Abstract
Magnetic magnesium-zinc spinel ferrite Mg1 − xZnxFe2O4 (where x = 0.4, 0.6, and 0.8) was investigated as adsorbent for the efficient removal of Sr(II) ions and salicylic acid (SA) contaminants from aqueous medium. The characterization of ferrites was carried out using XRD, VSM, BET, SEM, and EDS. The surface charge of magnetic adsorbents was measured by the drift method. The determination of SA and Sr(II) ion concentrations in the solution phase was carried out by UFLC and complexometry, respectively. It was shown that varying of the Zn(II) content affected the adsorption capacities of magnesium-zinc ferrites. The increasing of zinc content from x(Zn2+) = 0.4 to x(Zn2+) = 0.6 increased the adsorption of Sr(II) ions from 50 to 65 mg/g, and then it was decreased to 36 mg/g for the sample with x(Zn) = 0.8. The Mg0.4Zn0.6Fe2O4 sample demonstrated the maximum adsorption capacity of 74 mg/g. The adsorption isotherm for Sr(II) was fitted by the Dubinin-Radushkevich, Langmuir, Freundlich, and Sips models. The adsorption kinetics of Sr(II) was analyzed by PFO, PSO, and Elovich models. The adsorption kinetics of SA was also investigated. It was demonstrated that the Mg0.2Zn0.8Fe2O4 sample exhibited 90% removal of salicylic acid from the water solutions. The results demonstrated that magnetic Mg-Zn ferrites with spinel structure are good sorbents for the removal of SA and Sr(II) ions from aqueous solution.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Magnetic materials are increasingly used in various scientific and technological processes (Sharma et al. 2017; Kothawale et al. 2019; Satheeshkumar et al. 2019; Tsay et al. 2019; Hua et al. 2020). The use of such materials in adsorption processes makes them possible to significantly simplify the complicated procedure for separating powdered adsorbents from solution using an external magnetic field (Tatarchuk et al. 2017, 2019a, d; Deepty et al. 2018). One of the environmental problems is the contamination of territories with radionuclides, originated from nuclear power plants. A characteristic feature of all nuclear power facilities is the presence of radiation sources, which under certain conditions can lead to negative effects on humans and the environment. Strontium and cesium are the often identifed species in water and soils close to the nuclear plant facilities. Strontium has high toxicity due to its ability to actively engage in the biological cycle of substances. Strontium is an analog of calcium and easily enters the metabolic processes of animals and humans (Alby et al. 2018).
One of the most promising routes for the strontium removal is adsorption technology due to its simplicity of equipment design, low energy consumption, accessibility, and high effectiveness (Ainscough et al. 2017; Mironyuk et al. 2019a, b; Mohanraj et al. 2019). Advantages of adsorption are that it allows the separation of selected compounds from solutions (Ng et al. 2018; Mohanraj et al. 2019; Zhang et al. 2020). The design and operation of adsorption equipment are relatively simple. The adsorption demonstrates high performance and favorable speed and is insensitive to toxic substances (Mohamed et al. 2020). In addition, adsorbed compounds can be recovered by desorbing agents, leaching, biological processes, and heat treatment. The use of adsorption processes is especially relevant when large volumes of liquid radioactive waste have to be cleaned (Faisal et al. 2020). For example, in the aftermath of accidents at nuclear facilities (during the release of radioactive substances into the environment), the decommissioning of nuclear power plants, the reclamation of places, contaminated by acts of nuclear terrorism, etc. adsorption processes are avaiable for this purposes. In such case, the use of magnetic adsorbents is very attractive (Reddy and Yun 2016; Tatarchuk et al. 2019e).
Non-magnetic cations are often introduced into the spinel structure in order to enhance the magnetic properties of spinels (Alla et al. 2018). For example, Mg2+ ions can be introduced into non-magnetic zinc ferrite in order to raise magnetic properties and to get stronger magnetically controlled samples. Such systems are widely studied. Magnesium-zinc ferrites with chemical formula Mg1−xZnxFe2O4 (x = 0.4–0.7) were obtained by the hydrothermal method (Tsay et al. 2019). The spinel-type core-shell Zn-Mg-Fe microspheres were synthesized by the solvothermal method and tested as photocatalysts in the degradation of 1,2-dichlorobenzene gas (o-DCB) under the sunlight (Hu et al. 2019). The solution combustion route followed by sintering has been used to magnesium-zinc ferrites with chemical formula Mg1−xZnxFe2O4 (x = 0.00…1.00, step 0.25) and crystallite sizes in the range of 47–80 nm (Choodamani et al. 2016). The Mg1 − xZnxFe2O4 nanoparticles (x = 0–0.9) with quasispherical morphology and average core diameter about 15 nm for magnetic hyperthermia applications were obtained by the sol-gel method (Reyes-Rodríguez et al. 2017). The magnesium-zinc ferrites can be obtained by other methods: auto combustion method (John and Mathew 2019), microwave-assisted sol-gel combustion method (Gore et al. 2018), co-precipitation (Zaki et al. 2015; Sharma et al. 2016; Liu et al. 2019), electrospinning technique (Ghazi et al. 2018), plasma spray (Liu et al. 2014), etc. The studies on the use of spinel ferrite nanoparticles to purify water and wastewater from organic and inorganic contaminants have proven their benefits (Kefeni et al. 2017). In particular, magnetic spinel nanoparticles are very stable and can be regenerated and reused over several cycles without losing their properties (Tatarchuk et al. 2019b). The presence of magnetic properties allows for the effective separation of solid and liquid phases under the impact of an external magnetic field, bypassing the process of filtration and/or centrifugation.
Magnetic materials based on nanoscale iron oxides are also widely used in the field of concentration and separation of various emerging contaminants from water mediums (Li 2014; Rodriguez-Narvaez et al. 2017; Sophia and Lima 2018). Among them is the salicylic acid as emerging pollutant, which is contained in the wastewater of the pharmaceutical industry and causes an urgent environmental problem (He et al. 2015). Salicylic acid and salicylates, as well as its esters (methyl salicylate) and other synthetic derivatives, have a pronounced anti-inflammatory effect (Arshadi et al. 2017). As a result, salicylic acid derivatives are widely used in medicine: sodium salicylate, salicylamide, and acetylsalicylic acid (aspirin)—as antipyretic, anti-rheumatic, anti-inflammatory, and painkillers; phenyl salicylate—as an antiseptic; and para-aminosalicylic acid—as a specific anti-tuberculosis drug. The production of these substances contributes to their contamination into the environment, mainly the aquatic environment. Salicylic acid is toxic in large doses. Thus, the removal of salicylic acid and its derivatives from the wastewater of the pharmaceutical industry is an urgent problem (Karunanayake et al. 2017; Xiao et al. 2017; Ghezzi et al. 2018; Martín et al. 2018).
