Abstract
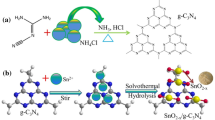
The low-cost composite of g-C3N4 modified by Zn-doped SnO2 nanoparticles was prepared for the first time in this work. The characterization results of XRD and SEM demonstrated that Zn was successfully doped into SnO2. The formed Sn-O-Zn bonds and interaction between the Zn-doped SnO2 sample and g-C3N4 in the composite were explored by FT-IR and XPS technologies. Photocatalytic degradation experiments showed that the as-prepared optimal composite photocatalyst displayed enhanced photocatalytic reactivity towards both dyes and antibiotics, which could degrade 85.6% of RhB and 86.8% of tetracycline within 30 and 90 min, respectively. The oxygen vacancies formed in SnO2 after Zn doping could capture the photogenerated electrons of g-C3N4, thereby promoting the separation of photogenerated electron-hole pairs, then more ·O2− and holes can be generated during the visible light-driven photocatalytic reaction, so that the composite of Zn-doped SnO2/g-C3N4 acquired higher photocatalytic activity and accelerated the degradation of target organics. Active species capturing experiments and ESR detection results also confirmed that ·O2− and holes were the main active species in the reaction process. This work developed a novel g-C3N4-based photocatalyst with no noble metal, low price, and high photocatalytic activity, which could provide a cost-effective and high-efficiency strategy for wastewater treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The problem of organic pollution is a negative impact on the environment caused by the rapid development of modern industry, which is extremely unfavorable to the sustainable development of the environment and human health. Therefore, exploring and developing efficient elimination approaches for organic pollutants is the priority (Xue et al., 2019). Semiconductor-based photocatalysis overcomes the disadvantages of insufficient degradation efficiency faced by traditional pollutant removal technologies such as adsorption and filtration which have been getting increasing attention (Balu et al., 2020). For photocatalysis, active free radicals will be generated depending on the conversion of photon energy by the photocatalyst, and the attack behavior of active free radicals on the structure of organic matter is the key and efficient way to decompose the targets.
Benefit from the characteristics of photocatalysis that converts light energy into chemical energy, it is considered a green and clean solar energy conversion technology, which has been widely applied in solving the current serious problems ranging from environmental pollution to energy shortage (Ismael, 2020). Recently, researches on photocatalysts have mainly focused on semiconductors that respond to visible light. Visible light-driven photocatalysts will undoubtedly expand the utilization of solar energy, thereby enhancing the photocatalytic activity of the photocatalyst at a high level. Among them, a polymeric semiconductor material, graphitic carbon nitride (g-C3N4) with intermediate bandgap (2.7 eV), has become the most famous photocatalyst in recent years.
g-C3N4 can be simply prepared by high-temperature condensation polymerization by melamine, dicyandiamide, and urea as the precursor material (Chen et al., 2020; Rattan Paul and Nehra, 2021). The unique and attractive characteristics of g-C3N4 including the simple reaction operation, the flexible two-dimensional (2D) layered structure, and the high thermal and chemical stability enable it to be extensively researched and applied in different fields such as contaminants degradation (Amedlous et al., 2021; Wang et al., 2020), sterilization (Ding et al., 2020), nitrogen fixation (Li et al., 2021), CO2 and NO conversion (Guo et al., 2021; Liu et al., 2021a), and energy production (Feng et al., 2021; Wang et al., 2021a). However, the structural defects in g-C3N4 would easily lead to the rapid recombination of photogenerated carriers, which greatly inhibits its photocatalytic activity. This will hinder the path of g-C3N4 from laboratory research to practical application. Many studies reported that the usage of introducing metallic (Paul et al., 2020b) or non-metallic elements (Chu et al., 2020) into the structure of g-C3N4, or the formation of heterojunctions by combining g-C3N4 with other semiconductor photocatalysts such as TiO2 (Sun et al., 2019), SiO2 (Sun et al., 2021), ZnO (Paul et al., 2020a), and Bi2MoO6 (Liu et al., 2021), can effectively enhance the photocatalytic activity of g-C3N4. Combination with semiconductors could improve the interface structure and promote the separation of photogenerated carriers in g-C3N4, which is an effective strategy employed to enhance the photocatalytic activity of g-C3N4 (Li et al., 2020).
Among various diversified semiconductors, the low-cost SnO2 with a bandgap of about 3.6 eV has received great attention owing to its non-toxicity, chemical stability, and electrical conductivity. SnO2 has mostly been used for the preparation of gas sensors (Phuoc et al., 2020), solar cells (Huang et al., 2020), photocatalyst (Liu et al., 2021b; Yakout, 2021), etc. While the large bandgap of SnO2 limits the utilization of solar energy, it is unreachable for it to perform photocatalytic application under visible light. As a useful improvement measure, doping metal ions such as Ga (Sivakumar et al., 2021), Nd (Song et al., 2021), Ce (Pacheco-Salazar et al., 2020), Ni, and Co (Matussin et al., 2020) into SnO2 is an important means. The conductivity and optical absorption of SnO2 can be improved by metal doping. Moreover, the surface activity of SnO2 can get enhanced, thereby the better performance of it can be acquired.
Some literature reported that when Zn was incorporated into SnO, the microstructure and the defect chemistry of the SnO2 could be modified (Ma and Wei, 2018; Wu et al., 2021). On account of the similar ion radius of Zn2+ and Sn4+ (0.074 nm for Zn2+, 0.071 nm for Sn4+), the introduction of Zn will cause the generation of oxygen vacancies for charge compensation while maintaining the integrity of SnO2 lattice (Zhao et al., 2015). It also has the advantage of increasing adsorption sites, which is beneficial for photocatalysis (Adhikari et al., 2018). Therefore, the introduction of Zn-doped SnO2 into g-C3N4 is expected to improve the separation efficiency of photogenerated carriers of g-C3N4, which can contribute to the improvement of the photocatalytic activity of the photocatalyst.
