Abstract
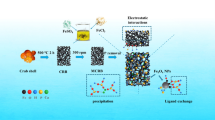
Guided by the concept of treating the wastes with wastes, the efficient use of bone residuals as separation materials is worthy of study. Since bone chars (BCs) are composed of hydroxyapatite and carbon matrix, it is desired to extend the carbon component with improved pore structure and abundant modified groups further, which is favorable to capture metal ions. In this work, phosphorus-modified BCs (PBCs) were fabricated by pretreating bone residuals with phytic acid, achieving improved surface areas (208.7–517.6 m2/g, 37.9–8.2-fold of enhancement) and abundant surface phosphorus contents (5.63–7.54 at.%, 2.8–5.8-fold of enhancement) than BCs. PBCs could adsorb heavy metals with fast kinetics (10.0 h) and excellent maximum capacities (463.9, 156.5, and 80.9 mg/g for Pb(II), Cu(II), and Cd(II)). Spectroscopic results demonstrated that the formation of precipitation was crucial for the enrichment of Pb(II). Moreover, the coordination with functional groups (O-/reductive N-species), the cation exchange with inorganic Ca2+, the electrostatic attraction with deprotonated O−, and the cation-π coordination should also be considered for the sorption. Our study facilitated the application of activated bone wastes as a promising candidate to remediate aquatic heavy metals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the development of industrialization, rigorous anthropogenic activities including large-scale mining, smelting of ores, and combustion of fossil fuels have aroused the wide existence of heavy metals (Briffa et al. 2020). More than 40% of lakes and rivers on the earth have been contaminated. Heavy metals exhibit physical properties such as bioaccumulation and nondegradability (El-Sherif et al. 2013; Vardhan et al. 2019). These attributes collectively contribute to their capability to disrupt fundamental biological processes, resulting in a range of adverse health reactions, including carcinogenicity, neurotoxicity, and reproductive disorders (Ijomone et al. 2020; Meunier et al. 2006; Fu and Wang 2006; Liu and Chen 2021; Hahladakis et al. 2013; Hu et al. 2015). Given that the discharge of wastewater from human-driven industries, such as mining or agricultural facilities lacking environmentally conscious production methods, coupled with the counterbalancing influence of natural phenomena like volcanoes, earthquakes, or storms, it becomes imperative to devise effective strategies to manage aquatic heavy metals. Recent studies have extensively explored four prominent methods to remove heavy metals. These methods encompass membrane-based processes, chemical treatments, electric methods, sorption, and photocatalytic-based treatments. Membrane-based filtration and separation technique is restricted by the high cost of maintenance and replacement (Abdullah et al. 2019; Abdullahi et al. 2021). Electric separation necessitates costly equipment and chemicals and generates hazardous byproducts such as chlorine gas and ozone, and a rising production of sludge potentially (Liu et al. 2020; Liu and Chen 2021; Ingelsson et al. 2020). Since the photocatalysis-based method is still in the laboratory stage, its research depth has not reached the level of widespread application. Additionally, its effectiveness in removing various substances from complex wastewater mixtures is not yet distinct, indicating the need for further refinement (Dong et al. 2021; Crini and Lichtfouse 2019). Sorption technique with low input costs, non-hazardous secondary products, ease of operation, and recyclability offers an efficient solution (Onotri et al. 2017; Qasem et al. 2021; Yang et al. 2019). Numerous sorbent materials have been developed including traditional ones (e.g., clays, minerals, oxide) and advanced ones (e.g., carbon nanotube, graphene) (Yang et al. 2020; Du et al. 2018; Krishnan et al. 2020; Dong et al. 2018; He et al. 2020; Kim et al. 2013; Afshari et al. 2020; Efome et al. 2018). The former is restricted by low efficiency, while the latter is greatly constrained due to the complicated synthetic process. Moreover, many reported sorbent materials were in the form of powder, which were dispersed in aqueous solutions directly. The efficient solid/liquid separation of these powdered materials from solutions hinders their large-scale applications.