In this study, we presented the results of the use of magnetic spinel ferrites with chemical composition Mg1 − xZnxFe2O4 (where x = 0.4, 0.6, and 0.8) for the removal of Sr(II) ions and salicylic from aqueous medium. Isotherm and kinetic studies for the removal of these two pollutants were also performed.
Experimental
Sample synthesis
Mg-Zn ferrites were obtained by auto-combustion sol-gel method as described in our earlier report (Tatarchuk et al. 2019c). It was noticed that the combination of fuels (urea and β-alanine) makes it possible to carry out low-temperature synthesis of spinel ferrites without additional heat treatment. In brief, the precursors were magnesium nitrate Mg(NO3)2·6Н2О, zinc acetate Zn(CH3COO)2·2Н2О, and ferric nitrate Fe(NO3)3·9Н2О (the nitrates used as oxidizers). The mixture of alanine C3H7NO2 and urea CO(NH2)2 was used as reductant and fuel. In propellant chemistry, the total valence of the metal salts precursors must be balanced by the valence of the complexing agent (fuel). The charges of the chemical elements were taking into account (V(C) = +4; V(H) = +1; V(O) = −2; V(N) = 0; V(Me) = +2 or + 3 (for Me(II) or (Me(III) respectively)) and the calculated results are shown in Table 1. The nitrates of metals were incorporated into a distilled water and stirred for half an hour. Then the fuel mixture was introduced into the solution. After homogenization, the mixture was heated until the gel-sol formation. The further heating caused ignition reaction. The obtained samples (in powder form) were hand-crushed with a pestle.
Characterization methods
X-ray diffraction analysis was carried out on the STOE STADI P diffractometer (CuKα1 radiation, 15 ≤ 2θ ≤ 130° with a step of 0.015°). The XRD patterns were refined by the Rietveld method using the pseudo-Voigt profile function using FullProf.2k (version 5.60). The morphological peculiarities of the ferrites were determined using REMMA-102-02 scanning electron microscopy (SEM) (JCS SELMI, Ukraine). The specific surface area of samples was estimated by N2 adsorption-desorption isotherms using surface area analyzer Quantachrome Autosorb, Nova 2200e, at temperature 77 K. Magnetic properties were checked using vibrating sample magnetometer (VSM) at room temperature under an applied field of ± 3 Tesla.
Adsorption studies
pHPZC determination (pH-drift method)
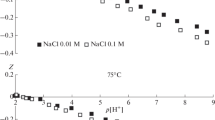
Initially, 0.1 M solutions of sodium chloride with different pH values were prepared. The pH was adjusted by adding a hydrochloric acid solution (pH < 7) or sodium hydroxide (pH > 7). Then, 75 mg of sample was added to 15 mL of each solution. The solutions were shaken at room temperature for 4 h and left for 24 h to establish equilibrium pH. The final pH was measured using a pH meter and ΔpH was plotted against pHo and then pHPZC was calculated.
Adsorption of Sr(II) ions
The adsorption of Sr(II) ions was investigated under static conditions by shaking 100 mg adsorbent with 5 mL Sr(II) solution of different concentrations: 0.001 to 0.1 M (87.6 to 8760 mg/L, respectively) for 6 h at 22 °C. The SrCl2 (Khimreaktyvy, Ukraine) was taken as source of Sr(II) ions. It was previously established that 6 h was sufficient to establish adsorption equilibrium in the system. The solutions were then decanted by magnetic separation, sampled, and the equilibrium concentration of Sr(II) was determined by a complexometric method (Harris 2007). The adsorption capacity was calculated by the following Eq.(1):
where Co and Ce are initial and equilibrium concentrations (mg/L) of Sr(II), respectively; m is the mass of adsorbent (g), and V is volume of strontium chloride solution (L).
Adsorption of salicylic acid
Salicylic acid (POCh, Gliwice, Poland) solution was first prepared in methanol (Merck, Darmstadt, Germany) due to its high solubility in methanol and then diluted in DMW from initial concentration of 100 mg/mL to 160.5 ng/mL. After that, sorbent (10 ± 0.2 mg) was placed in a separate centrifuge tube with 5 mL of the salicylic acid solution and mixed for 24 h using a mechanical shaker IntelliMixer (Elmi, Latvia). After 1, 2, 5, 10, and 24 h, the process was interrupted and a sample of 200 μL was withdrawn for liquid chromatography analysis, carried out on a Shimadzu UFLC Prominescence System (Shimadzu, Kyoto, Japan). The chromatograph consisted of a LC-20AB solvent delivery system equipped with a Rheodyne injection valve with a 20-μL loop, a fluorescence detector set at 332 and 450 nm, and a LC-Solution system software. Chromatographic separation was achieved at room temperature on a Supelco Discovery HS C18 (Supelco, Bellefonte, USA) column with the dimensions 4.6 mm I.D., 150 mm length, and 5 μm particle size. The efficiency of SA elimination from water solutions was estimated using Eqs.(1) and (2):
where C0 and Ce are the initial and equilibrium concentrations of SA (μg/L), respectively.
