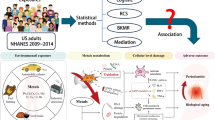
Abstract
The impact of environmental pollutant exposure on periodontitis has raised significant concerns. But the association between exposure to multiple polyaromatic hydrocarbons (PAHs) and periodontitis still remained unclear. Our study investigated the association of exposure to multiple PAHs with periodontitis. A total of 1880 participants from the National Health and Nutrition Examination Survey (NHANES) were included in this study. Urinary samples of the participants exposed to six PAHs, namely, 1-hydroxynaphthalene (1-OHN), 2-hydroxynaphthalene (2-OHN), 3-hydroxyfluorene (3-OHF), 2-hydroxyfluorene (2-OHF), 1-hydroxyphenanthrene (1-OHPhe), and 1-hydroxypyrene (1-OHPyr), were investigated. Multiple logistic regression, restricted cubic spline, and Bayesian kernel machine regression (BKMR) models were employed to identify the association between PAH exposures and periodontitis. The dose–response analysis exhibited a gradual increase in the periodontitis risk with an increase in multiple PAHs. After adjustment for several potential confounders, the odds ratio of the highest quartile (Quartile 4) was 1.648 (95% confidence interval (CI) 1.108–2.456, P = 0.014, P–t = 0.017) for 2-OHN, 2.046 (95%CI 1.352–3.104, P < 0.001, P–t = 0.005) for 3-OHF, 1.996 (95% CI 1.310–3.046, P = 0.001, P–t = 0.003) for 2-OHF, 1.789 (95% CI 1.230–2.604, P = 0.002, P–t = 0.003) for 1-OHPhe, and 1.494 (95% CI 1.025–2.181, P = 0.037, P–t = 0.021) for 1-OHPyr compared with that of the lowest quartile (Quartile 1). BKMR illustrated that the overall effect of the PAH mixture was positively related to periodontitis. Mediation analysis identified blood neutrophils as a partial mediator of 3-OHF and 2-OHF. Exposure to multiple PAHs was positively associated with periodontitis in US adults, and blood neutrophils mediate the effects of 3-OHF and 2-OHF therein.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Periodontitis is a crucial public health problem affecting the oral health of the world population, with an estimated 43% prevalence in US adults (Eke et al. 2018a; Eke et al. 2020). It is a complex infectious disease attributable to several etiologic and contributory factors. Among them, a dynamic and polymicrobial oral microbiome is a direct precursor (Lamont et al. 2018; Slots 2000). Periodontitis can eventually lead to tooth loss, a decline in chewing function, deformation, and a poor quality of life (Peres et al. 2019). The levels of inflammatory mediators, circulating hormones, immunosuppression, etc. can affect the susceptibility to periodontitis (Kinane et al. 2017). Dentists treating periodontitis aim to suppress or eradicate microbial pathogens in periodontal pockets and the adjacent gingiva and stick to periodontal maintenance therapy at regular intervals and long-term follow-ups (Kwon et al. 2021; Slots 2020).
Polycyclic aromatic hydrocarbons (PAHs) are ubiquitous organic pollutants. They are characterized by more than 2 fused benzene rings (Sahoo et al. 2020). They also have high lipid solubility, which allows them to easily pass through the human skin and mucous membrane system (Kim 2013). However, their aqueous solubility is poor and decreases for each additional ring system in their structural configurations (Sahoo et al. 2020). Some PAH metabolites are highly carcinogenic and teratogenic and damage cellular proteins, bind reactive oxygen species (ROS) to biomolecules, and prompt DNA adduct formation, which is associated with an increased risk of cancers (e.g., ovarian, uterine, breast, endometrial, cervical, and lung cancers) and various birth defects (e.g., congenital heart diseases, neural tube defects, and craniosynostosis) (Barbosa et al. 2023). Therefore, the impact of PAHs on human health has raised considerable public concerns.
On the whole, people might be exposed to the PAH mixture from various sources, including automobile exhaust, asphalt, coal tar, wildfires, agricultural combustion, chargrilled foods, and smoke from tobacco (“NHANES 2013–2014” n.d.). PAHs are ubiquitously distributed in the vapor phase, airborne particles, and settled dust (Ali et al. 2016). PAH metabolism has been widely researched (Gao et al. 2018). After entering the body, PAHs are easily metabolized through three major pathways (namely, diol-epoxide pathway, o-quinone pathway, and radical cation pathway) and transformed into biotransformation products that could destroy cellular structures. In particular, PAHs are nitrated through an ionic reaction before liver biotransformation (Miyanishi et al. 1996). Their metabolites are eventually eliminated through urine, thereby allowing feasibility for analysis (“NHANES 2013–2014” n.d.; Vondráček and Machala 2021).
Many studies have explained that PAHs can cause DNA damage (Miglani et al. 2019), oxidative stress (Zhang et al. 2021), the neutrophil influx, and other immune overreactions (Zhang et al. 2016), and these cellular and molecular alterations are confirmed to be associated with periodontitis onset (Cekici et al. 2014). Further to elucidate, DNA damage could lead to bone resorption in murine periodontitis models (Luo et al. 2023). And higher oxidative stress was shown to be associated with periodontitis in a population-based study (Qu 2023). The evidence from both in vivo and in vitro studies confirmed that in regard to inflammation response, inflammatory cells, especially neutrophils, could secrete cytokines and matrix metalloproteinases that contributed to the destruction of the extracellular matrix in periodontal tissues (Uitto et al. 2003). However, the association between PAH exposure and periodontitis has not been elucidated. Our study was therefore designed to investigate the association between exposure to PAH mixture and periodontitis in US adults. We also conducted the mediation effect analysis to investigate the mediation effects of blood neutrophils on the association, based on the data collected and issued by National Health and Nutrition Examination Survey (NHANES).
