Abstract
The accelerated accumulation of phthalate esters (PAEs) in paddy soils poses a serious threat to human health. However, related studies mainly focus on facility vegetable fields, drylands, and orchards, and little is known about paddy soils. In this study, 125 samples were collected from typical red paddy fields to investigate the pollution characteristics, sources, health risks, and main drivers of PAEs. Soil physicochemical properties, enzyme activity, and bacterial community composition were also measured simultaneously. The results showed that eight PAE congeners were detected ranging from 0.17 to 1.97 mg kg−1. Di-n-butyl phthalate (DBP), di-(2-ethylhexyl) phthalate (DEHP), and di-isobutyl phthalate (DIBP) were the most abundant PAE congeners, accounting for 81% of the total PAEs. DEHP exhibited a potential carcinogenic risk to humans through the intake route. The main PAEs were positively correlated with soil organic matter (SOM) and soil water content (SWC) contents. Low levels of PAEs increased bacterial abundance. Furthermore, most PAE congeners were positively correlated with hydrolase activity. Soil acidity and nutrient dynamics played a dominant role in the bacterial community composition, with PAE congeners playing a secondary role. These findings suggest that there may be a threshold response between PAEs and organic matter and nutrient transformation in red paddy soils, and that microbial community should be the key driver. Overall, this study deepens the understanding of ecological risks and microbial mechanisms of PAEs in red paddy soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phthalate esters (PAEs) are the most commonly used plasticizers in polyvinyl chloride (PVC) products to improve durability and plasticity (Tan et al. 2018). Over the past few decades, PAEs have been widely used in industry, agriculture, and even residential areas worldwide (Tran et al. 2022; Wu et al. 2023). According to statistics, global plastic production reached 359 million tons in 2018, and China accounted for 30% of the total production (Garcia and Robertson 2017; Plastics Europe 2020). In addition, PAEs are commonly used in non-plastic processing industries such as insect repellents, pesticide carriers for insecticides, synthetic rubber, and printing ink additives (Tyler et al. 1998). Due to extensive production and improper recycling, as well as the covalent bonding of PAEs to materials through hydrogen and van der Waals forces, it readily permeate into environment matrices through leaching, migration, and volatilization (Lee et al. 2019). The accumulation of PAEs in environmental media has become a global concern. PAEs have been reported to widespread in diverse environment matrices, including atmospheric particulate matter (Liu et al. 2022), wastewater (Tran et al. 2022), food (Ji et al. 2014), indoor air (Bu et al. 2016), greenhouse vegetable production bases (Zhou et al. 2020), open seas (Mi et al. 2019), and suspended particulate matter in water (He et al. 2019).
Severe PAE pollution poses a significant threat to human health through various exposure pathways such as intake, ingestion, inhalation, and dermal exposure. According to numerous epidemiological and clinical studies, PAEs may cause estrogenic endocrine disruption (Mu et al. 2015), reproductive toxicity (Arbuckle et al. 2018), and breast cancer (Benjamin et al. 2017). Several studies have assessed the risk of human health exposure to PAEs in farmland, parks, atmospheric particulate matter, and plastic film greenhouses (Lü et al. 2018). PAEs even occur in different types of foods such as fish and chicken (Ji et al. 2014). Niu et al. (2014) found the non-cancer and carcinogenic risks of phthalates to be within generally acceptable levels in China. Since the growing emphasis on soil PAE pollution, it is important to correctly master the distribution, source, and behavior of PAEs, and to establish an early warning mechanism.
Agricultural soils are major reservoirs of hydrophobic organic pollutants, especially PAEs (Zhou et al. 2020). The long-term applications of agricultural mulch, chemical fertilizer, compost, and irrigation wastewater accelerated the accumulation of soil PAEs (Zhang et al. 2015; Ye et al. 2021; Xu et al. 2022). Also, soil properties, microbial degradation, and meteorological conditions affect the accumulation and dissipation of PAEs (Ma et al. 2020; Dueñas-Moreno et al. 2022). Temperature and precipitation have been reported to impact PAE dispersion. In general, soil PAE concentrations are higher and composition is more complex in summer than in winter (Wang et al. 2021; Zhou et al. 2021; Xu et al. 2022). Heavy precipitation during the rainy season increases the leaching of PAEs (Wu et al. 2011). Besides, lower pH will promote PAE adsorption in soil through a combination of compound hydrophobicity and humic acid (Cheng et al. 2018; Xue et al. 2020). Soil-dissolved organic matter (DOM) can adsorb PAEs and increase the solubility and mobility of soil PAEs (Yang et al. 2013; Tang et al. 2020). PAEs have been shown to affect soil microbial community composition and enzyme activities related to carbon, nitrogen, and phosphorus cycles (Zhou et al. 2020; Tao et al. 2022). However, the above research results are still limited in open red paddy soils, and it is important to elucidate the biotic and abiotic factors affecting the distribution of PAEs in farmland to provide theoretical guidance for the remediation of PAE-contaminated soils.
