Abstract
Persistent organic pollutants (POPs) including chlorophenols (CPs) are increasing in water effluents, creating serious problems for both aquatic and terrestrial lives. Several research attempts have considered the removal of CPs by functionalised nanomaterials as adsorbents and catalysts. Besides the unique crystal structure, spinel ferrite nanomaterials (SFNs) own interesting optical and magnetic properties that give them the potential to be utilised in the removal of different types of CPs. In this review, we highlighted the recent research work that focused on the application of SFNs in the removal of different CP substances based on the number of chlorine atom attached to the phenolic compound. We have also discussed the structure and properties of SFN along with their numerous characterisation tools. We demonstrated the importance of identifying the structure, surface area, porosity, optical properties, etc. in the efficiency of the SFN during the CP removal process. The reviewed research efforts applied photocatalysis, wet peroxide oxidation (WPO), persulfate activated oxidation and adsorption. The studies presented different paths of enhancing the SFN ability to remove the CPs including doping (ion substitution), oxide composite structure and polymer composite structure. Experimental parameters such as temperature, dosage of CPs and SFN structure have shown to have a major effect in the CP removal efficiency. More attention is needed to investigate the different properties of SFN that can be tailored through different techniques and expected to have major role in the removal mechanism of CPs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The scarcity of water resources that coeval with water pollution emerged as a crisis threatening environmental ecosystems and human health (Alexandratos et al. 2019)(Mir et al. 2022). Along with the excessive industrial activities worldwide, water effluents become highly polluted with different types of organic and inorganic substances. Inorganic pollutants are mainly heavy metals ions such as lead, cadmium, chromium, copper and arsenic and also acids. In the other hand, most of organic pollutants that found in water are called persistent organic pollutants (POPs) that are extremely stable and resistant to degradation in nature (Nair and Kurian 2017b). Their molecules are easily transporting through water and air and tend to accumulate in fatty tissue, creating a serious intimidation to quality of life in Earth (Fei et al. 2022). POPs are related to significant health disorders including hypersensitivity, allergies, cancers and disease of endocrine, immune, reproductive, central and peripheral nervous systems (Sahoo and Prelot 2020) (Wang and Zhang 2020) (Mota et al. 2021). They are also causing abnormalities in land and sea wildlife species (Zhao et al. 2019) (Tkaczyk et al. 2020). POPs include organochlorine pesticides (OCPs) (Bersuder et al. 2020), polychlorinated biphenyls (PCBs) (Castiglioni et al. 2022) and polybrominated diphenyl ethers (PBDEs) (Rodriguez-Narvaez et al. 2019). The existence of a conventional water remediation tool of POPs is still a challenging issue; hence, researchers are investigating different and efficient pathways to eliminate POPs. The functionalisation of nanomaterials (NMs) adsorbents and catalysts is one of the main potential and highly effective pathways (Fei et al. 2022).
NMs are grabbing a great interest of researchers around the world due to their small size (1 to 100 nm), high surface area and enhanced mechanical, optical and magnetic properties which make them more effective in several reactions and applications (Villaseñor and Ríos 2018). Most materials are powerful and efficacious in their nanoscale as metal oxides (Huang et al. 2020), iron oxides (Shah et al. 2021), graphene oxides (Lim et al. 2020) and carbon-based compounds (Z. Duan et al. 2020). Therefore, they are applied widely in medical applications such as controlled drug delivery (Hareendran et al. 2022) and diagnostic tools (Khizar et al. 2020). NMs are also functionalised effectively in energy harvesting (Bandala and Berli 2019), environmental sensing (Afzal et al. 2020) and water treatment applications (Chakhtouna et al. 2021).
Among NMs, spinel ferrites nanoparticles (SFNs) raised as promising and efficient oxides due to their low cost, low toxicity, easy and various synthesis methods. SFNs can be produced in different sizes and morphologies (Kefeni and Mamba 2020; Rashdan and Hazeem 2020; Y. jia Sun et al. 2017). They are well-known because of their improved mechanical structure (Manasa et al. 2018), high stability (Swathi et al. 2021), magnetic behaviour (Kmita et al. 2021) and optical responsiveness (Jyothish and Jacob 2021). Accordingly, SFNs have shown great effects in different applications and in particular the removal of different pollutants from wastewater (Nambikkattu et al. 2020; Tatarchuk et al. 2020b; Vinosha et al. 2022; Xiang et al. 2020; You et al. 2021). Recent research work considered SFNs, doped SFNs and SFN composites in the removal of heavy metals and organic pollutants through adsorption and oxidative photocatalytic processes. Adsorption process depends on the surface interaction between the adsorbent and the pollutant due to possible physical and chemical sorption (Tatarchuk et al. 2021b). Differently, oxidation process involved a series of chemical reactions caused by the electrons and holes that produced when the SFN atoms are excited. These chemical series depend on the generation of different radicals, called reactive oxygen species (ROS), in the aqueous solution that can degrade the organic compounds into less harmful molecules (Ariza-Tarazona et al. 2020). For instance, ZnFe2O4 nanoparticles has been applied efficiently in the degradation of methylene orange and methylene blue organic dyes under visible irradiation in seawater (Al-Najar et al. 2022) while Mg-doped ZnFe2O4 were applied to adsorb Cr+6 and Ni2+ ions from wastewater samples with maximum adsorption capacity of 30.49 g/mg (Tatarchuk et al. 2021a). NiFe2O4, CoFe2O4 and MnFe2O4 nanoparticles were also used for successful adsorptive removal of Cr+6, Pb2+, Cd2+ and Zn2+ from wastewater (Asadi et al. 2020; Khoso et al. 2021). In addition, polymer @ SFN composites are functionalised as heterogenous structures that provide higher surface area and more electron-hole yield that assist the oxidation process. Nickle-zinc spinel ferrite linked to graphene oxides was recently applied as visible light driven photocatalyst (Javed et al. 2019) as well as zinc-cobalt ferrite and silver halide composite (Chnadel et al. 2020). Moreover, SFNs have shown great effect in degradation and adsorption of other complex chemical structures such as antibiotics (Chakhtouna et al. 2021; Y. Xiang et al. 2020) and phenolic compounds (Camacho-González et al. 2019; Othman et al. 2019). Recently, some research work investigated the effect of SFNs and their composites on the removal of chlorinated phenolic compounds, chlorophenols (CPs), which are very stable POPs. The potential of applying SFNs in removing such persistent molecules is not only because of their outstanding outcome in water remediation process, it is because of the uniqueness of the SFNs’ structure that can be easily optimised to meet the efficiency, economic and environmental considerations of the process.
In this review, we present SFNs as one of the sustainable and environmental materials that can be utilised efficiently in water remediation applications. We shall discuss the unique structure and controllable properties of the SFNs and the different experimental aspects affecting their efficiency in the removal of CPs from aqueous solutions.
Chlorophenols
Chlorophenols (CPs) are one of the significant classes of POPs that have been used widely in different industrial products including paper, textiles, pesticides, pharmaceuticals and dyes (Garba et al. 2019). They are formed by adding chlorine atoms to the phenol structure. There are 19 possible combinations that settle the chlorine atoms within the phenol structure depending on the number and the position of attached chlorine atoms. Figure 1 shows some examples of the possible CPs’ structure. All of these types are considered dangerous and toxic as they are related to several health and environmental risks (X. Duan et al. 2019) and they are found in air (Kayan et al. 2021), surface water (Gallego et al. 2018), ground water, soil, urine (Kallawar et al. 2021) and in animals’ bodies (Zada et al. 2021). As per the US Environmental Protection Agency and WHO, the amount of CPs must be lower than 0.5 μg/L in drinking water and 100 μg/L in industrial wastewater (Abu-Alsoud and Bottaro 2021). The toxicity of CPs is affected by the temperature (higher temperature increase inhale process), pH (increase in low pH) and sunlight which makes them occur in variable forms in nature (Zada et al. 2021).
Furthermore, CPs such as 2,4-dichlorophenol are highly transportable and persistent in water, and it is very difficult to be degraded due to its chemical stability. Reports considered them to be carcinogenic and endocrine-disrupting chemicals. They can get through food chain, causing serious harms to human health, animals and other species (Nair and Kurian 2017a). Reports also confirmed that CPs can cause serious damage to the human nervous system (Choi et al. 2019) and to the gene expression and pancreas of zebra fish embryos (Wilson et al. 2023). Most of CPs are found in water as a result of diffused pesticides; however, some CP compounds such as tris(4-chlorophenyl) methanol (TCPMOH) are found in water without a certain source which makes them very dangerous, and applying more toxicity assays is essential. Traces of TCPMOH have been found in human breast milk, which creates a great risk for developing infants (Navarrete et al. 2021). Therefore, researchers are investigating different ways of removing these hazardous compounds from different mediums.
Specifying aqueous medium, there are several techniques that applied to remediate wastewater from CPs including adsorption (Garba et al. 2019; Rangabhashiyam et al. 2022; Rezaei et al. 2021), filtration (Kim et al. 2022; Kotlhao et al. 2019), coagulation (Matin et al. 2020) and oxidation (Choi et al. 2019; Jiang et al. 2022; Kavitha et al. 2021; W. Li et al. 2021; Soltani and Lee 2017). Adsorption and oxidation are mainly governed by functional materials that attach with the pollutants or host the oxidation process on their surfaces. In the following section, we will discuss the structure and properties of SFNs that make them good candidates for CPs’ removal applications.
Spinel ferrites: structure and properties
The spinel ferrite structure has a formula of MFe2O4, (M = Co, Ni, Mn, Mg, Cu and Zn) and a face-centred cubic (fcc) crystal where oxygen and other metal ions are positioned in tetrahedral (A) and octahedral (B) sites, as shown in Fig. 2. In a single ferrite unit cell, there are a total of 64 A sites and 32 B sites, while the cations only occupy 8 and 16 position respectively. Thus, the positions of metals within the A and B sites of the ferrite structure own different affinities depending on crystal size, energy levels and even the synthesis route (Aisida et al. 2020a; Al Maashani et al. 2020; Bhushan Das et al. 2021) The cation distribution of the divalent and trivalent ions in a few representative spinel ferrites is shown in Table 1.
Crystal structure of spinel ferrites, the diagram reproduced from Hwang et al. (2020)
SFNs are well-known for the variety, easiness and low cost of their synthesis routes. They can be synthesised through different chemical routes including co-precipitation, sol-gel, microemulsion, solvo(hydro)thermal method and template method template. Physical routes such as microwave assisted and mechanical milling were also applied (Soufi et al. 2021). Applying different routes led to different size and shapes of SFNs including spheres (Yong Lee et al. 2022), rods (Sarac 2020), tubes (Maleki et al. 2019), think films (Soe et al. 2020) and flowers (Sanna Angotzi et al. 2020). The hierarchal structure of the ZnFe2O4 composite has also shown to improve its photocatalysis efficiency (Gu et al. 2020). Also, NiFe2O4 flower-like 3D design grains were synthesised through facile hydrothermal approach (B. Xiang et al. 2017), providing a great surface area that allows high adsorption for water pollutants. Budhiraja et al. (2019) have reported a strategy to synthesise different shapes of ZnFe2O4 whereas spheres, rods and flowers were successfully synthesised by applying certain chemical routes. Consequently, this results varies the morphology, band properties, surface area and also magnetic properties of the ZnFe2O4 structure (Budhiraja et al. 2019).
