Abstract
Recently, the reaction speed and cycle performance of hexavalent chromium reduction over microsized zero-valent iron (ZVI) with an Fe0 core and iron oxide (FeOx) shell structure have been improved by activating the Fe0-core electrons through electromagnetic coupling between Fe0-core electrons and charges (hexavalent chromium in solution, double-charge layers of the ZVI/solution interface). Herein, the abovementioned electromagnetic coupling was greatly increased by adding salt (CH3COONa, NaCl, NaNO3, and Na2SO4) in the hexavalent chromium solution to increase the charge response. Adding salt greatly improved the reaction speed and cycle performance of hexavalent chromium reduction. It took 8 min to reduce hexavalent chromium with CH3COONa to below the discharge standard of wastewater in the first cycle and 20 min after reducing for 20 cycles. The best apparent rate of constant value (0.416 (min)-1) is nearly four times larger than those without salts. X-ray diffraction and X-ray photoelectron spectroscopy revealed the production of amorphous iron oxide shell with salt. The salt improves the hexavalent chromium reduction speed and cycle performance and impedes the Fe0-core-electron transfer via the produced Fe2O3, resulting in existence of an optimized salt dosage. This work aims to provide an effective route for enhancing the removal efficiency and cycle performance of heavy-metal–ion reduction via Fe0. And this work also proposes a novel viewpoint that adding salt in waste water would increase the electromagnetic coupling between the charges in solution and Fe0-core electrons which could finally activate the redox reaction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nowadays, human activities have caused increased pollution, especially industrial pollution arising from effluents coming from industries such as leather manufacturing, metal processing, and electroplating (Chen et al. 2014; Bhaumik et al. 2012; Nguyen et al. 2021). Among the heavy metals found in industrial wastewater, chromium (VI) (hexavalent chromium) is one of the most toxic and dangerous (Banerjee et al. 2017). Hexavalent chromium is extensively used in industry with the most common sources coming from chrome plating, leather tanning and textiles (Saha et al. 2011; Saha and Orvig 2010). Hexavalent chromium exists as Cr2O72− in acidic conditions or CrO42− in neutral or alkaline conditions (Liu et al. 2003; Ambika and Nambi 2016). Thus, hexavalent chromium can be very mobile in the ecological environment (Liu et al. 2003). A highly soluble metal in water, hexavalent chromium may excessively accumulate, which may eventually lead to cancer in humans (Cheung and Gu 2003; Singh et al. 2015). Thus, hexavalent chromium has been classified as a B-grade carcinogen by the U.S. Environmental Protection Agency (Chen et al. 2014). Hence, it is crucial to find a way to safely remove hexavalent chromium from the ecological environment.
Meanwhile, chromium (III) (trivalent chromium), another primary form of Cr existing in solution, is far less toxic. Moreover, it is an essential element in humans (Liu et al. 2010). Hence, a feasible way to remove hexavalent chromium species is to reduce them to trivalent chromium to lessen the toxicity and subsequently remove them from solution (Nguyen et al. 2021; Cheung and Gu 2003; Zhu et al. 2018). Recently, zero-valent iron (Fe0, ZVI) has been used to reduce hexavalent chromium owing to its strong reducibility and nontoxicity (Ambika and Nambi 2016; Guo et al. 2017). However, the efficiency needs to be further improved and its cost needs to be lowered for application.
To address the abovementioned problem, in our previous work, tribocatalysis was conducted on wastewater to persistently remove hexavalent chromium by reducing it to trivalent chromium over iron turning (Fe0) with nearly complete hexavalent chromium removal (removal rate 98%) in 80 min (He et al. 2021). Hexavalent chromium was removed by absorbing on the surface of iron turning and precipitating as Cr2O3 and magnetic production such as FeOOH, followed by removal with a magnet. The iron turning was microsized which was easy to be removed from the solution. Despite being an environmentally friendly method, the process has unsatisfactory efficiency and cycle performance for application (Liu et al. 2003; Sun et al. 2011; Dalal and Reddy 2019). The residual Fe concentration in the treated solution was only slightly lower than the government standard (0.3 mg/L) (Liu et al. 2003). Thus, the efficiency and cycle performance need to be further improved and the residual Fe concentration should be lowered more.