The National Bureau of Statistics of China reported that 5.2–7.9 million tons of bone waste was generated in China. The random disposal of bone residuals would be imposed with massive threats and even violates the laws from the view of waste utilization and environmental sustainability. The application of bone chars derived from the pyrolysis of bone wastes with high efficiency in the remediation of pollutants in waters or soils would provide a promising alternative for the resourceful utilization of bone residuals, which is commonly utilized as the animal feed recently (Alkurdi et al. 2019). BCs were composed of carbon matrix loaded with hydroxyapatite (HAP). The sorption capacity of BCs for heavy metals could be further improved by modifying their carbon component with phosphate group (-PO4) since -PO4 could coordinate heavy metals efficiently (Zhao et al. 2017). Previous study has reported the development of carbon materials using natural phytic acid (PA). The hydrogen radicals released from PA extended the porosity of carbon, and the organic phosphate component decomposed from PA could graft onto the surface of carbon. Therefore, the pretreatment of bone residuals with PA would be a possible approach to prepare PO4-modified BCs with enhanced sorption performance for aquatic heavy metals.
In this work, PO4-functionalized BCs (PBCs) were fabricated with the pyrolysis of bone residuals pretreated by PA. The morphology, crystalline structure, and surface composition of PBCs were studied. The functions of hydrochemical parameters like kinetics, pH, ionic strength, coexisting humic acid (HA) and cations/anions, and sorption temperature on the sorption of heavy metals were systematically evaluated. Sorption mechanisms were elucidated by combining with the spectroscopic study and quantitative determination.
Experimental
Materials
Cow bone was collected from the local slaughter house of Liaocheng (Shandong, China). All the chemicals and reagents including phytic acid solution (70 wt%, C6H18O24P6) were obtained from Macklin Co. Ltd. (Shanghai, China). Aqueous solutions in this study were prepared using deionized (DI) water (18.2 MΩ·cm of resistance).
Preparation of PBCs
The collected cow bone residuals were grinded. Typically, 10-g bone powder was immersed into 20-g PA solution. After drying, these residuals were heated at 350 °C, 500 °C, and 650 °C respectively for 2 h with a N2 protection. Excessive PA residual was thoroughly rinsed. After the separation of solid/liquid through the centrifugation, the as-fabricated products were phosphate-modified bone chars (PBCs), which were named as PBC350, PBC500, and PBC650 respectively. Products pyrolyzed with no addition of PA were termed as BC350, BC500, and BC650.
Characterization
BCs and PBCs were pretreated to coat gold (4 mm of thickness) using a spraying device (Leica EM ACE200 coater, Wetzlar, Germany). Then the morphologies of samples were recorded using a scanning electron microscopy (SEM, Zeiss EVO LS-15). X-ray diffractometry (XRD, 7000 Shimadzu, Japan) measurement was performed to analyze the crystalline structure of BCs and PBCs. The porosity was determined using ASAP 2020 analyzer (Micromeritics, USA). Surface zeta potentials were recorded through a SZ-100 zeta analyzer (HORIBA, Japan) at pH 2.0–7.0. X-ray photoelectron spectroscopy (XPS) was measured in ESCALAB 250 (ThermoFisher SCIENTIFIC). Fourier transform infrared spectroscopy (FTIR) was performed on a Bruker ALPHA spectrometer.
Sorption experiment procedures
Functions of hydrochemical parameters, i.e., contact time (0.0–44.0 h), solution pH (2.0–7.0), ionic strength (0.001–0.2 mol/L), HA concentration (10–30 mg/L), coexisting ions (Na+, K+, Ca2+, Mg2+, Ba2+, NO3−, Cl−, ClO4−), initial concentration of heavy metals (10.0–100.0 mg/L), and temperature (298 K, 318 K, 338 K) were evaluated. Specifically, PBCs and BCs achieved the pre-equilibrium with the background solution of NaNO3 in 10-mL flasks. After adding the solution of heavy metals, the duration of oscillation was 24 h except for the kinetic investigation. After the solid/liquid separation, the concentrations of heavy metals were recorded by an atomic absorption spectrometry (Therml iCE 3500, U.S.A). The equilibrium sorption capacity (qe, mg/g) of heavy metals was calculated as qe = (C0 − Ce)/(m/V), where C0 and Ce (mg/L) are the initial and equilibrium concentration of heavy metals respectively. V (mL) is the suspension volume, and m (g) is the sorbent weight. More details of data analysis are described in Supplementary Materials.
Results and discussion
Characterization of PBCs
PBCs and BCs were presented as irregular particles as shown in Fig. 1. For BC350 to BC650, the compact and smooth surface changed to be more granulated. As the bone powder was pretreated with PA solution before the pyrolysis, more fine particles were observed. PA, an organic polymeric phosphoric acid, would decompose into phosphorous oxyacids with the release of some radicals simultaneously. These radicals including hydrogen protons could promote the oxidative decomposition and accelerate the carbonization at low temperature. The release of volatile matter from PBs during carbonization would result in the newly formed micropores and evolved mesopores which were derived from the expansion of existed microporous spaces between the C atomic layers. The protons derived from H3PO3 and H4P2O7 could catalyze and accelerate the decomposition and dehydration of biomass effectively. It can be deduced that the hydrogen protons released from PA could etch the pristine sawdust dramatically by cleaving the bond of alcohol or ether components in raw feedstock during the heating process (Sun et al. 2012).