Material and methods
Study population
NHANES, implemented by the National Center for Health Statistics, a branch of the US Centers for Disease Control and Prevention (CDC), is a nationwide program comprising a series of studies designed for evaluating the health status and nutritional condition of both adults and children. People can participate in NHANES only if they have received an invitation from NHANES. These participants were randomly selected through a statistical process using US census information. Once enrolled, a participant can represent 65,000 people with similar populational characteristics (“NHANES—Participants—Why I was selected” n.d.). The NHANES protocol was approved and authorized by the National Health Statistics Ethics Review Board of the CDC, and all participants had provided written informed consent. A complete case in data was adopted for statistical analyses. Among 19,931 individuals who participated in NHANES from 2011 to 2014, 1880 individuals, who are aged 30 years or older, with complete periodontal examination, urinary PAH assessment, segmented neutrophil count, and potential confounders (including age, sex, race, marital status, education level, annual family income, smoking status, drinking, physical activity, time since the last dental visit, abdominal adiposity, obesity, hypertension, dyslipidemia, dysglycemia, cardiovascular diseases, arthritis, and urinary creatine), were finally included as the study population (Fig. S1).
Measurement of PAH exposure
Professionally trained medical personnel collected biosamples from the participants at mobile examination centers by using special devices. Thereafter, the samples were well processed and stored. The urine samples were transported to the Laboratory Sciences Division of the CDC National Center for Environmental Health, while blood samples were delivered to the University of Minnesota for testing. Several specific analytical methods such as enzymatic hydrolysis, extraction, derivatization, and analysis by isotope dilution capillary gas chromatography-tandem mass spectrometry (GC–MS/MS) in 2011–2012 NHANES and isotope dilution high-performance liquid chromatography–tandem mass spectrometry (on-line SPE-HPLC–MS/MS) in 2013–2014 NHANES were used to quantify the concentrations of mono-hydroxylated PAH metabolites (OH-PAHs). The details of the laboratory methods used for detecting urinary PAHs can be found elsewhere at https://wwwn.cdc.gov/nchs/data/nhanes/2011-2012/labmethods/pah_g_met.pdf. During 2011–2014, a total of six PAH metabolites were measured in NHANES, including 1-hydroxynaphthalene (1-OHN), 2-hydroxynaphthalene (2-OHN), 3-hydroxyfluorene (3-OHF), 2-hydroxyfluorene (2-OHF), 1-hydroxyphenanthrene (1-OHPhe), and 1-hydroxypyrene (1-OHPyr). For PAHs with analytical results below their corresponding lower limit of detection (LLOD, in ng/L), the concentrations below LLOD were considered a constant value—LLOD divided by a square root of 2. In accordance with a previous study (Oskar et al. 2021), PAH metabolites with a detection rate above 70% were selected in our study.
Periodontal examination
Based on the probing depth (PD) and attachment loss (AL) at interproximal regions, the Centers for Disease Control and Prevention and the American Academy of Periodontology case definition were used to classify periodontitis severity in our study. According to the definition (Eke et al. 2018b), periodontitis in the study population was subdivided into four levels: “No,” participants do not have any physical signs of periodontitis; “Mild,” participants have two or more interproximal regions with AL ≥ 3 mm and two or more interproximal regions with PD ≥ 4 mm on different teeth or one site with PD ≥ 5 mm; “Moderate,” participants have two or more interproximal regions with AL ≥ 4 mm on different teeth or two or more interproximal regions with PD ≥ 5 mm on different teeth; and “Severe,” participants have two or more interproximal regions with AL ≥ 6 mm on different teeth and one or more interproximal site with PD ≥ 5 mm. Based on a previous study, periodontal health status was regrouped as “no or mild” and “moderate or severe” (Li et al. 2021a).
Potential confounders
To obtain the most realistic results possible, we investigated some sociodemographic and behavioral variables, along with the number of teeth present and seven systemic diseases associated with periodontitis, as potential confounding factors. Sociodemographic confounders were gender (male and female), age (30–49 years and 50–80 years), race (Mexican American, non-Hispanic Asian, non-Hispanic White, non-Hispanic Black, other Hispanic, and other races), marital status (separated, married, divorced, widowed, never married, and living with a partner), educational level (less than 11th grade, high school graduate, some college or AA degree, and college graduate or above), and family annual income (< $25,000, $25,000–$75,000, and ≥ $75,000). Behavioral confounders included smoking status (non-smoker, smoking less than 100 cigarettes during the lifetime; former smoker, smoking more than 100 cigarettes during the lifetime but less than 100 cigarettes during the past year; current smoker, smoking more than 100 cigarettes during both lifetime and the past year), drinking (no, having less than 12 alcohol drinks per year; yes, having at least 12 alcohol drinks per year), physical activity (vigorous, moderate, and no or lower), and time since last dental visit (less than 6 months and more than 6 months). Because both periodontitis and PAH exposure were associated with systemic inflammation, some systemic diseases (Ferrillo et al. 2023; Li et al. 2021b), encompassing abdominal adiposity, obesity, hypertension, dyslipidemia, dysglycemia, cardiovascular diseases, and arthritis, were included as confounders (Table S1). Urinary creatine was also incorporated as a confounder to avoid urine dilution. Given that both periodontitis and PAH exposure may be associated with the aforementioned factors, we decided to calibrate for these confounders in our analyses.
Statistical analysis
The data of the quadrennium (2011–2014), including socioeconomic information, periodontal examination, and PAH exposures, were combined and used for the analysis. The Kolmogorov–Smirnov test was performed to verify whether the continuous variables conformed to a normal distribution. Considering the right-skewed distribution, urinary PAH concentration underwent a logarithmical transformation to achieve an approximately normal distribution in the algorithm for the analysis (Zhang et al. 2019). Normally distributed continuous variables, presented as mean ± SD, were compared among the groups with different periodontal statuses by using a t-test. Non-normally distributed variables, presented as median [25% percentile, 75% percentile], were compared using the Mann–Whitney U-test. Categorical variables, described as numbers (percentage), were compared using the chi-square test. The Spearman test was used to determine the correlation among PAHs, where substances with a coefficient of association (r2) of > 0.80 were considered to be highly correlated (Schober et al. 2018). Previous studies (Korn and Graubard 1991; Graubard and Korn 1999) have demonstrated that when the primary variables, such as age, gender, and race, used to calculate the sample weights, are included as covariates in the model, the weighted results might introduce an overestimation bias. NHANES weights were therefore not used in our study. All statistical analyses and plotting were conducted in R software (version 4.1.3).