Results and discussion
Adsorbents characterization
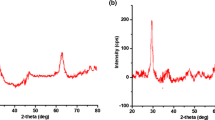
The deep characterization of magnesium-zinc ferrites has been already described in our previous paper (Tatarchuk et al. 2019c). It was shown that they have porous structure so they can be used as adsorbents for environmental remediation. Here, we reported the adsorption properties of three magnetic samples Mg1−xZnxFe2O4 (x= 0.4, 0.6, 0.8) toward Sr(II) ions and salicylic acid. The phase composition of the ferrite samples was identified by the XRD phase analysis (Fig. 1a). All diffraction peaks in the range of 15° to 130° belong to single spinel phase and corresponded to cubic spinel structure with space group Fd3m. The cell constants are shown in Table 2. The average crystallite size of the Mg1−xZnxFe2O4 samples was computed using Scherer formula (3):
where D is average crystallite size, β is line broadening in radians (FWHM) obtained from β1/2 = [(βmeasured)2−(βinstur)2]1/2, θ is Bragg angle, and λ is X-ray wavelength (λ = 0.1546 nm). The average crystallite size of the magnesium-zinc ferrite nanoparticles changed from 31 to 39 nm and are shown in Table 2.
a The XRD patterns of Mg1−xZnxFe2O4 (x = 0.4, 0.6, 0.8) samples [reprinted from Tatarchuk et al. 2019c, Copyright (2019), with permission from Elsevier]; b BET; c magnetization (MS) versus applied magnetic field (H) of the Mg1−xZnxFe2O4 samples at room temperature; d pHPZC of Mg1−xZnxFe2O4 ( x= 0.4, 0.6, 0.8) samples
It is known that the physicochemical characteristics of the adsorbent are primarily influenced by its texture and surface chemistry. The specific surface area of ferrite adsorbents was calculated using BET method (Fig. 1b). The results are presented in Fig. 1b which demonstrated that the samples have relatively small specific surface area from 6 to 11 m2/g.
The vibrational sample magnetometry was used to characterize the magnetization of the magnesium-zinc ferrites (the applied magnetic field up to 3 kOe) and results are shown in Fig. 1c. The magnetic parameters (saturation magnetization MS, remanence Mr, coercivity HC) for Mg1−xZnxFe2O4 samples are listed in Table 2. The saturation magnetization values were 40.1, 25.1, and 5.1 emu/g for Mg0.6Zn0.4Fe2O4, Mg0.4Zn0.6Fe2O4, and Mg0.2Zn0.8Fe2O4 samples, respectively. Thus, the substitution of Mg(II) ions by Zn(II) ions decreased the magnetization of ferrites. The remanence magnetization was decreased from 4.7 to 0.03 emu/g with increase in Zn content. The coercivity value was also decreased from 32.6 to 2.5 Oe with increase in Zn content. The magnitudes of experimental magnetic moment were estimated from Eq. (4):
where MS is saturation magnetization of the sample (emu/g) and M is molecular mass of the sample (mol/g). The experimental magnetic moments μexp are presented in Table 2. It can be seen that the increase in Zn content decreased the magnetization, but samples were still magnetic. The magnetic parameters confirmed the possibility of magnetic separation of magnesium-zinc ferrites after wastewater treatment.
Acid-base parameters are often used to describe the surface of solids, since their values indicate the presence of acid and basic Lewis and Bronsted centers directly involved in surface reactions. The acid-base parameter is a universal physicochemical characteristic of the solid surface, which depends on the material nature, synthesis method, chemical composition, the impurities of nature, and amount on the surface, etc. In this regard, we tried to trace the change in the acid-base state of the ferrite surface in order to identify the common peculiarities in the formation of acid-base surface centers. One of the methods for studying of acid-base surface properties is “drift pH method,” which allows to get the point of zero charge (pHPZC) parameter. For this purpose, the values of ΔpH = pHo − pHi were calculated and the curve ΔpH vs. pHo was drawn. The pHPZC was determined at the crossing of the curve with the axis (x) (Fig. 1d). In Fig. 1d, the dependence of the pHPZC vs. Zn(II) content for the magnetic samples is shown. The values of the point zero charges (pHPZC) indicated that at a pH lower than the pHPZC, the surface of the adsorbent was positively charged and adsorption of the anions took place on it. At higher pH, the surface has negative charge and the cation adsorption more preferably occurred on it. The points of zero charge for the studied magnetic samples was 10.4, 10.0, and 9.2 for Zn0.4Mg0.6Fe2O4, Zn0.6Mg0.4Fe2O4, and Zn0.8Mg0.2Fe2O4, respectively (Fig. 1d). The SEM of adsorbents showed that the structures of magnetic samples are represented by a large number of pores of 0.5–2 μm on their surfaces (Fig. 2). The results, which are presented in Fig. 2, indicated the difference in the porous structure of magnesium-zinc ferrite powders. Figure 2 shows the macropores that were represented by recesses with a spherical shape and a diameter of 0.5 to 2 μm. It can be seen that in Zn0.6Mg0.4Fe2O4 sample, there were pores with the smallest size (approximately 0.5 μm) compared to the pore sizes in the Zn0.4Mg0.6Fe2O4 and Zn0.8Mg0.2Fe2O4 samples.
Adsorption of Sr(II): isotherm modeling and kinetics
The adsorption capacity of magnetic adsorbents toward Sr(II) ions was determined from adsorption isotherms (Fig. 3). The isotherm at constant temperature relates the amount of adsorbed compound to the amount of absorbate in the equilibrium solution. The shape of the isotherm reflects the intensity of adsorption and the attraction between the adsorption and adsorbent. Isotherms can provide qualitative information about the adsorption process, as well as indicate the proportion of surface coverage. The adsorption activity of ferrite samples was studied in a batch mode in the aqueous solutions, containing Sr(II) ions. The data showed that Mg0.4Zn0.6Fe2O4 sample had the highest adsorption capacity which was 65 mg/g. The experiments showed that changes in the zinc content affected the adsorption efficiency of Sr(II) ions on the adsorbents surface. Figure 3 shows that the increasing of zinc content from x = 0.4 to x = 0.6 increased the adsorption of Sr(II) ions from 50 to 65 mg/g, and then the adsorption capacity was decreased to 36 mg/g for the sample with x = 0.8. This was due to the change in the morphological and structural properties of the adsorbent surface. It can be seen from Fig. 2 that sample x(Zn) = 0.6 showed the surface with smallest pores and has particles with the biggest crystallite sizes (Table 2). It could also be seen that observed adsorption was due to the presence of specific active sites on the adsorbent surface, able to react with adsorbate (Sr(II)), and does not determine by the specific surface area (Table 2).