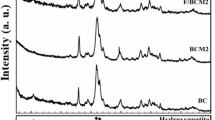
The crystal diffraction patterns of PBCs and BCs are illustrated in Fig. 2a. Five peaks corresponding to the reflections of (211), (310), (222), (213), and (004) of HAP were observed at 31.6°, 39.4°, 46.5°, 49.4°, and 53.0° respectively (JCPDS PDF 00–001–1008) (Xiao et al. 2020). Diffraction peaks at 25.6° of PBCs and BCs were corresponded to the aromatic carbon component which is different from the peak of HAP component at 25.2°. It is indicated that the crystal texture of HAP kept stable via the co-pyrolysis of bone residuals with PB solution.
The presence of mesoporous structure in BCs and PBCs was suggested by the type-IV isotherms of BET analysis as shown in Fig. 2b. The surface areas (SA) of PBCs (208.7, 448.2, and 517.6 m2/g for PBC350, PBC500, and PBC650 respectively) were distinctly improved compared to those of inactivated BCs (5.5, 29.5, and 63.1 m2/g for BC350, BC500, and BC650 respectively). The pore volumes of PBCs were 0.1056, 0.2478, and 0.3945 cm3/g for PBC350, PBC500, and PBC650, respectively. These pore volumes of PBCs were obviously larger than those of BCs (0.0183, 0.0873, and 0.1057 cm3/g for BC350, BC500, and BC650, respectively). The increasing pyrolysis temperature from 350 to 650 °C would favor the decomposition of oxygen- and nitrogen-containing ingredients in pristine biomass, promoting the development of porous structure. More micropores would be generated in the biochar matrix pyrolyzed at higher temperature, which were still blocked in the matrix and could not be detected by the SA analyzer. The presence of these surface groups could introduce strong repulsive electrical double layer forces to maintain the well suspension of PBCs at submicron sizes. The significant enhancement of SA (8.2–37.9 times) and porous volume (2.8–5.8 times) should be due to the formation of new micropores and their accordingly evolution to meso-/macro-pores.
The stretching vibrations of OH groups at 3420 cm−1 were clearly presented in FTIR spectra (Fig. 2c). Since OH groups are the representative functional groups of HAP and biochar, the C-H stretching vibration intensity of methylene group at 2925 cm−1 and 2852 cm−1 decreased gradually from PBC350 to PBC650, proving the improved aromaticity of PBCs with the pyrolyzed temperature of PA-treated bone residuals (Lyu et al. 2018). Bands of C = O/C = C, C-O, and C-O at 1612, 1452, and 1038 cm−1 were displayed. Bending vibrations of tetrahedral PO43− at 566 cm−1 were shown. The detected PO43− groups should be divided into two parts including PO43− groups of HAP component and organic PO43− component of PA.
Considering the protonation/deprotonation of surface species, it is necessary to investigate the surface charge on BCs and PBCs to evaluate its effect on the sorption behavior. The protonation and deprotonation of sorbent species in aqueous solutions would enable the surface with charges. For instance, hydroxyl group on the surface of PBCs would be protonated as ≡SOH + H+ → ≡SOH2+ at pH < pHpzc (point of zero charge) and deprotonated as ≡SOH → ≡SO− + H+ at pH > pHpzc, where ≡S refers to the surfaces of sorbents, and ≡SOH refers to the surfaces with hydroxyl groups. pHpzc values were 3.53, 4.33, and 5.12 for BC350, BC500, and BC650 (Fig. 2d). After the pretreatment of bone residuals with PA solution, pHpzc values of PBC350, PBC500, and PBC650 decreased to 2.63, 2.81, and 3.17, respectively. The grafted -PO4 induce the decline of surface positive charge, promoting the electrostatic attraction of heavy metals with PBCs (Table 1).