Furthermore, the size of SFNs showed to be sensitive to the synthesis route and its parameter conditions. As size is a very critical factor within the nonorange, researchers have been investigating the effect of the synthesis parameters of the SFNs on their size, along with their shape. For instance, the annealing temperature has a direct impact on the crystal growth and hence the grain size of the ferrite structure. As the annealing temperature increases, the SFN crystal grows, causing different cation locations within the unique cubic structure and accordingly affecting their magnetic and optical properties (Al-Najar et al. 2022; Amir et al. 2018). Moreover, other factors such as pH tuning and ammonia addition caused considerable variation in the SFNs’ grain size that led to different magnetisation and absorption values (Ait Kerroum et al. 2019; Al-Najar et al. 2016).
When the size of SFNs is varied, exchange interaction occurs between the valence electrons of cations located at A and B sites. Consequently, the cation composition and their distributions over A and B sites influence the optical, electrical, mechanical and magnetic properties of the spinel (Algarou et al. 2020; Fayazzadeh et al. 2020; Rahman et al. 2021; Soufi et al. 2021; Xie et al. 2021) Therefore, SFNs own unique structure and controllable properties that have been utilised in wide range of applications including medicine (Somvanshi et al. 2020)(Bououdina et al. 2019), environment (Taqvi et al. 2022) and industry (B. Verma and Balomajumder 2020b).
Considering environmental applications, SFNs were applied widely in sensors (Akhlaghi and Najafpour-Darzi 2021) (Wu et al. 2019a) and wastewater remediation from different pollutants (Mmelesi et al. 2021). Highlighting the water pollution issue, recent research work has shown a great involvement of SFNs in the removal of different pollutants. SFNs own high surface area, high stability and good optical properties which makes them suitable candidates for adsorption (Tatarchuk et al. 2021b) (Niu et al. 2020)(Narayana et al. 2021) and oxidation processes (Kefeni and Mamba 2020)(Ismael 2021)(Park et al. 2019). (Soufi et al. 2021). SFNs can be utilised as enhanced pure phase structure (Swathi et al. 2021) or as a part of composite which involves metal oxides (Tatarchuk et al. 2020a), graphene oxides (Park et al. 2019), carbon nanotubes (B. Verma and Balomajumder 2020a), biochars (Xiang et al. 2021) and polymers (Aisida et al. 2020b).
For instance, pure ZnFe2O4 was applied to degrade dyes under harsh environmental conditions by the aid of solar irradiation (Al-Najar et al. 2022). Also, green synthesised CoFe2O4 and ZnFe2O4 were used to degrade bisphenol under sunlight (Rani and Shanker 2020). MgFe2O4 nanoparticles were used as efficient adsorbent for Mn2+, Co2+, Ni2+ and Cu2+ metal ions (Ivanets et al. 2021a). Moreover, CoFe2O4@TiO2 nanoparticles were successfully applied to adsorb Congo red dye (Tatarchuk et al. 2020a). In addition, graphene oxide-based NiFe2O4 (Narayana et al. 2021) and MnFe2O4 (M. Verma et al. 2020) showed great ability to adsorb Pb(II) ions. Based on these vast applications on organic and inorganic pollutants removal, SFN has raised as a potential structure to eliminate more persistent pollutants such as CPs.
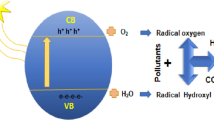
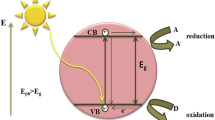
As mentioned before, SFNs are good candidates for photocatalysis applications. Photocatalysis is a physiochemical process that depends on semiconductor materials to initiate a series of chemical reactions that cause degradation of POPs. As shown in Fig. 3, the semiconductor atoms consisted of optical energy gap (Eg) that exists between the conduction band (CB) and valance band (VB). The amount of the Eg controls the absorption properties of the material, as the absorbed radiation should be more or equal to the energy gap of the material. When the radiation is absorbed, the electrons will be excited from VB to CB, as shown in Fig. 3, leaving holes (h+) in the CB while the VB will contain the electrons. The flow of these electrons and the abundancy of holes are considered as the trigger of the oxidation process within the water molecules that eventually causes different possible reactions that led to pollutant degradation.
Photocatalysis mechanism by SFN structure (Kefeni and Mamba 2020)
The efficiency of the photocatalysis process in removing pollutants depends on the amount of energy absorbed by the material as well as the number of electrons and holes generated. The recombination between the electrons and holes is the main cause of the photocatalysis suppression. Therefore, different approaches are applied to tune the energy levels within the materials to make them absorb the desired radiation as well as maintain the abundancy electron-hole pairs.
Considering the case of SFN, their relatively narrow energy gap can absorb larger irradiation spectrum including visible light. Figure 4 shows the optical band gaps of SFNs and other different semiconductors that can be functionalised efficiently in photocatalysis applications. Among them, TiO2 emerged as one of the most effective and widely use photocatalysis. We can notice here that it owns the largest bandgap of around 3.2 eV which means that electrons in its atoms can be excited from the CB to the VB with an amount of energy larger than 3.2 eV which corresponds to the UV region (3 to 124 eV). Therefore, TiO2 shows outstanding photocatalysis performance under UV irradiation.
Energy gaps for selected metal oxides and spinel ferrites, the diagram reproduced from Casbeer et al. (2012)
It is very important to note that the energy of electrons coming out from this reaction will carry at least 3.2 eV, which is the minimum excitation energy of TiO2. This gives the TiO2 good ability to degrade organic compounds as the dissociation energies of most organic compounds are in the range of 1 to 2 eV (Khatymov et al. 2003; Vannatta et al. 2020). However, solar light cannot be applied efficiently to activate TiO2 nanoparticles. This is because most of solar spectrum lay in the visible light range with wavelength of (0.4–0.7 μm, corresponding to energy range of 2–2.75 eV). Hence, it is difficult to activate the TiO2 bandgap through solar spectrum. With the recent consideration of energy efficiency and utilizing renewable energy, SFN ferrites become good candidates for solar photocatalysis as they own smaller bandgap than TiO2. As shown in Fig. 3, CaFe2O4, MgFe2O4 and ZnFe2O4 own band gaps of 1.9, 2.18 and 1.92 eV respectively. Such a small bandgap can be excited by lower energy visible light as well as UV. This gives them the privilege of conducting more sustainable water remediation process. However, the small band gaps allow faster recombination between holes and electrons and hence; efforts are made to tune the energy levels within the ferrites to create a slower path for electrons to re-join with holes. This is mainly done through metals defects (doping), self-defects (lattice vacancies) and creating heterogenous structure (composite) (Huang et al. 2020; Sahel et al. 2016; A. Sarkar and Khan 2019; Serpone 2018).
Generally, other factors such as SFN size, surface area, porosity and surface charge affect the removal ability (Ivanets et al. 2021b). The optimisation of all these factors is necessary to approach the optimum removal to certain pollutants. For the removal of CP substances from water, higher energy is required to overcome the persistent bonds in the CP molecules; hence, most conducted research considered doping to tune the SFN lattice. Moreover, additional factors were introduced to enhance the catalytic process such as oxidation substances and applying heat.
Characterisation of SFNs is very essential to choose the optimum sample for the application and to explain the removal mechanism. Most characterisation techniques include imaging using transition and scanning electron microscopes (TEM) and (SEM), crystal structure using X-ray diffraction spectroscopy (XRD), Fourier transform infrared (FTIR), Brunauer-Emmett-Teller (BET) analysis, vibrating sample magnetron (VSM), photoluminescence (PL) and ultraviolet visible light spectroscopy (UV-vis) spectroscopy. In the following sections, we shall discuss the recent research work that applied SFN structure in the removal of CP substances.
Chlorophenol removal applying spinel ferrite nanoparticles
In spite of the limited research attempts that related the spinel ferrites to chlorophenol removal, most of these efforts have shown high removal efficiency reaching almost 100% for different types of chlorophenol compounds. Authors demonstrated the influence of the ferrite structure and the experimental parameters on the chlorophenol removal efficiency. Table 2 includes details about types of ferrites applied, type of chlorophenol, removal efficiency and the optimum experimental conditions. The following sections will discuss some notable research work that successfully applied ferrite nanostructure to remove chlorophenols from aqueous solution samples.
Mochlorophenol removal
Among different types of monochlorophenol structure, 4-chlorophenol 4-CP is the most widely type investigated in literature. Most of recent research work applied the catalytic wet peroxide oxidation (WPO) to degrade 4-CP. Kurian and Nair (2015) applied the WPO for the degradation of 4-CP using Ni-dopped zinc ferrite (NixZn1−xFe2O4 where x = 0.0, 0.25, 0.5, 0.75, 1.0) that synthesised by sol-gel auto combustion method. Their studies demonstrated the effect of the ambient parameters (temperature, catalyst dose, H2O2 dose) on the removal of 4-CP as well as the COD reduction, which is a strong indication of the pollutants’ destruction. Interestingly, samples with different doping amount demonstrated different removal rates and efficiencies. This was also shown for the COD reduction and residual amount of H2O2 in the reaction. Authors had suggested a possible reaction mechanism, as shown in Fig. 5. They proposed two pathway mechanisms based on the intermediates obtained and the attack of OH. radicals. The first pathway showed substitution of Cl by the attacked OH. forming p-hydroxyphenol or hydroquinone. The obtained hydroquinone immediately forms 1,4-benzoquinone. The formed 1,4-benzoquinone is attacked by more hydroxyl radicals forming open-chain carboxylic acid derivatives and finally forming CO2 and H2O. In the second pathway, the hydroxyl radical first attacks on the ortho position to form 4-chlorobenzene-1,2-diol, which on dichlorination forms 2-hydroxyphenol or pyrocatechol in the second step. The pyrocatechol forms 2-hydroxy-1,4-benzoquinone which, under the attack of more OH. radicals, forms CO2 and H2O.
Proposed mechanism of 4-CP degradation by H2O2 on the surface of the spinel ferrite catalyst. Copyrights are obtained from Elsevier (Kurian and Nair 2015)
Zinc ferrite dopped with nitrogen and sulphur nanostructure was applied to remove 4-CP from water through oxidation process assisted by K2S2O8. Conventionally, ZnFe2O4 nanoparticles were prepared by sol-gel method with the addition of nitrogen and sulphur atoms during the preparation (Hareendran et al. 2022). The catalysis experiment showed a major role of persulfate in comparison to the applied ZnFe2O4 catalyst. Under certain experimental conditions (temperature = 30 °C, 4-CP/persulfate ratio = 1:3, catalyst dosage = 0.1 g, 4-CP concentration = 1 g/L, time = 60 min), the 4-CP conversion was found to be 87.25% with the absence of catalyst, while no conversion was obtained in the absence of persulfate (Hareendran et al. 2022).