Restricted cubic spline model
Compared with traditional regression models, restricted cubic spline (RCS) is a practicable tool for investigating the dose–response relationship and for minimizing residual confounding for continuous confounders (Desquilbet and Mariotti 2010). We hypothesized non-linear associations between PAHs and moderate/severe periodontitis. As recommended by Harrell (Harrell 2001), we used 4 knots in RCS to achieve better fitting by considering the smoothness of the curve and avoiding the loss of accuracy due to overfitting. RCS was conducted to determine the dose–response associations and avoid arbitrary PAH categorization in the logistic regression model.
Multiple logistic regression model
We used the multiple logistic regression model to quantify the association between exposure to each PAH and the risk of moderate/severe periodontitis. Odds ratio (OR) with a 95% confidence interval (CI) was used to measure the degrees of association. PAH exposure was assumed to be positively associated with moderate/severe periodontitis. Two models were constructed to address the potential bias introduced by these confounders. Model 1 was the crude model with none of the covariates adjusted, and Model 2 was adjusted for age, sex, race, marital status, education level, annual family income, smoking status, drinking, physical activity, time since the last dental visit, abdominal adiposity, obesity, hypertension, dyslipidemia, dysglycemia, cardiovascular diseases, arthritis, and urinary creatine. All urinary PAHs were subdivided into four quartiles. Of them, the first quartile acted as a reference. Trend tests were performed based on variables with median values for each PAH quartile included.
Bayesian kernel machine regression model
The Bayesian kernel machine regression (BKMR) model is centered on an algorithm in which a Markov chain Monte Carlo (MCMC) has been implemented (Bobb et al. 2015) based on a Gaussian kernel function (Weng et al. 2022). In our study, this model was used to investigate the overall effect of mixed PAHs on periodontitis and calibrate the possible deviation caused by highly correlated variables. This analysis was performed using “bkmr,” a R package, to determine the univariate, bivariate, and joint effects of PAHs, which were evaluated through “PredictorResponseUnivar,” “PredictorResponseBivar,” and “OverallRiskSummaries” functions, respectively. The formula for BKMR is as follows:
In the given equation, Y* is the outcome variable, ϵi is the normally distributed residual, x is the confounding factor, and β is the coefficient of x. Function h[·] displays the exposure–response correlations. Multiple PAHs were assumed to have accumulative effects on periodontitis and addictive interactions with each other. In each small of the panel interaction section, if the slope of the bivariate dose–response curve exhibited a clear change as the exposure 2 concentration increased, the interaction between exposure 1 and exposure 2 can be suggested to be significant (Lee et al. 2021).
Mediation effect analysis
Mediation effect analysis was employed to investigate whether blood neutrophils mediate the association between PAH exposure and the outcome according to the framework approach of mediation (Baron and Kenny 1986). We here assumed that blood neutrophils (M) mediate the influence of PAH exposure (X) on periodontitis (Y). The overall effects of each PAH on the outcome consist of two individual parts, namely, the direct and indirect effects (DE and IE, respectively). According to a previous study (Barth et al. 2016), a mediation effect exists when the following conditions are satisfied: (1) X must be significantly correlated with Y; (2) X must also be significantly correlated with M; (3) M must be significantly correlated with Y when the effect of X on Y has been controlled; and (4) after controlling for the mediator, X must be less significantly related to Y. Partial mediation is considered to exist when X remains significant after the mediator has been controlled.
Sensitivity analysis
A sensitivity analysis was performed to increase the reliability of our findings. First, to avoid potential selection bias, the baseline characteristics (gender and race) were compared between the participants included (n = 1880) and those excluded (n = 18,027). Age was not compared between the participants because only participants aged ≥ 30 years were eligible for periodontal examinations. Second, multiple imputations were conducted with random forests to fill in missing values of potential confounders. Random forest is a non-parametric method that can simultaneously cope with different types of variables by averaging over many unpruned classifications or regression trees. It can outperform other methods when potential interactions and non-linear associations are suspected (Stekhoven and Buhlmann 2012). Third, serum cotinine (non-smoker < 1 ng/mL, smoker ≥ 1 ng/mL), a nicotine metabolite and an indicator of tobacco smoke exposure (Hudson-Hanley et al. 2021), was used to replace smoking status in order to control potential confounding bias in tobacco smoke exposure, such as second-hand smoke. Fourth, considering PAHs might also derive from dietary intake, the 2015 Healthy Eating Index (HEI-2015), representing diet quality, was further adjusted in accordance with a previous study (Yang et al. 2022).
Results
Demographic characteristics
Table 1 lists the LLOD and detectable rates for urinary PAHs and PAH distribution between participants with different periodontal conditions. 1-OHPyr had a detectable rate of 83.99%, while other PAHs were almost completely detectable (approximately 100%). Therefore, they were all included in the study.
Among the 19,931 individuals who participated in the NHANES program from 2011 to 2014, 1880 participants with comprehensive data were finally included as the present study population. The proportion of participants with moderate/severe periodontitis was 45.69%. As shown in Table 2, the participants with moderate/severe periodontitis were compared with those without periodontitis or with mild periodontitis varied in gender, age, race, marital status, educational level, annual family income, smoking status, physical activity, time since last dental visit, abdominal adiposity, hypertension, dyslipidemia, dysglycemia, cardiovascular diseases, arthritis, the number of teeth present, urinary creatinine, blood neutrophil count, and PAH concentrations (all P values < 0.05).
Furthermore, the distribution characteristics of different PAH quartiles are probed in Table S2. The participants exposed to different quartiles varied in sex, age, race, marital status, educational level, annual family income, smoking status, physical activity, time since the last dental visit, abdominal adiposity, and obesity (all P values < 0.05).
Associations between exposure to individual PAH and periodontitis
Before the application of logistic regression models, we employed RCS regression models to clarify the dose–response associations between PAH exposure and the outcome and avoid arbitrary categorization of PAH concentrations. A potential non-linear association was observed between 1-OHPhe and periodontitis, but the association was not significant (P value for Pnonlinearity = 0.2874; Fig. 1). We observed generally linear dose–response associations between other PAHs and the periodontitis risk (all P values for Pnonlinearity > 0.05).
