Abstract
Glyphosate (GLY) exposure, both exogenous and endogenous, is a global concern. Multiple studies of model systems in vitro and in vivo have demonstrated the potential toxic effects of GLY exposure on human organs, particularly the liver and renal system. However, there is currently limited epidemiological evidence establishing a link between GLY exposure and hepatorenal function in the general population. In this study, a multivariable linear regression model and forest plots were employed to evaluate the connection between urinary GLY and biomarkers of hepatorenal function in 2241 participants from the National Health and Nutrition Examination Survey 2013–2016. Additionally, subgroup analyses were conducted based on age, gender, race, BMI, and chronic kidney disease (CKD). Alkaline phosphatase (ALP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), AST/ALT and fibrosis 4 score (FIB-4) all increased with elevated urinary GLY concentrations after adjusting for potential confounders, while albumin (ALB) exhibited the opposite trend, particularly among younger, female, non-Hispanic white, overweight, and CKD participants. Furthermore, individuals in the third tertile had a greater risk of liver dysfunction than those in the first tertile after categorizing urinary GLY concentrations. However, our study showed no proof that GLY exposure affects the ratio of urine albumin to creatinine (ACR) or serum creatinine levels. Overall, these results imply that GLY exposure may have adverse effects on human liver function.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background
Glyphosate (GLY), an efficient herbicide recognized for its broad-spectrum activity, has been extensively used in agricultural production, urban greening, residential gardens, and even waterways to eliminate weeds and exotic species (Botero-Coy et al. 2013; Bento et al. 2016). Presently, there are hundreds of GBHs available on the market globally under a variety of labels (Williams et al. 2000). As a consequence, GLY is detectable in the air, water, food supply, and therefore in biological fluids, including urine, blood, and breast milk, due to its widespread usage (Gillezeau et al. 2019). Despite the assertion that GLY exclusively interferes with the shikimate pathway, which is present in plants, bacteria, fungi, and protozoa but absent in humans (Steinrücken and Amrhein 1980), accumulated studies have demonstrated adverse effects of GLY in aquatic species, vertebrates, and invertebrates (Mesnage et al. 2017; Milić et al. 2018; Le Du-Carrée et al. 2021; Mutwedu et al. 2021). Therefore, concerns have grown within the scientific community regarding the possible toxicity and consequences of GLY exposure to human health.
The toxicity of GLY in target organs has been extensively investigated in numerous recent studies. One study revealed that GLY exposure may induce liver damage due to the increased production of oxidative stress (Soudani et al. 2019). Additionally, a rat model study reported the nephrotoxicity of GLY that results in the apoptosis of tubular cells (Gao et al. 2019). Moreover, case‒control studies also proved that GLY could pose an adverse effect on hepatic and renal function and work synergistically with other pollutants, in particular paraquat, to increase toxic effects, resulting in the epidemic of chronic kidney disease (CKD) of unknown etiology in some regions (Jayasumana et al. 2015; Zhang et al. 2017; Gunatilake et al. 2019). However, most of these studies were performed in workers or farmers with occupational exposure. There are few studies on the impacts of GLY exposure on hepatorenal function in the wider population.
The purpose of this study was to investigate the effects of varying levels of GLY exposure on hepatorenal function across a sizable population. To accomplish this objective, the current study examined the recently accessible data from the National Health and Nutrition Examination Survey (NHANES) (as of November 2022) to investigate potential associations between urinary GLY concentrations and hepatorenal function within a large population of the United States (US).
Methods
Study population
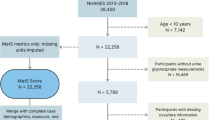
The NHANES is a population-based study that uses a complex, stepwise, and probabilistic sampling process to gather extensive data on nutrition and health in the general US population (Curtin et al. 2012). The 2013–2016 continuous cycle of the NHANES dataset was used for this study. The following patients were excluded from the 20146 eligible participants: 15408 with missing urine GLY data, 1237 under the age of 19, 101 with missing hepatorenal function or albumin to creatinine ratio (ACR) data, and 786 with hepatitis B or C (positive for HbsAg or HCV antibodies). In the end, 2241 individuals were involved in the study. Figure 1 depicts the process for sample selection.
Measurement of Urinary Glyphosate
After collecting the random urine samples, 2D-on-line ion chromatography, tandem mass spectrometry (IC-MS/MS), and isotope dilution quantification were used for analytical measurements, during which CLIA guidelines played a role in assuring accuracy and reliability (Schütze et al. 2021). The content of GLY in the urine had a 0.2 ng/mL lower limit of detection, and 1677 (74.83%) of the individuals were at or over the detection limit.