To date, there are rare works that have been reported about the Zn-doped SnO2/g-C3N4 composites and their photocatalytic performances. Therefore, the combination of Zn-doped SnO2 nanoparticles with g-C3N4 proposed here is an innovative work, which utilizes the oxygen vacancies generated by Zn-doped SnO2 to capture the photogenerated electrons of g-C3N4, and promotes the separation of photogenerated carriers of g-C3N4, finally improving the degradation efficiency of dyes and antibiotic contaminants represented by Rhodamine B (RhB) and tetracycline (TC). By combining the characterization results of the physicochemical properties of the as-prepared photocatalysts and the evaluation analysis of their photocatalytic activities, we revealed the mechanism of the Zn-doped SnO2/g-C3N4 composite photocatalyst with enhanced visible light-driven photocatalytic activity for contaminant degradation. Moreover, this work synthesized a photocatalyst with no noble metal, low price, and high photocatalytic activity, which could provide a cost-effective and high-efficiency strategy for wastewater treatment.
Material and methods
Materials
All the chemicals used in the work were of analytical grade.
The chemicals required for the material preparation process (including urea, Tin chloride pentahydrate (SnCl4·5H2O), zinc acetate dihydrate (Zn(CH3COO)2·2H2O), NH3·H2O, and absolute ethyl alcohol) and the target pollutants of RhB were all purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Tetracycline hydrochloride was purchased from Shanghai Meryer Chemical Technology Co., Ltd. (Shanghai, China).
Preparation of g-C3N4
g-C3N4 was fabricated by thermal condensation reaction. The product was obtained by heating urea to 530 °C for 2 h. After cooling, the light yellow product was placed in a mortar and ground to a fine powder to obtain the g-C3N4 sample.
Preparation of SnO2 and Zn-doped SnO2 materials
Hydrothermal synthesis was used to prepare SnO2 and Zn-doped SnO2. Twelve millimoles of SnCl4·5H2O was dissolved in 50 mL solution containing NH3•H2O and deionized (DI) water (NH3•H2O: DI water = 1:1, v/v), and then different amounts of Zn(CH3COO)2·2H2O (0, 0.12, 0.24, 0.36 mmol) were added into the above mixture respectively by vigorous stirring for 2 h. Then the different mixture was transferred into 100 mL Teflon-lined autoclaves and reacted at 180 °C for 12 h. When the autoclaves were cooled to room temperature, the white products were collected at the bottom by centrifugation and washed with DI water and ethanol repeatedly. After drying them at 80 °C overnight, Zn-doped SnO2 samples with a different molar ratio of Zn and Sn were obtained. And we abbreviated them as SnO2, 1% ZS, 2% ZS, and 3% ZS, respectively.
Preparation of SnO2/g-C3N4 and Zn-doped SnO2/g-C3N4 composites
Solvent mixing followed by the co-calcination method was selected for the synthesis of SnO2/g-C3N4 and Zn-doped SnO2/g-C3N4. Specifically, put g-C3N4 (0.2 g) and different amounts of 2% ZS sample (0.01, 0.03, and 0.05 g) into 10 mL solution of absolute ethyl alcohol and DI water (1:1, v/v), and then use a magnetic stirring device to continuously stir the above suspension for 2 h. Next, the above-mixed solution was placed in an oven and dried at 80 °C for 12 h. Finally, the mixed dry samples were calcined in a muffle furnace for 2 h at 400°C to obtain 2% ZS/g-C3N4 composites in mass ratios. The composites were designated as 2% ZS/CN-5, 2% ZS/CN-15, and 2% ZS/CN-25, respectively. SnO2/CN-15, 1% ZS/CN-15, and 3% ZS/CN-15 samples were prepared by using the same method.
Characterization
The crystal phases of the as-prepared catalysts were analyzed by X-ray diffractometer (XRD, RU-200B/D/MAX-RB, Japan). The morphologies and structures of the samples were examined by field emission scanning electron microscope (FESEM, Zeiss Ultra Plus, Germany) and field emission high-resolution transmission electron microscope (FHTEM, JEM-2100F, Japan). Fourier transform infrared (FTIR) spectra of the samples were acquired on an FTIR instrument (Nicolet 6700, USA) in the range of 400–4000 cm−1. UV-vis absorption spectra of the samples were examined by a UV-vis spectrophotometer (PerkinElmer Lambda 750 S, USA). The X-ray photoelectron spectroscopy (XPS, Thermo ESCALAB 250 Xi, USA) was performed for analysis of elemental composition and valence states. Photoluminescence (PL) spectra of the materials were acquired by a fluorescence spectrophotometer (Gangdong F-380, China) equipped with a 300W Xe arc lamp at the excitation wavelength of 325 nm. The active free radicals were measured by an ESR spectrometer (Bruker A300, Germany).
Evaluation of photocatalytic activity
The photocatalytic performance of the as-prepared g-C3N4, 2% ZS, SnO2/CN-15, 1% ZS/CN-15, 2% ZS/CN-15, and 3% ZS/CN-15 composites was evaluated by using RhB and TC as the model organic contaminants. For the degradation of RhB, 20 mg of as-prepared material was added into 100 mL RhB aqueous solution (10 mg/L), and for the degradation of TC, 50 mg photocatalyst was added into 100 mL TC aqueous solution with the concentration of 30 mg/L. Then the formed RhB (or TC) suspension was stirred in the dark circumstances for 30 min to establish an adsorption/desorption equilibrium. After that, the photodegradation was performed under visible light irradiation by using a 150 W Xe lamp with a 420-nm cut-off filter. During the photocatalytic process, 3 mL of sample solution was taken out every 5 min for RhB (10 min for TC solution), and it was filtered through a filter membrane (0.22 μm, Jinteng, China) to obtain a clear solution. Finally, the concentration of RhB (or TC) in the sample was determined by Shimadzu UV-vis spectrophotometer (mini 1240, Japan) at 554 nm (or 357 nm). The degradation efficiency is defined as C/C0, where C0 is the concentration of the RhB (or TC) solution when photodegradation starts, and C is the concentration of the RhB (or TC) at different irradiation times.
Results and discussion
XRD analysis
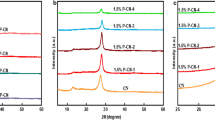
XRD was utilized to acquire the crystal phase information of the as-prepared samples. The XRD patterns of SnO2, 1% ZS, 2% ZS, and 3% ZS are shown in Fig. 1a. The diffraction peaks at 26.6°, 33.9°, 39.0°, 51.8°, and 54.8° of these samples can be assigned to (110), (101), (200), (211), and (220) facets of the rutile SnO2 (JCPDS No. 41-1445), which acted as the host material. It can be found that, when the concentration of the doped Zn2+ increased from 1 to 3%, the diffraction peaks of Zn-doped SnO2 shifted 0.1° towards a lower diffraction angle compared with undoped SnO2. This indicates that the doping of Zn affects the lattice parameters of SnO2 (Soltan et al., 2017). Fig. 1b shows the XRD patterns of g-C3N4 and 2% ZS/CN composites, and the diffraction peaks located at 12.9° and 27.6° in these samples accord with the (100) and (002) planes of g-C3N4 (Liu et al., 2021c; Paul et al., 2019a). The characteristic diffraction peaks of SnO2 also can be observed in the 2% ZS/CN composite, and their intensity gradually increases when the content of 2% ZS increases in the composite.