The attempts were made to apply some of adsorption models (Langmuir, Freindlich, Dubinin-Radushkevich, and Sips) in order to quantify the main characteristics of the interaction of Sr(II) ions with ferrite nanoparticles. Nowadays, the two approaches can be used to determine adsorption parameters: non-linear regression (Dotto et al. 2012; Marques et al. 2018) and linearization (Tran et al. 2017). The linearization was used to determine adsorption parameters in the present work. The Langmuir model is based on the concept of a monomolecular layer of adsorbed particles formed due to the short-range nature of surface forces. The Langmuir isotherms are based on physical assumptions that all surface states have the same adsorption energies, that atoms do not interact with each other, and that only one atom or molecule can accommodate on one surface center. The change in energy during adsorption or desorption is the same (Tran et al. 2017). The Langmuir equation in the linear form can be expressed as Eq. (5):
where qe (mg/g) is adsorption capacity, qmax (mg/g) is the maximum adsorption capacity, Ce (mg/L) is the strontium amount at equilibrium, and KL (L/mg) is a Langmuir constant. The parameters qmax and KL were obtained from a plot of Ce/qe vs. Ce. The separation factor RL (dimensionless constant characterizing the Langmuir isotherm) was computed by Eq. (6):
where RL is a constant separation factor (dimensionless) of the solid-liquid adsorption system, KL is the Langmuir equilibrium constant, and Co (mg/L) is the initial Sr(II) concentration (Tran et al. 2017). RL is related to the nature of adsorbent/adsorbate interaction and isotherm type: unfavorable (RL > 1), linear (RL = 1), favorable (0 < RL < 1), or irreversible (RL = 0).
In fact, if the surface of the adsorbent is heterogeneous, an interaction takes place between the adsorbed particles, the active centers are not completely independent of each other, etc. All these factors complicate the form of the isotherm equation. The Freundlich model is used to describe the adsorption on a heterogeneous surface and there is no saturation zone on the Freindlich isotherm. The Freundlich equation in the linear form can be expressed as Eq. (7):
where qe (mg/g) is the equilibrium adsorption capacity; Ce (mg/L) is the equilibrium concentration of Sr(II); KF ((mg g−1)(mg L−1)–1/nF) is Freundlich constant; and 1/nF is the heterogeneity factor.
The Langmuir and Freundlich models are widely used, but they do not explain the adsorption mechanism. To study the mechanism of the adsorption process, we verified the equilibrium data using the Dubinin-Radushkevich (D-R) isotherm model. The D-R model does not imply surface homogeneity and is used to differentiate the chemical and physical adsorption. The Dubinin-Radushkevich model determines the nature of adsorption and can be used to calculate the average free adsorption energy. The linear form of the Dubinin-Radushkevich model can be expressed as Eq. (8):
where qDR (mol/g) is the adsorption capacity, KDR (mol2/kJ2) is a constant related to the adsorption energy, R is the gas constant (R = 8314 × 10−3 kJ/(mol × К)), T is the temperature (in Kelvin), qe (mg/g) is the equilibrium adsorption capacity, and Ce (mg/L) is the equilibrium concentration of Sr(II). The parameters qDR and KDR were obtained from a plot of lnqe versus \( {R}^2{T}^2{\mathit{\ln}}^2\left(1+{C}_e^{-1}\right). \) The slope and intercept were equal to -KDR and ln(qRD), respectively. Thus the mean adsorption energy E (kJ/mol) was calculated using Eq. (9):
The value of KDR allows one to make a conclusion about the nature of the interaction forces between strontium ions and active surface centers of the adsorbents in order to answer the question whether the binding of strontium ions to the ferrite surface is a physical process or a chemical process. It is believed that if the value of E is higher than 8 kJ/mol, the adsorption process is chemosorption and if this value is less than 8 kJ/mol, the adsorption process has physical nature.
The Sips model is based on the Langmuir equation, but takes into account the attractive forces between the adsorbate molecules. The Sips equation has three parameters and it combines the Freundlich and Langmuir equations. The linear form of the Sips equation can be written as Eq. (10):
where qe (mg/g) is the Sr(II) amount adsorbed at equilibrium, qms (mg/g) is the maximum adsorption capacity, Ce (mg/L) is the adsorbate concentration at equilibrium, KS (L/mg) is the Sips equilibrium constant, and nS is the Sips parameter. The difference between the Sips and Langmuir equations lies in the additional parameter ns. It can be considered as a parameter that characterizes the system’s heterogeneity and it comes from either adsorbent or adsorbate or a combination of both. The Sips model describes the adsorption processes in a wider range of concentrations compared to Freundlich model.
The linearized forms of Langmuir, Freindlich, Dubinin-Radushkevich, and Sips models are presented in Fig. 4. The calculated model parameters as well as the correlation coefficients are presented in Table 3.
The values of the correlation coefficients (R2) obtained from linearized forms of isotherm showed that the adsorption of Sr(II) ions by magnetic ferrite nanoparticles was best described by the Langmuir model. The Langmuir equation describes localized adsorption. Therefore, it can be assumed that Sr(II) ions were quite strongly fixed on the ferrite surface due to chemical interactions. The maximum theoretical adsorption capacities of magnesium-zinc ferrite, obtained from Langmuir model, were 57, 74, and 43 mg/g for x = 0.4, x = 0.6, and x = 0.8 samples, respectively. The adsorption energy (E) obtained from Dubinin-Radushkevich isotherm model can define adsorption processes. The calculations showed that the values of the adsorption free energy was 10.1–11.3 kJ/mol (Table 3), which indicated the chemical interaction of the adsorbate with the adsorbent. Thus, the adsorption of Sr(II) ions on the ferrite surface was chemosorption in nature. For the sample with x = 0.6, the Sips model described adsorption most accurately. Since the Sips model is a combination of the Langmuir and Freindlich models, the application of this model to the obtained experimental results indicated the surface heterogeneity of the synthesized materials on the one hand, and monolayer adsorption on the other hand. It should be noted that there is no correlation of qmax with the specific surface area of synthesized materials. Hence, it can be assumed that the nature of the active centers of adsorbents contributed to the effective adsorption of Sr(II) ions on magnetic adsorbents. The spinel ferrite nanoparticles contain surface-active cations, which could attract the Sr(II) ions. Based on the anti-structural modeling (see below), the magnesium ions were the ones that were interacted with Sr(II) ions.