Study variables
The following factors were analyzed in the current study: age, gender, race, education level, body mass index (BMI), income-poverty ratio, lifetime smoking, alcohol drinking times in the last 12 months, albumin (ALB), alkaline phosphatase (ALP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), alanine aminotransferase (ALT), AST/ALT ratio, fibrosis 4 score (FIB-4), serum creatinine, ACR, estimated glomerular filtration rate (eGFR, using the CKD Epidemiology Collaboration creatinine equation (Levey et al. 2009)), hypertension, and CKD. FIB-4 = (age (year) × AST (U/L)) / [PLT (109/L) × ALT1/2(U/L)] (Hou et al. 2023). The younger group and elderly group were divided according to the 60-year-old cutoff and CKD was defined by either an eGFR < 60 ml/min/1.73 m2 or a urine ACR ≥ 30 mg/g (Stevens and Levin 2013).
Statistical analysis
According to the recommendations of the Centers for Disease Control and Prevention (CDC), all statistical analyses were carried out using the appropriate NHANES sample weights, taking complicated multistage cluster surveys into account. Means with standard errors (SE) were used to summarize continuous variables, while proportions were used to display categorical characteristics. Either a weighted linear regression model or weighted chi-square test was employed for continuous/categorical variables, thus evaluating the variations between individuals as classified by GLY tertiles. Multivariate linear regression analysis was used to obtain β values and 95% confidence intervals (CIs). Three models were built as multivariate tests: Model 1: no variables adjusted; Model 2: age, gender, race, and education level adjusted; Model 3: age, gender, race, education level, income-poverty ratio, BMI, lifetime smoking, alcohol drinking times, hypertension, diabetes, and CKD.
Stratified analyses were conducted by age (< 60 or ≥ 60), gender (male or female), race (non-Hispanic white, non-Hispanic black, Hispanic or other groups), BMI (< 25 or ≥ 25 kg/m2), and CKD (yes or no). To estimate the significance of interactions between urinary GLY concentrations and stratification variables, P values for each product term were recorded. R software (version 4.1.3) and EmpowerStats (version 2.0) were used during the statistical computing and graphics analysis process. When statistically significant, P < 0.05 was recorded.
Results
Demographic and clinical characteristics of participants
Based on the above criteria, a total of 2241 adults were included in this study, with an average age of 50.09 ± 16.75 years. Among these participants, 49.15% were men, and 50.85% were women; 66.60% were non-Hispanic white, 10.42% were non-Hispanic black, 6.38% were Hispanic, and 16.61% were from other races; 14.77% were less than high school, 22.04% were high school, and 63.19% were more than high school. Based on the levels of urinary GLY concentration, the individuals' weighted features were split into three tertiles (Tertile 1: ≤ 0.262 ng/ml; Tertile 2: 0.263 ~ 0.495 ng/ml; Tertile 3: ≥ 0.496 ng/ml), as shown in Table 1. Of all the participants, the data revealed significant differences (p < 0.05) between individuals among the three tertiles in the distribution of age, gender, alcohol drinking times, hypertension, diabetes, income-poverty ratio, BMI, ALB, ALP, ALT, AST, FIB-4, serum creatinine, and eGFR.
Higher urinary glyphosate exposure is associated with a higher likelihood of liver dysfunction
Regarding the relationship between several biomarkers of hepatorenal function and GLY, the findings of the multivariate regression analyses are shown in Table 2. In the unadjusted model, albumin had a negative relationship with GLY (β = -0.424, 95% CI [-0.644, -0.204], P < 0.001). This strong correlation persisted even after covariate adjustment in Model 2 (β = -0.349, 95% CI [-0.555, -0.144], P < 0.001) and Model 3 (β = -0.560, 95% CI [-0.813, -0.307], P < 0.001). Similarly, although not statistically significant, inverse relationships were identified between levels of serum creatinine, ACR, and urinary GLY levels after adjusting for all covariates (β = -0.02, 95% CI [-0.04, 0.00], P = 0.052; β = -27.70, 95% CI [-59.45, 4.04], P = 0.087). In contrast, ALP was positively linked with GLY in the unadjusted model (β = 2.99, 95% CI [1.59, 4.39], P < 0.001), model 2 (β = 2.82, 95% CI [1.44, 4.20], P < 0.001) and model 3 (β = 3.27, 95% CI [1.55, 4.98], P < 0.001). Similarly, ALT, AST, AST/ALT and FIB-4 showed significantly positive links with GLY in these three models, and the figures in model 3 are listed (β = 2.25, 95% CI [0.85, 3.65], P = 0.002; β = 6.80, 95% CI [5.50, 8.11], P < 0.001; β = 0.05, 95% CI [0.03, 0.08], P < 0.001; β = 0.23, 95% CI [0.17, 0.28], P < 0.001).