Morphological characteristics
SEM was conducted to determine the microstructure and morphologies of g-C3N4, SnO2, 2% ZS, and 2% ZS/CN-15 samples, and Fig. 2 shows their SEM images. The slightly curled multiple nanosheet structures exhibited in Fig. 2a are a characteristic structure of 2D polymerized g-C3N4 prepared by urea. Fig. 2b displays nanoparticle morphologies of the as-prepared 2% ZS, and the diameter of it ranged from 200 to 400 nm. The structure image of the composite of 2% ZS/CN-15 composite (Fig. 2c) was almost identical with the morphology of g-C3N4, owing to the small size and low content. The surface element distribution detection of the 2% ZS/CN-15 composite sample (Fig. 2c) was executed by EDS. We can observe that all the elements of C, N, O, Sn, and Zn exist in the 2% ZS/CN-15 sample, which is shown in Fig. 2d, Fig. 2e, Fig. 2f, Fig. 2g, and Fig. 2h, respectively.
The structures of g-C3N4 and the 2% ZS/CN-15 composite were further identified by employing TEM (Fig. 3). As shown in Fig. 3a, g-C3N4 displays a stretchable, thin, and malleable structure similar to that of graphite. Different from SEM image, the TEM of 2% ZS/CN-15 sample listed in Fig. 3b exhibits the morphology that 2% ZS nanoparticles are evenly dispersive and wrapped by g-C3N4. Moreover, we can also find out from Fig. 3c that the 2% ZS nanoparticles are aggregated by very small nanoparticles with a diameter of about 5 nm. The HR-TEM image of the 2% ZS sample shown in Fig. 3d uncovers the lattice fringes corresponding to the (110) and (101) facets of SnO2 crystal, with the interplanar spacing of 0.33 nm and 0.26 nm, respectively. The visualized SEM and EDS mapping as well as TEM images not only assist in the analysis of the structure of the samples but also verify the successful synthesis of the material.
XPS measurement
Relying on the determination of XPS, the surface components and the chemical element valence information of the materials were further investigated. Fig. 4 shows the related spectra. The Sn 3d spectra of SnO2, 2% ZS, and 2% ZS/CN-15 are exhibited in Fig. 4a. The peaks at the binding energy of 486.7 eV and 495.1 eV belong to the Sn 3d5/2 and Sn 3d3/2 of SnO2, respectively. The 2% ZS sample displays a slight shift of the Sn 3d peak to the lower binding energy position. This change in binding energy is due to the formed oxygen vacancies by Zn doping into SnO2 weakening the binding energy of the Sn4+ oxidation state (Lu et al., 2018), while for 2% ZS/CN-15 composite, the binding energy peak position of Sn 3d5/2 and Sn 3d3/2 shift to 486.1 eV and 494.5 eV, which can be attributed to the interaction between 2% ZS and g-C3N4.
From the O 1s spectra of SnO2, 2% ZS, and 2% ZS/CN-15 samples displayed in Fig. 4b, we can observe that, compared with SnO2, the O 1s peak in 2% ZS sample is divided into two peaks, which are located at 530.1 eV and 531.3 eV, respectively, while in the 2% ZS/CN-15 sample, the O 1s peaks change to 530.9 eV and 533.2 eV, respectively. The shift of O 1s implies that there were two different kinds of chemical environments in 2% ZS and 2% ZS/CN-15 samples. Beyond that, the interaction between 2% ZS and g-C3N4 in 2% ZS/CN-15 composite also could cause a shift of O 1s peaks. O 1s peaks at 530.1 eV and 530.9 eV in 2% ZS and 2% ZS/CN-15 express the coordination of oxygen originating from the Sn-O-Sn bond, while peaks at 531.3 eV and 533.2 eV denote the coordination of oxygen in Sn-O-Zn introduced by Zn doping (Ma and Wei, 2018).
The Zn 2p spectra for the 2% ZS sample can be seen in Fig. 4c. The peaks located at binding energy of 1021.9 eV and 1044.8 eV represent Zn 2p3/2 and Zn 2p1/2 orbital, respectively, which means that Zn2+ was successfully doped into SnO2.
The C 1s and N 1s spectra of 2% ZS/CN-15 are shown in Fig. 4d and Fig. 4e, respectively. The C 1s peaks located at binding energy of 284.5 eV, 286.6 eV, and 287.8 eV represent the extra carbon added to the instrument, carbon atoms in C-(N)3 groups, and carbon atoms from N-C=N in the aromatic ring structure, respectively (Zhang et al., 2021).
For N 1s spectrum, three peaks at 397.9 eV, 398.8 eV, and 402.3 eV are attributed to the nitrogen in triazine rings which contains C-N=C bond, the nitrogen atoms in N-(C)3 groups, and nitrogen in -NH2 groups, respectively (Zhu et al., 2021a). The results of XPS detection once again proved that the materials were successfully synthesized and there was strong interaction in the 2% ZS/CN-15 composite.
FT-IR analysis
The functional groups in the materials were tested by FT-IR technology. Fig. S1 displays the FT-IR spectra of SnO2, 2% ZS, g-C3N4, SnO2/CN-15, and 2% ZS/CN-15 samples. For the SnO2 sample, the stretching vibration and bending vibration of the O-H bond in the hydroxyl groups of the adsorbed water can be observed at 3416 cm−1 and 1630 cm−1. The peak at about 620 cm−1 is the presentation of the Sn-O stretching vibration (Gao et al., 2018). For the 2% ZS sample, in addition to the peak that appeared at 620 cm−1 that represents the vibration absorption of Sn-O, the slight absorption peak at 580 cm−1 can be found as well, which belongs to the stretching vibrations of Zn-O. It is proved that Zn2+ was successfully doped into SnO2 material (Shanmugam et al., 2016). For g-C3N4, the stretching vibrations absorption of N-H bonds is reflected in peaks at 3250 cm−1, 3161 cm−1, and 3081 cm−1. Peaks at 1638 cm−1 and 1236 cm−1 refer to the vibration absorption of C=N and C-N bonds from the heterocyclic rings in g-C3N4 (Paul et al., 2019b). The strong absorption peak at 810 cm−1 is the response to the stretching vibrations of triazine units in the structure of g-C3N4 (Wang et al., 2021c). It can be observed that SnO2/CN-15 and 2% ZS/CN-15 samples show absorption spectra similar to that of g-C3N4, due to the low content of SnO2 in the composites.
