfer rate and restrain the recombination of carriers. With increasing TiO2 amount, the PL strength had almost negligible change. It could be concluded that TiO2 amount has a tiny impact on the PL strength of xTiO2/g-C3N4, showing that the carries of the composites were easy to be separated.
EIS and transient photocurrent response of the samples were further carried out to analyze the transfer and divorce efficiencies of carriers (Fan et al. 2016). From Fig. 5c, it was shown that the photocurrent intensities of 0.5TiO2/g-C3N4 and 0.1TiO2/g-C3N4 were both significantly superior to pure g-C3N4. Nonetheless, photocurrent intensity of 0.15TiO2/g-C3N4 was close to pure g-C3N4, signifying that excessive loading of TiO2 could hinder the transfer and separation of carries due to its damaged heterojunction structure. And 0.1TiO2/g-C3N4 had the highest photocurrent intensity in all samples, further proving the highest separation and migration rate of carriers attributed to the outstanding synergistic effect from applicable core–shell heterojunction, which was agreed with the result in Fig. 5b. The arc radius of EIS Nyquist plots in Fig. 5b reflected the surface reaction velocity (Vr) and interfacial charge transfer resistance (Rc) of the photocatalyst. Generally, the charge transfer resistance would be lower with smaller arc radius, which was more conductive to the charge move across the surface. Therefore, arc radius of xTiO2/g-C3N4 were smaller than single g-C3N4 along with a faster charge transfer rate which clarified the rapid charge transfer of the composites owing to the construction of heterojunctions. Moreover, with raising TiO2 amount, the arc radius of the composites gradually reduced then rapidly enlarged, demonstrating that TiO2 amount had a vital impact on the charge transfer efficiency of xTiO2/g-C3N4. Among them, 0.1TiO2/g-C3N4 presented the smallest arc radius, indicating that 0.1TiO2/g-C3N4 had the lowest Rc value and the fastest Vr. These above results demonstrated that 0.1TiO2/g-C3N4 had the longest carrier lifetime and the fastest separation efficiency, leading to the best degradation ability of TC on account of the formation of an optimal heterojunction structure and acquisition of more active sites. In addition, in order to study the active radicals which played a major effect in the process of TC degradation, different inhibitors were added for experiments. In a word, the BQ, EDTA, and IPA were used as the scavengers to determine the effect of \({\cdot {O}_{2}}^{-}\), h+, and \(\cdot OH\) in the degradation process, respectively (Zhu et al. 2019). Figure 5d shows the removal efficiency of 0.1TiO2/g-C3N4 distinctly decreased after the addition of BQ and EDTA, while the removal efficiency displayed a minor decline when adding IPA compared with the condition without scavengers, indicating the free radicals (\({\cdot {O}_{2}}^{-}\)) and h+ played crucial roles in photocatalytic process.
Fig. S5 presents the photocatalytic cyclic curves and removal efficiencies of 0.1TiO2/g-C3N4 at the typical conditions. It could be seen that the sample maintained high photocatalytic degradation efficiency of about 99% after four cycles, which indicated that the sample had excellent cycle stability and photocorrosion resistance. It was expected that the hierarchical and coated composites had good application prospects in degrading antibiotics. Fig. S5(c) shows the XRD pattern before and after four times of photocatalysis. After cycles, no obvious changes were in spectrum and no diffraction peaks of other impurities were found which meant synthesized TiO2/g-C3N4 composite had extremely high stability.
TRPL was used to analyze the average lifetime of the photogenerated carrier of sample in Fig. S6. The average lifetime of g-C3N4, TiO2, and 0.1TiO2/g-C3N4 were 5.26, 1.07, and 1.68 ns when the sample was excited at 337 nm and calculated by double exponential fitting. As a rule, the longer the material life meant, the lower the recombination rate of photogenerated carriers, and the lifetime of TiO2 after loading g-C3N4 was significantly enhanced, thus corroborating the construction of heterojunction significantly accelerated the division and transfer of photoinduced carriers. The recombination of electron and hole was inhibited, and the photocatalytic activity was enhanced.