The rate at which thermodynamic equilibrium is reached in equilibrium is usually described by kinetics. The adsorption process continues until equilibrium is reached. Adsorption, physical or chemical, involves the mass transfer of the adsorbent from solution to the surface of the adsorbent. The adsorption kinetics of magnesium-zinc ferrites are presented in Fig. 5. The results showed that the synthesized ferrites effectively adsorb Sr(II) ions. It can be seen that the time necessary to reach adsorption equilibrium was approximately 3 h. A further increase in adsorption time to 5 h does not lead to a significant change in the concentration of Sr(II) in the solutions. The adsorption under static conditions proceeds at a high rate for the first 30 min and reaches its maximum value after 180 min. The increase in the Zn content from 0.4 to 0.6 mol leads to a noticeable change in the adsorption activity, but the further increase in zinc content from 0.6 to 0.8 mol significantly slowed down the adsorption process.
In this study, experimental data was compared with various kinetics models, including pseudo-first-order, pseudo-second-order, intra-particle diffusion, and Elovich models. The kinetic curves were drawn in corresponding coordinates: log(qe −qt) versus t (min) for pseudo-first-order; t/qe versus t (min) for pseudo-second-order; qt versus t1/2 for interparticle diffusion interaction model; and qt versus ln(t) for Elovich model, where qe is equilibrium amount of Sr2+ (mg/g) and qt is amount of Sr2+ (mg/g) at time t, min. The plots of these models are shown in Fig. 6 and the parameters of all kinetic models are given in Table 4. The highest values of correlation coefficient R2 for pseudo-second-order model mean that the adsorption process depends not only on the constant of the adsorption rate but also on the initial concentration of Sr(II) ions. The sample Mg0.4Zn0.6Fe2O4 was the most active adsorbent and the PSO rate constant h has the highest value in comparison to the other samples.
To confirm the presence of Sr(II) ions on the adsorbents surface, magnetic samples were analyzed by the energy-dispersion analysis after Sr(II) adsorption. Figure 7 shows the SEM and EDX spectrum of Mg0.4Zn0.6Fe2O4 and Mg0.2Zn0.8Fe2O4 samples after adsorption process. The selected samples were washed with distilled water after adsorption of Sr(II) ions, and dried in air. It can be seen that Sr(II) ions were present on the sample surface. The porous structures of adsorbents were still without significant changes.
The surface of any material can be largely inhomogeneous due to the existing various crystallographic planes, atoms, the presence of structural defects (vacancies, dislocations, etc.), the presence of various functional groups, and also physically and chemically adsorbed impurities. The formation of structural defects on the surface leads to the formation of active surface centers with different energies. This determines the possibility of physical and/or chemical adsorption of various molecules, as well as the reactivity of compounds, i.e., surface chemistry. Antistructural model (Kumar et al. 2018; Prabukanthan et al. 2018; Rajesh Kumar et al. 2018; Theerthagiri et al. 2019) gives a better comprehension on the lattice defects formation and the types of surface active centers:
where ● is an excess positive charge; ′ is an excess negative charge; V is a cationic/anionic vacancy; A and B are indices of cationic tetrahedral and octahedral positions, respectively, and O is anion position in the spinel lattice.
During the interaction of Sr(II) with the sorbent surface, the partial adsorption of Sr(II) occurred at certain active centers. From Table 5, we can see that the increase of zinc content led to decrease of concentration of negatively charged defect centers \( M{g}_B^{\prime } \) and positively charged defect centers \( {Fe}_{\mathrm{A}}^{\cdotp } \). Anti-structural modeling gives us new and clear information about active centers: \( {Zn}_A^{2+} \), \( {Mg}_A^{2+} \), and \( {Fe}_B^{3+} \) were not active centers (due to their effective zero charge), while \( {Fe}_A^{3+} \) and \( {Mg}_B^{2+} \) were active centers in the adsorption, catalytic, or other processes in “solid/gas” or “solid/liquid” systems. This is the reason that Sr(II) ions were better adsorbed on the samples with x = 0.4 and x = 0.6 due to higher content of negatively charged active defects \( M{g}_B^{\prime } \).
Adsorption of salicylic acid
The adsorption kinetics of salicylic acid was investigated at 24 h in order to find the equilibrium time for achieving the maximum adsorption capacity of magnesium-zinc ferrites. The kinetic curves are shown in Fig. 8a. As can be seen from Fig. 8a, the adsorption equilibrium for Mg0.6Zn0.4Fe2O4 sample was reached in 5 h and for Mg0.2Zn0.8Fe2O4, it was reached in 10 h. However, for the Mg0.4Zn0.6Fe2O4 sample, the equilibrium was established after 24 h. The adsorption capacity was increased with increasing the Zn content and more time was required for saturation of adsorbent surface.
The three kinetic models were involved in order to explain the adsorption mechanism of salicylic acid on magnesium-zinc ferrites: pseudo-first-order, pseudo-second-order, and Elovich models. PFO and PSO models were describing the physical adsorption, while the Elovich model describes some cases of heterogeneous chemosorption. The linearized forms of kinetic models are presented on Fig. 8b–d. The calculated model parameters as well as the correlation coefficients are described in Table 6.
For the sample with x = 0.4, the adsorption kinetics was well fitted by a pseudo-second-order model due to the better values of R2 (0.999) and the estimated values of the adsorption capacity were also close to those obtained experimentally. For the samples with x = 0.6 and x = 0.8, the adsorption kinetics was fitted by a pseudo-first-order model, since R2 was 0.949 and 0.992, respectively. The adsorption rate (h) was calculated from the values of the second-order rate constant which were equal to 0.496 and 0.435 μg/(g·min) for samples with x = 0.4 and x = 0.8, respectively. The sample with x = 0.6 has the lowest h = 0.127 μg/(g min). The rate constants (k2) were 0.63, 0.03, and 0.06 μg/(g min) for magnesium-zinc ferrites with x = 0.4, 0.6, and x = 0.8, respectively. It means that for Mg0.4Zn0.6Fe2O4 sample, the rate constant k2 has the lowest value. The rate constant for Mg0.6Zn0.4Fe2O4 sample was ten times higher than that of Mg0.2Zn0.8Fe2O4 sample and 20 times higher than that of Mg0.4Zn0.6Fe2O4 sample. As magnesium-zinc ferrite samples were fitted better by pseudo-second-order model, it can be concluded that the adsorption process was chemosorption. The negatively charged octahedral magnesium ions attract the SA molecules (based on the anti-structure modeling (Table 5)). It is advisable to use Mg0.2Zn0.8Fe2O4 sample for fast removing of salicylic acid from the water solutions. The parameters of the Elovich model are important for understanding the kinetics mechanism, for example, the constant α is the initial adsorption rate, and the constant β characterizes desorption process. The initial adsorption rate has the lowest value for Mg0.4Zn0.6Fe2O4 sample and the highest value for Mg0.6Zn0.4Fe2O4 sample. The desorption constant β was decreased from 0.242 to 0.054 g/μg with increasing zinc content. This was in a good agreement with the experimental data of the adsorption capacity. In addition, the removal rate of Mg0.2Zn0.8Fe2O4 sample toward SA was maintained above 90% after 24 h (Fig. 9). It can be concluded that uptake of salicylic acid molecules occurred on the surface of the magnetic sorbents due to hydrogen bonds appearing among the OH− groups of salicylic acid and the H+ ions of the ferrites.