Following the transformation of urine GLY concentrations from a continuous to a categorical variable (tertiles) for sensitivity analysis, the findings are shown in Table 3. Compared with the lowest GLY tertile (Tertile 1), participants in the top GLY tertile had 0.52 g/L lower ALB, 3.09 IU/L higher ALP, 2.33 IU/L higher AST and 0.12 higher FIB-4 than those in the bottom GLY tertile, and the P for trends were 0.012, 0.015, 0.005 and < 0.001, respectively. However, the association between ALT and GLY tertiles did not meet statistical significance (β = 1.36, 95% CI [-0.59, 3.31], P = 0.170). No significant difference between AST/ALT and urinary GLY concentrations was observed (β = 0.02, 95% CI [-0.02, 0.05], P = 0.396).
Subgroup analysis
The robustness of the relationship between several biomarkers of liver function and GLY was assessed by using subgroup analysis stratified by age, gender, race, BMI, and CKD (Fig. 2). In the younger subgroup stratified by age, only ALB exhibited a negative correlation with urinary GLY concentrations (β = -0.69, 95% CI [-1.01, -0.36], P < 0.001), while ALP, ALT, AST, AST/ALT and FIB-4 showed positive associations (all P < 0.05, Supplementary table). In addition, there was a positive association between FIB-4 and urinary GLY concentrations in the elderly group (β = 0.23, 95% CI [0.08, 0.38], P = 0.025).
Subgroup analyses of the association between GLY and ALB, ALP, ALT, AST, AST/ALT, FIB-4. Each subgroup analysis adjusted for all the factors (age, gender, race, education level, income-poverty ratio, BMI, lifetime smoking, alcohol drinking times, hypertension, diabetes, and CKD) except the stratification factor itself
In terms of subgroup analyses stratified by female and race, the negative correlation of ALB with urinary GLY concentrations was maintained in females (β = -0.78, 95% CI [-1.14, -0.42], P < 0.001) but not in males (β = -0.23, 95% CI [-0.58, 0.11], P = 0.186), as well as in non-Hispanic white individuals (β = -0.69, 95% CI [-1.03, -0.34], P < 0.001), but not in other races. However, our results showed that there was a positive association between urinary GLY and ALP, ALT, AST, AST/ALT and FIB-4 (all P < 0.05, Supplementary table).
With regard to subgroup analyses stratified by BMI, ALB was negatively linked with urinary GLY concentrations in both the low BMI group (β = -0.69, 95% CI [-1.16, -0.22], P = 0.004) and the high BMI group (β = -0.52, 95% CI [-0.82, -0.22], P < 0.001). Positive associations of ALP, ALT, AST, AST/ALT, FIB-4 and urinary GLY concentrations were observed in the high BMI group (β = 4.93, 95% CI [2.85, 7.01], P < 0.001; β = 3.13, 95% CI [1.25, 5.00], P < 0.001; β = 10.00, 95% CI [8.30, 11.69], P < 0.001; β = 0.07, 95% CI [0.04, 0.10], P < 0.001; β = 0.35, 95% CI [0.28, 0.42], P < 0.001).
With reference to CKD-stratified subgroup analyses, there was a negative correlation between ALB and urinary GLY concentrations in both the CKD and non-CKD groups (β = -0.32, 95% CI [-0.60, -0.04], P = 0.025; β = -1.46, 95% CI [-2.03, -0.89], P < 0.001), while FIB-4 showed a positive correlation with urinary GLY (β = 0.08, 95% CI [0.03, 0.14], P = 0.004; β = 0.68, 95% CI [0.53, 0.84], P < 0.001). For ALP, ALT, AST, and AST/ALT, this statistical significance was observed only in the CKD group (β = 4.93, 95% CI [2.85, 7.01], P < 0.001; β = 3.13, 95% CI [1.25, 5.00], P < 0.001; β = 10.00, 95% CI [8.30, 11.69], P < 0.001; β = 0.07, 95% CI [0.04, 0.10], P < 0.001).