In order to clarify the degradation pathway and the intermediates molecules generated during the degradation process of TC, the degradation products were collected from reaction mixtures at different irradiation times by LC–MS test. In conformity with the LC–MS spectra (Fig. S7), the m/z values of major intermediates were 459,301, 349, 279, 201, 149, and 159, respectively. By analyzing LC–MS spectra (Fig. S7) and referring to earlier reports (Dong et al. 2018), the decomposition pathway of TC and molecular structure of intermediate during degradation were listed (Table S1). TC degradation by TiO2/g-C3N4 had three main pathways (Fig. 6): First, for path I, TC (m/z = 445) was hydroxylated, then broken into P1 (m/z = 459). After the dehydroxylation reaction and deamination reaction, the c = c bonds formed P2 under \(\cdot OH\) and ·O2− attack (Gan et al. 2022). Alternatively, P2 (m/z = 301) could be obtained directly from path II by oxidation ring opening under the action of free radicals (Liu et al. 2020). Finally, the intermediates were broken down into smaller molecules (m/z = 149, 116, and 201). For path III, TC was attacked by \(\cdot OH\) and carbon atom ring was cleaved to achieve intermediate P6 (m/z = 349) (Bai et al. 2019), and then, after decarboxylation reaction, P7 (m/z = 279) and P8 (m/z = 279) were obtained under attack of reactive radical (Guo et al. 2021).
Figure S8 and the formula (3.2)–(3.6) present the feasible diagrammatic drawing of direct Z-type photocatalytic mechanism to explain the elevation of photocatalytic capability for 0.1TiO2/g-C3N4. First, TiO2 along with g-C3N4 both generated photo-induced electron–hole pairs via light irradiation. The photocatalytic reactions of direct Z-type TiO2/g-C3N4 composite were as followed. The CB of TiO2 generated electrons which could move to the VB of g-C3N4 and combined with holes in the VB of g-C3N4, ensuring the CB of g-C3N4 had strong reducing action electrons and the VB of TiO2 had high oxidation holes, which was identified as a direct Z-type charge divorce path. Concurrently, the CB of g-C3N4 could reduce O2 to form \({\cdot {O}_{2}}^{-}\) with electrons in it, and then, free radical \({\cdot {O}_{2}}^{-}\) could strongly oxidize TC to obtain small molecule products (e.g., CO2 and H2O) because g-C3N4 had more negative CB potential than that of O2/\({\cdot {O}_{2}}^{-}\) (− 0.33 eV vs. NHE). As a result, the free radicals (\({\cdot {O}_{2}}^{-}\)) and h+ participated in the oxidization reactions played the good roles in degrading TC. Thus, the VB of TiO2 could directly oxidize TC by h+ in it because the level of its VB was positive than the standard redox potential of OH−/\(\cdot OH\) (2.29 eV vs. NHE). However, the VB level of g-C3N4 was dissatisfied with the redox potential of OH−/\(\cdot OH\), suggesting that it was impossible transfer h+ from VB in TiO2 to VB in g-C3N4 (Jiang et al. 2017), indicating that direct Z-type photocatalytic mechanism was viable. In Z-type pathway, the composite with good light absorption ability was excited to obtain amounts of photo-generated carries, and then, these carriers were effectively separated and transferred and the composite retained the excellent oxidation property to degrade TC, which were ascribed to the synergistic effect of core–shell TiO2/g-C3N4 structure to form an optimized interface and abundant surface –active sites.
Conclusions
Briefly, we successfully prepared the hierarchical g-C3N4/TiO2 composites via template method followed by pyrolysis. The tight Z-type heterojunction was constructed via using TiO2 as thick core and g-C3N4 as ultra-thin shell. The g-C3N4/TiO2 composites had good ability to degrade TC under ultraviolet light (365 nm, 200–400 nm), especially in single-band 365 nm. During the degradation process, ·O2− and h+ played critical roles in improving TC degradation effect. Among these composites, 0.1TiO2/g-C3N4 had the finest photocatalytic degradation capability, and degradation rate constant K was 0.049, which was about 49 and 23 times superior to single g-C3N4 and TiO2, respectively. On account of construction of direct Z-type heterojunction with core–shell structure and favorable contact interface, 0.1 g-C3N4/TiO2 has high separation and transfer rate of carriers, resulting in its excellent photocatalytic performance. The core–shell TiO2/g-C3N4 Z-type photocatalyst shows the advantages of low cost, high activity, and superior stability, which can be expected for removing numerous refractory pollutants from pharmaceutical wastewater, dye wastewater, and pesticide wastewater.
Data Availability
The datasets used and/or analysed during the current study available from the corresponding author on request.