The employed iron turning is microsized zero-valent iron (MZVI) with a ZVI (Fe-0) core and an iron oxide (FeOx) shell structure. MZVI can be recycled with magnets and also separated during stirring, which can be suitable for large-scale applications. The mechanism of improving the hexavalent chromium-reduction speed and cycle performance by MVZI in our previous work is that the mechanical energy is coupled to the electrons of iron turning by stirring the solution and disturbing the charges (including hexavalent chromium ions in solution and the double-charge layers in the iron/hexavalent chromium solution interface). The motion of charges can produce an induced electromagnetic field, which may activate the electrons in MZVI to overcome the potential barrier produced by the oxide shell more easily, yielding higher hexavalent chromium reduction efficiency and better cycle performance. Based on this mechanism, an innovative way to further enhance the induced electromagnetic field is designed to further promote the reduction speed and cycle performance while reducing the Fe concentration to a much lower level in the treated solution. The charges of ions in solution and double layers on the interface of Fe0/FeOx is limited, affording limit variation of the electromagnetic field by stirring. Adding ions in hexavalent chromium solution would increase the ion density and therefore enhance the variation of the electromagnetic field by stirring, and it is expected that there is more electromagnetic field coupling with the electrons in MZVI and therefore more electrons react with hexavalent chromium, yielding an enhanced hexavalent chromium-reduction efficiency.
Herein, sodium salts, namely, CH3COONa (CNa), NaCl, NaNO3, and Na2SO4, were chosen to provide the ions for the hexavalent chromium solution to enhance the variation of the electromagnetic field by stirring the ions in the solution. It has been found that the added sodium salts have led to vastly enhanced hexavalent chromium removal efficiency and greatly improved cycle performance and simultaneous reduction of Fe concentration in the treated solution. In all treating cases of this work, the maximum removal rate of hexavalent chromium can reach to 99.9%, at which the lowest concentration of hexavalent chromium in waste water is much less than the discharge standard required by the government. The reaction equilibrium time (denoted as t100) with salt is 8 min compared to that (80 min) obtained in our previous work. The Fe concentration in the treated solution is also far below the drinking water limit. t100 is still 20 min at the 20th cycle.
The reactions between solid and liquid are always related to the variation of electric double layer (EDL) near the solid-liquid interface. In previous work, the effect of adding salt into the waste water is regarded as changing the EDL and the Zeta potential of the waste water-a viewpoint of electrochemistry (Zhang et al. 2019). In this work, the other effect of adding salt into the waste water has been proposed from the viewpoint of the energy conversion: EDL can assist to converse the non-chemical energy (like mechanical energy) near the solid-liquid interface into the chemical energy which could activate the redox reaction through producing electromagnetic induction by changing EDL. And it is pointed that this kind of electromagnetic induction can be increased by adding involving ions in solution, like adding salt in waste water. This is the innovativeness in this work. Also, this work provides an effective route to treat heavy-metal wastewater massively, simply, and environmentally friendly at a low cost.
Experiments
Materials
Iron turning, AR, was purchased from Tianjin Kermel Chemical Reagent Company, China. Potassium dichromate (K2Cr2O7), (AR, Chengdu Keshi Chemical Reagent Company, China) was used to prepare the hexavalent chromium solution. Sodium acetate anhydrous (CH3COONa, AR, Tianjin Zhiyuan Chemical Reagent Co., LTd.), sodium chloride (NaCl, AR, Tianjin Zhiyuan Chemical Reagent Co., LTd.), sodium nitrate (NaNO3, AR, Guangzhou Chemical Reagent Factory), and sodium sulfate anhydrous (Na2SO4, AR, Tianjin Zhiyuan Chemical Reagent Co., LTd.) were used as the assisted salts during experiments. Table 1 lists the various dosages of salts used, labeled Lv1-Lv6, according to the rank of the amounts.