A previous work has showed a better role of ZnFe2O4 as a part of the nanocomposite ZnFe2O4-Fe2O3-CeO2 that is fabricated through several chemical steps including green walnut hull extract (Shafiee et al. 2017). The authors applied the fabricated nanocomposite with the aid of irradiation of 500-W halide lamp and the addition of a certain amount of H2O2 activator. Under optimised parameters, 100% of 4-CP molecules were degraded within 250 min. Such an outstanding degradation behaviour was related to heterogenous structure that provides abundant electron-hole yield that initiates oxidative radicals that break the persistent chemical bonds in the 4-CP compound with the aid of H2O2. The addition of H2O2 is very important to establish enough radical yield for the degradation process, as results revealed almost no effect when the nanocomposites were applied alone (without H2O2). Furthermore, experiments showed that the single CeO2 did not initiate any degradation, even with the addition of H2O2 while ZnFe2O4 and Fe2O3 showed considerable removal efficiency, under the same condition.
Cobalt ferrites (CoFe2O4) doped with zirconium, nitrogen and sulphur were also applied to remove 4-CP (Kavitha et al. 2021). Authors investigated the effect of different experimental parameters on the efficiency of the degradation. As previous reports confirmed, temperature and the amount of H2O2 are very important to be introduced into the ferrite structure and 4-CP reaction. In this reported study, raising temperature from 30 to 50 °C has shown to enhance the removal efficiency of Zr-dopped CoFe2O4 from 48 to 100% respectively. The reaction also was enhanced as the molar ratio of 4-CP/H2O2 was increased, reaching its optimum at 1:15. With no ferrites applied, only 23% of 4-CP were degraded while the removal efficiency increased gradually with the amount of doped cobalt ferrite, reaching its maximum at 0.5 g/L. Under these optimum conditions, 100% degradation of 4-CP was obtained within 75 min. Authors have also demonstrated the effect of Zr doping within the CoFe2O4 lattice in the reaction. As seen in Fig. 6a, b, different amounts of Zr doping (25, 50 and 75% w/w) lead to different rates of 4-CP removal as faster rates are obtained with increasing the Zr doping amount, reaching its maximum rate at pure phase of ZrFe2O4. Furthermore, radical scavenging tests suggested that the hydroxide OH. radical has a major role in the reaction. Nevertheless, the COD removal was also affected by the doping amount as Co0.5Zr0.5Fe2O4 caused the highest rate of COD removal (Kavitha et al. 2021). It is very essential to assure the conversion of 4-CP to less harmful substances in order to achieve the objective of environmental remediations to pollutants. Therefore, the doping process and varying the lattice structure of the ferrite showed to play an important role in the enhancement of the 4-CP degradation process.
Effect of Zr doping in the (a) WPO of 4-CP and (b) the removal of COD under reaction parameters: 4-CP/H2O2 1:15, temperature 50 °C, 4-CP 1 g/L, catalyst 0.5 g/L and time 75 min. Copyrights are obtained from Elsevier (Kavitha et al. 2021)
Dichlorophenol removal
Copper ferrite (CuFe2O4) was utalised with Ag3PO4, NrGO and CuFe2O4 to form a novel nanocomposite applied effveintly to degrade 2,4-dichlorophenol through photocatalysis process (Wei et al. 2021). Due to their unique optical properties and in particular the size of their optical band gap, ferrite nanoparticles own great response to visible light. The composite was synthesised through three main chemical steps that combine the three oxides together. The optical band gap calculated from the absorption function curve decreased in the composite Ag3PO4/NrGO/CuFe2O4 composite (1.44 eV) in comparison to Ag3PO4 (2.3 eV) and CuFe2O4 (2.1 eV) alone, indicating an improved visible light absorption ability. The photoluminescence spectra also showed lower emission from the composite Ag3PO4/NrGO/CuFe2O4, in comparison with the oxides separately which led to better separation to electron-hole pairs. Therefore, photocatalysis experiments reflected the optical characterisations of each material, as the Ag3PO4/NrGO/CuFe2O4 demonstrated the maximum degradation rate of the 2,4-dichlorophenol under visible light source of 250 W (Wei et al. 2021). The authors of this work proposed the mechanism shown in Fig. 7 for the degradation process. Initially, one chloride atom is removed from the 2,4-DCP structure as a result of OH radical occurrence, generating 4-chlorocatechol. The latter will be subjected to OH attack too, producing 5-chlorobenzen-1,2,4-triol. •OH radicals will further degrade the product into benzene-1,2,4,5-tetrol and eventually produce maleic acid, oxalic acid and acetic acid (Wei et al. 2021).
Proposed degradation mechanism of 2,4 DCP applying Ag3PO4/NrGO/CuFe2O4 nanocomposite under visible light. Copyrights are obtained from Elsevier (Wei et al. 2021)
In addition, different sets of CoFe2O4 were fabricated through hydrothermal method and investigated for 2,4-DCP degradation with the aid of peroxymonosulphate (PMS) (Zhou et al. 2020). The authors aimed to create iron and oxygen vacancies within the spinel structure, in order to alter their properties and enhance their ability to create radicals during the degradation process. The alteration in crystal structure was confirmed by the XRD pattern as well as the d-spaces in the TEM images that are shown in Fig. 8a, b respectively. Raman spectra revealed oxygen vacancy-related bands in the samples (604, 991 and 1024 cm−1) which indicates the existence of peroxide (O22−), and superoxide complexes (O2−), that plays a vital role in oxidation process. This was further confirmed by XPS spectra. By optimizing oxidation experiment parameters, the researchers expressed the dual role of PMS and the spinel catalyst in the degradation of 2,4-DCP. As shown in Fig. 8c, d, both catalyst dose and concentration influence the degradation process, along with pH and 2,4-DCP concentration. At optimum conditions, the degradation of 2,4-DCP reached almost 100%; however, the existence of other inorganic anions, including CO32−, HCO3−, Cl−, NO3− and SO42−, demonstrated a considerable inhibition of the degradation process. This was explained by the low oxidation potential of these anions that eradicate the oxidation potential of the radicals created by the catalyst and PMS (OH and SO4-) (Zhou et al. 2020).
a XRD spectra of FeCo2O4−x spinel. b TEM image of FeCo2O4−x spinel. c Effect of FeCo2O4−x spinel dose on the degradation of 2,4-DCP. d Effect of PMS concentration on the degradation of 2,4-DCP. Copyrights are obtained from Elsevier (Zhou et al. 2020)
Furthermore, zinc ferrite nanoparticles were prepared by sol-gel autocombustion method and doped with different amounts of cobalt (CoxZn1-xFe2O4 with x = 0.0, 0.25, 0.50, 0.75, 1.0) that were prepared by sol-gel method. It showed efficient removal of 2,4 dichlorophenol from aqueous solutions (Nair and Kurian 2017b). Along with the increase of cobalt contribution within the zinc ferrite lattice, the crystallite size was found to change considerably from 14.4 to 25.3 nm, as confirmed by XRD analysis. A typical catalysis experimental setup was conducted, where a certain amount of catalyst was introduced to the 2,4-dichlorophenol solution with addition of H2O2 and applying heat that ranges from 298 to 353 K. Further increase in temperature to 353 K resulted in a drop in reaction efficiency to 72%. Such high influence of temperature in the wet peroxide reaction was confirmed for all samples of doped CoxZn1−xFe2O4. It is very essential to emphasise the role of the nanoparticles combined with raising temperature in the reaction, as the experiments confirmed that only 21.81% of 2,4 dichlorophenol was removed in the absence of nano catalyst (Nair and Kurian 2017b). The same group have also investigated the effect of chromium-dopped ferrite in 4-CP degradation (Nair and Kurian 2018). Similarly, the temperature and H2O2 addition influenced the reaction, greatly. They considered the effect of the structure of ZnFe2O4 as different doped chromium zinc ferrite CrxZn1−xFe2O4 (x = 0.0, 0.25, 0.5, 0.75, 1.0) was applied under ambient conditions as the 4-CP and 2,4 DCP removal rate increased gradually with chromium substitution and CrFe2O4 catalyst demonstrated 100% removal. On the other hand, the study also showed that the effect of temperature is not the same for all doped composites as well as the type of pollutants (4-CP and 2,4 DCP). The study included a large set of data that investigated ambient condition of temperature, pollutant type, pollutant dose, catalyst dose and catalyst type. This revealed the complexity of the degradation reaction of such organic pollutants as it needs several factors to be achieved (Nair and Kurian 2018). Nickle-doped zinc ferrite was also investigated by the same group under the same conditions, confirming the effect of doping and ambient conditions on the WPO process (Nair and Kurian 2017a)(Kurian and Nair 2015).