References
Bai X, Wang Y, Li Y, Wang X (2019) Adsorption–photocatalytical remediation for series of tetracycline contaminants with BiOCl–CdS composite under simulated sunlight. J Taiwan Inst Chem Eng 104:94–105. https://doi.org/10.1016/j.jtice.2019.08.016
Chen C, Zhang J, Xiong X, Lin J, Yang S, Xi J, Kong Z (2022) A novel Z-type multidimensional FeSe2/CuSe heterojunction photocatalyst with high photocatalytic and photoelectrochemical performance. Int J Hydrog Energy 47(67):28879–28893. https://doi.org/10.1016/j.ijhydene.2022.06.219
Dewil R, Mantzavinos D, Poulios I, Rodrigo MA (2017) New perspectives for advanced oxidation processes. J Environ Manage 195:93–99. https://doi.org/10.1016/j.jenvman.2017.04.010
Dong G, Huang L, Wu X, Wang C, Liu Y, Liu G, Wang L, Liu X, Xia H (2018) Effect and mechanism analysis of MnO2 on permeable reactive barrier (PRB) system for the removal of tetracycline. Chemosphere 193:702–710. https://doi.org/10.1016/j.chemosphere.2017.11.085
Fan D, Guo C, Ma H, Zhao D, Li Y, Wu D, Wei Q (2016) Facile fabrication of an aptasensor for thrombin based on graphitic carbon nitride/TiO2 with high visible-light photoelectrochemical activity. Biosens Bioelectron 75:116–122. https://doi.org/10.1016/j.bios.2015.08.029
Gan W, Fu X, Guo J, Zhang M, Li D, Ding C, Lu Y, Wang P, Sun Z (2022) Ag nanoparticles decorated 2D/2D TiO2/g-C3N4 heterojunction for efficient removal of tetracycline hydrochloride: synthesis, degradation pathways, and mechanism. Appl Surf Sci 606:154837. https://doi.org/10.1016/j.apsusc.2022.154837
Guo J, Jiang L, Liang J, Xu W, Yu H, Zhang J, Ye S, Xing W, Yuan X (2021) Photocatalytic degradation of tetracycline antibiotics using delafossite silver ferrite-based Z-scheme photocatalyst: pathways and mechanism insight. Chemosphere 270:128651. https://doi.org/10.1016/j.chemosphere.2020.128651
Hao J, Zhang S, Ren F, Wang Z, Lei J, Wang X, Cheng T, Li L (2017) Synthesis of TiO2@g-C3N4 core-shell nanorod arrays with Z-scheme enhanced photocatalytic activity under visible light. J Colloid Interface Sci 508:419–425. https://doi.org/10.1016/j.jcis.2017.08.065
Hao H, Wang Z, Shi J, Li X, Lang X (2018) Improving the visible light photocatalytic aerobic oxidation of sulfides into sulfoxides on dye-sensitized TiO2. ChemCatChem 10(20):4545–4554. https://doi.org/10.1002/cctc.201801304
Hunge YM, Yadav AA, Kang SW, Kim H (2021) Photocatalytic degradation of tetracycline antibiotics using hydrothermally synthesized two-dimensional molybdenum disulfide/titanium dioxide composites. J Colloid Interface Sci 606:454–463. https://doi.org/10.1016/j.jcis.2021.07.151
Jiang D, Xiao P, Shao L, Li D, Chen M (2017) RGO-promoted all-solid-state g-C3N4/BiVO4 Z-scheme heterostructure with enhanced photocatalytic activity toward the degradation of antibiotics. Ind Eng Chem Res 56(31):8823–8832. https://doi.org/10.1021/acs.iecr.7b01840
Jiang G, Geng K, Wu Y, Han Y, Shen X (2018a) High photocatalytic performance of ruthenium complexes sensitizing g-C3 N4 /TiO2 hybrid in visible light irradiation. Appl Catal B 227:366–375. https://doi.org/10.1016/j.apcatb.2018.01.034
Jiang X, Xing Q, Luo X, Li F, Zou J, Liu S, Li X, Wang X (2018b) Simultaneous photoreduction of Uranium(VI) and photooxidation of arsenic (III) in aqueous solution over g-C3 N4 /TiO2 heterostructured catalysts under simulated sunlight irradiation. Appl Catal B 228:29–38. https://doi.org/10.1016/j.apcatb.2018.01.062
Jo WK, Natarajan TS (2015) Influence of TiO2 morphology on the photocatalytic efficiency of direct Z-scheme g-C3N4/TiO2 photocatalysts for isoniazid degradation. Chem Eng J 281:549–565. https://doi.org/10.1016/j.cej.2015.06.120