In conclusion, the substantial connection with the p for interaction suggested that this association between biomarkers of liver function and GLY was certainly dependent on age, gender, race, BMI, and CKD (p for interaction < 0.05, Supplementary table).
Discussion
In our nationally representative sample of US adults, urinary GLY concentrations were positively connected with liver function but showed no significant association with renal function. In particular, our findings revealed that elevated GLY concentrations in urine were strongly linked to an increased risk of liver injury in specific subgroups, including younger participants, women, non-Hispanic whites, overweight individuals, and CKD patients.
The liver is the major organ affected by xenobiotic exposure. ALT, AST, and ALP activities serve as hepatotoxicity markers (McGill 2016). Previous studies illustrated that GLY could induce hepatic oxidative stress with altered AST, ALT, and ALP, evidenced by the upregulation of malondialdehyde, hydrogen peroxide and the downregulation of superoxide dismutase, glutathione, and vitamin C (Soudani et al. 2019; Abdelmagid et al. 2022). Transcriptome profile analysis also proved that the alterations in gene expression of the liver were consistent with fibrosis, necrosis, phospholipidosis, and mitochondrial membrane dysfunction following chronic low-dose GLY exposure (Mesnage et al. 2015). These studies may serve as useful information for explaining the results of the present study. The AST/ALT ratio was helpful in the detection of liver fibrosis or damage (Mansoor et al. 2015), and a high AST/ALT ratio may indicate hepatocyte necrosis (Fu et al. 2019). We found that GLY was significantly correlated with the AST/ALT ratio, which indicated a positive association between prolonged exposure to GLY and hepatocyte necrosis. A previous study showed that liver pathology confirmed the presence of inflammatory reaction with pathology involving hepatocyte necrosis in rats (Hamdaoui et al. 2019). FIB-4 is another diagnostic tool for predicting the stage of liver fibrosis (Itakura et al. 2021; Xu et al. 2022). As previously described, the notable dose-dependent rise in glyphosate exposure was along with an increase in fibrosis phases (Mills et al. 2020), that consistent with the positive relationship between urinary GLY concentrations and FIB-4 in our study. In addition, ALB is a marker of hepatic synthesis function and is involved in physiological processes, such as endothelium stabilization, molecular transportation, antioxidant, anti-inflammatory, and modulating capillary permeability (Sun et al. 2019). Our findings supported previous research that showed a negative relationship between serum albumin concentrations and GLY (Abdelmagid et al. 2022). In addition, a higher concentration of GLY altered the structure of albumin while significantly decreasing its antioxidant capability (Movaghati et al. 2023). More investigations are required to comprehend the mechanisms underlying the link between GLY exposure and albumin.
In the subgroup, we further investigated the association between GLY exposure and liver dysfunction. Age constitutes a pivotal risk factor contributing to an elevated prevalence of liver injury in the elderly population (Maeso-Díaz et al. 2018); however, our findings demonstrated that GLY exposure conferred a greater susceptibility to liver dysfunction in younger participants compared to elders, potentially attributable to heightened GLY exposure resulting from disparities in lifestyle habits and metabolic processes (Grau et al. 2022). The evidence from our study also strongly pointed to a greater risk for females to develop liver dysfunction than males, and the disparity was also discovered in other comparable studies (Colantoni et al. 2000; Wahlang et al. 2023). Recent epidemiological studies and studies performed in animals suggested that GLY could have endocrine-disrupting potentiality targeting sex hormones, which may be a significant contributing reason to women's increased susceptibility to liver dysfunction following GLY exposure (Lesseur et al. 2021; Maddalon et al. 2021; Geier and Geier 2023). Similar associations were also observed between GLY and biomarkers of liver function in non-Hispanic white individuals and the risks correlated with GLY exposure varied significantly by racial difference. It is anticipated that the observed substantial correlations might be impacted by genetic and/or biochemical variances (Strnad et al. 2010; Cirulli et al. 2019). However, no previous studies have demonstrated that liver dysfunction with regard to levels of GLY is attributed to racial differences and future studies are needed to focus on this issue. In addition, this study also revealed that the risk of liver dysfunction from GLY exposure was greater for overweight individuals. Overweight, especially progressing to obesity, is involved in the development of diseases linked to metabolic syndrome, which includes nonalcoholic fatty liver disease with an accumulation of fat in the liver. Hepatic steatosis subsequently causes inflammation and hepatocyte injury and even progresses to fibrosis (Weiß et al. 2014). Therefore, overweight individuals with exposure to GLY were more susceptible to liver injury and could even develop more severe fibrosis (Mills et al. 2020).