Optical absorption property
The optical absorption properties of the as-prepared samples were measured employing a UV-vis spectrometer. Fig. 5a shows the spectra of SnO2, 1–3% ZS, g-C3N4, and a series of 2% ZS/CN composites. The absorption curves of SnO2-based samples and g-C3N4-based composite can be distinguished. SnO2 and 1–3% ZS samples show strong absorption in the ultraviolet region between 200 and 400 nm, while for g-C3N4 and all 2% ZS/CN composites, they exhibit visible light absorption. The absorption curves of the composites are highly consistent with that of g-C3N4, which means that the combination with a small amount of Zn-doped SnO2 does not change the visible light absorption capacity of g-C3N4. Also, it can be found that a slight redshift of the absorption edges occurs from Zn-doped SnO2 samples. This indicates that the introduction of Zn2+ expands the light absorption range of SnO2.
According to the Kubelka-Munk equation (Ji et al., 2017; Shen et al., 2017):
The related curves were plotted and shown in Fig. 5b, from which the optical bandgaps of g-C3N4, SnO2, and 1–3% ZS samples were estimated. The calculated bandgaps of g-C3N4 and SnO2 were 2.70 eV and 3.40 eV, respectively. Besides, we can find from Fig. 5b that with the content of Zn2+ gradually increasing, the bandgap of Zn-doped SnO2 gradually decreases. The bandgaps of 1% ZS, 2% ZS, and 3% ZS were calculated as 3.35 eV, 3.20 eV, and 3.18 eV, respectively. The decreased band gap of ZS samples can be owing to the introduced Zn2+ which occupies part of the Sn4+, and participating in the SnO2 lattice network, making the formed impurity band merges into the conduction band, resulting in the narrower bandgap of the doped sample (Soltan et al., 2017).
Photoluminescence (PL) analysis
The separation and recombination of photogenerated carriers are closely related to the activity of the photocatalyst. Thus, we implemented PL experiments to explore the recombination circumstances of photogenerated electron-hole pairs in the as-synthesized photocatalysts. The PL curves were drawn based on the obtained data and shown in Fig. S2. The strongest peak centered at 460 nm can be interpreted as the fast recombination of photogenerated carriers promoting fluorescence emission in g-C3N4, while for both SnO2/CN-15 and 2% ZS/CN-15 composites, their PL signal strength is significantly lower than that of g-C3N4, which indicates that the modification of g-C3N4 by SnO2 could suppress the recombination of photogenerated electron-hole pairs. The PL peak intensity is the weakest for the 2% ZS/CN-15 sample, implying that the photogenerated carrier recombination rate is the lowest in this composite. The reason for this is that when Sn4+ in the SnO2 lattice was partly replaced by Zn2+, then the oxygen vacancy centers were formed (Wang et al., 2015) and could quickly capture photogenerated electrons of g-C3N4 in the composite, thereby promoting the transfer of photogenerated electrons and inhibiting the recombination of photogenerated carriers.
Photocatalytic activity evaluation
The application of photocatalysts to wastewater purification is of great significance to human health and safety. The degradation efficiency of pollutants depends on the photocatalytic activity of the photocatalyst. Therefore, the evaluation of the photocatalytic activities of the synthesized samples under visible light was launched by taking the dye of RhB and the antibiotic of TC as the target contaminants. Fig. 6 shows the degradation results. As shown in Fig. 6a, 2% ZS has no degradation activity on RhB oxidation under visible light that is because it could only respond to ultraviolet light, while 54.0% RhB can be degraded in 30 min by using g-C3N4, reflecting the better visible light catalytic activity of it. We can see that the composite samples formed by doped or undoped SnO2 and g-C3N4 exhibit higher RhB degradation efficiency than g-C3N4. In comparison, the degradation efficiency of the composite samples combined with Zn-doped SnO2 and g-C3N4 is higher than that of undoped SnO2/g-C3N4. The degradation efficiencies of RhB by 1% ZS/CN-15 and 3% ZS/CN-15 samples are similar. For 2% ZS/CN samples, it can be drawn from the degradation curves that as the content of 2% ZS in the composite increases from 5 to 15%, the degradation efficiency of RhB by the composite increases, and when the content of 2% ZS continues to increase from 15 to 25%, the degradation efficiency of RhB reduced. A total of 85.6% of RhB can be degraded within 30 min by 2% ZS/CN-15 composite, which showed the best degradation performance among the samples under visible light. And it is significantly higher than the 57.6% degradation efficiency for RhB by undoped SnO2/CN sample. For the degradation of tetracycline by the as-synthesized photocatalysts, the results are shown in Fig. 6c. Due to the poor ability to respond to visible light, 2% ZS could hardly degrade TC. Although pure g-C3N4 showed the activity of degrading TC, the degradation efficiency only reached 40.3% within 90 min. When using the composite of undoped SnO2 and g-C3N4 (SnO2/CN-15), the degradation efficiency increased, and 56.5% TC could be degraded in 90 min. While the degradation efficiencies of TC were further improved when the composites of Zn-doped SnO2 and g-C3N4 were used, the degradation efficiencies of TC by using 2% ZS/CN-5, 2% ZS/CN-15 and 2% ZS/CN-25 photocatalysts were 74.6%, 86.8%, and 80.3%, respectively. When the content of ZS in the composite is the same, but the doping concentration of Zn is different, the degradation efficiency of TC is also different. The degradation efficiencies of TC by using 1% ZS/CN-15, 2% ZS/CN-15, and 3% ZS/CN-15 were 60.7%, 86.8%, and 72.3%, respectively. The composite of 2% ZS/CN-15 displayed the best photocatalytic activity for both the dye of RhB and tetracycline pollutants. Based on PL spectra and analysis, the main reason for the improvement of the photocatalytic activity can be concluded as the formation of a suitable amount of oxygen vacancies in 2% ZS and the improved photogenerated carriers’ separation rate in the 2% ZS/CN-15 composite.