The surface composition and accordingly chemical states of PBCs were studied. C, N, O, Ca, and P are clearly exhibited in Fig. 3a. Four components were deconvoluted in C 1 s spectra, including sp2 C–C (284.0 eV), sp3 C–C (284.8 eV), C-O/sp2 C-N (286.0 eV), and C = O/sp3 C-N (287.6 eV) (Fig. 2b) (Puziy et al. 2002). The atomic ratio of sp3 C–C reduced for PBC350 (66.4%) to PBC650 (35.7%).The atomic ratio of sp2 C–C rose obviously for PBC350 (1.1%) to PBC650 (17.2%), and that of. For the deconvolution of O 1 s, two components including P = O/C = O (530.9 eV) and P-O/C-O at 532.9 eV were presented (Faghihian and Farsani 2013). The ratio of = O increased from 3.8 to 9.4%, indicating the enhanced pyrolyzation of carbon species in bone residuals with more aromatized structure. Moreover, quaternary, pyrrolic, amino, and pyridinic N at 401.7, 400.4, 399.2, and 398.2 eV formed N 1 s spectra of PBCs (Hu et al. 2020). The pretreatment of bone residuals with PA solution could introduce more phosphorus-containing function groups onto the surface of PBCs. Based on the analysis of physicochemical characteristics of PBCs, i.e., HAP component and carbon matrix modified with nitrogen-/phosphorus-containing groups, it would be expected to manage heavy metals in aqueous solutions by providing capture positions for heavy metals.
All the above characterization results including SEM images, XRD patterns, BET measurements, FTIR spectra, XPS analysis, and zeta potentials of PBCs and BCs indicated that phosphorus-modified PBCs were successfully fabricated by pretreating bone residuals with PA solution, achieving improved surface areas (208.7–517.6 m2/g and 37.9–8.2-fold of enhancement) and abundant surface phosphorus contents (5.63–7.54 at.% and 2.8–5.8-fold of enhancement) in comparison with BCs.
Effects of hydrochemical conditions on sorption
Kinetic study
Two periods of sorption kinetics on PBCs were observed (Fig. 4a–c), including a rapid enhancement of qe values from 0.0 to 8.0 h and the approximately balanced period from 8.0 to 44.0 h. The adequate capture positions of PBCs and sufficient local heavy metal ions allowed this first period. As the resistance of heavy metals diffused to the pores of PBCs rose with less available capture positions to occupy, the second period occurred. Meanwhile, PBCs showed nearly twice qe values on PBCs compared to untreated BCs pyrolyzed at the same temperature. Closer R2 values to 1.0 for the fitting of kinetics via the pseudo-second-order model (Fig. 4d–f and Fig. S1) demonstrated that the sorption process on PBC was primarily governed by the chemical sorption (Ambaye et al. 2021).
Function of pH and ionic strength
For pH 2.0–7.0, heavy metals Pb(II), Cu(II), and Cd(II) existed as positively charged heavy metals such as Pb2+, PbOH+, Cu2+, Cu2(OH)22+, and Cd2+ in the aqueous solutions. The surfaces of sorbents would also be charged with positive charges at pH 2.0-pHpzc due to the protonation and with negative charges at pH pHpzc-7.0 due to the deprotonation. Taking PBC650 as an example, for pH at 2.0–2.63, the positively charged surfaces of PBC650 would be electrostatically repulsed to these positively charged heavy metals existed in the aqueous solutions. For pH at 2.63–7.0, the negatively charged surfaces of PBC650 would be electrostatically attracted to these existed positively charged heavy metals. Therefore, a continuous rising tendency of qe values for the sorption of Pb(II), Cu(II), and Cd(II) is displayed in Fig. 5a–c. Functions of ionic strength (0.0001–0.2 mol/L NaNO3) on sorption were displayed (Fig. 5d–f). qe values for PBCs presented a slight change at 0.001–0.01 mol/L NaNO3, inferring the essential role of inner-sphere surface complexation at this ionic strength range. While qe values decreased distinctly at 0.01–0.2 mol/L NaNO3, suggesting the governed role of ion exchange/outer-sphere surface complexation at this ionic strength range (Xie et al. 2015).
Functions of HA and coexisting ions
HA substance always exerts a considerable effect on sorption behaviors of target pollutants in aquatic systems. HA molecule with negative charge would be attached or repulsed by sorbents. Since the surface charges of PBC350–PBC650 were negative at pH 5.0, qe values of Pb(II), Cu(II), and Cd(II) on PBCs reduced gradually with 10–30 mg/L HA because of the coordination of free HA molecule with heavy metals in aqueous solutions (Fig. 6a–c). As the functions of coexisting cations and anions on the sorption displayed in Fig. 6d–f, monovalent Na+ (1.02 Å) and K+ (1.38 Å) with small ionic radius presented slight interference, while divalent Mg2+ (0.72 Å), Ca2+ (1.00 Å), and Ba2+ (1.34 Å) with larger ionic radius restricted the qe values (Shannon 1976). Furthermore, the influence of anions (ClO4−, Cl−, and NO3−) was negligible interference.