Dose–response relationship between single PAHs and the risk of periodontitis. a 1-OHN; b 2-OHN; c 3-OHF; d 2-OHF; e 1-OHPhe; f 1-OHPyr; PAHs polyaromatic hydrocarbons, 1-OHN 1-hydroxynaphthalene, 2-OHN 2-hydroxynaphthalene, 3-OHF 3-hydroxyfluorene, 2-OHF 2-hydroxyfluorene, 1-OHPhe 1-hydroxyphenanthrene, 1-OHPyr 1-hydroxypyrene
Later, we constructed two multiple logistic regression models to determine the association between single PAH exposure and the outcome (Fig. 2). In Model 1, all PAHs were positively associated with an increased periodontitis risk. Compared with the reference (Quartile 1), crude ORs of the highest quartile (Quartile 4) were 2.537 (95% CI 1.953–3.305, P < 0.001, P–t < 0.001) for 1-OHN, 2.210 (95% CI 1.703–2.875, P < 0.001, P–t < 0.001) for 2-OHN, 2.900 (95% CI 2.228–3.786,, P < 0.001, P–t < 0.001) for 3-OHF, 2.855 (95% CI 2.194–3.726, P < 0.001, P–t < 0.001) for 2-OHF, 1.761 (95% CI 1.359–2.287, P < 0.001, P–t < 0.001) for 1-OHPhe, and 1.828 (95% CI 1.416–2.365, P < 0.001, P–t < 0.001) for 1-OHPyr. In Model 2, except for 1-OHN, positive associations between other PAHs and the risk of moderate/severe periodontitis exhibited no attenuation after adjustment for potential confounders. Compared with the reference (Quartile 1), the periodontitis risk increased by 1.648-fold (95% CI 1.108–2.456, P = 0.014, P–t = 0.017) for the highest quartile (Quartile 4) of 2-OHN. Compared with the reference (Quartile 1), the adjusted ORs of the highest quartile (Quartile 4) were 2.046 (95% CI 1.352–3.104, P < 0.001, P–t = 0.005) for 3-OHF, 1.996 (95% CI 1.310–3.046, P = 0.001, P–t = 0.003) for 2-OHF, 1.789 (95% CI 1.230–2.604, P = 0.002, P–t = 0.003) for 1-OHPhe, and 1.494 (95% CI 1.025–2.181, P = 0.037, P–t = 0.021) for 1-OHPyr.
Forest plot results for the association between single PAHs and the risk of periodontitis in Model 1 and Model 2. Model 1 was the crude model. Model 2 was adjusted for age, sex, race, marital status, education level, annual family income, smoking status, drinking, physical activity, time since the last dental visit, abdominal adiposity, obesity, hypertension, dyslipidemia, dysglycemia, cardiovascular diseases, arthritis, and urinary creatine; 1-OHN 1-hydroxynaphthalene, 2-OHN 2-hydroxynaphthalene, 3-OHF 3-hydroxyfluorene, 2-OHF 2-hydroxyfluorene, 1-OHPhe 1-hydroxyphenanthrene, 1-OHPyr 1-hydroxypyrene, OR odds ratio, CI confidence interval, P–t P for trend
Overall effects of the mixture of six PAHs on periodontitis
The Spearman test was used to determine the correlation among PAHs. A high correlation was observed between 3-OHF and 2-OHF (r2 = 0.95), and moderate correlations were observed among other PAHs (r2 = 0.50–0.80) (Fig. S2). A manual hierarchical selection in BKMR was conducted to inform the model, subgrouping PAHs in two clusters (Cluster 1 containing 3-OHF and 2-OHF and Cluster 2 containing others). Table S3 presents the significance of every cluster and substance in the periodontitis risk, which was quantified by posterior inclusion probabilities (PIPs) with a higher value indicating a more important role. With all other PAHs maintained at their median levels, the association between each PAH component and the outcome was presented in the univariate section (Fig. S3). The overall effects of the PAH mixture on the outcome are depicted in Fig. 3, where the risk of moderate/severe periodontitis increased with an increase in PAH concentrations with 95% CI constantly away from 0, thereby indicating a positive correlation between the PAH concentrations and moderate/severe periodontitis. For example, compared with the 25th percentile of PAH exposure, the 75th percentile increased the periodontitis risk by 9.34-fold. In the model, Cluster 1 played the most crucial role (PIPCluster 1 = 0.900), in which 2-OHF had a more indispensable role (PIP2-OHF = 0.693) than 3-OHF did (PIP3-OHF = 0.307), while 2-OHN functioned predominantly in Cluster 2 (PIP2-OHN = 0.794, PIPCluster 2 = 0.698). No significant interaction was observed among urinary PAHs (Fig. S4).
Overall effect of the PAHS mixture on risk of (95% CIs) periodontitis. Overall effect of the PAHS mixture on risk of (95% CIs) periodontitis. The model has been adjusted for age, sex, race, marital status, education level, annual family income, smoking status, drinking, physical activity, time since the last dental visit, abdominal adiposity, obesity, hypertension, dyslipidemia, dysglycemia, cardiovascular diseases, arthritis, and urinary creatine. The Y-axis represents the estimated change in the risk of the outcome when six PAHs were set at particular percentiles (ranging from 25 to 75th) compared to the 50th percentile of each PAH. Dots indicate the estimate, and black vertical lines represent 95% CIs
Blood neutrophils partially mediated the effects of PAH exposure on periodontal health
We performed the mediation effect analysis to determine whether neutrophils act as a mediator in the effects of PAHs on the periodontitis risk. 3-OHF and 2-OHF increased the periodontitis risk by affecting the blood neutrophil level (Fig. 4). To be more specific, neutrophil-mediated effect contributed to 3.9% of the total effect of 3-OHF exposure on periodontitis (IE 0.003, 95% CI = 0.000–0.010, P = 0.040; DE = 0.073, 95% CI 0.032–0.100, P < 0.001) and 6.0% of the total effect of 2-OHF exposure on periodontitis (IE 0.005, 95% CI = 0.002–0.010, P < 0.001; DE = 0.077, 95% CI 0.041–0.110, P < 0.001). Two multiple logistic regression models were also constructed before and after the calibration of the blood neutrophil count. As shown in Fig. S5, the associations between 3-OHF and 2-OHF exposure and the periodontitis risk weakened but remained significant after the blood neutrophil count was calibrated. Therefore, neutrophils were considered to partially mediate the associations.