The red soil hilly area is an important rice production base in southern China. Inorganic and organic pollution in soil is a serious threat to human health. Soil organic pollution (especially PAEs) is prominent (Zhang et al. 2020). However, the spatial distribution and major control factors of PAEs in paddy soils at the regional scale have not been thoroughly investigated. Taking Taihe County, Jiangxi Province, as a case, this study aimed to investigate the composition, spatial distribution, and health risks of PAEs in paddy soils in southern China, and to evaluate the relationship between soil PAEs and major biotic and abiotic factors.
Materials and methods
Study area description and soil sampling
The study area is located in Taihe County, Jiangxi Province, southern China (114°17″–115°20″E, 26°27″–26°58″N). The administrative division includes 21 townships with an area of 2,666 km2. It characterizes a subtropical monsoon climate. The mean annual temperature is 18.7 °C; the highest and lowest temperatures are 34.3 °C (in July) and 3.9 °C (in January), respectively. The mean annual precipitation is 1473 mm with 44% of them falling in between April and June, and drought is prone to occur from September to October (Yu et al. 2018). The study area is a typical red soil hilly area, with mountains, valley plains, and hills accounting for 15.9%, 29.6%, and 54.5% of the total area, respectively. The Jitai Basin is an important agricultural area and commercial grain production base in China, with rice cultivation accounting for about 93% of the total planting area. The study area is rich in mineral resources and tungsten mining is mainly concentrated in Xiaolong Town, which has caused serious pollution of the farmland soil in the area (Zhang et al. 2022).
In November 2020, four to seven representative farmlands far away from residential areas were selected in each township, and 125 sampling sites were located using GPS (Fig. 1). Five plots (20 m × 20 m) were established at each site. After removing gravels and plant debris, topsoil in the 0–20 cm depth was collected using a five-point sampling method. All soil samples were pooled together and immediately passed through a 2-mm sieve for homogenization. Here, each soil samples were divided into two subsamples: one for the determination of PAEs and general soil physicochemical properties, and the other for enzyme activity and high-throughput sequencing analysis. All samples were stored at – 20 °C until analysis.
Determination of PAEs
The extraction and purification of PAEs were performed according to Wei et al. (2020). Briefly, 10 g of soil was mixed with 30 ml of acetone/hexane (1: 1, v/v) in a glass centrifuge tube, vortexed for 1 min, and left overnight. The tubes were sonicated for 30 min and then centrifuged at 3000 rpm for 5 min, and this procedure was repeated three times to finally collect about 70 ml supernatant. The extract was purified on a glass column packed with 1 cm of anhydrous Na2SO4 and 4 cm of neutral silicone. The column was prewashed with 15 ml of hexane and 15 ml of hexane/acetone (4:1, v/v). The extract wash eluted with 40 ml of hexane/acetone (4:1, v/v). The final eluate was concentrated to 1 ml using a gentle stream of nitrogen gas. All solvents used were residue-analysis grade. All glassware used was soaked in potassium dichromate for 6 h, baked in a muffle furnace at 400 °C for 6 h, and cleaned with hexane and acetone before use to avoid potential phthalate contamination, and plastic utensils were strictly prohibited during the experiment. PAEs were analyzed using an Agilent 7890 GC equipped with an Agilent 5975 MS in selected ion monitoring (SIM) mode. A TD-5MS capillary column (30 m × 0.25 mm × 0.25 μm, Agilent Technologies) was used. Qualitative and quantitative analyses were summarized in Supplementary Material.
Health risk assessment
Phthalates are ubiquitous in the environment due to their extensive use. PAEs can migrate to rice, cucumber, and cabbage grown in agricultural soils (Wei et al. 2020; Wu et al. 2023). Consumption of contaminated crops, direct soil contact, inhalation, ingestion, and dermal contact are potential pathways of exposure to PAEs. Once in the body, PAEs are rapidly absorbed and metabolized. PAEs and their metabolites have been detected in humans (i.e., breast milk, blood, urine) (Deng et al. 2021). The carcinogenic and non-carcinogenic risks of PAEs to adults and children were assessed using the method recommended by USEPA (2013). Among the PAE congeners, dimethyl phthalate (DMP), diethyl phthalate (DEP), di-(n-butyl)-phthalate (DnBP), and di-n-octyl phthalate (DnOP) were non-carcinogens, whereas benzyl butyl phthalate (BBP) and di-(2-ethyl-hexyl) phthalate (DEHP) were carcinogens. The average daily dose (ADDintake, crops intake; ADDingest, soil ingestion; ADDdermal, dermal contact; ADDinhale, inhalation) was calculated using Eqs. (1) to (4). The hazard quotient (HQ) and carcinogenic risk (CR) representing the total health risks through multiple exposure pathways were calculated by Eqs. (5) and (6).