Characterization
X-ray diffraction (XRD, Bruker D8 Advance, Germany) using a Cu target (40 kV, 40 mA) within 2θ from10 to 80°and X-ray photoelectron spectroscopy using a Thermo Scientific K-Alpha XPS system (XPS, Thermo Fisher Scientific, USA) were used to analyze the phases and surface elements of iron turning and sediments.
Measurement of the removal rate of hexavalent chromium with assisted salts in the dark
Removal experiments were conducted using a mechanical stirrer (JJ-1A, Changzhou Chunqiu Electronic Instrument Co. Ltd., China) at a rotation speed of 360 rpm. In order to meet higher effluent discharge standard, wastewater with low concentration (2mg/L) is used as simulated contaminant which is more difficult to be removed. K2Cr2O7 was used to prepare 2 mg/L hexavalent chromium as simulated wastewater. During the experiments, ~1 g of iron turning and various amounts of different assisted salts (Lv1-Lv6, one kind each experiment) were put into 100 mL hexavalent chromium solution and 2 mL of solution was taken out at a certain time interval to test the hexavalent chromium concentration in the rest solution using a UV-vis spectrophotometer (UV-2600 Shimadzu, Japan) via the 1,5-diphenylcarbazide colorimetric method (Velegraki et al. 2018) analyzed at 540 nm. Cr and Fe concentrations in the treated solution were measured using an inductive-coupled plasma emission spectrometer (ICP, SPECTRO ARCOS MV, Germany). The pH values were measured using a conductivity meter (DDSJ-308F, Shanghai INESA Scientific Instrument Co., Ltd.).
Results and discussion
Improved hexavalent chromium-reduction speed and cycle performance by MZVI with sodium salt
Figure 1(a)-(d) displays the hexavalent chromium removal rates using MZVI with different amounts of CH3COONa, NaCl, NaNO3, and Na2SO4, respectively. The removal rates in all cases finally reached 99.9%, and the t100 values varied with the added salt types and dosages, among which the shortest times were 8, 8, 8, and 7 min and the longest times were 12, 14, 14, and 14 min for CH3COONa, NaCl, NaNO3, and Na2SO4, respectively. Bioremediation is also a useful method for hexavalent chromium removal. Previous works have demonstrated good performances in removing hexavalent chromium by bioremediation in high concentration (Saha et al. 2013a; Saha and Saha 2014; Saha et al. 2013b). This study aims to further process the low-concentration hexavalent-chromium waste water, i.e., the post processing hexavalent-chromium waste water, to meet the high emission standard.
Table 2 lists the k values of the removal rate–time curves. The k values were obtained through fitting and calculating according to the pseudo-first-order model (Sun et al. 2015). From Table 2, all k values were larger than 0.08 (min)-1 which obtained without assisted salt reported in our previous work (He et al. 2021). In addition, the best k values of CH3COONa, NaCl, NaNO3, and Na2SO4 are 0.266 (min)-1 at Lv4, 0.325 (min)-1 at Lv3, 0.322 (min)-1 at Lv5, and 0.416 (min)-1 at Lv5, respectively. The best k (0.416 (min)-1) is nearly four times larger than that without salt reported in our previous work (He et al. 2021). It can be concluded that different salts possessed different optimum dosages during the removal process. In other words, both salt type and dosage are factors that can influence the removal efficiency.
To examine if the treated wastewater meets the effluent discharge standard, the Cr and Fe concentrations in the solution after treatment assisted by CH3COONa with Lv1 to Lv5 dosages were measured using ICP. As shown in Table 3, the Cr and Fe concentrations in the solution after treatment were far below the drinking water limit (0.05 and 0.3 mg/L, respectively) (Liu et al. 2003) and those of our previous work (0.0499 and 0.277 mg/L) (He et al. 2021) in all cases. Hence, the hexavalent chromium removal rate could be regarded as 100% after the treatment in the cases mentioned. Moreover, at Lv4, the lowest hexavalent chromium concentration was obtained in the solution after treatment.