Mediation analysis of neutrophil on the interaction between PAH mixtures and prevalence of periodontitis. (A) Mediation effects of blood neutrophil on the association between 1-OHN and periodontitis; (B) mediation effects of blood neutrophil on the association between 2-OHN and periodontitis; (C) mediation effects of blood neutrophil on the association between 3-OHF and periodontitis; (D) mediation effects of blood neutrophil on the association between 2-OHF and periodontitis; (E) mediation effects of blood neutrophil on the association between 1-OHPhe and periodontitis; (F) mediation effects of blood neutrophil on the association between 1-OHPyr and periodontitis. PAHs polyaromatic hydrocarbons, 1-OHN 1-hydroxynaphthalene, 2-OHN 2-hydroxynaphthalene, 3-OHF 3-hydroxyfluorene, 2-OHF 2-hydroxyfluorene, 1-OHPhe 1-hydroxyphenanthrene, 1-OHPyr 1-hydroxypyrene; *P < 0.05; **P < 0.01; ***P < 0.001
Sensitivity analysis
First, the excluded and included populations did not vary significantly in gender and race (Table S4). Among 18,027 participants excluded, 15.9% were Mexican American, 32.4% were non-Hispanic White, 25.1% were non-Hispanic Black, 10.3% were other Hispanic, and 16.3% belonged to other races. Male participants accounted for 49.2% in the excluded population, while female accounted for 50.8%. Second, by including participants with missing values of potential confounders, 2177 participants were enrolled in the study. The inclusion of the previously excluded population did not significantly change the results (Fig. S6). However, after adjustment for the potential confounders, the association between Quartile 3 of 2-OHN, 3-OHF, and the outcome became significant (OR 1.401, 95% CI 1.027–1.912, P = 0.034 for Quartile 3 of 2-OHN; OR 1.394, 95% CI 1.018–1.910, P = 0.039 for Quartile 3 of 3-OHF). Third, after adjustment for serum cotinine as the replacement of smoke status, the results remained generally robust, except for Quartile 2 of 2-OHN (OR 1.383, 95% CI 1.006,1.903, P = 0.046) and 1-OHPyr (OR 1.357, 95% CI 0.995–1.851, P = 0.054) (Table S5). Fourth, after further adjustment for HEI-2015, almost all results remained robust, except for Quartile 2 of 2-OHF (OR 1.389, 95% CI 1.001–1.929, P = 0.050) (Table S6).
Discussion
This is the first study exploring the association of PAH mixture with moderate/severe periodontitis in the US population. Multiple PAHs might increase the risk of moderate/severe periodontitis. Among PAHs, 2-OHF was the most crucial. The overall effects of PAH exposure were identified as statistically significant by the BKMR model. No non-linearity was observed between PAH exposure and the risk of the outcome. Blood neutrophils partially mediated the effects of 3-OHF and 2-OHF exposure on periodontal health.
To the best of our knowledge, clarity about the association between PAH exposure and periodontal health is lacking, but some evidence has indirectly supported the results we observed. Sutton et al. reported that a 28% increase in the periodontitis risk among 4,329 non-smokers was associated with environmental tobacco smoke (Sutton et al. 2017), where PAHs might be produced through combustion and play a role in periodontitis pathogenesis. Another study by Shiue (Shiue 2015) demonstrated that PAH exposure is linked to the loosening of teeth in adults, aching, and poor tooth health.
The pathogenic mechanisms of PAHs have been reported in some studies, and we speculated that some of these mechanisms might be related to periodontitis occurrence. PAH metabolites can directly contribute to excessive ROS production (Libalova et al. 2018) and lead to oxidative stress in gingiva and alveolar bone (Javed et al. 2019), which is associated with periodontitis pathogenesis (Wang et al. 2017). On the one hand, such oxidative stress can damage the DNA structure and trigger DNA abduct formation, which is determined by the presence of the electron-dense region (e.g., K region in phenanthrene) in the structure (Sahoo et al. 2020). In an observational study (Rao et al. 2020), DNA damage was higher in patients with chronic periodontitis. On the other hand, PAHs can activate the aryl hydrocarbon receptor (AHR) of immune cells and disturb immune homeostasis (O’Driscoll et al. 2018). Periodontitis could occur when the balance between the microbial biofilm and the host’s immunity is disequilibrated (Kinane et al. 2017). AHR regulates neutrophils, which can then participate in the formation of bactericidal ROS and proinflammatory stimulation (Bock 2020). Neutrophils overwhelmed by several persistent microbial biofilms can lead to severe, chronic inflammation by releasing matrix metalloproteinase-8 and IL-1 (a destructive cytokine), thereby contributing to chronic periodontitis (Gemmell et al. 1997; Sorsa et al. 2016).
Our study has some strengths. This is the first study investigating the association between multiple PAHs and periodontitis. Moreover, it is also the first study analyzing airborne environmental pollutants as potential pathogenic factors of periodontitis by using a mixed exposure model. PAH exposures were assessed using urinary metabolites, which are comprehensive indicators for diverse sources of exposure. Several potential confounders associated with exposure and the outcome were also included to address confounding bias. Some study limitations that must be noted are as follows. First, because NHANES is a cross-sectional study where exposure assessment is measured using one-shot urinary samples, drawing a clear causal relationship that long-term exposure to PAHs could lead to periodontitis is difficult and needs to be further validated in future cohort studies. Second, since PAHs have relative short half-lives (Miyanishi et al. 1996), it is difficult to obtain an accurate measurement of PAH concentrations in the human body. Third, the potential confounders adjusted in this study when establishing models might not be sufficient. For instance, pregnancy and menopause statuses should have been included in our study because hormones during specific physiological periods, such as menopause and pregnancy, can influence dental health in a woman (Akesson et al. 2004; Nordin et al. 2004). Additionally, apart from tobacco smoke and dietary intake, there might be other source of PAH exposures, such as cooking (Wang et al. 2022) and traffic (Wei et al. 2023), which was also not included in the study. Because of the public non-availability of the data, the confounders mentioned above were not included in our study.