The photocatalytic degradation kinetics of the as-prepared photocatalysts was studied by pseudo-first-order reaction with a simplified Langmuir-Hinshelwood:
And the corresponding rate curves were plotted and the rate constant k was calculated as well. Fig. 6b and d display the results, and from which, we can find that the k value for 2% ZS/g-C3N4-15 (6.27×10−2 min−1) was 2.4 times than that of g-C3N4 (2.57×10−2 min−1) and 2.2 times than that of undoped SnO2/CN-15 samples (2.85×10−2 min−1) for RhB degradation. And for TC degradation, the k value for ZS/g-C3N4-15 (2.32×10−2 min−1) was 4.0 times than that of g-C3N4 (5.77×10−3 min−1) and 2.4 times than that of undoped SnO2/CN-15 samples (9.47×10−3 min−1). We could have concluded that the degradation kinetic constant k increased with the modification of g-C3N4 by Zn-doped SnO2, and when the optimal mass ratio was 15% for 2% ZS sample and g-C3N4, kinetic constant k for RhB and TC degradation reached the highest.
Then the durability performance of the prepared Zn-doped SnO2/g-C3N4 composite photocatalyst was studied. And 4 cycles of degradation experiments were carried out under visible light by using the 2% ZS/CN-15 composite. The composite photocatalyst was applied to degrade RhB (or TC) until the degradation was completed. After thoroughly cleaning and drying, the composite continued to be used for the degradation of a fresh RhB (or TC) solution. And a total of 4 rounds of degradation experiments have proceeded in this way; Fig. S3a and b show the cyclic degradation results of RhB and TC. In the fourth application on RhB degradation, the 2% ZS/CN-15 composite still maintained high degradation efficiency with an RhB elimination efficiency of 81.7%. And after four consecutive degradations of TC, the degradation efficiency can still reach 82.2%. The cyclic degradation data confirms that the as-prepared low-cost composite has good recyclability. Furthermore, the photocatalytic degradation performances of RhB and TC by this work and other works were compared and summarized, which is shown in Table 1. It can be seen from Table 1 that compared to other previous works, the degradation efficiency and rate constants of RhB and TC in this work are at high levels. Therefore, it is proved that this Zn-doped SnO2/g-C3N4 composite proposed in this work has a satisfactory effect on the degradation of organic pollutants, which can be regarded as a promising candidate for wastewater treatment.
Trapping experiments
The activity of the photocatalyst is inseparable from the active species it produces. Designing and executing the active species trapping experiments is beneficial to explore the role of the generated active species during the RhB and TC degradation process. In the process of photocatalytic reaction by using the 2% ZS/CN-15 composite, we utilized isopropanol (IPA), triethanolamine (TEOA), and nitrogen (N2) as the scavengers of hydroxyl radical (·OH), holes (h+), and superoxide radical (·O2−), respectively. The role of each active species is confirmed by the consumption of the corresponding scavenger. And the results are shown in Fig. 7a and b.
For RhB degradation, the addition of IPA did not significantly inhibit the degradation, indicating that the role of hydroxyl radicals in RhB degradation is negligible. The removal efficiencies of RhB dropped to 15.0% when TEOA was added to the reaction, suggesting that h+ provided an important role in the oxidation of RhB. When N2 was brought into the photocatalytic process, the degradation efficiency of RhB was drastically reduced to 4.6%, revealing that ·O2− was the main active species produced during the photodegradation of RhB by 2% ZS/CN-15 composite photocatalyst. For the degradation of TC, when IPA was added, the degradation efficiency of TC decreased slightly, and 74.1% of TC was degraded. However, when TEOA and N2 were introduced, the degradation efficiency of TC was inhibited, and only 24.1% and 7.4% of TC were degraded. It shows that in the degradation of TC, the role of the scavenger is the same as in the degradation of RhB. It can be concluded that O2 is the dominant active species, h+ also plays an important role, and the effect of ·OH is not significant in the photocatalytic reaction carried out by the 2% ZS/CN composite photocatalyst.
ESR technology was employed to detect ·O2−, ·OH, and oxygen vacancy in photocatalytic reaction over the 2% ZS/CN-15 composite sample to further verify the existence of free radicals and oxygen vacancies, and the results are displayed in Fig. 7c, Fig. 7d, and Fig. 7e, respectively. Fig. 7c shows a strong signal of ·O2−, indicating that the composite sample generated active species dominated by ·O2− under visible light irradiation, while the ·OH signal captured by ESR (Fig. 7d) was much weaker than that of ·O2−, and even ·O2− signals appeared in it, showing that the composite sample generated very few ·OH under visible light, and these ·OH may be formed from the reaction of ·O2- and H2O. The results from trapping experiments and ESR tests are identical with each other, which once again illustrates the main role of ·O2− in the degradation process.
Fig. 7e shows the signals of trapped oxygen vacancy centered at g = 2.004. It demonstrates the presence of oxygen vacancies in the composite sample (Wu et al., 2021). The doped Zn2+ replaced a part of Sn4+ in the SnO2 lattice so that oxygen vacancies appeared, then the local electrons of the oxygen vacancies can be trapped to form ESR signal peaks. Active species trapping experiments and ESR detections proved that under visible light irradiation, there are a large number of ·O2−, few ·OH, and some oxygen vacancies existed in the photocatalytic system. That will conduce to understand the photocatalytic oxidation mechanism of RhB and TC by 2% ZS/CN composite photocatalyst.
Photodegradation mechanism
The analysis of the bandgap structures of g-C3N4 and 2% ZS samples can be useful for realizing the photocatalytic reaction mechanism for RhB and TC degradation by the composite photocatalyst proposed. From the previous study, we know the information that the bandgap of g-C3N4 and 2% ZS are 2.70 and 3.20 eV, respectively. The valence band (VB) and conduction band (CB) edge potentials of g-C3N4 were estimated according to the empirical equations (Su et al., 2021) below:
And by substituting the value of E0 = 4.5 and the χ value for g-C3N4 is 4.73 (Zhang et al., 2018), the calculated VB and CB for g-C3N4 are 1.58 eV and −1.12 eV, respectively. The EVB of 2% ZS was calculated as 3.30 eV from the VB-XPS spectrum shown in Fig. 4f. According to Equation (4), the ECB of 2% ZS can be calculated as 0.1 eV.