Ultimately, a recent study investigated the effect of combining novel polyaniline-co-o-toluidine (POAT) with NiFe2O4 in 2,4-DCP adsorption (Fathy et al. 2022). NiFe2O4 nanoparticles were prepared through conventional chemical routs and then combined with POAT through chemical polymerisation method. The formation of the composite was confirmed through XRF, FTIR and HRTEM. NiFe2O4/PAOT nanocomposite showed enhanced removal of 2,4-DCP reaching 83% in 120 min, in comparison with only 24% for the pristine NiFe2O4. The adsorption experimental data were fitted to both Langmuir and Freundlich isotherms indicating the formation of non-ideal monolayer on the adsorbent surface. The process showed high sensitivity to the medium pH as it worked only in acidic medium (pH from 2 to 4) as shown in Fig. 9a. This was related to the abundancy of the nitrogen atoms of the amine and imine groups that created in lower pH medium. It was also related to the surface charge of the composite as higher values of charges were obtained at lower pH values, as shown in Fig. 9b. The authors suggested a possible mechanism for the adsorption as illustrated in Fig. 9c (Fathy et al. 2022)
Schematic 2D simulation for the adsorption mechanism of 2,4-DCP on NiFe2O4 nanoparticles and NiFe2O4/PAOT nanocomposite (Fathy et al. 2022)
Trichlorophenol removal
Recently, a nanocomposite including ZnFe2O4 has been applied successfully to degrade 2,4,6-TCP in water (P. Sarkar et al. 2022) The graphitic g-C3N4?/ZnFe2O4/Bi2S3 composite was prepared through a facile microwave-assisted synthesis method that combines the previously prepared ZnFe2O4/g-C3N4 (facile thermal polycondensation method) to different weights of Bi2S3 (5, 10, 15 and 20 wt%). As shown in Fig. 10a, TEM images confirmed the production of the nanocomposite as the related lattice spaces were identified in the image. All samples (along with single gCN, ZnFe2O4 and Bi2S3) were intensively characterised, and the foremost sample was chosen for the PMS mediated degradation experiment. In terms of optical properties, g-C3N4/ZnFe2O4/Bi2S3 (10 wt%) showed the strongest photocurrent response in the light radiation cycle and weakest PL intensity among samples indicating a high response to radiation and high electron hole separation which are important factors in photocatalytic reaction. BET surface area calculations also showed that g-C3N4/ZnFe2O4/Bi2S3 (10 wt%) owns the highest surface area (10.6 m2 g1) among the prepared samples which is an important factor too, as reaction active sites are expected to increase with surface area. PMS-assisted photocatalysis experiment was carried out under visible light intensity of 80 W only. As the best nanocomposite was chosen carefully in this study, an efficient result was obtained resulting in 100% removal of 2,4,6-TCP within 1 h. Typical degradation parameters were adjusted, and the reaction showed a good efficiency in tap water, groundwater and lake water as shown in Fig. 10b. This study also highlighted the importance of adjusting the PMS dose to the catalyst dose to get the optimum reaction, as excessive PMS dose may decrease the reaction efficiency (P. Sarkar et al. 2022). The authors suggested the mechanism of 2,4,6-TCP degradation with the aid of PMS and visible light on the surface of the ferrite catalyst as follows:
a TEM images of gCN/ZnFe2O4/Bi2S3 nanocomposite and its b degradation efficiency of 2,4,6-TCP in different type of aqueous solutions. c The mechanism of photocatalysis process. Copyrights obtained from Elsevier (P. Sarkar et al. 2022)
The mechanism of the photocatalysis process is also represented in Fig. 8c, d where two configurations of electron transfer are presented. Figure 10c shows dual type II heterojunction where the electrons are excited as a result of solar irradiation and takes the path from the CBs of both g-C3N4 and ZnFe2O4 to the CB of Bi2S3. Such electron path configuration prevents the electron-hole recombination that occurs in the general type heterojunction as shown in Fig. 10d. With the aid of scavenging, the dual type II heterojunction has more affinity to occur causing considerable degradation to the 2,4,6-TCP molecules (P. Sarkar et al. 2022). This study confirms the influence of heterogenous structure that involves ferrites to assure wide range of irradiation absorption that includes solar radiation where the electron-hole recombination can be reduced/prevented.
Challenges and considerations
The exiguous literature resources that discussed the effect of SFNs in the removal of CPs have shown almost similar removal approaches and considerations which will be discussed as follows:
Enhancement of SFN structure
As per our search in the literature, no work on pure phase SFNs for the removal of CPs has been reported recently. SFNs own relatively small bandgap that makes them absorb radiation with wavelengths that range within the visible light region (R. Li et al. 2020)(Khadgi and Upreti 2019)(Jahanara and Farhadi 2019) and hence can be excited easily through visible light. However, due to the small band gap, the electron hole recombination occurs faster, which lead to lower oxidation ability. Therefore, enhancing SFN structure by including it as a part of metal oxide or polymeric composite is very essential to obtain good oxidation properties (M. Verma et al. 2020)(J. Sun et al. 2020). Within the composite, the ration of the combined chemical structure has shown to alter the composite properties (Dippong et al. 2020). Moreover, researchers are adopting the doping strategy (creating lattice defects) of the SFNs by introducing another metal ion substitution within the SFN crystal, to create additional subenergy levels that provide more electron-hole separation (Yang et al. 2019). These defects are rather internal such as oxygen vacancies (Wu et al. 2019b) or by adding a certain amount of metals to create the lattice defect (Jyothish and Jacob 2021). Accordingly, the behaviour of the ferrite within the application could be altered or controlled based on the required scenario. It is very important to mention here that doping SFN has shown effects not only on the mechanism route, but also on the COD removal during the oxidation process (Kavitha et al. 2021). The unique structure of ferrite allows wide variety of structure arrangements that still have not been fully investigated experimentally and specifically, in the removal of CP application. Further research in different strategies in enhancing the SFN structure and its effect on CPs’ removal process is essential to create a platform of the optimum features of SFNs that are needed to degrade CPs. Recent reviewed studies included a remarkable SFNs’ characterisation profile; however, stronger link between the properties of SFNs and their application in CP removal is indispensable.
Ambient experimental parameters
Research attempts on SFNs for CP removal emphasised the role of ambient parameters in the efficacy of CP degradation. As shown in Table 2, detailed work of varying all ambient parameters including temperature, catalyst dose, CP dose and pH was investigated, confirming the importance of tuning all of these parameters (Kurian and Nair 2015). This indicates the complexity of achieving degradation of CPs (Barzegar et al. 2018), as even the mount of activation radicals (H2O2 and PM) has noticeable effect on the degradation process. In some research attempts, temperature seems to have a great effect during the oxidation process (Nair and Kurian 2017b), while other work did not apply any temperature, taking the advantage of other parameters such Fenton’s (Kavitha et al. 2021) and/or complex heterogenous structure (P. Sarkar et al. 2022) (Fathy et al. 2022). All the reviewed studies attempt a combination of external experimental enhancers as well as structure enhancement to ferrite chemical phase itself, as shown in Table 2.
Removal approach for different chlorophenols
Most of reported research work focused on degradation of CPs applying SFN structure through photocatalysis and oxidation process. These two processes showed great efficiencies in CP removal reaching 100%. However, additional heat, chemicals and irradiation are required to maintain robust and efficient degradation process, which is considered to be cost and energy consumable. In the other hand, very few reports considered the adsorption of CPs on the surface of SFNs (Fathy et al. 2022), though SFNs are generally well-known for their good adsorption ability in terms of surface charges and chemical groups (Amrutha et al. 2023; Lingamdinne et al. 2018; Punia et al. 2022; Xiang et al. 2021). Therefore, there is a great potential to apply SFNs, or/and composites including SFNs, as efficient adsorbents to CPs.
As shown in our review, 4-CP substance removal was mostly fulfilled through WPO route, while 2,4-CP substances were degraded through PMS activation and photocatalysis. This might be related to the mechanism of degradation of both CPs, as 4-CP depends more in OH radicals in its degradation (Hareendran et al. 2022), while 2,4-CP needs both O and OH radicals (Wei et al. 2021). The mechanism of other CP substances that can be degraded applying SFNs still needs to be investigated, as research attempts only considered 4-CP for monochlorophenols and 2,4-CP for dichlorphenols. Other oxide nanoparticles such as ZnO, TiO2, SnO2, and Fe3O4 (Chen et al. 2022; Choi et al. 2017; X. Duan et al. 2019; Kotlhao et al. 2019) were applied to degrade other types of phenols such as 2-CP and 3-CP (Soltani and Lee 2017). Thus, there is a wide research possibility in this aspect, considering that SFN composites own potential properties to remove different types of CPs.
In view of the above aspects, it seems that all of them are essential to achieve CP removal, starting from the choice of SFN structure until the strong effect of ambient parameters. Therefore, more research in this area, investigating more SFN structure, more types of CPs and more removal approaches are highly recommended to create a solid understanding to this specific research area.
Toxicity and environmental considerations
As the use of NPs is spreading into wide range of applications, the toxicity and environmental aspects become very important. Mainly, the chemical composition, morphology, size and concentration of the NPs are the main factors that affect their toxicity toward certain biological cells (Samei et al. 2019). Though most of the reviewed research efforts have described SFNs as environmentally friendly, excessive sets of cytotoxicity assays are very essential to assure safety of the synthesised SFNs that vary in morphology and chemical composition. The assays include structural and functional investigations on a certain biological cell line. As the SFNs are effective in water remediation applications, their toxicity into aquatic life is a great concern. Therefore, most of research experiments are conducted using marine organism cell line models, to provide an indication of the SFNs’ effect on marine species (Saxena et al. 2020). Toxicity assessment including growth and oxidative stress shows negligible toxicity effect of ZnFe2O4-Ag/rGO composite of the cells of Daphnia magna, a marine organism (Khadgi and Upreti 2019). Also, SnFe2O4/ZnFe2O4 structure was confirmed to be non-toxic based on the Escherichia coli bacterial growth (Wang et al. 2020). Furthermore, it is reported that different ferrites (Zn, Cr, Cu, Ni and Co) that have a particle size ranging from 30 to 50 nm caused different inhibition rates of the growth of Picochlorum sp. marine microalgae. Within a certain concentration, the toxic effect of these SFNs can be neglected (Rashdan and Hazeem 2020). The size is a very important factor too, as smaller size may cause more toxic effect. Smaller sizes of ZnFe2O4 structure (10 to 12 nm) have shown to cause more oxidation stress than larger ZnFe2O4 (20 to 25 nm). This is attributed to the ability of the smaller size particles to invade the cell membrane of the microalgae species (Al-Najar et al. 2022). Mainly, the concern is more related to the SFNs that contain heavier metallic components such as CoFe2O4 have shown to cause toxic reactions in organisms. CoFe2O4 can cause noticeable oxidative reactions in organism cells which led to cell membrane destruction as well as genotoxicity (Mmelesi et al. 2021).
In general, though some SFNs are considered as environmentally friendly, not all the SFNs can be considered as completely safe and non-toxic, and more investigations should be conducted. Aspects of size, concentration and chemical composition should be taken into account. The evaluation of the suitable SFNs for water treatment application should consider toxicity to marine and human life as an important factor along with its efficiency in removing pollutants. The size of NPs has a great role in initiating the oxidative stress within the biological cells, and hence, the size should be optimised not only for the efficient application but also for less harmfulness to environment and health.
Conclusion
Chlorophenols (CPs) are extremely hazardous and persistent organic compounds (POPs) that threat human life. Removal of such compounds is therefore very urgent and requires the fabrication of new functional nano-photocatalysts to deal accordingly. Their removal is further regarded in the presence of different soluble salts usually present in the effluents of many industries. It is therefore highly required to direct future research towards the fabrication of novel photocatalysts to deal with all these problems. Ferrite materials (spinel/inverse spinel or perovskite) have attracted significant scientific attention in heterogeneous photocatalyst research thanks to their excellent properties such as relatively small bandgaps, eco-friendly and low cost. Combination of ferrites with other photocatalysts leads to enhance photocatalytic activity performance. The synergistic effect and the efficient charge separation offered by the formation of the heterojunction structure are responsible for the photocatalytic activity improvement, resulting in increasing their photocatalytic activity. Besides, ferrite photocatalysts are suggested to be an effective photocatalyst when applied alone or with oxidants such as H2O2 in the presence of electromagnetic radiations such as UV/visible for the degradation of various organic pollutants.