Kayal A, Mandal S (2022) Microbial degradation of antibiotic: future possibility of mitigating antibiotic pollution. Environ Monit Assess 194(9):639. https://doi.org/10.1007/s10661-022-10314-2
Kong L, Zhang X, Wang C, Xu J, Du X, Li L (2018) Ti3+ defect mediated g-C3N4/TiO2 Z-scheme system for enhanced photocatalytic redox performance. Appl Surf Sci 448:188–296. https://doi.org/10.1016/j.apsusc.2018.04.011
Li J, Zhang M, Li X, Li Q, Yang J (2017) Effect of the calcination temperature on the visible light photocatalytic activity of direct contact Z-scheme g-C3N4-TiO2 heterojunction. Appl Catal B 212:106–114. https://doi.org/10.1016/j.apcatb.2017.04.061
Li Y, Chang H, Wang Z, Shen Q, Liu X, Xue J, Jia H (2022) A 3D C@TiO2 multishell nanoframe for simultaneous photothermal catalytic hydrogen generation and organic pollutant degradation. J Colloid Interface Sci 609:536–546. https://doi.org/10.1016/j.jcis.2021.11.052
Liu F, Nguyen TP, Wang Q, Massuyeau F, Dan Y, Jiang L (2019) Construction of Z-scheme g-C3N4/Ag/P3HT heterojunction for enhanced visible-light photocatalytic degradation of tetracycline (TC) and methyl orange (MO). Appl Surf Sci 496:143653. https://doi.org/10.1016/j.apsusc.2019.143653
Liu B, Sun Y, Zheng T, He X, Wang P, Wang J (2020) Wang Regeneration of carbon nanotube saturated with tetracycline by microwave-ultraviolet system: performance and degradation pathway. Chem Eng J 394:124752. https://doi.org/10.1016/j.cej.2020.124752
Maziarz W (2019) TiO2/SnO2 and TiO2/CuO thin film nano-heterostructures as gas sensors. Appl Surf Sci 480:361–370. https://doi.org/10.1016/j.apsusc.2019.02.139
Meng A, Zhang L, Cheng B, Yu J (2019) Dual cocatalysts in TiO2 photocatalysis. Adv Mater 31(30):1807660. https://doi.org/10.1002/adma.201807660
Milh H, Yu X, Cabooter D, Dewil R (2021) Degradation of ciprofloxacin using UV-based advanced removal processes: comparison of persulfate-based advanced oxidation and sulfite-based advanced reduction processes. Sci Total Environ 764:144510. https://doi.org/10.1016/j.scitotenv.2020.144510
Monga D, Basu S (2019) Enhanced photocatalytic degradation of industrial dye by g-C3N4/TiO2 nanocomposite: role of shape of TiO2. Adv Powder Technol 30(5):1089–1098. https://doi.org/10.1016/j.apt.2019.03.004
Pan J, Zhang Y, Guan Y, Yan Y, Tang H, Liu X, Wang M, Wei X (2022) Multifunctional Ni nanoparticles decorated SiC nanofibers/g-C3N4 nanosheets heterojunctions for drastically increased LED-light-driven hydrogen generation. Appl Surf Sci 579:152171. https://doi.org/10.1016/j.apsusc.2021.152171
Pan J, Guan Y, Zhang Y, Xu Z, Han S, Tang H, Yan X, Liu H, Lu Q (2023) Near-infrared-induced photothermal enhanced photocatalytic H2 production for 3D/2D heterojunctions of snowflake-like CuS/g-C3N4 nanosheets. Inorg Chem 62(1):624–635. https://doi.org/10.1021/acs.inorgchem.2c04000
Perumal K, Shanavas S, Ahamad T, Karthigeyan A, Murugakoothan P (2023) Construction of Ag2CO3/BiOBr/CdS ternary composite photocatalyst with improved visible-light photocatalytic activity on tetracycline molecule degradation. J Environ Sci-China 125:47–60. https://doi.org/10.1016/j.jes.2021.10.021
Qiu J, Feng Y, Zhang X, Zhang X, Jia M, Yao J (2018) Facile stir-dried preparation of g-C3N4/TiO2 homogeneous composites with enhanced photocatalytic activity. RSC Adv 7(18):10668–10674. https://doi.org/10.1039/c7ra00050b
Rehman ZU, Bilal M, Hou J, Butt FK, Ahmad J, Ali S, Hussain A (2022) Photocatalytic CO2 reduction using TiO2-based photocatalysts and TiO2 Z-scheme heterojunction composites: a review. Molecules 27(7):2069. https://doi.org/10.3390/molecules27072069