where Csoil was the average concentration of target phthalates in soil (mg kg−1); BAF was the bioaccumulation factor of individual PAEs from soil to food (vegetables and grains); IRF was the daily food intake of residents (mg day−1), where the IRF of children was supposed to be 1/3 that of adults; IRS was the daily soil intake of residents (mg day−1); EF was exposure frequency (days year−1); ED was exposure duration (year); BW was body weight (kg); AT was average lifespan (days); CF was conversion factor (kg mg−1); SA was skin surface area (cm2 day−1); AF was soil adherence factor (mg cm2); ABS was the proportion of pollutants absorbed from soil through the skin; Ij was respiratory rate (m3 day−1); PEF was particulate emission factor (m3 kg−1). CFS was the carcinogenicity rate (mg kg−1 day−1), and RfDi indicated the average recommended daily dose of non-carcinogenic substances (mg kg−1 day−1). Parameters for non-cancer and carcinogenic risks were presented in Table S2. CR was considered very low or negligible if it was below 10−6, acceptable if 10−6 < CR < 10−4, and high if it was above 10−4. As for HQ, humans were considered to be at non-cancer risk if the value was greater than 1.
Determination of soil physicochemical properties and enzyme activity
Soil pH and electrical conductivity (EC) were measured at a soil/water ratio of 1:2.5 using a pH and conductivity meter (OHAUS Model ST300), respectively. Soil cation exchange capacity (CEC) was determined using the ammonium acetate exchange method. The concentrations of NH4+–N and NO3−–N in soil were analyzed by colorimetry on a continuous flow auto analyzer (AA3, SEAL Company, Germany). Total carbon (TC) and total nitrogen (TN) were determined by dry combustion method on a CN analyzer (vario EL III, Elementa, Germany). Soil water content (SWC) was determined using the oven-drying method (105 °C, 24 h). Also, hydrolase and oxidoreductase activities were determined using a modified microplate fluorometric protocol (Saiya-Cork et al. 2002). Seven hydrolases (i.e., β-glucosidase (βG), α-1,4-glucosidase (αG), acid phosphatase (AP), cellobiohydrolase (CB), β-1,4-xylosidase (βX), β-N-acetylgluco-saminidase (NAG), and leucine aminopeptidase (LAP)), and two oxidases (i.e., polyphenol oxidase (PhOx), peroxidase (Perox)) were considered. The fluorescence and absorbance were measured on a microplate reader (SynergyH4, BioTek).
Determination of bacterial community composition
Total DNA was extracted from 0.5 g soil using the Fast DNA™ Spin Kit for Soil (MP Biomedicals, USA). The concentration and quality of the extracted DNA were determined by spectrophotometer analysis (NanoDrop 2000, Thermo Fisher Scientific, USA). The bacterial V3V4 region gene was amplified using the universal primer pairs 338F (338F: ACTCCTACGGGAGGCAGCA)/806R (GGACTACHVGGGTWTCTAAT). The purified PCR amplicons were sequenced and quantified on the Illumina MiSeq platform. Raw sequencing data were filtered and analyzed using QIIME2 (Shetty and Lahti 2019), and DADA2 was used for primer removal, denoise, filtering, splicing, and chimera removal to generate high-quality amplicon sequence variants (ASVs) (Callahan et al. 2016). ASV affiliations were assigned using the Silva_123 database (Kõljalg et al. 2013). The raw sequencing data obtained in this study had been uploaded to the NCBI Sequence Read Archive under the accession number PRJNA853870.
Statistical analysis
Before statistical analysis, Shapiro-Wilk’s test and Levene’s test were used to assess the normality and homogeneity of variance, respectively. The data that did not follow a normal distribution were log-transformed before analysis. Descriptive statistics of soil PAEs, physicochemical properties, and enzyme activities were performed using SPSS 22.0 software (SPSS Inc., Chicago, IL, USA). The spatial distribution of soil individual and total PAEs was mapped using kriging interpolation in ArcGIS 10.6 (Esri Corp., Redlands, CA, USA). Hierarchical cluster analysis was used to investigate the correlations between soil PAEs and soil enzymes, physicochemical properties, and bacterial diversity by the “pheatmap” package for R. Principal component analysis (PCA) was estimated from the “NbClust” and “stats” packages to analyze the source of individual PAEs. Redundancy analysis (RDA) was performed to visualize the dominant factors of soil PAEs using the “vegan” R package based on the Bray-Curtis distance. Before analysis, forward selection was performed using the “packfor” package.