Based on the large-scale population from NHANES, we found that high exposure to PAHs was associated with an increased periodontitis risk. Moreover, neutrophils acted as a mediator of this association. Furthermore, our study bridges the gap in the etiology of periodontitis. Urinary assessment of PAHs may facilitate the diagnosis and treatment of unexplained periodontitis. Longitudinal cohort studies are urgently required to further validate the accumulated adverse effects of multiple PAHs on periodontal health over time and discover additional environmental pollutants that can cause periodontitis.
Conclusion
Using single exposure models (RCS and multivariate logistic regression models), mixed exposure models (BKMR models), and mediation effect models, our cross-sectional study illustrates that exposure to multiple PAHs is associated with an increased periodontitis risk in US adults, and blood neutrophils mediate the effects of 3-OHF and 2-OHF on periodontitis. Moreover, our findings offer a novel insight into the etiology of unexplained periodontitis and suggest early prevention for the cause-specific periodontal health problem.
Data availability
The data supporting this study’s findings are available at https://www.cdc.gov/nchs/nhanes/index.htm.
Abbreviations
- PAHs :
-
Polyaromatic hydrocarbons
- NHANES :
-
National Health and Nutrition Examination Survey
- 1-OHN :
-
1-Hydroxynaphthalene
- 2-OHN :
-
2-Hydroxynaphthalene
- 3-OHF :
-
3-Hydroxyfluorene
- 2-OHF :
-
2-Hydroxyfluorene
- 1-OHPhe :
-
1-Hydroxyphenanthrene
- 1-OHPyr :
-
1-Hydroxypyrene
- RCS :
-
Restricted cubic spline
- BKMR :
-
Bayesian kernel machine regression
- WHtR :
-
Waist-to-height ratio
- PIP :
-
Posterior inclusion probability
- HEI-2015 :
-
The 2015 Healthy Eating Index
- OR :
-
Odds ratio
- 95% CI :
-
95% Confidence interval
- P -t :
-
P For trend
References
Akesson A, Vahter M, Berglund M, Eklöf T, Bremme K, Bjellerup P (2004) Bone turnover from early pregnancy to postweaning. Acta Obstet Gynecol Scand 83:1049–1055. https://doi.org/10.1111/j.0001-6349.2004.00428.x
Ali N, Ismail IMI, Khoder M, Shamy M, Alghamdi M, Costa M, Ali LN, Wang W, Eqani SAMAS (2016) Polycyclic aromatic hydrocarbons (PAHs) in indoor dust samples from cities of Jeddah and Kuwait: levels, sources and non-dietary human exposure. Sci Total Environ 573:1607–1614. https://doi.org/10.1016/j.scitotenv.2016.09.134
Barbosa F, Rocha BA, Souza MCO, Bocato MZ, Azevedo LF, Adeyemi JA, Santana A, Campiglia AD (2023) Polycyclic aromatic hydrocarbons (PAHs): updated aspects of their determination, kinetics in the human body, and toxicity. J Toxicol Environ Health, Part B 26:28–65. https://doi.org/10.1080/10937404.2022.2164390
Baron RM, Kenny DA (1986) The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol 51(6): 1173–1182. https://doi.org/10.1037//0022-3514.51.6.1173
Barth M, Kriston L, Klostermann S, Barbui C, Cipriani A, Linde K (2016) Efficacy of selective serotonin reuptake inhibitors and adverse events: meta-regression and mediation analysis of placebo-controlled trials. Br J Psychiatry 208:114–119. https://doi.org/10.1192/bjp.bp.114.150136
Bobb JF, Valeri L, Claus Henn B, Christiani DC, Wright RO, Mazumdar M, Godleski JJ, Coull BA (2015) Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics 16:493–508. https://doi.org/10.1093/biostatistics/kxu058
Bock KW (2020) Aryl hydrocarbon receptor (AHR) functions: balancing opposing processes including inflammatory reactions. Biochem Pharmacol 178:114093. https://doi.org/10.1016/j.bcp.2020.114093
Cekici A, Kantarci A, Hasturk H, Van Dyke TE (2014) Inflammatory and immune pathways in the pathogenesis of periodontal disease: inflammatory and immune pathways in periodontal disease. Periodontol 2000(64):57–80. https://doi.org/10.1111/prd.12002
Desquilbet L, Mariotti F (2010) Dose-response analyses using restricted cubic spline functions in public health research. Statist Med n/a-n/a. https://doi.org/10.1002/sim.3841
Eke PI, Thornton-Evans GO, Wei L, Borgnakke WS, Dye BA, Genco RJ (2018b) Periodontitis in US adults. J Am Dent Assoc 149:576-588.e6. https://doi.org/10.1016/j.adaj.2018.04.023
Eke PI, Borgnakke WS, Genco RJ (2020) Recent epidemiologic trends in periodontitis in the USA. Periodontol 2000(82):257–267. https://doi.org/10.1111/prd.12323
Eke PI, Page RC, Wei L, Thornton-Evans G, Genco RJ (2018a) Update of the case definitions for population-based surveillance of periodontitis. J Periodontol 83(12):1449–1454. https://doi.org/10.1902/jop.2012.110664
Ferrillo M, Giudice A, Migliario M, Renó F, Lippi L, Calafiore D, Marotta N, de Sire R, Fortunato L, Ammendolia A, Invernizzi M, de Sire A (2023) Oral–gut microbiota, periodontal diseases, and arthritis: literature overview on the role of probiotics. IJMS 24:4626. https://doi.org/10.3390/ijms24054626
Gao P, da Silva E, Hou L, Denslow ND, Xiang P, Ma LQ (2018) Human exposure to polycyclic aromatic hydrocarbons: metabolomics perspective. Environ Int 119:466–477. https://doi.org/10.1016/j.envint.2018.07.017
Gemmell E, Marshall RI, Seymour GJ (1997) Cytokines and prostaglandins in immune homeostasis and tissue destruction in periodontal disease. Periodontol 2000(14):112–143. https://doi.org/10.1111/j.1600-0757.1997.tb00194.x
Graubard BI, Korn EL (1999) Analyzing health surveys for cancer-related objectives. JNCI J Natl Cancer Inst 91:1005–1016. https://doi.org/10.1093/jnci/91.12.1005