Isotherms and thermodynamic evaluation
Sorption isotherms of heavy metals on PBCs are displayed in Fig. 7. Typical L-shape curves with an increasing tendency demonstrated that the formation of precipitation should be present to adsorb heavy metals (Alqadami et al. 2018). As shown in Fig. 7, fittings of Langmuir model and Freundlich model were marked as solid line and dash line respectively. As the simulated parameters listed in Table S2, the obtained correlation coefficients (R2) of Langmuir fittings were higher than those of Freundlich fittings. Considering the more appropriate description of sorption isotherms by Langmuir model, the enrichment of heavy metals on PBCs should occur as a monolayer coverage. The maximum uptake capacity (qmax) values obtained from Langmuir fitting were 463.9, 156.5, and 80.9 mg/g for the sorption of Pb(II), Cu(II), and Cd(II) on PBC650. Furthermore, we made a comparison of qmax values on PBCs with previous sorbents such as biochars, cellulose, and chitosan as shown in Table S3, presenting a relative excellent performance than others. It is demonstrated that PBCs were promising separation materials to control the concentrations of heavy metals. qe values on PBCs presented an obvious rising trend with the water temperature for the same initial concentration of heavy metal. From the parameters listed in Table S4, the standard enthalpy change (ΔH°) values (e.g., 15.08–25.20 kJ/mol for the sorption of Pb(II) on PBC350–PBC650) and the standard entropy change (ΔS°) values (i.e., 109.45–162.51 J/mol·K for the sorption of Pb(II) on PBC350–PBC650) were positive, verifying the endothermicity of these sorption process. Moreover, the more negative standard free energy change (ΔG°) values (e.g., − 17.55, − 19.34, − 21.93 kJ/mol for the sorption of Pb(II) on PBC350 at 298–338 K) suggested the spontaneity of these enrichment processes.
Elucidation of sorption mechanism
Both XRD and XPS techniques were applied to analyze the heavy metal-adsorbed PBCs to elucidate the mechanism. No distinct difference in XRD patterns of Cu(II)-adsorbed and Cd(II)-adsorbed PBC650. It is interesting to note that three new peaks at 2θ = 21.45°, 30.90°, and 40.60° corresponding to (PbxCa1‒x)5(PO4)3(OH) (JCPDS code 01–087-2477) were observed (Zhao et al. 2018). HAP component would dissolute and react with dissolved Pb2+ by forming the precipitation of hydroxypyromorphite. Meanwhile, Ca2+ ions in HAP lattice would be released into the aquatic system to exchange with Pb2+. Double peaks of Pb 4f were clearly illustrated (Fig. 8b). The atomic ratios of -O on Pb(II)-adsorbed PBCs decreased significantly from 90.6 to 23.7% for PBC350, 92.8 to 53.2% for PBC500, and 91.3 to 88.6% for PBC650 (Fig. 8c). Pyrrolic, amino, and pyridinic N could coordinate with metal ions by sharing the lone pair electrons, showing a reductive property. The reductive at.% of N on PBC350 decreased from 84.3 to 67.1% after the sorption of Pb(II). It is verified that O- and reductive N heteroatoms exerted a non-negligible role in the capture process. SEM and EDS analyses of PBCs after the sorption of heavy metals were commonly carried out to investigate the potential mechanism. Taking PBC650 as an example, no obvious difference was observed in the SEM images of PBC650 before and after the sorption of Pb(II) (Fig. 8e). EDS in Fig. 8f showed that the peaks of O, C, P, and Ca were the principle components of PBC650 before the sorption of heavy metals, while the distinct difference presented in the EDS spectra was the presence of Pb element on Pb(II)-adsorbed PBC650, Cu element on Cu(II)-adsorbed PBC650, and Cd element on Cd(II)-adsorbed PBC650, which suggested the distribution of Pb(II), Cu(II), and Cd(II) on PBCs.
According to the spectroscopic analysis for PBCs after the sorption of heavy metals including XRD, XPS, SEM, and EDS analysis as shown in Fig. 9, the sorption mechanisms were summarized. The sorption of Pb(II) should be governed via forming the hydroxypyromorphite precipitation of Pb(II) with HAP component (proved by XRD). Other four types of mechanisms should be combined together for the capture of Pb(II), Cu(II), and Cd(II), including their coordination with O-/reductive N groups (proved by XPS), the electrostatic attraction of deprotonated O−, the cation exchange with Ca2+ ion, and the coordination of π-electrons with aromatic carbon in PBCs.