Results
Presence of soil PAEs
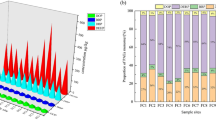
Eight PAE congeners were detected in paddy soils in Taihe County, including DMP, DBP, DEHP, DEP, bis(2-ethoxyethyl) phthalate (DEEP), diisobutyl phthalate (DIBP), bis(2-methoxyethyl) phthalate (DMEP), and BBP. The first six PAEs (i.e., DMP, DBP, DEHP, DEP, DEEP, and DIBP) were detected in all soil samples, with 94% and 55.2% detection rates for BBP and DMEP, respectively. Total PAE concentrations ranged from 0.17 to 1.97 mg kg−1 with a mean of 0.94 mg kg−1 (Fig. 2). DBP, DEHP, and DIBP were the dominant PAE species with an average of 0.41 mg kg−1, 0.22 mg kg−1, and 0.16 mg kg−1, respectively, accounting for 81% of the total PAEs (Fig. 2). ∑PAES was the sum of DMP, DBP, DEHP, DEP, and BBP concentrations, which accounted for 78.9% of the total PAEs on average (Fig. 2). In contrast, the concentrations of DEP, DMEP, and BBP were typically below 0.06 mg kg−1, with mean values of 0.014 mg kg−1, 0.005 mg kg−1, and 0.033 mg kg−1, respectively (Fig. 2).
Violin plot of the distribution of eight PAE concentrations in paddy soils. ∑6PAEs represents the sum of the six dominant PAE congeners, and total represents the total concentration of PAEs in paddy soils. The inside of the violin plot is a boxplot (median represented by a white dot); the outside is wrapped in a kernel density plot, where wider shapes indicate higher probability of values
Kriging interpolation was used to map the spatial distribution of eight PAEs, all of which exhibited high spatial heterogeneity (Fig. 3). The distribution of DMP, DEP, DIBP, and DBP concentrations showed a similar spatial pattern, i.e., the highest concentration was in the east of Taihe City, followed by the west, and the lowest in the central (Fig. 3a–d). In contrast, the spatial distribution of DEHP, DMEP, and DEEP concentrations was relatively uniform and dispersed (Fig. 3e–g). Also, the high values of BBP concentrations were in the eastern and western Taihe County, whereas the low values covered the central part (Fig. 3h). The different spatial patterns of PAE congeners could suggest their different origins.
Spatial distribution of the concentrations of various PAE congeners in paddy soils in Taihe County. a Dimethyl phthalate (DMP); b diethyl phthalate (DEP); c diisobutyl phthalate (DIBP); d di-(n-butyl)-phthalate (DBP); e di-(2-methoxyethyl) phthalate (DMEP); f bis(2-ethoxyethyl) phthalate (DEEP); g di-(2-ethyl-hexyl) phthalate (DEHP); h butyl benzyl phthalate (BBP)
Principal component analysis (PCA) and correlation analysis (CA) were performed to determine the source of PAEs. In this study, the PAEs were classified into three groups (k = 3), indicating that there were three main sources of PAEs in paddy soils in Taihe County. To maximize the sum of variances of the loading vectors, two principal components (PCs) were extracted by varimax rotation, explaining 52.7% and 29.1% of the total variation, respectively (Fig. 4). The first principal component (PC1) was related to DEP, DBP, DIBP, and DMP, indicating similar environmental origins and behaviors. The correlation coefficients between them were all greater than 0.6 (P < 0.01) (Table S4). The second principal component (PC2) was highly related to DMEP, DEEP, and DEHP (Fig. 4). The correlation coefficient between DMEP and DEEP was 0.97 (P < 0.01) (Table S4). However, soil BBP concentrations were negatively correlated with DMEP and DEEP concentrations, respectively (RDMEP = − 0.28, RDEEP = − 0.29; P < 0.01) (Table S4). This was consistent with the position vector of BBP in Fig. 4.
Non-cancer and carcinogenic risks
The non-cancer and carcinogenic risks to local populations were assessed by the concentrations of PAEs in soil. The results of risk assessment are summarized in Table 1. Dietary intake was the main route of exposure to PAEs for both children and adults, with their HQ values two or more orders of magnitude higher than the other three routes, but all were below the threshold (HQ = 1). Children and adults had the highest HQ for DEHP intake, up to 0.16 and 0.10, respectively (Table 1). Furthermore, the HQ values for ingestion and dermal intake were one order of magnitude greater in children than in adults (Table 1). For carcinogenic risk, the CR values for BBP were less than 10−6, which was considered a “very low or negligible” level. In contrast, the CRs for DEHP ingestion were 4 × 10−6 and 1 × 10−5, indicating a low cancer risk for human exposure to DEHP. Therefore, DEHP exhibited potential carcinogenicity to humans through the intake route.