Considering the 100% removal rate of Cr and low Fe concentration in the treated solution, CH3COONa with an Lv4 dosage was chosen in the next cycling experiments at room temperature (25°C). Figure S1(a) shows the “removal rate vs time” curves in 20 cycles in case of CH3COONa at Lv4 dosage. The “removal rate vs time” curves in 5 circles without CH3COONa obtained in our previous work (He et al. 2021) are also given in Fig. S1(b) as comparison. In order to clearly show the cycle performance, a column diagram presenting “t100-cycle number” relations was drawn based on Fig. S1(a) and shown in Fig. 2. Figure 2 illustrates that increasing the cycles took a longer time to thoroughly remove hexavalent chromium. However, the t100-s varied with cycles with the nonmonotonic law, which can possibly be attributed to the irregular drop of compounds forming on the surface of iron turning during the treatment. Nevertheless, the t100 of the 20th cycle was 20 min and the longest one was 24 min at the 18th cycle, both far more quickly than those in our previous work (He et al. 2021). In summary, the assisted salts can greatly enhance the cycle performance of hexavalent chromium reduction over MZVI.
Chemical reaction route in assisted salt
To investigate the possible chemical reaction route of hexavalent chromium reduction over MZVI with sodium salt, XRD and XPS were conducted to measure the characteristics of MZVI at every stage and sediment collected from the solution after treatment with CNa, an example of sodium salt (He et al. 2021). The phases, element, and chemical bonds of the surface on MZVI and the collected sediment are shown in Figs. S2 and S3. Analysis of the Fig.s revealed that there were Fe0, FeO, and amorphous Fe2O3 on the MZVI surface after CNa treatment. Moreover, the following materials existed in the sediments after hexavalent chromium reduction with CNa based on the reducing content order: FeOOH > Fe0 > FeO > Cr2O3. The added sodium salt triggered a corrosion reaction of MZVI, yielding more amorphous Fe2O3 on the MZVI surface.
From Figs. S2 and S3, the possible mechanism at pH 6-7 involved in the removal treatment is given below (Ambika and Nambi 2016; He et al. 2021).
Mechanism
As stated in the Introduction, sodium salt increases the charges involved in electromagnetic field induction by stirring. Therefore, the electromagnetic field coupling with the electrons in MZVI increases, along with the electrons participating in hexavalent chromium reduction.
Figure 3 displays the comparison of hexavalent chromium-removal rates among different cases. In the figure, the removal rates are nearly the same both in the cases that without stirring. It means that applying no stirring has no effect on the hexavalent chromium-reduction speed with CNa, which is almost the same as that without CNa. In another word, stirring plays a significant role in the process.
Figure 4(a) and (b) illustrate the mechanism increasing the induced electromagnetic field by adding salt in wastewater when stirring. The effect of the added salt could be contributed by cations (Fig. 4(a)) such as Na+ and anions (Fig. 4(b)) such as Cl− and NO3−. Figure 4 illustrates the top view of the hexavalent chromium reduction system, which includes a container holding wastewater, MZVI (schematically shown by a black square in Fig. 4), and a stir bar. Without stirring, Na+ and anions maintain a static state and no induced electromagnetic field is produced. Hence, no effect from the added salt is observed (Fig. 3). Applying rotation, as shown in Figs. 4(a) and (b), both cations and anions rotate along with the rotation of the solution. Suppose that the solution rotates in a clockwise manner (Fig. 4(a)), the cations will produce a current in the direction of rotation, producing a magnetic field with a direction perpendicular to the top view plane and inward (with the symbol ×), determined using the right-hand screw rule. Under this magnetic field, an additional Lorentz force w will be applied on the electrons in the MZVI core in the direction of pulling the electrons out of MZVI. The Lorentz force pushes the electrons in MZVI to go over the potential barrier near the interface of Fe0/FeOx to the outside of the MZVI, as shown in Fig. 5, increasing the number of electrons reacting with hexavalent chromium in the solution. These electrons will enhance the removal efficiency.