Fig. 8 describes the possible mechanism of the photocatalytic degradation over 2% ZS/CN-15 composite based on the bandgap structures of g-C3N4 and 2% ZS. Since g-C3N4 could respond to visible light, when exposed to visible light, electrons on the VB of g-C3N4 could absorb light energy and leap onto the CB of it, forming photogenerated holes and electrons. The CB position of g-C3N4 is −1.12 eV, which is more negative than the potential of O2/·O2− (−0.13 eV vs. NHE, at pH = 7) (Zhang et al., 2020), allowing the photogenerated electrons on CB of g-C3N4 to reduce O2 to generate ·O2−. While the VB potential of g-C3N4 is negative than that of OH−/·OH (1.99 eV vs. NHE, at pH = 7) (Zhu et al., 2021b), ·OH cannot be generated via the oxidation of OH− by holes. The ·OH in the photocatalytic system should be derived from the reaction of ·O2− with H2O. For 2% ZS, according to the UV-vis absorption curve, it could only absorb ultraviolet light and cannot be excited by visible light. However, due to the doping of Zn2+, oxygen vacancies were generated near the CB of the 2% ZS sample, which could capture electrons. When 2% ZS and g-C3N4 are combined, the electrons of g-C3N4 which is generated by the excitation of visible light migrate to the CB of the 2% ZS sample, and are captured by oxygen vacancies. This behavior can promote the separation of photogenerated carriers and inhibit the recombination of photogenerated electron-hole pairs in g-C3N4, thereby it further increases the generation rate of ·O2− and h+, then the target pollutants of RhB and TC can be oxidized by ·O2− and h+. This is why the 2% ZS/g-C3N4 composite photocatalyst has enhanced photocatalytic activity under visible light.
Conclusions
We reported the synthesis of Zn-doped SnO2 nanoparticles modified g-C3N4 composite photocatalyst. By applying it to the degradation of organic pollutants of RhB and TC, it is found that the composite exhibited enhanced visible-light photocatalytic activity. The characterization results of XRD and SEM confirmed that Zn2+ had been doped into the SnO2 lattice. The chemical bond and interaction formed between g-C3N4 and ZS sample are also verified by FT-IR and XPS. Active species trapping experiments showed that ·O2− plays a leading role in the degradation process under visible light. Also, the results of ESR tests proved the existence of oxygen vacancies in the composite sample. In addition, the modification of g-C3N4 with Zn-doped SnO2 exhibited a lower photogenerated carrier recombination phenomenon than g-C3N4. This is attributed to the capture of photogenerated electrons by oxygen vacancies, which promotes the separation of photogenerated electron-hole pairs in the system. In summary, in this work, metal oxides doped with non-precious metals were used to modify g-C3N4, and the oxygen vacancies formed in the metal oxides were utilized to provide a transfer path for the photogenerated electron of g-C3N4. The formed composite photocatalyst showed enhanced performance for organics degradation. This work could provide a low-cost and high-efficiency treatment strategy for wastewater purification.
References
Adhikari AD, Oraon R, Tiwari SK, Saren P, Maity CK, Lee JH, Hoon Kim N, Nayak GC (2018) Zn-doped SnO2 nano-urchin-enriched 3D carbonaceous framework for supercapacitor application. New J. Chem. 42:955–963
Ali G, Zaidi SJA, Basit MA, Park TJ (2021) Synergetic performance of systematically designed g-C3N4/rGO/SnO2 nanocomposite for photodegradation of Rhodamine-B dye. Appl. Surf. Sci. 570:151140
Amedlous A, Majdoub M, Amaterz E, Anfar Z, Benlhachemi A (2021) Synergistic effect of g-C3N4 nanosheets/Ag3PO4 microcubes as efficient n-p-type heterostructure based photoanode for photoelectrocatalytic dye degradation. J. Photoch. Photobio. A. 409:113127
Balu S, Chen YL, Juang RC, Yang TC, Juan JC (2020) Morphology-controlled synthesis of alpha-Fe2O3 nanocrystals impregnated on g-C3N4-SO3H with ultrafast charge separation for photoreduction of Cr (VI) under visible light. Environ. Pollut. 267:115491
Chen Z, Zhang S, Liu Y, Alharbi NS, Rabah SO, Wang S, Wang X (2020) Synthesis and fabrication of g-C3N4-based materials and their application in elimination of pollutants. Sci. Total. Environ. 731:139054
Chu YC, Lin TJ, Lin YR, Chiu WL, Nguyen BS, Hu C (2020) Influence of P, S, O-Doping on g-C3N4 for hydrogel formation and photocatalysis: An experimental and theoretical study. Carbon. 169:338–348
Jesus ETD, Moreira AJ, Sá MC, Freschi GPG, Joya MR, Li MS, Paris EC (2021) Potential of Nb2O5 nanofibers in photocatalytic degradation of organic pollutants. Environ. Sci. Pollut. R. 28:69401–69415
Ding H, Han D, Han Y, Liang Y, Liu X, Li Z, Zhu S, Wu S (2020) Visible light responsive CuS/protonated g-C3N4 heterostructure for rapid sterilization. J. Hazard. Mater. 393:122423
Feng C, Tang L, Deng Y, Wang J, Liu Y, Ouyang X, Yang H, Yu J, Wang J (2021) A novel sulfur-assisted annealing method of g-C3N4 nanosheet compensates for the loss of light absorption with further promoted charge transfer for photocatalytic production of H2 and H2O2. Appl. Catal. B Environ. 281:119539
Gao WY, Zhao Y, Mao Z, Bi D, Chen J, Wang D (2018) Enhanced visible light photocatalytic activity for g-C3N4/SnO2: Sb composites induced by Sb doping. J. Mater. Sci. 53:9473–9485