Conclusion
The magnetic spinel ferrite Mg1 − xZnxFe2O4 (where x = 0.4; 0.6 and 0.8) was tested for the removal of Sr(II) ions and SA as emerging contaminant from water medium. The structure of magnetic samples was characterized by XRD; the morphology and surface were characterized by SEM, BET, and pHpzc; magnetic properties were characterized by VSM. The samples exhibited good affinity toward Sr(II) due to strong adsorbent–adsorbate interactions in the wide concentration range. It was concluded that the adsorption of Sr(II) ions on the ferrite surface was due to chemosorption. The spinel ferrite nanoparticles contain surface-active cations, which could attract the Sr(II) ions. Based on the anti-structural modeling, it was noted that magnesium ions were the one which were interacted with Sr(II) ions. The maximum theoretical adsorption capacities of magnesium-zinc ferrite, obtained from Langmuir model, were 57, 74, and 43 mg/g for x = 0.4, x = 0.6, and x = 0.8 samples, respectively. The adsorption of SA was 90% onto Mg0.2Zn0.8Fe2O4 sample after 24 h. The adsorption of SA molecules involves the hydrogen bonds, which were formed between the hydroxyl groups of salicylic acid and the H+ ions of the ferrite surface. The studies of the Sr(II) and SA adsorption are important for the development of the adsorbent for industrial applications and environmental applications.
References
Ainscough TJ, Alagappan P, Oatley-Radcliffe DL, Barron AR (2017) A hybrid super hydrophilic ceramic membrane and carbon nanotube adsorption process for clean water production and heavy metal removal and recovery in remote locations. J Water Process Eng 19:220–230. https://doi.org/10.1016/J.JWPE.2017.08.006
Alby D, Charnay C, Heran M, Prelot B, Zajac J (2018) Recent developments in nanostructured inorganic materials for sorption of cesium and strontium: synthesis and shaping, sorption capacity, mechanisms, and selectivity—a review. J Hazard Mater 344:511–530. https://doi.org/10.1016/J.JHAZMAT.2017.10.047
Alla SK, Kollu P, Meena SS, Poswal HK, Mandal RK, Prasad NK (2018) Mn-substituted cerium oxide nanostructures and their magnetic properties. Mater Res Bull 104:65–71. https://doi.org/10.1016/J.MATERRESBULL.2018.04.008
Arshadi M, Mousavinia F, Abdolmaleki MK, Amiri MJ, Khalafi-Nezhad A (2017) Removal of salicylic acid as an emerging contaminant by a polar nano-dendritic adsorbent from aqueous media. J Colloid Interface Sci 493:138–149. https://doi.org/10.1016/j.jcis.2017.01.017
Choodamani C, Rudraswamy B, Chandrappa GT (2016) Structural, electrical, and magnetic properties of Zn substituted magnesium ferrite. Ceram Int 42:10565–10571. https://doi.org/10.1016/J.CERAMINT.2016.03.120
Deepty M, Srinivas C, Vijaya Babu K, Ranjith Kumar E, Singh Meena S, Prajapat CL, Krisha Mohan N, Sastry DL (2018) Structural and electron spin resonance spectroscopic studies of MnxZn1−xFe2O4 (x = 0.5, 0.6, 0.7) nanoferrites synthesized by sol-gel auto combustion method. J Magn Magn Mater 466:60–68. https://doi.org/10.1016/J.JMMM.2018.06.078
Dotto GL, Cadaval TRS, Pinto LAA (2012) Preparation of bionanoparticles derived from Spirulina platensis and its application for Cr (VI) removal from aqueous solutions. J Ind Eng Chem 18:1925–1930. https://doi.org/10.1016/j.jiec.2012.05.005
Faisal AAH, Al-Wakel SFA, Assi HA et al (2020) Waterworks sludge-filter sand permeable reactive barrier for removal of toxic lead ions from contaminated groundwater. J Water Process Eng 33:101112. https://doi.org/10.1016/J.JWPE.2019.101112
Ghazi N, Mahmoudi Chenari H, Ghodsi FE (2018) Rietveld refinement, morphology analysis, optical and magnetic properties of magnesium-zinc ferrite nanofibers. J Magn Magn Mater 468:132–140. https://doi.org/10.1016/J.JMMM.2018.07.084
Ghezzi L, Spepi A, Agnolucci M, Cristani C, Giovannetti M, Tiné MR, Duce C (2018) Kinetics of release and antibacterial activity of salicylic acid loaded into halloysite nanotubes. Appl Clay Sci 160:88–94. https://doi.org/10.1016/j.clay.2017.11.041
Gore SK, Tumberphale UB, Jadhav SS, Kawale RS, Naushad M, Mane RS (2018) Microwave-assisted synthesis and magneto-electrical properties of Mg-Zn ferrimagnetic oxide nanostructures. Phys B Condens Matter 530:177–182. https://doi.org/10.1016/J.PHYSB.2017.11.044
Harris DC (2007) Quantitative chemical analysis. Seventh, New York
He Z, Meng M, Yan L, Zhu W, Sun F, Yan Y, Liu Y, Liu S (2015) Fabrication of new cellulose acetate blend imprinted membrane assisted with ionic liquid ([BMIM]Cl) for selective adsorption of salicylic acid from industrial wastewater. Sep Purif Technol 145:63–74. https://doi.org/10.1016/j.seppur.2015.03.005
Hu X, Liu B, Liu J, Qin J, Zhao W, Lam KH (2019) Construction of zinc-magnesium-iron multinary spinel core-shell microspheres with enhanced photocatalytic properties of 1, 2-dichlorobenzene toxic species. J Photochem Photobiol A Chem 382:111903. https://doi.org/10.1016/J.JPHOTOCHEM.2019.111903
Hua P, Sellaoui L, Franco D, Netto MS, Luiz Dotto G, Bajahzar A, Belmabrouk H, Bonilla-Petriciolet A, Li Z (2020) Adsorption of acid green and procion red on a magnetic geopolymer based adsorbent: experiments, characterization and theoretical treatment. Chem Eng J 383:123113. https://doi.org/10.1016/J.CEJ.2019.123113
John SP, Mathew J (2019) Determination of ferromagnetic, superparamagnetic and paramagnetic components of magnetization and the effect of magnesium substitution on structural, magnetic and hyperfine properties of zinc ferrite nanoparticles. J Magn Magn Mater 475:160–170. https://doi.org/10.1016/J.JMMM.2018.11.030
Karunanayake AG, Todd OA, Crowley ML, et al (2017) Rapid removal of salicylic acid, 4-nitroaniline, benzoic acid and phthalic acid from wastewater using magnetized fast pyrolysis biochar from waste Douglas fir. Chem Eng J 319:75. https://doi.org/10.1016/j.cej.2017.02.116.