Although previous studies in humans and animals have reported that chronic exposure to GLY is nephrotoxic (Jayasumana et al. 2015; Zhang et al. 2017; Gunatilake et al. 2019), serum creatinine and ACR were not found to have significant associations with GLY exposure in this study. As a possible explanation, participants were those from general populations, not as observation subjects suffering from long-term uninterrupted GLY exposure in previous studies; thus, the average concentration of GLY in the recruited participants may not be high enough to cause impaired renal function (Gillezeau et al. 2019). However, we found that participants with CKD had a higher risk of liver dysfunction than non-CKD participants. This phenomenon could be explained by the fact that the liver is one of the main organs where ingested GLY accumulates (Williams et al. 2000), and its clearance capacity decreases in the presence of renal impairment (Tokunaga et al. 2020). Therefore, more attention should be given to the risk of GLY for individuals with CKD.
The current study has several limitations that need to be acknowledged. First, as this analysis was cross-sectional, we cannot establish causality or determine the temporal relationship between variables. To address this limitation, future research should consider longitudinal designs. Second, we were unable to completely control for all potential confounding variables, such as dietary habits and living and working environments. Therefore, caution should be exercised when interpreting our results. Future studies should aim to include a more comprehensive assessment of confounding factors. Third, the measurement of GLY exposure was based on the concentration of GLY in random urine samples. This method may introduce variability in the test outcomes, and first-morning void or 24-h urine samples might yield more precise statistical data. Future studies should consider using these alternative sampling methods to improve the accuracy of GLY exposure measurements. In addition, the equation for estimation of eGFR only incorporates creatinine while omitting cystatin C in the current study. However, the eGFR equation with the combination of creatinine and cystatin C provides a more accurate assessment than the equation with creatinine alone. Furthermore, cystatin C levels exhibit an earlier increase than creatinine levels, making it a more sensitive marker for diagnosing kidney repairment in the early stages. Whereas, there are no assay values for cystatin C in the 2013–2016 NHANES datasets. Last, glyphosate-based herbicide (GBH) formulations encompass additional chemicals in addition to glyphosate. The precise attribution of adverse effects to either glyphosate or the combined components remains uncertain. Consequently, NHANES should furnish comprehensive information concerning GBH formulations to facilitate further investigation into their potential impact on human health. In spite of these constraints, our research presented several benefits. First, we utilized a nationally representative sample, generalizability of our findings to the diverse adult population in the US, encompassing various ethnicities and genders. Additionally, the inclusion of a significant number of participants in our study enabled us to conduct subgroup analysis, providing valuable insights into specific subpopulations.
Conclusions
This study revealed significant associations between GLY exposure and biomarkers of liver dysfunction among US adults, suggesting that avoiding exposure can assist in lessening the harmful effects of GLY on health. Considering the limited availability of data in the literature and the constraints of our study, additional research is required to validate our findings concerning the effects of environmental GLY exposure on liver injury.
Data availability
Both the data and analysis materials are freely downloaded from National Health and Nutritional Examination Survey. (https://www.cdc.gov/nchs/nhanes).