Mechanism of increasing the induced electromagnetic field with stirring the added salt: (a) the effect from cations; (b) the effect from anions. F represents the Lorentz force in MZVI, v refers to the speed of solution, × means that the direction of the induced electromagnetic field is perpendicular to the paper plane and inward, and ● means that the direction of the induced electromagnetic field is perpendicular to the paper plane and outward
Likewise, the anions in the solution would produce a Lorentz force on the electrons in Fe0, pulling those electrons out of the MZVI (Fig. 4(b)). Although the direction of the Lorentz force from the rotated anions is opposite to that from the rotated cations, the ultimate effect of the Lorentz force is to pull the electrons out of MZVI, causing similar effects.
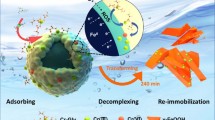
Based on double-charge layer theory, the whole removal process of hexavalent chromium may be summarized. As shown in Fig. 6, at first, hexavalent chromium in the solution may diffuse towards iron turning under the influence of rotation. Then hexavalent chromium may be physically adsorbed on the surface of iron turning. The addition of sodium salts may induce a stronger electromagnetic field generated by stirring the solution, which may attract more electrons inside iron turning to migrate to the outside, and react with hexavalent chromium. And the process of charges transfer was accelerated. Thus, the improved removal performance was achieved. Subsequently, trivalent chromium reduced from hexavalent chromium may desorb from the surface of iron turning and be removed from solution in the form of sediments. The present of sodium salts may also accelerate the desorption of trivalent chromium, which may also improve the removal performance of hexavalent chromium.
Adding sodium salts may thinning the double-charge layer at the interface between iron turning and solution and lead to the decrease of induced electromagnetic field, which may slightly affect the hexavalent chromium reduction. However, this influence may be ignored comparing to the effects caused by the stronger electromagnetic field generated by stirring the solution.
However, the added sodium salt would induce other complicated effects. As stated in the supplementary materials (Figs. S2 and S3), the added sodium salt produced Fe2O3 on the MZVI surface owing to the corrosion reaction of MZVI with sodium salt, which would induce more impediment for the electron transferring from the core to the shell surface of MZVI. Meanwhile, the corrosion would change the microstructure on the MZVI surface, accelerating the collapse of the employed MZVI shell. This collapse of the employed MZVI shell benefits the electrons to expose to the hexavalent chromium solution. The synthetic effects of added sodium salts are complicated. Thus, the improved hexavalent chromium-reduction speed cannot monotonically increase with the amount of added salt, i.e., there is an optimized amount for each salt.
Moreover, the improved hexavalent chromium-reduction efficiency and the collapse of the MZVI shell can account for improved cycle performance. The shorter time when hexavalent chromium is completely removed had led to the less Fe0 oxidation, therefore led to less FeOx to deposit on the surface of MZVI. Meanwhile, the greater collapse of the MZVI shell due to corrosion is good for cycle performance.
Conclusions
This work proposed a novel mechanism to enhance the electromagnetic coupling between the electrons of the core of Fe0 and ions in solution by adding sodium salts, namely, CH3COONa, NaCl, NaNO3, and Na2SO4, in hexavalent chromium solution. The added salts could provide more ions in the hexavalent chromium solution, providing more electromagnetic response and consequently more electromagnetically coupling. It was found that the reaction speed and cycle performance of hexavalent chromium reduction were greatly enhanced with the added salts. The experiments found that t100 decreased to 8 min from 80 min and the remaining Fe in the treated solution was far below the drinking water limit compared to that from our previous work. Moreover, the best k (0.416 (min)-1) was nearly four times larger than those without assisted salts in our previous work. Hexavalent chromium was 100% removed in 20 min with CNa even after 20 cycles, resulting mainly from the accelerating hexavalent chromium-reduction speed and accelerating collapse of the MZVI shell owing to the corrosion of MZVI with salt. The synthetic effects of added sodium salts have led to improved hexavalent chromium-reduction speed, which could not be monotonically increased with the amount of added salt, i.e., there was an optimized amount for each salt. This work may provide a very effective route for enhancing the reaction speed and cycle performance of heavy-metal reduction by MZVI.