Kefeni KK, Mamba BB, Msagati TAM (2017) Application of spinel ferrite nanoparticles in water and wastewater treatment: a review. Sep Purif Technol 188:399–422. https://doi.org/10.1016/J.SEPPUR.2017.07.015
Kothawale MM, Tangsali RB, Meena SS, Prasad NK, Gangwar A (2019) Mössbauer study and curie temperature configuration on sintering Nano-Ni-Zn ferrite powder. J Supercond Nov Magn 32:2141–2147. https://doi.org/10.1007/s10948-018-4935-x
Kumar TR, Prabukanthan P, Harichandran G, Theerthagiri J, Moydeen AM, Durai G, Kuppusami P, Tatarchuk T (2018) Comparative study of structural, optical and electrical properties of electrochemically deposited Eu, Sm and Gd doped ZnSe thin films. J Mater Sci Mater Electron 29:5638–5648. https://doi.org/10.1007/s10854-018-8533-2
Li WC (2014) Occurrence, sources, and fate of pharmaceuticals in aquatic environment and soil. Environ Pollut 187:193–201. https://doi.org/10.1016/J.ENVPOL.2014.01.015
Liu Y, Wei S, Tian H, Tong H, Xu B (2014) Characterization of soft magnetic spinel ferrite coating prepared by plasma spray. Surf Coat Technol 258:189–199. https://doi.org/10.1016/J.SURFCOAT.2014.09.029
Liu H, Li A, Ding X, Yang F, Sun K (2019) Magnetic induction heating properties of Mg1-xZnxFe2O4 ferrites synthesized by co-precipitation method. Solid State Sci 93:101–108. https://doi.org/10.1016/J.SOLIDSTATESCIENCES.2019.05.005
Marques JL, Lütke SF, Frantz TS et al (2018) Removal of Al (III) and Fe (III) from binary system and industrial effluent using chitosan films. Int J Biol Macromol 120:1667–1673. https://doi.org/10.1016/j.ijbiomac.2018.09.135
Martín J, del MM O, Medina-Carrasco S et al (2018) Removal of priority and emerging pollutants from aqueous media by adsorption onto synthetic organo-funtionalized high-charge swelling micas. Environ Res 164:488–494. https://doi.org/10.1016/j.envres.2018.03.037
Mironyuk I, Tatarchuk T, Naushad M, Vasylyeva H, Mykytyn I (2019a) Highly efficient adsorption of strontium ions by carbonated mesoporous TiO2. J Mol Liq 285:742–753. https://doi.org/10.1016/J.MOLLIQ.2019.04.111
Mironyuk I, Tatarchuk T, Vasylyeva H, Naushad M, Mykytyn I (2019b) Adsorption of Sr(II) cations onto phosphated mesoporous titanium dioxide: mechanism, isotherm and kinetics studies. J Environ Chem Eng 7:103430. https://doi.org/10.1016/j.jece.2019.103430
Mohamed EA, Selim AQ, Ahmed SA, Sellaoui L, Bonilla-Petriciolet A, Erto A, Li Z, Li Y, Seliem MK (2020) H2O2-activated anthracite impregnated with chitosan as a novel composite for Cr(VI) and methyl orange adsorption in single-compound and binary systems: modeling and mechanism interpretation. Chem Eng J 380:122445. https://doi.org/10.1016/J.CEJ.2019.122445
Mohanraj J, Durgalakshmi D, Balakumar S, Aruna P, Ganesan S, Rajendran S, Naushad M (2019) Low cost and quick time absorption of organic dye pollutants under ambient condition using partially exfoliated graphite J Water Process Eng 101078. https://doi.org/10.1016/J.JWPE.2019.101078
Ng LY, Ng CY, Mahmoudi E, Ong CB, Mohammad AW (2018) A review of the management of inflow water, wastewater and water reuse by membrane technology for a sustainable production in shrimp farming. J Water Process Eng 23:27–44. https://doi.org/10.1016/J.JWPE.2018.02.020
Prabukanthan P, Lakshmi R, Harichandran G, Tatarchuk T (2018) Photovoltaic device performance of pure, manganese (Mn2+) doped and irradiated CuInSe2 thin films. New J Chem 42:11642–11652. https://doi.org/10.1039/c8nj01056k
Rajesh Kumar T, Prabukanthan P, Harichandran G, Theerthagiri J, Tatarchuk T, Maiyalagan T, Maia G, Bououdina M (2018) Physicochemical and electrochemical properties of Gd3+−doped ZnSe thin films fabricated by single-step electrochemical deposition process. J Solid State Electrochem 22:1197–1207. https://doi.org/10.1007/s10008-017-3865-z
Reddy DHK, Yun Y-S (2016) Spinel ferrite magnetic adsorbents: alternative future materials for water purification? Coord Chem Rev 315:90–111. https://doi.org/10.1016/J.CCR.2016.01.012
Reyes-Rodríguez PY, Cortés-Hernández DA, Escobedo-Bocardo JC, Almanza-Robles JM, Sánchez-Fuentes HJ, Jasso-Terán A, de León-Prado LE, Méndez-Nonell J, Hurtado-López GF (2017) Structural and magnetic properties of Mg-Zn ferrites (Mg1−xZnxFe2O4) prepared by sol-gel method. J Magn Magn Mater 427:268–271. https://doi.org/10.1016/J.JMMM.2016.10.078
Rodriguez-Narvaez OM, Peralta-Hernandez JM, Goonetilleke A, Bandala ER (2017) Treatment technologies for emerging contaminants in water: a review. Chem Eng J 323:361–380. https://doi.org/10.1016/J.CEJ.2017.04.106