Bacterial composition and diversity
The rarefaction curves showed near saturation at each sampling site, indicating sufficient sequencing depth (Fig. S1). Various alpha-diversity metrics (e.g. Chao1 index, observed species index, Shannon index, and Simpson index) were calculated to evaluate the richness and diversity of bacterial communities. The spatial distribution of Chao 1 index and observed species was similar to the total PAE concentrations (Fig. S2), i.e. the highest in the eastern region, followed by the western region, and the lowest in the central region (Fig. S2). Taxonomy was assigned to amplicon sequence variants (ASVs) using the q2-feature-classifier classify-sklearn Naive Bayes taxonomy classifier. The composition of soil bacterial community at different sampling sites was similar, but the proportion of individual species at the phylum level varied. The dominant bacterial phyla were Proteobacteria, Chloroflexi, Acidobacteria, Nitrospirae, Actinobacteria, and Bacteroidetes, accounting for 89% of the total bacterial species (Fig. S3).
Soil abiotic and biotic factors
Soil general properties were determined for 125 paddy soil samples (Table S3). Soil water content (SWC) ranged from 12.58 to 57.67% with an average of 26.85%, consistent with less precipitation at the time of sampling. The soil was generally acidic, and soil pH ranged from 4.36 to 5.88 with an average of 4.89. The mean values of EC and CEC were 86.82 ms cm−1 and 5.53 cmol kg−1, respectively. SOM content varied from 11.12 to 79.19 g kg−1, with an average of 41.43 g kg−1. Soil TN content varied from 0.56 to 3.18 g kg−1, with an average of 41.43 g kg−1. Soil C/N ratio averaged 13.55, indicating nitrogen limitation. Soil DOC content averaged 42.14 mg kg−1, accounting for only 0.18% of total SOC. Soil-dissolved N was dominated by NO3−–N, averaging 12.80 mg kg−1 and accounting for 49.48% of total dissolved N content. Soil DON and NH4+–N contents accounted for 40.28% and 8.35% of the dissolved N content on average, respectively. The soil texture was sandy loam, and the average proportions of sand, silt, and clay were 17.41%, 80.75%, and 1.85%, respectively.
Seven hydrolase and two oxidase activities were measured (Table 2). The activities of AP, βG, and NAG belonged to the first echelon, with an average of 2041.40 nmol g soil−1 day−1, 547.18 nmol g soil−1 day−1, and 154.39 nmol g soil−1 day−1, respectively. βX, CB, αG, and LAP activities belonged to the second echelon, with an average of 94.01 nmol g soil−1 day−1, 49.77 nmol g soil−1 day−1, 23.86 nmol g soil−1 day−1, and 56.26 nmol g soil−1 day−1. The average PPO and PER concentrations were 1.64 μmol g soil−1 day−1 and 1.21 μmol g soil−1 day−1, which were higher than the hydrolase activities (except AP) (Table 2). Overall, the average alpha-diversity indices (Chao1, Observed species, Shannon, and Simpson) of bacterial communities were all significantly higher than those of fungal communities, whereas their coefficients of variations (CVs) were opposite (Table 2).
Relationship between PAEs and soil variables
Cluster analysis classified soil PAEs into five groups. BBP concentrations were negatively correlated with soil hydrolase activities, especially with CB, NAG, and αG (Fig. 5). In contrast, DMEP, DEEP, DEHP, ΣPAEs, and total PAEs were strongly positively correlated with most hydrolase activities (except CB) (Fig. 5). In addition, soil oxidase activities (PPO, PER) were negatively correlated with individual PAE concentrations, but not statistically significant (Fig. 5). For soil properties, DMP, DIBP, DBP, DEHP, ΣPAEs, and total PAEs were positively correlated with SOM and SWC contents. Soil DOC (dissolved organic carbon) concentrations were negatively correlated with DMEP and DEEP, but positively correlated with BBP, DBP, and ΣPAEs (Fig. 5). Most PAE species were significantly and positively correlated with the Chao1 and Observed species indices, but not with Shannon and Simpson (Fig. 5).
Redundancy analysis (RDA) was performed to investigate the relationships between soil properties, PAEs, and bacterial communities. Based on the 12 selected soil variables, the first and second axes of the RDA explained 22.1% and 6.4% of the total variation in bacterial community distribution, respectively (Fig. 6). Soil physiochemical properties such as pH, CEC, AP, and TDN had strong effects on the spatial distribution of bacterial communities (Fig. 6), indicating the dominant role of soil acidity and nutrient dynamics. In contrast, BBP and DMEP contributed the most to the second RDA axis (Fig. 6), indicating that the dominant PAE species had a secondary role in the spatial distribution of bacterial communities.