In this review, we tried to elaborate the removal of different chlorophenols using ferrite nanoparticles either by photodegradation or adsorption. However, huge research is underway and every day, new literatures are introduced in different journals dealing with the different removal methods of chlorophenols from wastewater. Therefore, the day is not far away that advancement will be made in the field of photocatalysis to effectively remove toxic pollutants and safeguard environment for the future generation. The thermal and chemical stability of ferrite-based materials is less tackled, and future research should focus on ferrite stability, which will be the main challenge for the development of photocatalysts. All published articles which deal with photocatalysis application of ferrites operated at the lab scale and showed an excellent photocatalytic activity; their functional applicability on an industrial scale has not yet been published. This field, therefore, involves engineering action to establish the correct configuration of the reactor that will fit for realistic operation on an industrial scale. We hope that this review will provide a sound knowledge to the viewers about the importance of using ferrites and their role in the removal of chlorophenols in the environment.
Data availability
I hereby consent that this manuscript did not use any data.
References
Abu-Alsoud GF, Bottaro CS (2021) Porous thin-film molecularly imprinted polymer device for simultaneous determination of phenol, alkylphenol and chlorophenol compounds in water. Talanta 223(P2):121727. https://doi.org/10.1016/j.talanta.2020.121727
Afzal A, Mujahid A, Iqbal N, Javaid R (2020) Enhanced. High-Temperature (600 ° C) NO 2 Response of ZnFe 2 O 4 Nanoparticle-Based Exhaust Gas Sensors 3(2):1–14
Aisida SO, Ahmad I, Ezema FI (2020a) Effect of calcination on the microstructural and magnetic properties of PVA, PVP and PEG assisted zinc ferrite nanoparticles. Phys B Condens Matter 579:411907. https://doi.org/10.1016/j.physb.2019.411907
Aisida SO, Alnasir MH, Botha S, Bashir AKH, Bucher R, Ahmad I, Zhao T, Maaza M, Ezema FI (2020b) The role of polyethylene glycol on the microstructural, magnetic and specific absorption rate in thermoablation properties of Mn-Zn ferrite nanoparticles by sol–gel protocol. Eur Polym J 132(April):109739. https://doi.org/10.1016/j.eurpolymj.2020.109739
Ait Kerroum MA, Essyed A, Iacovita C, Baaziz W, Ihiawakrim D, Mounkachi O, Hamedoun M, Benyoussef A, Benaissa M, Ersen O (2019) The effect of basic pH on the elaboration of ZnFe 2 O 4 nanoparticles by co-precipitation method: structural, magnetic and hyperthermia characterization. J Magn Magn Mater 478:239–246. https://doi.org/10.1016/j.jmmm.2019.01.081
Akhlaghi N, Najafpour-Darzi G (2021) Manganese ferrite (MnFe2O4) nanoparticles: from synthesis to application—a review. J Ind Eng Chem 103:292–304. https://doi.org/10.1016/j.jiec.2021.07.043
Al-Najar B, Khezami L, Judith Vijaya J, Lemine OM, Bououdina M (2016) Effect of synthesis route on the uptake of Ni and Cd by MgFe2O4 nanopowders. Applied Physics A 123(1):100. https://doi.org/10.1007/s00339-016-0710-7
Al-Najar B, Younis A, Hazeem L, Sehar S, Rashdan S, Shaikh MN, Albuflasa H, Hankins NP (2022) Thermally induced oxygen related defects in eco-friendly ZnFe2O4 nanoparticles for enhanced wastewater treatment efficiencies. Chemosphere 288(P2):132525. https://doi.org/10.1016/j.chemosphere.2021.132525
Al Maashani MS, Khalaf KA, Gismelseed AM, Al-Omari IA (2020) The structural and magnetic properties of the nano-CoFe2O4 ferrite prepared by sol-gel auto-combustion technique. J Alloys Compd 817:152786. https://doi.org/10.1016/j.jallcom.2019.152786
Alexandratos SD, Barak N, Bauer D, Davidson FT, Gibney BR, Hubbard SS, Taft HL, Westerhof P (2019) Sustaining water resources: environmental and economic impact. ACS Sustain Chem Eng 7(3):2879–2888. https://doi.org/10.1021/acssuschemeng.8b05859
Algarou, N. A., Slimani, Y., Almessiere, M. A., Sadaqat, A., Trukhanov, A. V, Gondal, M. A., & Hakeem, A. S. (2020). Hard – Soft Ferrite Nanocomposites : Structure, Magnetic and Microwave Properties.
Amir M, Gungunes H, Baykal A, Almessiere MA, Sözeri H, Ercan I, Sertkol M, Asiri S, Manikandan A (2018) Effect of annealing temperature on magnetic and mössbauer properties of ZnFe2O4 Nanoparticles By Sol-Gel Approach. J Supercond Nov Magn 31(10):3347–3356. https://doi.org/10.1007/s10948-018-4610-2
Amrutha JG, Girish CR, Prabhu B, Mayer K (2023) Multi-component adsorption isotherms: review and modeling studies. In: Environmental Processes, vol 10. Springer International Publishing. https://doi.org/10.1007/s40710-023-00631-0
Ariza-Tarazona MC, Villarreal-Chiu JF, Hernández-López JM, Rivera De la Rosa J, Barbieri V, Siligardi C, Cedillo-González EI (2020) Microplastic pollution reduction by a carbon and nitrogen-doped TiO2: effect of pH and temperature in the photocatalytic degradation process. J Hazard Mater 395:122632. https://doi.org/10.1016/j.jhazmat.2020.122632
Asadi R, Abdollahi H, Gharabaghi M, Boroumand Z (2020) Effective removal of Zn (II) ions from aqueous solution by the magnetic MnFe2O4 and CoFe2O4 spinel ferrite nanoparticles with focuses on synthesis, characterization, adsorption, and desorption. Adv Powder Technol 31(4):1480–1489. https://doi.org/10.1016/j.apt.2020.01.028
Bandala ER, Berli M (2019) Engineered nanomaterials (ENMs) and their role at the nexus of food, energy, and water. Mater Sci Energ Technol 2(1):29–40. https://doi.org/10.1016/j.mset.2018.09.004
Barzegar G, Jorfi S, Zarezade V, Khatebasreh M, Mehdipour F, Ghanbari F (2018) 4-Chlorophenol degradation using ultrasound/peroxymonosulfate/nanoscale zero valent iron: reusability, identification of degradation intermediates and potential application for real wastewater. Chemosphere 201:370–379. https://doi.org/10.1016/j.chemosphere.2018.02.143
Bersuder P, Smith AJ, Hynes C, Warford L, Barber JL, Losada S, Limpenny C, Khamis AS, Abdulla KH, Le Quesne WJF, Lyons BP (2020) Baseline survey of marine sediments collected from the Kingdom of Bahrain: PAHs, PCBs, organochlorine pesticides, perfluoroalkyl substances, dioxins, brominated flame retardants and metal contamination. Mar Pollut Bull 161:111734. https://doi.org/10.1016/j.marpolbul.2020.111734
Bhushan Das S, Kumar Singh R, Kumar V, Kumar N, Kumar S (2021) Tailoring the structural, optical and multiferroic properties of low temperature synthesized cobalt ferrite nanomaterials, by citrate precursor method. Mater Today: Proc, xxxx 1–7. https://doi.org/10.1016/j.matpr.2021.04.001
Bououdina M, Al-Najar B, Falamarzi L, Judith Vijaya J, Shaikh MN, Bellucci S (2019) Effect of annealing on phase formation, microstructure and magnetic properties of MgFe2O4 nanoparticles for hyperthermia. Eur Phys J Plus 134(3):84. https://doi.org/10.1140/epjp/i2019-12485-5
Camacho-González MA, Quezada-Cruz M, Cerón-Montes GI, Ramírez-Ayala MF, Hernández-Cruz LE, Garrido-Hernández A (2019) Synthesis and characterization of magnetic zinc-copper ferrites: antibacterial activity, photodegradation study and heavy metals removal evaluation. Mater Chem Phys 236. https://doi.org/10.1016/j.matchemphys.2019.121808
Casbeer E, Sharma VK, Li XZ (2012) Synthesis and photocatalytic activity of ferrites under visible light: a review. Sep Purif Technol 87:1–14. https://doi.org/10.1016/j.seppur.2011.11.034
Castiglioni M, Rivoira L, Ingrando I, Meucci L, Binetti R, Fungi M, El-Ghadraoui A, Bakari Z, Del Bubba M, Bruzzoniti MC (2022) Biochars intended for water filtration: a comparative study with activated carbons of their physicochemical properties and removal efficiency towards neutral and anionic organic pollutants. Chemosphere 288(P2):132538. https://doi.org/10.1016/j.chemosphere.2021.132538
Chakhtouna H, Benzeid H, Zari N, Bouhfid R (2021) Functional CoFe2O4-modified biochar derived from banana pseudostem as an efficient adsorbent for the removal of amoxicillin from water. Sep Purif Technol 266(March):118592. https://doi.org/10.1016/j.seppur.2021.118592
Chen Z, Yao J, Ma B, Liu B, Kim J, Li H, Zhu X, Zhao C, Amde M (2022) A robust biocatalyst based on laccase immobilized superparamagnetic Fe3O4@SiO2–NH2 nanoparticles and its application for degradation of chlorophenols. Chemosphere 291. https://doi.org/10.1016/j.chemosphere.2021.132727
Chnadel N, Dutta V, Sharma S, Raizada P, Hosseini-Bandegharaei A, Kumar R, Singh P, Thakur VK (2020) Z-scheme photocatalytic dye degradation on AgBr/Zn(Co)Fe2O4 photocatalysts supported on nitrogen-doped graphene. Mater Today Sustain 9:100043. https://doi.org/10.1016/j.mtsust.2020.100043
Choi KH, Min J, Park SY, Park BJ, Jung JS (2019) Enhanced photocatalytic degradation of tri-chlorophenol by Fe 3 O 4 @TiO 2 @Au photocatalyst under visible-light. Ceram Int 45(7):9477–9482. https://doi.org/10.1016/j.ceramint.2018.09.104
Choi KH, Park SY, Park BJ, Jung JS (2017) Recyclable Ag-coated Fe3O4@TiO2 for efficient photocatalytic oxidation of chlorophenol. Surf Coat Technol 320:240–245. https://doi.org/10.1016/j.surfcoat.2017.01.029
Dippong T, Cadar O, Deac IG, Lazar M, Borodi G, Levei EA (2020) Influence of ferrite to silica ratio and thermal treatment on porosity, surface, microstructure and magnetic properties of Zn0.5Ni0.5Fe2O4/SiO2 nanocomposites. J Alloys Compd 828:154409. https://doi.org/10.1016/j.jallcom.2020.154409