Guo H, Wan S, Wang Y, Ma W, Zhong Q, Ding J (2021) Enhanced photocatalytic CO2 reduction over direct Z-scheme NiTiO3/g-C3N4 nanocomposite promoted by efficient interfacial charge transfer. Chem. Eng. J. 412:128646
Hu C, Liu ZT, Yang PC, Ding YX, Lin KYA, Nguyen BS (2021) Self-assembly L-cysteine based 2D g-C3N4 nanoflakes for light-dependent degradation of rhodamine B and tetracycline through photocatalysis. J. Taiwan Inst. Chem. E. 123:219–227
Huang S, Ali N, Huai Z, Ren J, Sun Y, Zhao X, Fu G, Kong W, Yang S (2020) A facile strategy for enhanced performance of inverted organic solar cells based on low-temperature solution-processed SnO2 electron transport layer. Org. Electron. 78:105555
Ismael M (2020) A review on graphitic carbon nitride (g-C3N4) based nanocomposites: synthesis, categories, and their application in photocatalysis. J. Alloy. Compd. 846:156446
Ji HY, Fan Y, Yan J, Xu Y, She X, Gu J, Fei T, Xu H, Li H (2017) Construction of SnO2/graphene-like g-C3N4 with enhanced visible light photocatalytic activity. RSC Adv. 7:36101–36111
Jing H, Ou R, Yu H, Zhao Y, Lu Y, Huo M, Huo H, Wang X (2021) Engineering of g-C3N4 nanoparticles/WO3 hollow microspheres photocatalyst with Z-scheme heterostructure for boosting tetracycline hydrochloride degradation. Sep. Purif. Technol. 255:117646
Li Y, Ti M, Zhao D, Zhang Y, Wu L, He Y (2021) Facile synthesis of nitrogen-vacancy pothole-rich few-layer g-C3N4 for photocatalytic nitrogen fixation into nitrate and ammonia. J. Alloy. Compd. 870:159298
Li Y, Zhang Q, Lu Y, Song Z, Wang C, Li D, Tang X, Zhou X (2022) Surface hydroxylation of TiO2/g-C3N4 photocatalyst for photo-Fenton degradation of tetracycline. Ceram. Int. 48:1306–1313
Li Y, Zhou M, Cheng B, Shao Y (2020) Recent advances in g-C3N4-based heterojunction photocatalysts. J. Mater. Sci. Technol. 56:1–17
Liu G, Huang Y, Lv H, Wang H, Zeng Y, Yuan M, Meng Q, Wang C (2021a) Confining single-atom Pd on g-C3N4 with carbon vacancies towards enhanced photocatalytic NO conversion. Appl. Catal. B Environ. 284:119683
Liu J, Zhang Q, Tian X, Hong Y, Nie Y, Su N, Jin G, Zhai Z, Fu C (2021b) Highly efficient photocatalytic degradation of oil pollutants by oxygen deficient SnO2 quantum dots for water remediation. Chem. Eng. J. 404:127146
Liu W, Li Z, Kang Q, Wen L (2021c) Efficient photocatalytic degradation of doxycycline by coupling α-Bi2O3/g-C3N4 composite and H2O2 under visible light. Environ. Res. 197:110925
Liu W, Zhou J, Zhou Y, Liu D (2021) Peroxymonosulfate-assisted g-C3N4@Bi2MoO6 photocatalytic system for degradation of nimesulide through phenyl ether bond cleavage under visible light irradiation. Sep. Purif. Technol. 264:118288
Lu C, Wang J, Xu F, Wang A, Meng D (2018) Zn-doped SnO2 hierarchical structures formed by a hydrothermal route with remarkably enhanced photocatalytic performance. Ceram. Int. 44:15145–15152
Ma LA, Wei ZH (2018) Effect of synthesis conditions on the morphology and field-emission properties of hydrothermally grown Zn-doped SnO2 nanorods. CrystEngComm. 20:1422–1430
Matussin SN, Harunsani MH, Tan AL, Cho MH, Khan MM (2020) Effect of Co2+ and Ni2+ co-doping on SnO2 synthesized via phytogenic method for photoantioxidant studies and photoconversion of 4-nitrophenol. Mater. Today Commun. 25:101677
Munawar T, Mukhtar F, Yasmeen S, Naveed-Ur-Rehman M, Nadeem MS, Riaz M, Mansoor M, Iqbal F (2021) Sunlight-induced photocatalytic degradation of various dyes and bacterial inactivation using CuO-MgO-ZnO nanocomposite. Environ. Sci. Pollut. R. 28:42243–42260
Pacheco-Salazar DG, Aragón FFH, Villegas-Lelovsky L, Ortiz de Zevallos A, Marques GE, Coaquira JAH (2020) Engineering of the band gap induced by Ce surface enrichment in Ce-doped SnO2 nanocrystals. Appl. Surf. Sci. 527:146794
Paul DR, Gautam S, Panchal P, Nehra SP, Choudhary P, Sharma A (2020a) ZnO-Modified g-C3N4: a potential photocatalyst for environmental application. ACS Omega. 5:3828–3838
Paul DR, Sharma R, Nehra SP, Sharma A (2019a) Effect of calcination temperature, pH and catalyst loading on photodegradation efficiency of urea derived graphitic carbon nitride towards methylene blue dye solution. RSC Adv. 9:15381–15391
Paul DR, Sharma R, Panchal P, Malik R, Sharma A, Tomer VK, Nehra SP (2019b) Silver doped graphitic carbon nitride for the enhanced photocatalytic activity towards organic dyes. J. Nanosci. Nanotechno. 19:5241–5248
Paul DR, Sharma R, Panchal P, Nehra SP, Gupta AP, Sharma A (2020b) Synthesis, characterization and application of silver doped graphitic carbon nitride as photocatalyst towards visible light photocatalytic hydrogen evolution. Int. J. Hydrogen Energ. 45:23937–23946
Paul DR, Nehra SP (2021) Graphitic carbon nitride: a sustainable photocatalyst for organic pollutant degradation and antibacterial applications. Environ. Sci. Pollut. R. 28:3888–3896
Phuoc PH, Hung CM, Van Toan N, Van Duy N, Hoa ND, Van Hieu N (2020) One-step fabrication of SnO2 porous nanofiber gas sensors for sub-ppm H2S detection. Sensor. Actuat. A Phys. 303:111722
Shanmugam N, Sathya T, Viruthagiri G, Kalyanasundaram C, Gobi R, Ragupathy S (2016) Photocatalytic degradation of brilliant green using undoped and Zn doped SnO2 nanoparticles under sunlight irradiation. Appl. Surf. Sci. 360:283–290
Shen H, Zhao X, Duan L, Liu R, Li H (2017) Enhanced visible light photocatalytic activity in SnO2@g-C3N4 core-shell structures. Mat. Sci. Eng. B. 218:23–30