This work proposes a viewpoint that adding salt in waste water would increase the electromagnetic coupling between the charges in solution and Fe0-core electrons which could finally activate the redox reaction. It is of great significance for treating heavy-metal wastewater and completely understanding the effect of EDL on the redox reaction at the solid-liquid interface.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Ambika S, Nambi IM (2016) Optimized synthesis of methanol-assisted nZVI for assessing reactivity by systematic chemical speciation approach at neutral and alkaline conditions. J Water Process Eng 13:107–116. https://doi.org/10.1016/j.jwpe.2016.08.011
Banerjee S, Joshi SR, Mandal T, Halder G (2017) Insight into Cr6+ hexavalent chromium reduction efficiency of Rhodococcus erythropolis isolated from coalmine waste water. Chemosphere 167:269–281. https://doi.org/10.1016/j.chemosphere.2016.10.012
Bhaumik M, Maity A, Srinivasu VV, Onyango MS (2012) Removal of hexavalent chromium from aqueous solution using polypyrrole-polyaniline nanofibers. Chem Eng J 181-182:323–333. https://doi.org/10.1016/j.cej.2011.11.088
Chen SS, Huang YC, Chen TY (2014) Reduction of hexavalent chromium in water using iron-aluminum bimetallic particles. Adv Mater Res 849:142–146. https://doi.org/10.4028/www.scientific.net/AMR.849.142
Cheung KH, Gu JD (2003) Reduction of chromate (CrO42-) by an enrichment consortium and an isolate of marine sulfate-reducing bacteria. Chemosphere 52:1523–1529. https://doi.org/10.1016/S0045-6535(03)00491-0
Dalal U, Reddy SN (2019) A novel nano zero-valent iron biomaterial for chromium (Cr6+ hexavalent chromium to Cr3+) reduction. Environ Sci Pollut Res 26:10631–10640. https://doi.org/10.1007/s11356-019-04528-0
Guo J, Kang Y, Feng Y (2017) Bioassessment of heavy metal toxicity and enhancement of heavy metal removal by sulfate-reducing bacteria in the presence of zero valent iron. J Environ Manag 203:278–285. https://doi.org/10.1016/j.jenvman.2017.07.075
He JF, Zhai WJ, Wang SF, Wang YZ, Li W, He GN, Hou XH, Liu JM, He QY (2021) Persistently high Cr6+ hexavalent chromium removal rate of centi-sized iron turning owing to tribocatalysis. Mater Today Phys 19:100408. https://doi.org/10.1016/j.mtphys.2021.100408
Liu F, Lu YM, Chen HH, Liu YG (2003) Removal of Cr6+ hexavalent chromium from groundwater using zerovalence iron in the laboratory. Chem Speciat Bioavailab 14(1-4):75–77. https://doi.org/10.3184/095422902782775290
Liu PS, Cai WP, Li ZG, Zeng HB, Hu JL (2010) Optical study of the reduction of hexavalent chromium by iron-based nanoparticles. J Nanosci Nanotechnol 10:5389–5392. https://doi.org/10.1166/jnn.2010.1933
Nguyen QA, Kim B, Chung HY, Nguyen AQK, Kim J, Kim K (2021) Reductive transformation of hexavalent chromium by ferrous ions in a frozen environment: Mechanism, kinetics, and environmental implications. Ecotoxicol Environ Saf 208:111735. https://doi.org/10.1016/j.ecoenv.2020.111735
Saha B, Orvig C (2010) Biosorbents for hexavalent chromium elimination from industrial and municipal effluents. Coord Chem Rev 254:2959–2972. https://doi.org/10.1016/j.ccr.2010.06.005
Saha R, Nandi R, Saha B (2011) Sources and toxicity of hexavalent chromium. J Coord Chem 64:1782–1806. https://doi.org/10.1080/00958972.2011.583646