The regeneration of heavy metal-adsorbed PBCs was commonly performed via the elution using phosphate solution pickling. In this study, desorption experiments of Pb(II), Cu(II), and Cd(II) from Pb(II), Cu(II), and Cd(II)-adsorbed PBC350, PBC500, and PBC650 were performed using 0.1 mol/L KH2PO4 solution. After agitation at 100 rpm for 6 h at T 298 K, the sorbent samples were gathered by centrifugation, then thoroughly washed 2 times using DI water, and dried at T 333 K overnight. As shown in Fig. S2, qe values for the sorption of Pb(II) on PBCs decreased significantly from the first to the second round (declined ratios of 22.8–25.6%), which should be ascribed to the formation of stable hydroxypyromorphite precipitation. qe values for the sorption of Pb(II) on PBCs kept around 64.2–65.8% after six cycles. qe values for the sorption of Cu(II) and Cd(II) on PBCs kept around 80.1–81.5% after six cycles. The recycling study shed light on that PBCs could be favorable sorbents to remove Pb(II), Cu(II), and Cd(II) from aqueous solutions repeatedly.
Conclusions
In summary, PBCs with improved porosity (208.7–517.6 m2/g, 37.9–8.2 time higher than BCs) and phosphorus contents (5.63–7.54 at.%, 2.8–5.8-fold of enhancement than BCs) were fabricated by immersing bone residuals into PA solution prior to the pyrolysis. PBCs could adsorb heavy metals with fast kinetics (10.0 h) and excellent capacities (463.9, 156.5, and 80.9 mg/g for Pb(II), Cu(II), and Cd(II) on PBC650). Based on the spectroscopic analysis, the hydroxypyromorphite precipitation played a crucial role in the capture of Pb(II). Furthermore, the cation exchange with inorganic Ca2+, the electrostatic attraction with deprotonated O−, the cation-π coordination, and the coordination with reductive N- and O-containing groups should also be considered. This work introduced a new insight to efficiently use bone waste in the management of aquatic contamination.
Data Availability
The data are available from the corresponding author on reasonable request.
References
Abdullah N, Yusof N, Lau WJ, Jaafar J, Ismail AF (2019) Recent trends of heavy metal removal from water/wastewater by membrane technologies. J Ind Eng Chem 76:17–38. https://doi.org/10.1016/j.jiec.2019.03.029
Abdullahi N, Igwe EC, Dandago MA, Umar NB (2021) Heavy metals in food crops: ideal sources and roles of urban agriculture in facilitating their consumption-a review. Fudma J Sci 5:34–45. https://doi.org/10.33003/fjs-2021-0502-520
Afshari M, Dinari M, Zargoosh K, Moradi H (2020) Novel triazine-based covalent organic framework as a superadsorbent for the removal of mercury(II) from aqueous solutions. Ind Eng Chem Res 59:9116–9126. https://doi.org/10.1021/acs.iecr.0c00953
Alkurdi SS, Al-Juboori RA, Bundschuh J, Hamawand I (2019) Bone char as a green sorbent for removing health threatening fluoride from drinking water. Environ Int 127:704–719. https://doi.org/10.1016/j.envint.2019.03.065
Alqadami AA, Khan MA, Otero M, Siddiqui MR, Jeon BH, Batoo KM (2018) A magnetic nanocomposite produced from camel bones for an efficient adsorption of toxic metals from water. J Clean Prod 178:293–304. https://doi.org/10.1016/j.jclepro.2018.01.023
Ambaye TG, Vaccari M, van Hullebusch ED, Amrane A, Rtimi S (2021) Mechanisms and adsorption capacities of biochar for the removal of organic and inorganic pollutants from industrial wastewater. Int J Environ Sci Technol 18:3273–3294. https://doi.org/10.1007/s13762-020-03060-w
Briffa J, Sinagra E, Blundell R (2020) Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 6:04691. https://doi.org/10.1016/j.heliyon.2020.e04691
Crini G, Lichtfouse E (2019) Advantages and disadvantages of techniques used for wastewater treatment. Environ Chem Lett 17:145–155. https://doi.org/10.1007/s10311-018-0785-9
Dong I, Hou L, Wang Z, Gu P, Chen G, Jiang R (2018) A new function of spent activated carbon in BAC process: removing heavy metals by ion exchange mechanism. J Hazard Mater 359:76–84. https://doi.org/10.1016/j.jhazmat.2018.07.030