Redundancy analysis (RDA) of bacterial community structure and a subset of 12 environmental variables including silt, pH, CEC, SWC, EC, SOM, AP, TP, TDN, NH4+, DMEP, and BBP. Black dots represent microbial community composition in a multidimensional space. Arrows represent environmental variables. The longer the arrow, the stronger the effect of the environmental variable on microorganism
Discussion
Distribution of PAEs in paddy soils
According to the guidelines developed by the New York State Department of Environmental Conservation (2010), the concentrations of DMP and DBP exceeded the thresholds by three to five times, indicating that the paddy soils in Taihe County were heavily polluted by PAEs. Clarifying the sources of PAEs is important to understand their environmental fate (Lü et al. 2018; Li et al. 2023). The use of chemical fertilizers, pesticides, and herbicides is an important means to increase crop yield in modern agriculture. PAEs are added to commoditized pesticides as solvents and even dissolved from the pesticide packaging (Wang et al. 2013a, 2013b). PAEs were detected in 22 fertilizers widely used in China ranging from 1.2 to 2795 μg kg−1, with DBP and DEHP having the highest content (Mo et al. 2008). Wei et al. (2020) found that the higher the fertilizer application, the higher the accumulation of PAE in the soil. Rice seedlings are planted using mulch or seedling trays. It is well known that seedling trays are made of polyethylene (PE), and BBP is the main plasticizer for PE (Zhang and Choudhury 2017). Plasticizers usually form weak physical interactions with polymers and are easily released into the soil (Ma et al. 2020; Li et al. 2023). This process is accompanied with the production of plastic debris. More and more PAEs remained in the soil due to aging and fragmentation during the agricultural activities. Literature also reveals that PAEs are natural-origin products in wood, lignin oxidation products, microorganisms, and humic compounds (Mathur 1974). DnOP was not detected in this study. DEHP and DnOP are often present in chemical fertilizers but are not added together (Mo et al. 2008; Kaewlaoyoong et al. 2018). Koniecki et al. (2011) reported that DEP was detected in almost all cosmetic and personal care products at levels up to 255 mg kg−1. Also, DnBP and DIBP were added in epoxy resin, cellulose ester, and adhesive (Daiem et al. 2012).
A high heterogeneity in the distribution of soil PAEs was observed, with the highest concentrations of total PAEs in the eastern region (Fig. 3). This could be due to decades of tungsten mining and heavy metal accumulation in this region (Guo et al. 2022). Heavy metal Cd2+/Cu2+ can enhance the adsorption capacity of DEHP in soil, especially chemisorption (Stoppa et al. 2017; Zhang et al. 2021). Furthermore, heavy metal enrichment inhibited the degradation rate of soil PAEs (Chang et al. 2005). The concentrations of PAEs were significantly higher in the east and west of the country than in the central part of county. The distribution of PAEs was inconsistent with gross domestic product (GDP) (Fig. S4). In general, relatively high levels of PAEs are observed in industrial and business centers. However, in the present study, the regions with high GDP were less polluted, which could be due to the limitation of our study scale. Wang et al. (2018) also reported that the concentrations of Σ6PAEs (six priority pollutants) increased with the distance from urban areas. DEHP and DBP were the most abundant phthalate esters in atmospheric fine particles and have been widely reported. This is a consequence of their widespread use in the urbanization and industrial discharges (Lü et al. 2018). Atmospheric dry and wet depositions were non-negligible sources of PAEs in remote regions, as confirmed by the occurrence of PAEs in the Arctic Ocean and the Tibetan Plateau (Routti et al. 2021; Zhang et al. 2022). In addition, the logarithm octanol-air partition coefficients (logKoa) of DEHP and BBP are 12.5 and 9.0, respectively (Staples et al. 1997), suggesting that they are more likely to adhere to particular matter and dust and eventually fall to the soil or migrate elsewhere through dry and wet deposition. This process was accompanied by the separation of PAEs (Ma et al. 2020), which could be the main reason of high-low-high spatial distribution pattern from west to east. This was further supported by the industry distribution.
Risk assessment of exposure to PAEs
Due to the ubiquity of PAEs, exposure to phthalates is inevitable (Lü et al. 2018; Li et al. 2023). Humans are exposed to PAEs through dietary and non-dietary pathways. The HQ values of DEHP, DnBP, BBP, DEP, and DMP for children and adults decreased sequentially, and similar results were observed in Xi’an, China (Wang et al. 2018). The CRintake of DEHP were all higher than 1 × 10−6, indicating a potential carcinogenic risk to humans. Dermal absorption was the dominant source of exposure for DBP, BBP, and DEHP, whereas inhalation was the main source of exposure for DEHP and DBP. These were consistent with Wang et al. (2017). Becker et al. (2009) showed that metabolite concentrations decreased with age through biomonitoring. Children are particularly vulnerable to environmental PAEs through mouthing plastic products and ingesting dust directly during hand-to-mouth contact (Li et al. 2023). DIBP also deserved special attention. Although DIBP has not been listed as a priority pollutant by authorities such as US EPA, it has been reported to have hormonal effects similar to DBP (Borch et al. 2006).