Harrell FE (2001) Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis, Springer Series in Statistics. Springer New York, New York, NY. https://doi.org/10.1007/978-1-4757-3462-1
Hudson-Hanley B, Smit E, Branscum A, Hystad P, Kile ML (2021) Trends in urinary metabolites of polycyclic aromatic hydrocarbons (PAHs) in the non-smoking U.S. population, NHANES 2001–2014. Chemosphere 276:130211. https://doi.org/10.1016/j.chemosphere.2021.130211
Javed F, Rahman I, Romanos GE (2019) Tobacco-product usage as a risk factor for dental implants. Periodontol 2000(81):48–56. https://doi.org/10.1111/prd.12282
Kim K-H (2013) A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects. Environ Int 60(2013):71–80. https://doi.org/10.1016/j.envint.2013.07.019
Kinane DF, Stathopoulou PG, Papapanou PN (2017) Periodontal diseases. Nat Rev Dis Primers 3:17038. https://doi.org/10.1038/nrdp.2017.38
Korn EL, Graubard BI (1991) Epidemiologic studies utilizing surveys: accounting for the sampling design. Am J Public Health 81:1166–1173. https://doi.org/10.2105/AJPH.81.9.1166
Kwon T, Lamster IB, Levin L (2021) Current concepts in the management of periodontitis. Int Dent J 71:462–476. https://doi.org/10.1111/idj.12630
Lamont RJ, Koo H, Hajishengallis G (2018) The oral microbiota: dynamic communities and host interactions. Nat Rev Microbiol 16:745–759. https://doi.org/10.1038/s41579-018-0089-x
Lee M-S, Eum K-D, Golam M, Quamruzzaman Q, Kile ML, Mazumdar M, Christiani DC (2021) Umbilical cord blood metal mixtures and birth size in bangladeshi children. Environ Health Perspect 129(EHP7502):057006. https://doi.org/10.1289/EHP7502
Li A, Chen Y, Schuller AA, Sluis LWM, Tjakkes GE (2021a) Dietary inflammatory potential is associated with poor periodontal health: a population-based study. J Clin Periodontol 48:907–918. https://doi.org/10.1111/jcpe.13472
Li K, Yin R, Wang Y, Zhao D (2021b) Associations between exposure to polycyclic aromatic hydrocarbons and metabolic syndrome in U.S. adolescents: cross-sectional results from the National Health and Nutrition Examination Survey (2003–2016) data. Environ Res 202:111747. https://doi.org/10.1016/j.envres.2021.111747
Libalova H, Milcova A, Cervena T, Vrbova K, Rossnerova A, Novakova Z, Topinka J, Rossner P (2018) Kinetics of ROS generation induced by polycyclic aromatic hydrocarbons and organic extracts from ambient air particulate matter in model human lung cell lines. Mutat Res/Genet Toxicol Environ Mutagen 827:50–58. https://doi.org/10.1016/j.mrgentox.2018.01.006
Luo Y, Yang B, Dong W, Yu W, Jia M, Wang J (2023) DNA damage-inducible transcript 3 deficiency promotes bone resorption in murine periodontitis models. J Periodontal Res 58:841–851. https://doi.org/10.1111/jre.13142
Miglani K, Kumar S, Yadav A, Aggarwal N, Ahmad I, Gupta R (2019) A multibiomarker approach to evaluate the effect of polyaromatic hydrocarbon exposure on oxidative and genotoxic damage in tandoor workers. Toxicol Ind Health 35:486–496. https://doi.org/10.1177/0748233719862728
Miyanishi K, Kinouchi T, Kataoka K, Kanoh T, Ohnishi Y (1996) In vivo formation of mutagens by intraperitoneal administration of polycyclic aromatic hydrocarbons in animals during exposure to nitrogen dioxide. Carcinogenesis 17:1483–1490. https://doi.org/10.1093/carcin/17.7.1483
NHANES - participants - why I was selected [WWW Document] (n.d) URL https://www.cdc.gov/nchs/nhanes/participant/participant-selected.htm Accessed 4.5.23
NHANES 2013–2014: Polycyclic aromatic hydrocarbons (PAH) - urine data documentation, codebook, and frequencies [WWW Document] (n.d.) URL https://wwwn.cdc.gov/Nchs/Nhanes/2013-2014/PAH_H.htm Accessed 9.1.22
Nordin BEC, Wishart JM, Clifton PM, McArthur R, Scopacasa F, Need AG, Morris HA, O’Loughlin PD, Horowitz M (2004) A longitudinal study of bone-related biochemical changes at the menopause. Clin Endocrinol 61:123–130. https://doi.org/10.1111/j.1365-2265.2004.02066.x
O’Driscoll CA, Gallo ME, Hoffmann EJ, Fechner JH, Schauer JJ, Bradfield CA, Mezrich JD (2018) Polycyclic aromatic hydrocarbons (PAHs) present in ambient urban dust drive proinflammatory T cell and dendritic cell responses via the aryl hydrocarbon receptor (AHR) in vitro. PLoS One 13:e0209690. https://doi.org/10.1371/journal.pone.0209690
Oskar S, Wolff MS, Teitelbaum SL, Stingone JA (2021) Identifying environmental exposure profiles associated with timing of menarche: a two-step machine learning approach to examine multiple environmental exposures. Environ Res 195:110524. https://doi.org/10.1016/j.envres.2020.110524
Peres MA, Macpherson LMD, Weyant RJ, Daly B, Venturelli R, Mathur MR, Listl S, Celeste RK, Guarnizo-Herreño CC, Kearns C, Benzian H, Allison P, Watt RG (2019) Oral diseases: a global public health challenge. The Lancet 394:249–260. https://doi.org/10.1016/S0140-6736(19)31146-8
Qu H (2023) The association between oxidative balance score and periodontitis in adults: a population-based study. Front Nutr 10:1138488. https://doi.org/10.3389/fnut.2023.1138488
Rao A, Thomas B, Prasad R, Kumari S, Vishakh R, Subba T (2020) A comparative evaluation of the DNA damage in the serum of chronic periodontitis patients with and without diabetes mellitus type II. Indian J Dent Res 31:169. https://doi.org/10.4103/ijdr.IJDR_503_17
Sahoo BM, Ravi Kumar BVV, Banik BK, Borah P (2020) Polyaromatic hydrocarbons (PAHs): structures, synthesis and their biological profile. COS 17:625–640. https://doi.org/10.2174/1570179417666200713182441