Dong C, Qiao T, Huang Y, Yuan X, Lian J, Duan T, Zhu W, He R (2021) Efficient photocatalytic extraction of uranium over ethylenediamine capped cadmium sulfide telluride nanobelts. ACS Appl Mater Interfaces 13:11968–11976. https://doi.org/10.1021/acsami.0c22800
Du H, Huang Q, Peacock CL, Tie B, Lei M, Liu X, Wei X (2018) Competitive binding of Cd, Ni and Cu on goethite organo–mineral composites made with soil bacteria. Environ Pollut 243:444–452. https://doi.org/10.1016/j.envpol.2018.08.087
Efome JE, Rana D, Matsuura T, Lan CQ (2018) Insight studies on metal-organic framework nanofibrous membrane adsorption and activation for heavy metal ions removal from aqueous solution. ACS Appl Mater Inter 10:18619–18629. https://doi.org/10.1021/acsami.8b01454
El-Sherif IY, Tolani S, Ofosu K, Mohamed OA, Wanekaya AK (2013) Polymeric nanofibers for the removal of Cr (III) from tannery waste water. J Environ Manage 129:410–413. https://doi.org/10.1016/j.jenvman.2013.08.004
Faghihian H, Farsani SN (2013) Modification of polyacrylamide-β-zeolite composite by phytic acid for the removal of lead from aqueous solutions. Pol J Chem Technol 15:1–6. https://doi.org/10.2478/pjct-2013-0001
Fu F, Wang Q (2006) Removal of heavy metal ions from wastewaters: a review. J Environ Manag 92:407–418. https://doi.org/10.1016/j.jenvman.2010.11.011
Hahladakis J, Smaragdaki E, Vasilaki G, Gidarakos E (2013) Use of sediment quality guidelines and pollution indicators for the assessment of heavy metal and PAH contamination in Greek surficial sea and lake sediments. Environ Monit Assess 185:2843–2853. https://doi.org/10.1007/s10661-012-2754-2
He M, Wang L, Zhang Z, Zhang Y, Zhu J, Wang X, Lv Y, Miao R (2020) Stable forward osmosis nanocomposite membrane doped with sulfonated graphene oxide@metal-organic frameworks for heavy metal removal. ACS Appl Mater Inter 12:57102–57116. https://doi.org/10.1021/acsami.0c17405
Hu R, Wang X, Dai S, Shao D, Hayat T, Alsaedi A (2015) Application of graphitic carbon nitride for the removal of Pb(II) and aniline from aqueous solution. Chem Eng J 260:469–477. https://doi.org/10.1016/j.cej.2014.09.013
Hu R, Xiao J, Wang T, Gong Y, Chen G, Chen L, Tian X (2020) Highly concentrated amino-modified biochars using a plasma: evolution of surface composition and porosity for heavy metal capture. Carbon 168:515–527. https://doi.org/10.1016/j.carbon.2020.07.012
Ijomone OM, Ifenatuoha CW, Aluko OM, Ijomone OK, Aschner M (2020) The aging brain: impact of heavy metal neurotoxicity. Crit Rev Toxicol 50:801–814. https://doi.org/10.1080/10408444.2020.1838441
Ingelsson M, Yasri N, Roberts EPL (2020) Electrode passivation, faradaic efficiency, and performance enhancement strategies in electrocoagulation—a review. Water Res 187:116433. https://doi.org/10.1016/j.watres.2020.116433
Kim SA, Kamala-Kannan S, Lee KJ, Park YJ, Shea PJ, Lee WH, Kim HM, Oh BT (2013) Removal of Pb(II) from aqueous solution by a zeolite-nanoscale zero-valent iron composite. Chem Eng J 271:54–60. https://doi.org/10.1016/j.cej.2012.11.097
Krishnan S, Chatterjee S, Solanki A, Guha N, Singh MK, Gupta AK, Rai DK (2020) Aminotetrazole-functionalized SiO2 coated MgO nanoparticle composites for removal of acid fuchsin dye and detection of heavy metal ions. ACS Appl Nano Mater 3:11203–11216. https://doi.org/10.1021/acsanm.0c02351
Liu T, Chen J (2021) Extraction and separation of heavy rare earth elements: a review. Sep Purif Technol 276:119263. https://doi.org/10.1016/j.seppur.2021.119263
Liu L, Chen H, Yang X, Tan W, Liu C, Dang Z, Qiu G (2020) High-efficiency As(III) oxidation and electrocoagulation removal using hematite with a charge−discharge technique. Sci Total Environ 703:135678. https://doi.org/10.1016/j.scitotenv.2019.135678