Several studies have attempted to find appropriate thresholds for plant and soil safety. Based on ecotoxicology and environmental chemistry data, the environmental risk limits for DEHP and DnBP in sediment and fresh soil with 10% organic matter are 1.0 mg kg−1 and 0.7 mg kg−1, respectively (Van Wezel et al. 2000). Wei et al. (2020) found that vegetables with lipid content < 0.21% in PAE-contamination soils with TOC < 1.77% were safe. For example, according to the maximum concentrations of food contaminants defined by the European Commission, BBP, DEHP, and DBP are 30 mg kg−1 food, 1.5 mg kg−1 food, and 0.3 mg kg−1 food, respectively (Petersen and Jensen 2010). PAEs accumulated in soil may be absorbed by crops and vegetables and subsequently transferred to humans through the food chain. Meanwhile, rice consumption has been identified as the main pathway of human exposure to PAEs in China (Wu et al. 2018). Therefore, effective measures are needed to inhibit the transport of PAEs from soil to plants, especially DEHP and DBP.
Relationship between PAE concentrations and bacterial communities
The residues of soil PAEs are closely related to their own physicochemical properties and soil properties (e.g., pH, SOM, etc.) (Yang et al. 2013; Bai et al. 2021). However, our results showed that soil PAE concentrations were weakly correlated with soil pH and SOM (Fig. 5). On the one hand, this may be due to the continuous influx of new PAEs into the soil, which has not yet reached a certain equilibrium (Wang et al. 2017). On the other hand, the long-term high temperature and high humidity environment favors soil organic carbon mineralization, thereby inhibiting the adsorption of hydrophobic organic compounds by SOM (Leirós et al. 1999).
Specific EEAs were involved in carbon and nitrogen cycling and decomposed different substrates. Nitrogen cycling-related enzymes include NAG and LAP, which target chitin and protein, respectively. Carbon cycling-related enzymes include αG, BG, CBH, and BX, which target on cellulose of varying complexity. The results showed that high molecular weight phthalates (DMEP, DEHP, and DEEP) were positively correlated with βG, αG, NAG, and LAP activities, indicating that increasing soil contamination concentrations would promote the decomposition of C- and N-substrates. Studies have shown that bacterial amoA gene abundance increased in the microplastic treatments, suggesting that PAEs enhanced soil nitrification potential (Seeley et al. 2020). Zhu et al. (2022) demonstrated that the impact of plasticized PVC microplastics on soil microbiome and nitrogen cycle was mainly attributed to the added phthalates. Similarly, Wang et al. (2018) reported that the abundance of N-cycle functional genes increased significantly with increasing DMP concentrations. In this study, no significant correlation was observed between PAEs and Shannon and Simpson indices, but there were positive correlations between PAEs and Chao1 and Observed species (Fig. 5). These results indicated that PAEs could increase bacterial abundance rather than diversity. Once low concentrations of chemical contaminants enter the soil, microorganisms expand and reproduce faster to resist exogenous pollutants (Shen et al. 2019). This could explain the increase in bacterial abundance. Soil microbial communities play a vital role in maintaining ecosystem processes such as nutrient cycling and pollution degradation (Delgado-Baquerizo 2019). Bacterial community composition was closely related to soil properties such as pH, CEC, and AP, which was consistent with the findings of Wang et al. (2019). Our research found that although the concentrations of BBP and DMEP were relatively low, they had a significant effect on the microbial community, implying that the type of phthalate rather than the concentration was the main factor affecting microorganisms (Zhou et al. 2020). BBP can increase the abundance of functional microorganisms such as Proteiniphilum, Petrimonas, and Syntrophomonas in waste activated sludge (Tang et al. 2021). Numerous studies have shown that the dissipation of PAEs is mainly driven by various indigenous bacteria (Hu et al. 2021), such as Proteobacteria and Acinobacteria, whereas fungi are hardly involved in degradation (Zhu et al. 2018). Anthropogenic pollutants are by-products of agricultural activities and have limited impact on bacterial community composition (Sun et al. 2018).
Conclusions
This study focused on the pollution characteristics, sources, human health risk, and drivers of PAEs in red paddy soils in southern China. DBP, DEHP, and DIBP were the major PAE congeners. BBP and DMEP had a greater impact on indigenous bacterial communities than other PAEs. Several types of PAEs exhibited high spatial heterogeneity, indicating different origins and environmental drivers including tungsten mining, atmospheric deposition, and industrial sources. Soil intake was the main exposure pathway, and the carcinogenic risk of DEHP ingestion had exceeded the threshold. The behavior of PAEs in red paddy soils was closely related to the dynamics of SOM, nutrients, and bacterial communities. Low levels of PAE pollution increased bacterial richness and promoted carbon and nitrogen transformation in paddy soils. Soil acidity and nutrient dynamics dominated the spatial distribution of bacterial communities, with the major PAE congeners playing a secondary role. Overall, more attention should be paid to PAEs in paddy soils in southern China. Our study provided theoretical guidance for the remediation of PAEs in red paddy soils.