Sivakumar P, Akkera HS, Ranjeth Kumar Reddy T, Srinivas Reddy G, Kambhala N, Nanda Kumar Reddy N (2021) Influence of Ga doping on structural, optical and electrical properties of transparent conducting SnO2 thin films. Optik. 226:165859
Soltan WB, Ammar S, Olivier C, Toupance T (2017) Influence of zinc doping on the photocatalytic activity of nanocrystalline SnO2 particles synthesized by the polyol method for enhanced degradation of organic dyes. J. Alloy. Compd. 729:638–647
Song YF, Liu JM, Ge F, Huang X, Zhang Y, Ge HH, Meng XJ, Zhao YZ (2021) Influence of Nd-doping on the degradation performance of Ti/Sb-SnO2 electrode. J. Environ. Chem. Eng. 9:105409
Su X, Chen W, Han Y, Wang D, Yao J (2021) In-situ synthesis of Cu2O on cotton fibers with antibacterial properties and reusable photocatalytic degradation of dyes. Appl. Surf. Sci. 536:147945
Sun Q, Hu X, Zheng S, Zhang J, Sheng J (2019) Effect of calcination on structure and photocatalytic property of N-TiO2/g-C3N4@diatomite hybrid photocatalyst for improving reduction of Cr(VI). Environ. Pollut. 245:53–62
Sun S, Li C, Sun Z, Wang J, Wang X, Ding H (2021) In-situ design of efficient hydroxylated SiO2/g-C3N4 composite photocatalyst: synergistic effect of compounding and surface hydroxylation. Chem. Eng. J. 416:129107
Wang J, Fan H, Yu H (2015) Synthesis of hierarchical porous Zn-Doped SnO2 spheres and their photocatalytic properties. J. Mater. Eng. Perform. 24:4260–4266
Wang W, Niu Q, Zeng G, Zhang C, Huang D, Shao B, Zhou C, Yang Y, Liu Y, Guo H, Xiong W, Lei L, Liu S, Yi H, Chen S, Tang X (2020) 1D porous tubular g-C3N4 capture black phosphorus quantum dots as 1D/0D metal-free photocatalysts for oxytetracycline hydrochloride degradation and hexavalent chromium reduction. Appl. Catal. B Environ. 273:119051
Wang W, Tao Y, Du L, Wei Z, Yan Z, Chan WK, Lian Z, Zhu R, Phillips DL, Li G (2021a) Femtosecond time-resolved spectroscopic observation of long-lived charge separation in bimetallic sulfide/g-C3N4 for boosting photocatalytic H2 evolution. Appl. Catal. B Environ. 282:119568
Wang Y, Huang H, Zhang Z, Wang C, Yang Y, Li Q, Xu D (2021c) Lead-free perovskite Cs2AgBiBr6@g-C3N4 Z-scheme system for improving CH4 production in photocatalytic CO2 reduction. Appl. Catal. B Environ. 282:119570
Wu H, Wang J, Chen R, Yuan C, Zhang J, Zhang Y, Sheng J, Dong F (2021) Zn-doping mediated formation of oxygen vacancies in SnO2 with unique electronic structure for efficient and stable photocatalytic toluene degradation. Chinese J. Catal. 42:1195–1204
Xue S, Wu C, Pu S, Hou Y, Tong T, Yang G, Qin Z, Wang Z, Bao J (2019) Direct Z-Scheme charge transfer in heterostructured MoO3/g-C3N4 photocatalysts and the generation of active radicals in photocatalytic dye degradations. Environ. Pollut. 250:338–345
Yakout SM (2021) Robust ferromagnetic and fast sunlight photocatalytic properties of nanocrystalline SnO2: Co/Cu codoping. Ceram. Int. 47:10104–10112
Zhang J, Xin B, Shan C, Zhang W, Dionysiou DD, Pan B (2021) Roles of oxygen-containing functional groups of O-doped g-C3N4 in catalytic ozonation: quantitative relationship and first-principles investigation. Appl. Catal. B Environ. 292:120155
Zhang L, Wang G, Xiong Z, Tang H, Jiang C (2018) Fabrication of flower-like direct Z-scheme β-Bi2O3/g-C3N4 photocatalyst with enhanced visible light photoactivity for Rhodamine B degradation. Appl. Surf. Sci. 436:162–171
Zhang T, Wang X, Sun Z, Liang Q, Zhou M, Xu S, Li Z, Sun D (2020) Constructing Z-scheme based BiOI/CdS heterojunction with efficient visible-light photocatalytic dye degradation. Solid State Sci. 107:106350
Zhao QQ, Deng X, Ding M, Gan L, Zhai T, Xu X (2015) One-pot synthesis of Zn-doped SnO2 nanosheet-based hierarchical architectures as a glycol gas sensor and photocatalyst. CrystEngComm. 17:4394–4401
Zhu X, Wang Y, Guo Y, Wan J, Yan Y, Zhou Y, Sun C (2021a) Environmental-friendly synthesis of heterojunction photocatalysts g-C3N4/BiPO4 with enhanced photocatalytic performance. Appl. Surf. Sci. 544:148872
Zhu Y, Cui Y, Xiao B, Ou-yang J, Li H, Chen Z (2021b) Z-scheme 2D/2D g-C3N4/Sn3O4 heterojunction for enhanced visible-light photocatalytic H2 evolution and degradation of ciprofloxacin. Mat. Sci. Semicon. Proc. 129:105767
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Funding
This work was supported by the Hubei Provincial Key Research and Development Program (2020BCB067) and Research and Development of New Integrated Equipment for Rural Sewage Treatment (2021BCA129).
Author information
Authors and Affiliations
Contributions
Wei Liu: writing—original draft, data curation. Qun Kang: funding acquisition, methodology. Ling Wang: investigation. Lilian Wen: investigation. Zhaohua Li: project administration. All authors read and contributed to the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent to publish
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Sami Rtimi
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 173 kb)
Rights and permissions
About this article
Cite this article
Liu, W., Kang, Q., Wang, L. et al. Improved performance of Zn-doped SnO2 modified g-C3N4 for visible light-driven photocatalysis. Environ Sci Pollut Res 29, 51989–52002 (2022). https://doi.org/10.1007/s11356-022-19581-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-19581-5