Satheeshkumar MK, Ranjith Kumar E, Srinivas C, Prasad G, Meena SS, Pradeep I, Suriyanarayanan N, Sastry DL (2019) Structural and magnetic properties of CuFe2O4 ferrite nanoparticles synthesized by cow urine assisted combustion method. J Magn Magn Mater 484:120–125. https://doi.org/10.1016/J.JMMM.2019.03.128
Sharma R, Thakur P, Kumar M, Thakur N, Negi NS, Sharma P, Sharma V (2016) Improvement in magnetic behaviour of cobalt doped magnesium zinc nano-ferrites via co-precipitation route. J Alloys Compd 684:569–581. https://doi.org/10.1016/J.JALLCOM.2016.05.200
Sharma R, Thakur P, Sharma P, Sharma V (2017) Ferrimagnetic Ni2+ doped Mg-Zn spinel ferrite nanoparticles for high density information storage. J Alloys Compd 704:7–17. https://doi.org/10.1016/J.JALLCOM.2017.02.021
Sophia AC, Lima EC (2018) Removal of emerging contaminants from the environment by adsorption. Ecotoxicol Environ Saf 150:1–17. https://doi.org/10.1016/J.ECOENV.2017.12.026
Tatarchuk T, Bououdina M, Judith Vijaya J, John Kennedy L (2017) Spinel ferrite nanoparticles: synthesis, crystal structure, properties, and perspective applications. In: Fesenko O, Yatsenko L (eds) Nanophysics, nanomaterials, interface studies, and applications. NANO 2016. Springer Proceedings in Physics. Springer, Cham, pp 305–325
Tatarchuk T, Al-Najar B, Bououdina M, Ahmed MAA (2019a) Catalytic and photocatalytic properties of oxide spinels. In: Martínez L, Kharissova O, Kharisov B (eds) Handbook of ecomaterials. Pp 1701–1750
Tatarchuk T, Bououdina M, Al-Najar B, Bitra RB (2019b) Green and ecofriendly materials for the remediation of inorganic and organic pollutants in water. In: Naushad M (ed) A new generation material graphene: applications in water technology. Springer, Cham, pp 69–110
Tatarchuk T, Myslin M, Mironyuk I, et al (2019c) Synthesis, morphology, crystallite size and adsorption properties of nanostructured Mg–Zn ferrites with enhanced porous structure. J Alloys Compd 152945. https://doi.org/10.1016/J.JALLCOM.2019.152945
Tatarchuk T, Paliychuk N, Bitra RB et al (2019d) Adsorptive removal of toxic Methylene blue and Acid Orange 7 dyes from aqueous medium using cobalt-zinc ferrite nanoadsorbents. Desalin Water Treat 150:374–385. https://doi.org/10.5004/dwt.2019.23751
Tatarchuk T, Shyichuk A, Mironyuk I, Naushad M (2019e) A review on removal of uranium(VI) ions using titanium dioxide based sorbents. J Mol Liq 293:111563. https://doi.org/10.1016/j.molliq.2019.111563
Theerthagiri J, Durai G, Tatarchuk T, Sumathi M, Kuppusami P, Qin J, Choi MY (2019) Synthesis of hierarchical structured rare earth metal–doped Co3O4 by polymer combustion method for high performance electrochemical supercapacitor electrode materials. Ionics (Kiel) 26:2051–2061. https://doi.org/10.1007/s11581-019-03330-9
Tran HN, You S-J, Hosseini-Bandegharaei A, Chao H-P (2017) Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: a critical review. Water Res 120:88–116. https://doi.org/10.1016/J.WATRES.2017.04.014
Tsay C-Y, Chiu Y-C, Tseng Y-K (2019) Investigation on structural, magnetic, and FMR properties for hydrothermally-synthesized magnesium-zinc ferrite nanoparticles. Phys B Condens Matter 570:29–34. https://doi.org/10.1016/J.PHYSB.2019.05.037
Xiao G, Wen R, Liu A, He G, Wu D (2017) Adsorption performance of salicylic acid on a novel resin with distinctive double pore structure. J Hazard Mater 329:77–83. https://doi.org/10.1016/j.jhazmat.2017.01.030
Zaki HM, Al-Heniti SH, Elmosalami TA (2015) Structural, magnetic and dielectric studies of copper substituted nano-crystalline spinel magnesium zinc ferrite. J Alloys Compd 633:104–114. https://doi.org/10.1016/J.JALLCOM.2015.01.304
Zhang L, Sellaoui L, Franco D, Dotto GL, Bajahzar A, Belmabrouk H, Bonilla-Petriciolet A, Oliveira MLS, Li Z (2020) Adsorption of dyes brilliant blue, sunset yellow and tartrazine from aqueous solution on chitosan: analytical interpretation via multilayer statistical physics model. Chem Eng J 382:122952. https://doi.org/10.1016/J.CEJ.2019.122952
Funding
The Ministry of Education and Science of Ukraine (project number 0118U000258) financially supported this research work. One of the authors (Mu. Naushad) is grateful to the Researchers Supporting Project number RSP-2019/8, King Saud University, Riyadh, Saudi Arabia for the financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Tito Roberto Cadaval Jr
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tatarchuk, T., Naushad, M., Tomaszewska, J. et al. Adsorption of Sr(II) ions and salicylic acid onto magnetic magnesium-zinc ferrites: isotherms and kinetic studies. Environ Sci Pollut Res 27, 26681–26693 (2020). https://doi.org/10.1007/s11356-020-09043-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-09043-1