Tang R, Gong D, Deng Y, Xiong S, Zheng F, Li L, Zhou Z, Su L, Zhao J (2021) π-π stacking derived from graphene-like biochar/g-C3 N4 with tunable band structure for photocatalytic antibiotics degradation via peroxymonosulfate activation. J Hazard Mater 423:126944. https://doi.org/10.1016/j.jhazmat.2021.126944
Xu W, Lai S, Pillai SC, Chu W, Hu Y, Jiang X, Fu M, Wu X, Li F, Wang H (2020) Visible light photocatalytic degradation of tetracycline with porous Ag/graphite carbon nitride plasmonic composite: degradation pathways and mechanism. J Colloid Interface Sci 574:110–121. https://doi.org/10.1016/j.jcis.2020.04.038
Yu J, Wang S, Low J, Xiao W (2013) Enhanced photocatalytic performance of direct Z-scheme g-C3N4-TiO2 photocatalysts for the decomposition of formaldehyde in air. Phys Chem Chem Phys 15(39):16883–16890. https://doi.org/10.1039/c3cp53131g
Yu H, Wang D, Zhao B, Lu Y, Wang X, Zhu S, Qin W, Huo M (2020) Enhanced photocatalytic degradation of tetracycline under visible light by using a ternary photocatalyst of Ag3PO4/AgBr/g-C3N4 with dual Z-scheme heterojunction. Sep Purif Technol 237:116365. https://doi.org/10.1016/j.seppur.2019.116365
Zhang B, He X, Yu C, Liu G, Ma D, Cui C, Yan Q, Zhang Y, Zhang G, Ma J, Xin Y (2022) Degradation of tetracycline hydrochloride by ultrafine TiO2 nanoparticles modified g-C3N4 heterojunction photocatalyst: influencing factors, products and mechanism insight. Chin Chem Lett 33(3):1337–1342. https://doi.org/10.1016/j.cclet.2021.08.008
Zhu Q, Sun Y, Xu S, Li Y, Lin X, Qin Y (2019) Rational design of 3D/2D In2O3 nanocube/ZnIn2S4 nanosheet heterojunction photocatalyst with large-area “high-speed channels” for photocatalytic oxidation of 2,4-dichlorophenol under visible light. J Hazard Mater 382:121098. https://doi.org/10.1016/j.jhazmat.2019.121098
Zong H, Zhao T, Zhou G, Qian R, Feng T, Pan J (2019) Revisiting structural and photocatalytic properties of g-C3N4/TiO2: is surface modification of TiO2 by calcination with urea an effective route to “solar” photocatalyst? Catal Today 335:252–261. https://doi.org/10.1016/j.cattod.2018.12.015
Zuo G, Wang Y, Teo W, Xian Q, Zhao Y (2021) Direct Z-scheme TiO2–ZnIn2S4 nanoflowers for cocatalyst-free photocatalytic water splitting. Appl Catal B 291:120126. https://doi.org/10.1016/j.apcatb.2021.120126
Funding
This work was supported by the National Natural Science Foundation of China (No. 51502116), the Special Funding of China Postdoctoral Science Foundation (No. 2016T90425), and the Innovative Practice Postdoctoral Project Foundation of Jiangmen City in China (No. JMBSH2021A03).
Author information
Authors and Affiliations
Contributions
Jianmei Pan: conceptualization, methodology, software, resources, project administration, writing — review and editing, supervision. Hu Liu: methodology, data curation, writing — original draft, software, writing — review and editing. Keyu E: visualization, investigation, software. Yi Guan: supervision. Wenbo Gou: software, validation. Peng Wang: investigation, validation. Ze Du: formal analysis. Chenfei Ma: software.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
The authors affirm that human research participants provided informed consent for publication of the images in Fig. 1 a, b, and c.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: George Z. Kyzas
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, H., Pan, J., E, K. et al. Selective efficient photocatalytic degradation of antibiotics and direct Z-type migration pathway for hierarchical core–shell TiO2/g-C3N4 composites. Environ Sci Pollut Res 31, 4582–4594 (2024). https://doi.org/10.1007/s11356-023-31358-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-31358-y