Duan X, Sui X, Wang W, Bai W, Chang L (2019) Fabrication of PbO2/SnO2 composite anode for electrochemical degradation of 3-chlorophenol in aqueous solution. Appl Surf Sci 494:211–222. https://doi.org/10.1016/j.apsusc.2019.07.161
Duan Z, Zhang W, Lu M, Shao Z, Huang W, Li J, Li Y, Mo J, Li Y, Chen C (2020) Magnetic Fe3O4/activated carbon for combined adsorption and Fenton oxidation of 4-chlorophenol. Carbon 167:351–363. https://doi.org/10.1016/j.carbon.2020.05.106
Fathy MA, Kamel AH, Hassan SSM (2022) Novel magnetic nickel ferrite nanoparticles modified with poly(aniline- co-o -toluidine) for the removal of hazardous 2,4-dichlorophenol pollutant from aqueous solutions. RSC Adv 12(12):7433–7445. https://doi.org/10.1039/d2ra00034b
Fayazzadeh S, Khodaei M, Arani M, Mahdavi SR, Nizamov T, Majouga A (2020) Magnetic Properties and magnetic hyperthermia of cobalt ferrite nanoparticles synthesized by hydrothermal method. J Supercond Nov Magn. https://doi.org/10.1007/s10948-020-05490-6
Fei L, Bilal M, Qamar SA, Imran HM, Riasat A, Jahangeer M, Ghafoor M, Ali N, Iqbal HMN (2022) Nano-remediation technologies for the sustainable mitigation of persistent organic pollutants. Environ Res 211:113060. https://doi.org/10.1016/j.envres.2022.113060
Gallego A, Laurino Soulé J, Napolitano H, Rossi SL, Vescina C, Korol SE (2018) Biodegradability of chlorophenols in surface waters from the urban area of Buenos Aires. Bull Environ Contam Toxicol 100(4):541–547. https://doi.org/10.1007/s00128-018-2300-1
Garba ZN, Zhou W, Lawan I, Xiao W, Zhang M, Wang L, Chen L, Yuan Z (2019) An overview of chlorophenols as contaminants and their removal from wastewater by adsorption: a review. J Environ Manag 241:59–75. https://doi.org/10.1016/j.jenvman.2019.04.004
Gu Y, Teng G, Jin X, Wang L, Qiang Z, Ma W, Zhang C (2020) Shape-Controlled Synthesis of coral-like ZnO/C-ZnFe2O4 hierarchical structures and their improved photocatalytic antibacterial efficiency under visible light illumination. Ind Eng Chem Res 59(24):11219–11231. https://doi.org/10.1021/acs.iecr.0c00939
Hareendran A, Dais E, Shinoy D, Srikripa S, Shibu GM, Kurian M (2022) Nitrogen- and sulfur-doped zinc ferrite nanoparticles as efficient heterogeneous catalysts in advanced oxidation processes. J Phys Chem Solids 161:110398. https://doi.org/10.1016/j.jpcs.2021.110398
Huang Y, Yu Y, Yu Y, Zhang B (2020) Oxygen vacancy engineering in photocatalysis. Solar RRL 4(8):1–14. https://doi.org/10.1002/solr.202000037
Hwang JA, Choi M, Shin HS, Ju BK, Chun MP (2020) Structural and magnetic properties of NiZn ferrite nanoparticles synthesized by a thermal decomposition method. Appl Sci (Switzerland) 10(18). https://doi.org/10.3390/APP10186279
Ismael M (2021) Ferrites as solar photocatalytic materials and their activities in solar energy conversion and environmental protection: a review. Sol Energy Mater Sol Cells 219:110786. https://doi.org/10.1016/j.solmat.2020.110786
Ivanets A, Prozorovich V, Kouznetsova T, Dontsova T, Yanushevska O, Hosseini-Bandegharaei A, Srivastava V, Sillanpää M (2021a) Effect of Mg2+ ions on competitive metal ions adsorption/desorption on magnesium ferrite: mechanism, reusability and stability studies. J Hazard Mater 411:1–9. https://doi.org/10.1016/j.jhazmat.2020.124902
Ivanets A, Prozorovich V, Roshchina M, Kouznetsova T, Budeiko N, Kulbitskaya L, Hosseini-Bandegharaei A, Masindi V, Pankov V (2021b) A comparative study on the synthesis of magnesium ferrite for the adsorption of metal ions: insights into the essential role of crystallite size and surface hydroxyl groups. Chem Eng J 411:128523. https://doi.org/10.1016/j.cej.2021.128523
Jahanara K, Farhadi S (2019) A magnetically separable plate-like cadmium titanate-copper ferrite nanocomposite with enhanced visible-light photocatalytic degradation performance for organic contaminants. RSC Adv 9(27):15615–15628. https://doi.org/10.1039/c9ra01968e
Javed H, Rehman A, Mussadiq S, Shahid M, Khan MA, Shakir I, Agboola PO, Aboud MFA, Warsi MF (2019) Reduced graphene oxide-spinel ferrite nano-hybrids as magnetically separable and recyclable visible light driven photocatalyst. Synth Met 254:1–9. https://doi.org/10.1016/j.synthmet.2019.05.013
Jiang T, Wang Y, Guo Z, Luo H, Zhan C, Wang Y, Wang Z, Jiang F, Chen H (2022) Bi25FeO40/Bi2O2CO3 piezoelectric catalyst with built-in electric fields that was prepared via photochemical self-etching of Bi25FeO40 for 4-chlorophenol degradation. J Clean Prod 341:130908. https://doi.org/10.1016/j.jclepro.2022.130908
Jyothish B, Jacob J (2021) Al-doped zinc ferrite nanoparticles: preparation and evaluation of thermal, structural, morphological and anticancer properties. J Alloys Compd 863. https://doi.org/10.1016/j.jallcom.2020.158352
Kallawar GA, Barai DP, Bhanvase BA (2021) Bismuth titanate based photocatalysts for degradation of persistent organic compounds in wastewater: a comprehensive review on synthesis methods, performance as photocatalyst and challenges. J Clean Prod 318:128563. https://doi.org/10.1016/j.jclepro.2021.128563
Kavitha S, Hareendran A, Kurian M (2021) Efficient degradation of 4-chlorophenol over zirconium, nitrogen and sulphur doped cobalt nanoferrite catalysts. Environ Nanotechnol , Monitor Manag 16(June):100540. https://doi.org/10.1016/j.enmm.2021.100540
Kayan I, Oz NA, Kantar C (2021) Comparison of treatability of four different chlorophenol-containing wastewater by pyrite-Fenton process combined with aerobic biodegradation: role of sludge acclimation. J Environ Manag 279:111781. https://doi.org/10.1016/j.jenvman.2020.111781
Kefeni KK, Mamba BB (2020) Photocatalytic application of spinel ferrite nanoparticles and nanocomposites in wastewater treatment: review. Sustain Mater Technol 23:e00140. https://doi.org/10.1016/j.susmat.2019.e00140
Khadgi N, Upreti AR (2019) Photocatalytic degradation of Microcystin-LR by visible light active and magnetic, ZnFe2O4-Ag/rGO nanocomposite and toxicity assessment of the intermediates. Chemosphere 221:441–451. https://doi.org/10.1016/j.chemosphere.2019.01.046
Khatymov RV, Muftakhov MV, Mazunov VA (2003) Phenol, chlorobenzene and chlorophenol isomers: resonant states and dissociative electron attachment. Rapid Commun Mass Spectrom 17(20):2327–2336. https://doi.org/10.1002/rcm.1197
Khizar S, Ahmad NM, Ahmed N, Manzoor S, Hamayun MA, Naseer N, Tenório MKL, Lebaz N, Elaissari A (2020) Aminodextran coated CoFe2O4 nanoparticles for combined magnetic resonance imaging and hyperthermia. Nanomaterials 10(11):1–16. https://doi.org/10.3390/nano10112182
Khoso WA, Haleem N, Baig MA, Jamal Y (2021) Synthesis, characterization and heavy metal removal efficiency of nickel ferrite nanoparticles (NFNs). Sci Rep 11(1):1–10. https://doi.org/10.1038/s41598-021-83363-1
Kim S, Nam SN, Jang A, Jang M, Park CM, Son A, Her N, Heo J, Yoon Y (2022) Review of adsorption–membrane hybrid systems for water and wastewater treatment. Chemosphere 286(P3):131916. https://doi.org/10.1016/j.chemosphere.2021.131916
Kmita A, Żukrowski J, Kuciakowski J, Marciszko-Wiąckowska M, Żywczak A, Lachowicz D, Gajewska M, Sikora M (2021) Effect of thermal treatment at inert atmosphere on structural and magnetic properties of non-stoichiometric zinc ferrite nanoparticles. Metall Mater Trans A Phys Metall Mater Sci 52(5):1632–1648. https://doi.org/10.1007/s11661-021-06154-3
Kotlhao K, Lawal IA, Moutloali RM, Klink MJ (2019) Antifouling properties of silver-zinc oxide polyamide thin film composite membrane and rejection of 2-chlorophenol and 2,4-dichlorophenol. Membranes 9(8). https://doi.org/10.3390/membranes9080096
Kurian M, Nair DS (2014) On the efficiency of cobalt zinc ferrite nanoparticles for catalytic wet peroxide oxidation of 4-chlorophenol. J Environ Chem Eng 2(1):63–69. https://doi.org/10.1016/j.jece.2013.11.026
Kurian M, Nair DS (2015) Heterogeneous Fenton behavior of nano nickel zinc ferrite catalysts in the degradation of 4-chlorophenol from water under neutral conditions. J Water Proc Eng 8:e37–e49. https://doi.org/10.1016/j.jwpe.2014.10.011
Li R, Hu H, Ma Y, Liu X, Zhang L, Zhou S, Deng B, Lin H, Zhang H (2020) Persulfate enhanced photocatalytic degradation of bisphenol A over wasted batteries-derived ZnFe2O4 under visible light. J Clean Prod 276:124246. https://doi.org/10.1016/j.jclepro.2020.124246
Li W, Wang Z, Liao H, Liu X, Zhou L, Lan Y, Zhang J (2021) Enhanced degradation of 2,4,6-trichlorophenol by activated peroxymonosulfate with sulfur doped copper manganese bimetallic oxides. Chem Eng J 417:128121. https://doi.org/10.1016/j.cej.2020.128121
Lim S, Park KH, Tran VH, Akther N, Phuntsho S, Choi JY, Shon HK (2020) Size-controlled graphene oxide for highly permeable and fouling-resistant outer-selective hollow fiber thin-film composite membranes for forward osmosis. J Membr Sci 118171. https://doi.org/10.1016/j.memsci.2020.118171
Lingamdinne LP, Koduru JR, Chang YY, Karri RR (2018) Process optimization and adsorption modeling of Pb(II) on nickel ferrite-reduced graphene oxide nano-composite. J Mol Liq 250:202–211. https://doi.org/10.1016/j.molliq.2017.11.174
Maleki A, Hajizadeh Z, Salehi P (2019) Mesoporous halloysite nanotubes modified by CuFe 2 O 4 spinel ferrite nanoparticles and study of its application as a novel and efficient heterogeneous catalyst in the synthesis of pyrazolopyridine derivatives. Sci Rep 9(1):1–8. https://doi.org/10.1038/s41598-019-42126-9