Lyu H, Gao B, He F, Zimmerman AR, Ding C, Tang J, Crittenden JC (2018) Experimental and modeling investigations of ball-milled biochar for the removal of aqueous methylene blue. Chem Eng J 335:110–119. https://doi.org/10.1016/j.cej.2017.10.130
Meunier N, Drogui P, Montane C, Hausler R, Mercier G, Blais JF (2006) Comparison between electrocoagulation and chemical precipitation for metals removal from acidic soil leachate. J Hazard Mater 137:581–590. https://doi.org/10.1016/j.jhzamat.2006.02.050
Onotri L, Race M, Clarizia L, Guida M, Alfè M, Andreozzi R, Marotta R (2017) Solar photocatalytic processes for treatment of soil washing wastewater. Chem Eng J 318:10–18. https://doi.org/10.1016/j.cej.2016.04.053
Puziy AM, Poddubnaya OI, Martínez-Alonso A, Suárez-García F, Tascón JMD (2002) Synthetic carbons activated with phosphoric acid: I. Surface chemistry and ion binding properties. Carbon 40:1493–1505. https://doi.org/10.1016/S0008-6223(01)00317-7
Qasem NAA, Mohammed RH, Lawal DU (2021) Removal of heavy metal ions from wastewater: a comprehensive and critical review. NPJ Clean Water 4:36. https://doi.org/10.1038/s41545-021-00127-0
Shannon RD (1976) Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst A32:751–767. https://doi.org/10.1107/S0567739476001551
Sun Y, Yue Q, Gao B, Huang L, Xu X, Li Q (2012) Comparative study on characterization and adsorption properties of activated carbons with H3PO4 and H4P2O7 activation employing Cyperus alternifoliusas precursor. Chem Eng J 181:790–797. https://doi.org/10.1016/j.cej.2011.11.098
Vardhan KH, Kumar PS, Panda RC (2019) A review on heavy metal pollution, toxicity and remedial measures: current trends and future perspectives. J Mol Liq 290:111197. https://doi.org/10.1016/j.molliq.2019.111197
Xiao J, Hu R, Chen G (2020) Micro-nano-engineered nitrogenous bone biochar developed with a ball milling technique for high-efficiency removal of aquatic Cd(II), Cu(II) and Pb(II). J Hazard Mater 387:121980. https://doi.org/10.1016/j.jhazmat.2019.121980
Xie J, Gu X, Tong F, Zhao Y, Tan Y (2015) Surface complexation modeling of Cr(VI) adsorption at the goethite–water interface. J Colloid Interf Sci 455:55–62. https://doi.org/10.1016/j.jcis.2015.05.041
Yang X, Wan Y, Zheng Y, He F, Yu Z, Huang J, Wang H, Ok YS, Jiang Y, Gao B (2019) Surface functional groups of carbon-based adsorbents and their roles in the removal of heavy metals from aqueous solutions: a critical review. Chem Eng J 366:608–621. https://doi.org/10.1016/j.cej.2019.02.119
Yang S, Liao S, Ren X, Li Y, Ma Y, Zhang Z (2020) Highly selective enrichment of radioactive cesium from solution by using zinc hexacyanoferrate(III)- functionalized magnetic bentonite. J Colloid Interf Sci 580:171–179. https://doi.org/10.1016/j.jcis.2020.06.115
Zhao L, Zheng W, Mašek O, Chen X, Gu B, Sharma BK, Cao X (2017) Roles of phosphoric acid in biochar formation: synchronously improving carbon retention and sorption capacity. J Environ Qual 46:393–401. https://doi.org/10.2134/jeq2016.09.0344
Zhao J, Giammar DE, Pasteris JD, Dai C, Bae Y, Hu Y (2018) Formation and aggregation of lead phosphate particles: implications for lead immobilization in water supply systems. Environ Sci Technol 52:12612–12623. https://doi.org/10.1021/acs.est.8b02788
Author information
Authors and Affiliations
Contributions
Zihao Wang: conceptualization, investigation, data curation, methodology, characterization, writing—original draft, writing—review and editing.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Competing interests
The author declares no competing interests.
Additional information
Responsible Editor: Tito Roberto Cadaval Jr
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Z. Phosphorus-modified bone chars with developed porosity for efficient removal of Pb(II), Cu(II), and Cd(II). Environ Sci Pollut Res 30, 123796–123807 (2023). https://doi.org/10.1007/s11356-023-31080-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-31080-9