References
Abdelmagid AD, Said AM, Abd El-Gawad EA, Shalaby SA, Dawood MAO (2022) Glyphosate-induced liver and kidney dysfunction, oxidative stress, immunosuppression in Nile tilapia, but ginger showed a protection role. Vet Res Commun 47:445–455. https://doi.org/10.1007/s11259-022-09961-0
Bento CPM, Yang X, Gort G, Xue S, van Dam R, Zomer P, Mol HGJ, Ritsema CJ, Geissen V (2016) Persistence of glyphosate and aminomethylphosphonic acid in loess soil under different combinations of temperature, soil moisture and light/darkness. Sci Total Environ 572:301–311. https://doi.org/10.1016/j.scitotenv.2016.07.215
Botero-Coy AM, Ibáñez M, Sancho JV, Hernández F (2013) Improvements in the analytical methodology for the residue determination of the herbicide glyphosate in soils by liquid chromatography coupled to mass spectrometry. J Chromatogr A 1292:132–141. https://doi.org/10.1016/j.chroma.2012.12.007
Cirulli ET, Nicoletti P, Abramson K, Andrade RJ, Bjornsson ES, Chalasani N, Fontana RJ, Hallberg P, Li YJ, Lucena MI, Long N, Molokhia M, Nelson MR, Odin JA, Pirmohamed M, Rafnar T, Serrano J, Stefánsson K, Stolz A, Daly AK, Aithal GP, Watkins PB (2019) A Missense variant in PTPN22 is a risk factor for drug-induced liver injury. Gastroenterology 156(6):1707-1716.e1702. https://doi.org/10.1053/j.gastro.2019.01.034
Colantoni A, La Paglia N, De Maria N, Emanuele MA, Emanuele NV, Idilman R, Harig J, Van Thiel DH (2000) Influence of sex hormonal status on alcohol-induced oxidative injury in male and female rat liver. Alcohol Clin Exp Res 24(9):1467–1473. https://doi.org/10.1111/j.1530-0277.2000.tb02118.x
Curtin LR, Mohadjer LK, Dohrmann SM, Montaquila JM, Kruszan-Moran D, Mirel LB, Carroll MD, Hirsch R, Schober S, Johnson CL (2012) The National Health and Nutrition Examination survey: sample design, 1999–2006. Vital Health Stat 2(155):1–39. https://doi.org/10.1002/9781118190180.ch20
Fu S, Wu D, Jiang W, Li J, Long J, Jia C, Zhou T (2019) Molecular biomarkers in drug-induced liver Injury: challenges and future perspectives. Front Pharmacol 10:1667. https://doi.org/10.3389/fphar.2019.01667
Gao H, Chen J, Ding F, Chou X, Zhang X, Wan Y, Hu J, Wu Q (2019) Activation of the N-methyl-d-aspartate receptor is involved in glyphosate-induced renal proximal tubule cell apoptosis. J Appl Toxicol 39(8):1096–1107. https://doi.org/10.1002/jat.3795
Geier DA, Geier MR (2023) Urine glyphosate exposure and serum sex hormone disruption within the 2013–2014 National Health and Nutrition Examination survey (NHANES). Chemosphere 316:137796. https://doi.org/10.1016/j.chemosphere.2023.137796
Gillezeau C, van Gerwen M, Shaffer RM, Rana I, Zhang L, Sheppard L, Taioli E (2019) The evidence of human exposure to glyphosate: a review. Environ Health 18(1):2. https://doi.org/10.48048/wjst.2020.7232
Grau D, Grau N, Gascuel Q, Paroissin C, Stratonovitch C, Lairon D, Devault DA, Di Cristofaro J (2022) Quantifiable urine glyphosate levels detected in 99% of the French population, with higher values in men, in younger people, and in farmers. Environ Sci Pollut Res Int 29(22):32882–32893. https://doi.org/10.1007/s11356-021-18110-0
Gunatilake S, Seneff S, Orlando L (2019) Glyphosate’s synergistic toxicity in Combination with other factors as a cause of chronic kidney disease of unknown origin. Int J Environ Res Public Health 16(15):2734. https://doi.org/10.3390/ijerph16152734
Hamdaoui L, Naifar M, Rahmouni F, Ayadi F, Rebai T (2019) Sub-chronic exposure to Kalach 360 SL-induced damage in rats’ liver and hematological system. Environ Sci Pollut Res Int 26(36):36634–36646. https://doi.org/10.1007/s11356-019-06491-2
Hou X, Mao Z, Song X, Li R, Liao W, Kang N, Zhang C, Liu X, Chen R, Huo W, Wang C, Hou J (2023) Synergistic association of long-term ozone exposure and solid fuel use with biomarkers of advanced fibrosis. Environ Sci Pollut Res Int 30(36):85318–85329. https://doi.org/10.1007/s11356-023-28337-8