Manasa R, Sambhudevan S, Shankar B (2018) Mechanical and solvent transport properties of nickel ferrite embedded natural rubber nanocomposites. Mater Today: Proc 5(9):20572–20579. https://doi.org/10.1016/j.matpr.2018.06.436
Matin AA, Biparva P, Gheshlaghi M, Khosrowshahi EM, Farhadi K (2020) Monolithic mixed matrix membrane based on polyethersulfone/functionalized MWCNTs nanocomposite as an SPME fiber: application to extract chlorophenols from human urine and serum samples followed by GC-ECD. J Chromatogr B Anal Technol Biomed Life Sci 1150:122190. https://doi.org/10.1016/j.jchromb.2020.122190
Mir R, Azizyan G, Massah A, Gohari A (2022) Fossil water: last resort to resolve long-standing water scarcity? Agric Water Manag 261:107358. https://doi.org/10.1016/j.agwat.2021.107358
Mmelesi OK, Masunga N, Kuvarega A, Nkambule TT, Mamba BB, Kefeni KK (2021) Cobalt ferrite nanoparticles and nanocomposites: photocatalytic, antimicrobial activity and toxicity in water treatment. Mater Sci Semicond Process 123:105523. https://doi.org/10.1016/j.mssp.2020.105523
Mota IG, Neves RA, Nascimento SS, Maciel BL, Morais AH, Passos TS (2021) Artificial dyes: health risks and the need for revision of international regulations. Food Rev Intl 00(00):1–16. https://doi.org/10.1080/87559129.2021.1934694
Nair DS, Kurian M (2017a) Catalytic peroxide oxidation of persistent chlorinated organics over nickel-zinc ferrite nanocomposites. J Water Proc Eng 16:69–80. https://doi.org/10.1016/j.jwpe.2016.12.010
Nair DS, Kurian M (2017b) Heterogeneous catalytic oxidation of persistent chlorinated organics over cobalt substituted zinc ferrite nanoparticles at mild conditions: reaction kinetics and catalyst reusability studies. J Environ Chem Eng 5(1):964–974. https://doi.org/10.1016/j.jece.2017.01.021
Nair DS, Kurian M (2018) Chromium-zinc ferrite nanocomposites for the catalytic abatement of toxic environmental pollutants under ambient conditions. J Hazard Mater 344:925–941. https://doi.org/10.1016/j.jhazmat.2017.11.045
Nambikkattu J, Kaleekkal NJ, Jacob JP (2020) Metal ferrite incorporated polysulfone thin-film nanocomposite membranes for wastewater treatment. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-020-08024-8
Narayana PL, Lingamdinne LP, Karri RR, Devanesan S, AlSalhi MS, Reddy NS, Chang Y-Y, Koduru JR (2021) Predictive capability evaluation and optimization of Pb(II) removal by reduced graphene oxide-based inverse spinel nickel ferrite nanocomposite. Environ Res 204(PA):112029. https://doi.org/10.1016/j.envres.2021.112029
Navarrete J, Wilson P, Allsing N, Gordon C, Margolis R, Schwartz AV, Cho C, Rogowski B, Topps J, George UZ, Sant KE (2021) The ecotoxicological contaminant tris(4-chlorophenyl)methanol (TCPMOH) impacts embryonic development in zebrafish (Danio rerio). Aquat Toxicol 235:105815. https://doi.org/10.1016/j.aquatox.2021.105815
Niu Z, Feng W, Huang H, Wang B, Chen L, Miao Y, Su S (2020) Green synthesis of a novel Mn–Zn ferrite/biochar composite from waste batteries and pine sawdust for Pb2+ removal. Chemosphere 252:126529. https://doi.org/10.1016/j.chemosphere.2020.126529
Othman I, Abu Haija M, Ismail I, Zain JH, Banat F (2019) Preparation and catalytic performance of CuFe2O4 nanoparticles supported on reduced graphene oxide (CuFe2O4/rGO) for phenol degradation. Mater Chem Phys 238:121931. https://doi.org/10.1016/j.matchemphys.2019.121931
Park CM, Kim YM, Kim KH, Wang D, Su C, Yoon Y (2019) Potential utility of graphene-based nano spinel ferrites as adsorbent and photocatalyst for removing organic/inorganic contaminants from aqueous solutions: a mini review. Chemosphere 221:392–402. https://doi.org/10.1016/j.chemosphere.2019.01.063
Punia P, Aggarwal RK, Kumar R, Dhar R, Thakur P, Thakur A (2022) Adsorption of Cd and Cr ions from industrial wastewater using Ca doped Ni–Zn nanoferrites: synthesis, characterization and isotherm analysis. Ceram Int 48(13):18048–18056. https://doi.org/10.1016/j.ceramint.2022.02.234
Rahman MA, Islam MT, Singh MSJ, Samsuzzaman M, Chowdhury MEH (2021) Synthesis and characterization of Mg–Zn ferrite based flexible microwave composites and its application as SNG metamaterial. Sci Rep 11(1):1–14. https://doi.org/10.1038/s41598-021-87100-6
Rangabhashiyam S, dos Santos Lins PV, de Magalhães Oliveira LM, Sepulveda P, Ighalo JO, Rajapaksha AU, Meili L (2022) Sewage sludge-derived biochar for the adsorptive removal of wastewater pollutants: a critical review. Environ Pollut 293. https://doi.org/10.1016/j.envpol.2021.118581
Rani M, Shanker U (2020) Efficient photocatalytic degradation of bisphenol A by metal ferrites nanoparticles under sunlight. Environ Technol Innov 19(203):100792. https://doi.org/10.1016/j.eti.2020.100792
Rashdan SA, Hazeem LJ (2020) Synthesis of spinel ferrites nanoparticles and investigating their effect on the growth of microalgae Picochlorum sp. Arab J Basic Appl Sci 27(1):134–141. https://doi.org/10.1080/25765299.2020.1733174
Rezaei A, Rezaei MR, Sayadi MH (2021) 3D network structure graphene hydrogel-Fe3O4@SnO2/Ag via an adsorption/photocatalysis synergy for removal of 2,4 dichlorophenol. J Taiwan Inst Chem Eng 121:154–167. https://doi.org/10.1016/j.jtice.2021.03.048
Rodriguez-Narvaez OM, Peralta-Hernandez JM, Goonetilleke A, Bandala ER (2019) Biochar-supported nanomaterials for environmental applications. J Ind Eng Chem 78:21–33. https://doi.org/10.1016/j.jiec.2019.06.008
Sahel K, Elsellami L, Mirali I, Dappozze F, Bouhent M, Guillard C (2016) Hydrogen peroxide and photocatalysis. Appl Catal B Environ 188:106–112. https://doi.org/10.1016/j.apcatb.2015.12.044
Sahoo TR, Prelot B (2020) Adsorption processes for the removal of contaminants from wastewater: the perspective role of nanomaterials and nanotechnology. In: Nanomaterials for the Detection and Removal of Wastewater Pollutants. Elsevier Inc. https://doi.org/10.1016/B978-0-12-818489-9.00007-4
Samei M, Sarrafzadeh MH, Faramarzi MA (2019) The impact of morphology and size of zinc oxide nanoparticles on its toxicity to the freshwater microalga, Raphidocelis subcapitata. Environ Sci Pollut Res 26(3):2409–2420. https://doi.org/10.1007/s11356-018-3787-z
Sanna Angotzi M, Mameli V, Cara C, Grillo V, Enzo S, Musinu A, Cannas C (2020) Defect-assisted synthesis of magneto-plasmonic silver-spinel ferrite heterostructures in a flower-like architecture. Sci Rep 10(1):1–13. https://doi.org/10.1038/s41598-020-73502-5
Budhiraja N, Kumar V, Singh SK (2019) Shape-controlled synthesis of superparamagnetic ZnFe2O4 hierarchical structures and their comparative structural, optical and magnetic properties. Ceram Int 45(1):1067–1076. https://doi.org/10.1016/j.ceramint.2018.09.286
Sarac MF (2020) Magnetic, structural, and optical properties of gadolinium-substituted Co0.5Ni0.5Fe2O4 spinel ferrite nanostructures. J Supercond Nov Magn 33(2):397–406. https://doi.org/10.1007/s10948-019-05359-3
Sarkar A, Khan GG (2019) The formation and detection techniques of oxygen vacancies in titanium oxide-based nanostructures. Nanoscale 11(8):3414–3444. https://doi.org/10.1039/c8nr09666j
Sarkar P, De S, Neogi S (2022) Microwave assisted facile fabrication of dual Z-scheme g-C3N4/ZnFe2O4/Bi2S3 photocatalyst for peroxymonosulphate mediated degradation of 2,4,6-Trichlorophenol: The mechanistic insights. Appl Catal B Environ 307:121165. https://doi.org/10.1016/j.apcatb.2022.121165
Saxena P, Sangela V, Harish. (2020) Toxicity evaluation of iron oxide nanoparticles and accumulation by microalgae Coelastrella terrestris. Environ Sci Pollut Res 27(16):19650–19660. https://doi.org/10.1007/s11356-020-08441-9
Serpone N (2018) Heterogeneous photocatalysis and prospects of TiO2-based photocatalytic DeNOxing the atmospheric environment. Catalysts 8(11). https://doi.org/10.3390/catal8110553
Shafiee MRM, Sadeghian M, Kargar M (2017) ZnFe2O4-Fe2O3-CeO2 composite nanopowder: Preparation, magnetic properties, and 4-chlorophenol removal characterizations. Ceram Int 43(16):14068–14073. https://doi.org/10.1016/j.ceramint.2017.07.142
Shah J, Jain S, Gahtori B, Sharma C, Kotnala RK (2021) Water splitting on the mesoporous surface and oxygen vacancies of iron oxide generates electricity by hydroelectric cell. Mater Chem Phys 258:123981. https://doi.org/10.1016/j.matchemphys.2020.123981
Soe T, Jityen A, Kongkaew T, Subannajui K, Sinsarp A, Osotchan T (2020) Atomic structure of cobalt doped copper ferrite thin film. Mater Today: Proc 23:752–756. https://doi.org/10.1016/j.matpr.2019.12.269
Soltani T, Lee BK (2017) Enhanced formation of sulfate radicals by metal-doped BiFeO3 under visible light for improving photo-Fenton catalytic degradation of 2-chlorophenol. Chem Eng J 313:1258–1268. https://doi.org/10.1016/j.cej.2016.11.016
Somvanshi SB, Kharat PB, Khedkar MV, Jadhav KM (2020) Hydrophobic to hydrophilic surface transformation of nano-scale zinc ferrite via oleic acid coating: Magnetic hyperthermia study towards biomedical applications. Ceram Int 46(6):7642–7653. https://doi.org/10.1016/j.ceramint.2019.11.265
Soufi A, Hajjaoui H, Elmoubarki R, Abdennouri M, Qourzal S, Barka N (2021) Spinel ferrites nanoparticles: synthesis methods and application in heterogeneous Fenton oxidation of organic pollutants—a review. Appl Surf Sci Adv 6. https://doi.org/10.1016/j.apsadv.2021.100145