Schober P, Boer C, Schwarte LA (2018) Correlation coefficients: appropriate use and interpretation. Anesth Analg 126:1763–1768. https://doi.org/10.1213/ANE.0000000000002864
Shiue I (2015) Urinary heavy metals, phthalates, phenols, thiocyanate, parabens, pesticides, polyaromatic hydrocarbons but not arsenic or polyfluorinated compounds are associated with adult oral health: USA NHANES, 2011–2012. Environ Sci Pollut Res 22:15636–15645. https://doi.org/10.1007/s11356-015-4749-3
Slots J (2020) Primer on etiology and treatment of progressive/severe periodontitis: a systemic health perspective. Periodontol 2000(83):272–276. https://doi.org/10.1111/prd.12325
Slots J (2000) Periodontitis: facts, fallacies and the future. Periodontology 75(1):7–23. https://doi.org/10.1111/prd.12221
Sorsa T, Gursoy UK, Nwhator S, Hernandez M, Tervahartiala T, Leppilahti J, Gursoy M, Könönen E, Emingil G, Pussinen PJ, Mäntylä P (2016) Analysis of matrix metalloproteinases, especially MMP-8, in gingival crevicular fluid, mouthrinse and saliva for monitoring periodontal diseases. Periodontol 2000(70):142–163. https://doi.org/10.1111/prd.12101
Stekhoven DJ, Buhlmann P (2012) MissForest–non-parametric missing value imputation for mixed-type data. Bioinformatics 28:112–118. https://doi.org/10.1093/bioinformatics/btr597
Sutton JD, Salas Martinez ML, Gerkovich MM (2017) Environmental tobacco smoke and periodontitis in United States non-smokers, 2009 to 2012. J Periodontol 88:565–574. https://doi.org/10.1902/jop.2017.160725
Uitto VJ, Overall CM, McCulloch C (2003) Proteolytic host cell enzymes in gingival crevice fluid. Periodontol 2000 31:77–104 (n.d.)
Vondráček J, Machala M (2021) The role of metabolism in toxicity of polycyclic aromatic hydrocarbons and their non-genotoxic modes of action. CDM 22:584–595. https://doi.org/10.2174/1389200221999201125205725
Wang Y, Andrukhov O, Rausch-Fan X (2017) Oxidative stress and antioxidant system in periodontitis. Front Physiol 8:910. https://doi.org/10.3389/fphys.2017.00910
Wang J, Du W, Chen Y, Lei Y, Chen L, Shen G, Pan B, Tao S (2022) Nitrated and oxygenated polycyclic aromatic hydrocarbons emissions from solid fuel combustion in rural China: database of 12 real-world scenarios for residential cooking and heating activities. Sci Total Environ 852:158501. https://doi.org/10.1016/j.scitotenv.2022.158501
Wei L, Yu Z, Zhu C, Chen Y, Pei Z, Li Y, Yang R, Zhang Q, Jiang G (2023) An evaluation of the impact of traffic on the distribution of PAHs and oxygenated PAHs in the soils and moss of the southeast Tibetan Plateau. Sci Total Environ 862:160938. https://doi.org/10.1016/j.scitotenv.2022.160938
Weng X, Tan Y, Fei Q, Yao H, Fu Y, Wu X, Zeng H, Yang Z, Zeng Z, Liang H, Wu Y, Wen L, Jing C (2022) Association between mixed exposure of phthalates and cognitive function among the U.S. elderly from NHANES 2011–2014: three statistical models. Sci Total Environ 828:154362. https://doi.org/10.1016/j.scitotenv.2022.154362
Yang X, Xue Q, Wen Y, Huang Y, Wang Yi, Mahai G, Yan T, Liu Y, Rong T, Wang Yixin, Chen D, Zeng S, Yang C-X, Pan X-F (2022) Environmental polycyclic aromatic hydrocarbon exposure in relation to metabolic syndrome in US adults. Sci Total Environ 840:156673. https://doi.org/10.1016/j.scitotenv.2022.156673
Zhang Y, Dong S, Wang H, Tao S, Kiyama R (2016) Biological impact of environmental polycyclic aromatic hydrocarbons (ePAHs) as endocrine disruptors. Environ Pollut 213:809–824. https://doi.org/10.1016/j.envpol.2016.03.050
Zhang Y, Dong T, Hu W, Wang Xu, Xu B, Lin Z, Hofer T, Stefanoff P, Chen Y, Wang X, Xia Y (2019) Association between exposure to a mixture of phenols, pesticides, and phthalates and obesity: comparison of three statistical models. Environ Int 123:325–336. https://doi.org/10.1016/j.envint.2018.11.076
Zhang Y-J, Huang C, Lv Y-S, Ma S-X, Guo Y, Zeng EY (2021) Polycyclic aromatic hydrocarbon exposure, oxidative potential in dust, and their relationships to oxidative stress in human body: a case study in the indoor environment of Guangzhou, South China. Environ Int 149:106405. https://doi.org/10.1016/j.envint.2021.106405
Acknowledgements
The authors thank the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention (CDC) for sharing the data. Thanks are given to Zhang Jing (Shanghai Tongren Hospital) for his work on the NHANES database. His outstanding work, “nhanesR” package and webpage, makes it easier for us to explore the NHANES database.
Funding
This work is sponsored by grant from the Fujian Natural Science Foundation Project (2022J01746).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Fei Lin, Huaicheng Wang, Xuefei Wang, and Yihong Fang. The first draft of the manuscript was written by Fei Lin, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
Study protocols for NHANES were approved by the NCHS ethnics review board (Protocol #2011–17, https://www.cdc.gov/nchs/nhanes/irba98.htm), and NHANES was conducted in accordance with the Helsinki Declaration of 1975, as revised in 2013.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Lotfi Aleya
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lin, F., Wang, H., Wang, X. et al. Association between exposure to multiple polyaromatic hydrocarbons and periodontitis: findings from a cross-sectional study. Environ Sci Pollut Res 30, 112611–112624 (2023). https://doi.org/10.1007/s11356-023-29421-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-29421-9