Itakura J, Kurosaki M, Setoyama H, Simakami T, Oza N, Korenaga M, Tanaka M, Torimura T, Sakamoto N, Enomoto N, Ueno Y, Kawada N, Kaneko S, Nishiguchi S, Chayama K, Tanaka J, Izumi N, Kanto T (2021) Applicability of APRI and FIB-4 as a transition indicator of liver fibrosis in patients with chronic viral hepatitis. J Gastroenterol 56(5):470–478. https://doi.org/10.1007/s00535-021-01782-3
Jayasumana C, Gunatilake S, Siribaddana S (2015) Simultaneous exposure to multiple heavy metals and glyphosate may contribute to Sri Lankan agricultural nephropathy. BMC Nephrol 16:103. https://doi.org/10.1186/s12882-015-0109-2
Le Du-Carrée J, Boukhari R, Cachot J, Cabon J, Louboutin L, Morin T, Danion M (2021) Generational effects of a chronic exposure to a low environmentally relevant concentration of glyphosate on rainbow trout, Oncorhynchus mykiss. Sci Total Environ 801:149462. https://doi.org/10.1016/j.scitotenv.2021.149462
Lesseur C, Pirrotte P, Pathak KV, Manservisi F, Mandrioli D, Belpoggi F, Panzacchi S, Li Q, Barrett ES, Nguyen RHN, Sathyanarayana S, Swan SH, Chen J (2021) Maternal urinary levels of glyphosate during pregnancy and anogenital distance in newborns in a US multicenter pregnancy cohort. Environ Pollut 280:117002. https://doi.org/10.1016/j.envpol.2021.117002
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150(9):604–612. https://doi.org/10.7326/0003-4819-150-9-200905050-00006
Maddalon A, Galbiati V, Colosio C, Mandić-Rajčević S, Corsini E (2021) Glyphosate-based herbicides: Evidence of immune-endocrine alteration. Toxicology 459:152851. https://doi.org/10.1016/j.tox.2021.152851
Maeso-Díaz R, Ortega-Ribera M, Fernández-Iglesias A, Hide D, Muñoz L, Hessheimer AJ, Vila S, Francés R, Fondevila C, Albillos A, Peralta C, Bosch J, Tacke F, Cogger VC, Gracia-Sancho J (2018) Effects of aging on liver microcirculatory function and sinusoidal phenotype. Aging Cell 17(6):e12829. https://doi.org/10.1111/acel.12829
Mansoor S, Collyer E, Alkhouri N (2015) A comprehensive review of noninvasive liver fibrosis tests in pediatric nonalcoholic Fatty liver disease. Curr Gastroenterol Rep 17(6):23. https://doi.org/10.1007/s11894-015-0447-z
McGill MR (2016) The past and present of serum aminotransferases and the future of liver injury biomarkers. Excli J 15:817–828. https://doi.org/10.17179/excli2016-800
Mesnage R, Arno M, Costanzo M, Malatesta M, Séralini GE, Antoniou MN (2015) Transcriptome profile analysis reflects rat liver and kidney damage following chronic ultra-low dose Roundup exposure. Environ Health 14:70. https://doi.org/10.1186/s12940-015-0056-1
Mesnage R, Renney G, Séralini GE, Ward M, Antoniou MN (2017) Multiomics reveal non-alcoholic fatty liver disease in rats following chronic exposure to an ultra-low dose of Roundup herbicide. Sci Rep 7:39328. https://doi.org/10.1038/srep39328
Milić M, Žunec S, Micek V, Kašuba V, Mikolić A, Lovaković BT, Semren T, Pavičić I, Čermak AMM, Pizent A, Vrdoljak AL, Valencia-Quintana R, Sánchez-Alarcón J, Želježić D (2018) Oxidative stress, cholinesterase activity, and DNA damage in the liver, whole blood, and plasma of Wistar rats following a 28-day exposure to glyphosate. Arh Hig Rada Toksikol 69(2):154–168. https://doi.org/10.2478/aiht-2018-69-3114
Mills PJ, Caussy C, Loomba R (2020) Glyphosate Excretion is Associated With Steatohepatitis and advanced liver fibrosis in patients with fatty liver disease. Clin Gastroenterol Hepatol 18(3):741–743. https://doi.org/10.1016/j.cgh.2019.03.045
Movaghati S, Delphi L, Disfani F, Moosavi-Movahedi AA (2023) The role of surface activity on the amyloid fibrillation pathway of bovine serum albumin upon interaction with glyphosate. Int J Biol Macromol 226:1166–1177. https://doi.org/10.1016/j.ijbiomac.2022.11.230
Mutwedu VB, Nyongesa AW, Azine PC, Chiregereza DK, Ngoumtsop VH, Mugumaarhahama Y, Ayagirwe RBB (2021) Growth performance and reproductive function impairment of glyphosate-based herbicide in male guinea pig (Cavia porcellus). Vet Med Sci 7(3):1047–1055. https://doi.org/10.1002/vms3.443