Sun J, Lin X, Xie J, Zhang Y, Wang Q, Ying Z (2020) Facile synthesis of novel ternary g-C3N4/ferrite/biochar hybrid photocatalyst for efficient degradation of methylene blue under visible-light irradiation. Colloids Surf A Physicochem Eng Asp 606:125556. https://doi.org/10.1016/j.colsurfa.2020.125556
Sun Y, Diao YF, Wang HG, Chen G, Zhang M, Guo M (2017) Synthesis, structure and magnetic properties of spinel ferrite (Ni, Cu, Co)Fe2O4 from low nickel matte. Ceram Int 43(18):16474–16481. https://doi.org/10.1016/j.ceramint.2017.09.029
Swathi S, Yuvakkumar R, Kumar PS, Ravi G, Velauthapillai D (2021) Annealing temperature effect on cobalt ferrite nanoparticles for photocatalytic degradation. Chemosphere 281:130903. https://doi.org/10.1016/j.chemosphere.2021.130903
Taqvi SIH, Solangi AR, Buledi JA, Khand NH, Junejo B, Memon AF, Ameen S, Bhatti A, Show PL, Vasseghian Y, Karimi-Maleh H (2022) Plant extract-based green fabrication of nickel ferrite (NiFe2O4) nanoparticles: an operative platform for non-enzymatic determination of pentachlorophenol. Chemosphere 294:133760. https://doi.org/10.1016/j.chemosphere.2022.133760
Tatarchuk T, Mironyuk I, Kotsyubynsky V, Shyichuk A, Myslin M, Boychuk V (2020a) Structure, morphology and adsorption properties of titania shell immobilized onto cobalt ferrite nanoparticle core. J Mol Liq 297:111757. https://doi.org/10.1016/j.molliq.2019.111757
Tatarchuk T, Myslin M, Lapchuk I, Shyichuk A, Murthy AP, Gargula R, Kurzydło P, Bogacz BF, Pędziwiatr AT (2021a) Magnesium-zinc ferrites as magnetic adsorbents for Cr(VI) and Ni(II) ions removal: cation distribution and antistructure modeling. Chemosphere 270. https://doi.org/10.1016/j.chemosphere.2020.129414
Tatarchuk T, Naushad M, Tomaszewska J, Kosobucki P, Myslin M, Vasylyeva H, Ścigalski P (2020b) Adsorption of Sr(II) ions and salicylic acid onto magnetic magnesium-zinc ferrites: isotherms and kinetic studies. Environ Sci Pollut Res 27(21):26681–26693. https://doi.org/10.1007/s11356-020-09043-1
Tatarchuk T, Shyichuk A, Sojka Z, Gryboś J, Naushad M, Kotsyubynsky V, Kowalska M, Kwiatkowska-Marks S, Danyliuk N (2021b) Green synthesis, structure, cations distribution and bonding characteristics of superparamagnetic cobalt-zinc ferrites nanoparticles for Pb(II) adsorption and magnetic hyperthermia applications. J Mol Liq 328. https://doi.org/10.1016/j.molliq.2021.115375
Tkaczyk A, Mitrowska K, Posyniak A (2020) Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: a review. Sci Total Environ 717:137222. https://doi.org/10.1016/j.scitotenv.2020.137222
Vannatta PE, Ramirez DA, Velarde AR, Ali G, Kieber-Emmons MT (2020) Exceptionally high O-H bond dissociation free energy of a dicopper(II) μ-hydroxo complex and insights into the geometric and electronic structure origins Thereof. J Am Chem Soc 142(38):16292–16312. https://doi.org/10.1021/jacs.0c06425
Verma B, Balomajumder C (2020a) Magnetic magnesium ferrite–doped multi-walled carbon nanotubes: an advanced treatment of chromium-containing wastewater. Environ Sci Pollut Res 27(12):13844–13854. https://doi.org/10.1007/s11356-020-07988-x
Verma B, Balomajumder C (2020b) Synthesis of magnetic nickel ferrites nanocomposites: an advanced remediation of electroplating wastewater. J Taiwan Inst Chem Eng 112:106–115. https://doi.org/10.1016/j.jtice.2020.07.006
Verma M, Kumar A, Singh KP, Kumar R, Kumar V, Srivastava CM, Rawat V, Rao G, Kumari S, Sharma P, Kim H (2020) Graphene oxide-manganese ferrite (GO-MnFe2O4) nanocomposite: one-pot hydrothermal synthesis and its use for adsorptive removal of Pb2+ ions from aqueous medium. J Mol Liq 315:113769. https://doi.org/10.1016/j.molliq.2020.113769
Villaseñor MJ, Ríos Á (2018) Nanomaterials for water cleaning and desalination, energy production, disinfection, agriculture and green chemistry. Environ Chem Lett 16(1):11–34. https://doi.org/10.1007/s10311-017-0656-9
Vinosha PA, Vinsla JVA, Madhavan J, Devanesan S, AlSalhi MS, Nicoletti M, Xavier B (2022) Impact of dysprosium doped (Dy) zinc ferrite (ZnFe2o4) nanocrystals in photo- fenton exclusion of recalcitrant organic pollutant. Environ Res 203. https://doi.org/10.1016/j.envres.2021.111913
Wang J, Zhang M (2020) Adsorption characteristics and mechanism of bisphenol a by magnetic biochar. Int J Environ Res Public Health 17(3). https://doi.org/10.3390/ijerph17031075
Wang J, Zhang Q, Deng F, Luo X, Dionysiou DD (2020) Rapid toxicity elimination of organic pollutants by the photocatalysis of environment-friendly and magnetically recoverable step-scheme SnFe2O4/ZnFe2O4 nano-heterojunctions. Chem Eng J 379:122264. https://doi.org/10.1016/j.cej.2019.122264
Wei X, Yang X, Xu X, Liu Z, Naraginti S, Wan J (2021) Novel magnetically separable tetrahedral Ag3PO4/NrGO/CuFe2O4 photocatalyst for efficient detoxification of 2,4-dichlorophenol. Environ Res 201:111519. https://doi.org/10.1016/j.envres.2021.111519
Wilson PW, Cho C, Allsing N, Khanum S, Bose P, Grubschmidt A, Sant KE (2023) Tris(4-chlorophenyl)methane and tris(4-chlorophenyl)methanol disrupt pancreatic organogenesis and gene expression in zebrafish embryos. Birth Defects Res 115(4):458–473. https://doi.org/10.1002/bdr2.2132
Wu K, Li J, Zhang C (2019a) Zinc ferrite based gas sensors: a review. Ceram Int 45(9):11143–11157. https://doi.org/10.1016/j.ceramint.2019.03.086
Wu L, Zhang Q, Hong J, Dong Z, Wang J (2019b) Degradation of bisphenol A by persulfate activation via oxygen vacancy-rich CoFe2O4–x. Chemosphere 221:412–422. https://doi.org/10.1016/j.chemosphere.2019.01.049
Xiang B, Ling D, Lou H, Gu H (2017) 3D hierarchical flower-like nickel ferrite/manganese dioxide toward lead (II) removal from aqueous water. J Hazard Mater 325:178–188. https://doi.org/10.1016/j.jhazmat.2016.11.011
Xiang Y, Yang X, Xu Z, Hu W, Zhou Y, Wan Z, Yang Y, Wei Y, Yang J, Tsang DCW (2020) Fabrication of sustainable manganese ferrite modified biochar from vinasse for enhanced adsorption of fluoroquinolone antibiotics: effects and mechanisms. Sci Total Environ 709:136079. https://doi.org/10.1016/j.scitotenv.2019.136079
Xie F, Liu H, Bai M, Wen S, Xu F, Zhao J, Liu W (2021) Flexible LiZnTiMn ferrite/PDMS composites with enhanced magnetic-dielectric properties for miniaturized application. Ceram Int 47(1):1121–1125. https://doi.org/10.1016/j.ceramint.2020.08.228
Xu Z, Xiang Y, Zhou H, Yang J, He Y, Zhu Z, Zhou Y (2021) Manganese ferrite modified biochar from vinasse for enhanced adsorption of levofloxacin: effects and mechanisms. Environ Pollut 272:115968. https://doi.org/10.1016/j.envpol.2020.115968
Yang T, Wei J, Guo Y, Lv Z, Xu Z, Cheng Z (2019) Manipulation of oxygen vacancy for high photovoltaic output in bismuth ferrite films [Research-article]. ACS Appl Mater Interfaces 11(26):23372–23381. https://doi.org/10.1021/acsami.9b06704
Yong Lee S, Kim H, Jang H, Hwang M-J, Bong Lee K, Choi J-W, Jung K-W (2022) Fabrication of manganese ferrite (MnFe2O4) microsphere-coated magnetic biochar composite for antimonate sequestration: characterization, adsorption behavior, and mechanistic understanding. Appl Surf Sci 578:152005. https://doi.org/10.1016/j.apsusc.2021.152005
You Y, Shi Z, Li Y, Zhao Z, He B, Cheng X (2021) Magnetic cobalt ferrite biochar composite as peroxymonosulfate activator for removal of lomefloxacin hydrochloride. Sep Purif Technol 272. https://doi.org/10.1016/j.seppur.2021.118889
Zada A, Khan M, Khan MA, Khan Q, Habibi-Yangjeh A, Dang A, Maqbool M (2021) Review on the hazardous applications and photodegradation mechanisms of chlorophenols over different photocatalysts. Environ Res 195:110742. https://doi.org/10.1016/j.envres.2021.110742
Zhao W, Chen IW, Huang F (2019) Toward large-scale water treatment using nanomaterials. Nano Today 27:11–27. https://doi.org/10.1016/j.nantod.2019.05.003
Zhou Y, Zhang Y, Hu X (2020) Enhanced activation of peroxymonosulfate using oxygen vacancy-enriched FeCo2O4−x spinel for 2,4-dichlorophenol removal: singlet oxygen-dominated nonradical process. Colloids Surf A Physicochem Eng Asp 597:124568. https://doi.org/10.1016/j.colsurfa.2020.124568
Author information
Authors and Affiliations
Contributions
Hereby, I declare that the author contribution in this manuscript is as follows: Dr. Basma Al-Najar: writing the first draft of the manuscript. Prof. Ayman H Kamel: the idea, design and structure of the manuscript. Dr. Hanan Albuflasa: supervision and revision. Dr. Nicholas P. Hankins: supervision and revision.
Corresponding author
Ethics declarations
Ethics approval
I hereby consent that this manuscript is not applicable for ethical approval as it does not contain any human or animal material and data.
Consent to participate
I hereby consent that this manuscript is not applicable for consent to participate as it does not contain any human or animal material and data.
Consent for publication
I hereby consent that this manuscript is not applicable for consent for publication as it does not contain any human or animal material and data.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Angeles Blanco
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Al-Najar, ., Kamel, A.H., Albuflasa, H. et al. Spinel ferrite nanoparticles as potential materials in chlorophenol removal from wastewater. Environ Sci Pollut Res 30, 104976–104997 (2023). https://doi.org/10.1007/s11356-023-29809-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-29809-7