Saha R, Mukherjee K, Saha I, Ghosh A, Ghosh SK, Saha B (2013a) Removal of hexavalent chromium from water by adsorption on mosambi (Citrus limetta) peel. Res Chem Intermed 39:2245–2257. https://doi.org/10.1007/s11164-012-0754-z
Saha R, Saha I, Nandi R, Ghosh A, Basu A, Ghosh SK, Saha B (2013b) Application of Chattim tree (devil tree, Alstonia scholaris) saw dust as a biosorbent for removal of hexavalent chromium from contaminated water. 91, 814-821 https://doi.org/10.1002/cjce.21703
Saha R, Saha B (2014) Removal of hexavalent chromium from contaminated water by adsorption using mango leaves (Mangiferaindica). Desalin Water Treat 52:1928–1936. https://doi.org/10.1080/19443994.2013.804458
Singh R, Dong HL, Liu D, Zhao LD, Marts AR, Farquhar E, Tierney DL, Almquist CB, Briggs BR (2015) Reduction of hexavalent chromium by the thermophilic methanogen Methanothermobacter thermautotrophicus. Geochim Cosmochim Acta 148:442–456. https://doi.org/10.1016/j.gca.2014.10.012
Sun L, Zhang LD, Liang CH, Yuan ZG, Zhang Y, Xu W, Zhang JX, Chen YZ (2011) Chitosan modified Fe0 nanowires in porous anodic alumina and their application for the removal of hexavalent chromium from water. J Mater Chem 21:5877. https://doi.org/10.1039/C1JM10205B
Sun Q, Hu XL, Zheng SL, Sun ZM (2015) Influence of calcination temperature on the structural, adsorption and photocatalytic properties of TiO2 nanoparticles supported on natural zeolite. Powder Technol 274:88–97. https://doi.org/10.1016/j.powtec.2014.12.052
Velegraki G, Miao JW, Drivas C, Liu B, Kennou S, Armatas GS (2018) Fabrication of 3D mesoporous networks of assembled CoO nanoparticles for efficient photocatalytic reduction of aqueous Cr (VI). Appl Catal B 221:635–644. https://doi.org/10.1016/j.apcatb.2017.09.064
Zhang C, Hutter J, Sprik M (2019) Coupling of surface chemistry and electric double layer at TiO2 electrochemical interfaces. J Phys Chem Lett 10:3871–3876. https://doi.org/10.48550/arXiv.1902.00802
Zhu SS, Huang XC, Wang DW, Wang L, Ma F (2018) Enhanced hexavalent chromium removal performance and stabilization by magnetic iron nanoparticles assisted biochar in aqueous solution: Mechanisms and application potential. Chemosphere 207:50–59. https://doi.org/10.1016/j.chemosphere.2018.05.046
Funding
This work was supported by the National Natural Science Foundation of China (Grant No.51972122 and 51672090).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Junfeng He. Investigation was performed by Yuheng Liang. Resources was performed by Hao Huang. Methodology was performed by Wangjian Zhai. The first draft of the manuscript was written by Junfeng He and the edited draft of the manuscript was written by Junfeng He and Qinyu He. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to publish
Not applicable.
Consent to participate
Not applicable.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Responsible Editor: Ioannis A. Katsoyiannis
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 412 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
He, J., Liang, Y., Huang, H. et al. Improved reduction efficiency, cycling performance, and removal rate of hexavalent chromium by adding water-soluble salts. Environ Sci Pollut Res 30, 113553–113560 (2023). https://doi.org/10.1007/s11356-023-30138-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-30138-y







 ), with CNa and stirring (
), with CNa and stirring (
 )
)