Schütze A, Morales-Agudelo P, Vidal M, Calafat AM, Ospina M (2021) Quantification of glyphosate and other organophosphorus compounds in human urine via ion chromatography isotope dilution tandem mass spectrometry. Chemosphere 274:129427. https://doi.org/10.1016/j.chemosphere.2020.129427
Soudani N, Chaâbane M, Ghorbel I, Elwej A, Boudawara T, Zeghal N (2019) Glyphosate disrupts redox status and up-regulates metallothionein I and II genes expression in the liver of adult rats. Alleviation by quercetin. Gen Physiol Biophys 38(2):123–134. https://doi.org/10.1016/j.chemosphere.2020.129427
Steinrücken HC, Amrhein N (1980) The herbicide glyphosate is a potent inhibitor of 5-enolpyruvyl-shikimic acid-3-phosphate synthase. Biochem Biophys Res Commun 94(4):1207–1212. https://doi.org/10.1016/0006-291x(80)90547-1
Stevens PE, Levin A (2013) Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med 158(11):825–830. https://doi.org/10.7326/0003-4819-158-11-201306040-00007
Strnad P, Zhou Q, Hanada S, Lazzeroni LC, Zhong BH, So P, Davern TJ, Lee WM, Omary MB (2010) Keratin variants predispose to acute liver failure and adverse outcome: race and ethnic associations. Gastroenterology 139(3):828-835.e821-823. https://doi.org/10.1053/j.gastro.2010.06.007
Sun L, Yin H, Liu M, Xu G, Zhou X, Ge P, Yang H, Mao Y (2019) Impaired albumin function: a novel potential indicator for liver function damage? Ann Med 51(7–8):333–344. https://doi.org/10.1080/07853890.2019.1693056
Tokunaga A, Miyamoto H, Fumoto S, Nishida K (2020) Effect of chronic kidney disease on hepatic clearance of drugs in rats. Biol Pharm Bull 43(9):1324–1330. https://doi.org/10.1248/bpb.b20-00124
Wahlang B, Gao H, Rai SN, Keith RJ, McClain CJ, Srivastava S, Cave MC, Bhatnagar A (2023) Associations between residential volatile organic compound exposures and liver injury markers: The role of biological sex and race. Environ Res 221:115228. https://doi.org/10.1016/j.envres.2023.115228
Weiß J, Rau M, Geier A (2014) Non-alcoholic fatty liver disease: epidemiology, clinical course, investigation, and treatment. Dtsch Arztebl Int 111(26):447–452. https://doi.org/10.3238/arztebl.2014.0447
Williams GM, Kroes R, Munro IC (2000) Safety evaluation and risk assessment of the herbicide Roundup and its active ingredient, glyphosate, for humans. Regul Toxicol Pharmacol 31(2 Pt 1):117–165. https://doi.org/10.1006/rtph.1999.1371
Xu XL, Jiang LS, Wu CS, Pan LY, Lou ZQ, Peng CT, Dong Y, Ruan B (2022) The role of fibrosis index FIB-4 in predicting liver fibrosis stage and clinical prognosis: A diagnostic or screening tool? J Formos Med Assoc 121(2):454–466. https://doi.org/10.1016/j.jfma.2021.07.013
Zhang F, Pan LP, Ding EM, Ge QJ, Zhang ZH, Xu JN, Zhang L, Zhu BL (2017) Study of the effect of occupational exposure to glyphosate on hepatorenal function. Zhonghua Yu Fang Yi Xue Za Zhi 51(7):615–620. https://doi.org/10.3760/cma.j.issn.0253-9624.2017.07.008
Funding
The authors declare that no funds were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Tuo Xiao: study design, statistical analysis, investigation, writing—original draft, review editing. Yue Xu and Yanqi Song: data collection and management. Yuhao Chen: data curation and statistical analysis. Xuejing Ren, Wenjuan Wang, and Kaiting Zhuang: writing—review and editing. Xiangmei Chen: project supervision. Guangyan Cai: project supervision, writing—review and editing. All authors have read and approved the final article.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The CDC/NCHS Ethics Review Board approved the NHANES study and all participants provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Responsible Editor: Lotfi Aleya
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xiao, T., Chen, Y., Xu, Y. et al. Higher urinary glyphosate exposure is associated with increased risk of liver dysfunction in adults: An analysis of NHANES, 2013–2016. Environ Sci Pollut Res (2023). https://doi.org/10.1007/s11356-023-30463-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11356-023-30463-2