Data availability
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
References
Arbuckle TE, Agarwal A, MacPherson SH, Fraser WD, Sathyanarayana S, Ramsay T, Dodds L, Muckle G, Fisher M, Foster W, Walker M, Monnier P (2018) Prenatal exposure to phthalates and phenols and infant endocrine-sensitive outcomes: the MIREC study. Environ Int 120:572–583. https://doi.org/10.1016/j.envint.2018.08.034
Bai H, Lu P, Li Y, Wang J, Zhao H (2021) Prediction of phthalate acid esters degradation in soil using QSAR model: a combined consideration of soil properties and quantum chemical parameters. Ecotoxicol Environ Saf 226:112830. https://doi.org/10.1016/j.ecoenv.2021.112830
Becker K, Goen T, Seiwert M, Conrad A, Pick-Fuss H, Muller J, Wittassek M, Schulz C, Kolossa-Gehring M (2009) GerES IV: phthalate metabolites and bisphenol A in urine of German children. Int J Hyg Environ Health 212(6):685–692. https://doi.org/10.1016/j.ijheh.2009.08.002
Benjamin S, Masai E, Kamimura N, Takahashi K, Anderson RC, Faisal PA (2017) Phthalates impact human health: epidemiological evidences and plausible mechanism of action. J Hazard Mater 340:360–383. https://doi.org/10.1016/j.jhazmat.2017.06.036
Borch J, Axelstad M, Vinggaard AM, Dalgaard M (2006) Diisobutyl phthalate has comparable anti-androgenic effects to di-n-butyl phthalate in fetal rat testis. Toxicol Lett 163:183–190. https://doi.org/10.1016/j.toxlet.2005.10.020
Bu Z, Zhang Y, Mmereki D, Yu W, Li B (2016) Indoor phthalate concentration in residential apartments in Chongqing, China: implications for preschool children's exposure and risk assessment. Atmospheric Environ 127:34–45. https://doi.org/10.1016/j.atmosenv.2015.12.010
Callahan BJ, Sankaran K, Fukuyama JA, McMurdie PJ, Holmes SP (2016) Bioconductor workflow for microbiome data analysis: from raw reads to community analyses. F1000Res 5:1492. https://doi.org/10.12688/f1000research.8986.2
Chang B, Liao C, Yuan S (2005) Anaerobic degradation of diethyl phthalate, di-n-butyl phthalate, and di-(2-ethylhexyl) phthalate from river sediment in Taiwan. Chemosphere 58:1601–1607. https://doi.org/10.1016/j.chemosphere.2004.11.031
Cheng J, Liu Y, Wan Q, Yuan L, Yu X (2018) Degradation of dibutyl phthalate in two contrasting agricultural soils and its long-term effects on soil microbial community. Sci Total Environ 640:821–829. https://doi.org/10.1016/j.scitotenv.2018.05.336
Daiem MMA, Rivera-Utrilla J, Ocampo-Perez R, Mendez-Diaz JD, Sanchez-Polo M (2012) Environmental impact of phthalic acid esters and their removal from water and sediments by different technologies-a review. J Environ Econ Manage 109:164–178. https://doi.org/10.1016/j.jenvman.2012.05.014
Delgado-Baquerizo M (2019) Obscure soil microbes and where to find them. ISME J 13:2120–2124. https://doi.org/10.1038/s41396-019-0405-0
Deng M, Liang X, Du B, Luo D, Chen H, Zhu C, Zeng L (2021) Beyond classic phthalates: Occurrence of multiple emerging phthalate alternatives and their metabolites in human milk and implications for combined exposure in infants. Environ Sci Technol Lett 8(8):705–712. https://doi.org/10.1021/acs.estlett.1c00476
Dueñas-Moreno J, Mora A, Cervantes-Avilés P, Mahlknecht J (2022) Groundwater contamination pathways of phthalates and bisphenol A: origin, characteristics, transport, and fate – a review. Environ Int 170(107550):107550. https://doi.org/10.1016/j.envint.2022.107550
Garcia JM, Robertson ML (2017) The future of plastics recycling chemical advances are increasing the proportion of polymer waste that can be recycled. Science 358:870–872. https://doi.org/10.1126/science.aaq0324
Guo Y, Cheng S, Fang H, Yang Y, Li Y, Zhou Y (2022) Responses of soil fungal taxonomic attributes and enzyme activities to copper and cadmium co-contamination in paddy soils. Sci Total Environ 844:157119. https://doi.org/10.1016/j.scitotenv.2022.157119
He Y, Wang Q, He W, Xu F (2019) The occurrence, composition and partitioning of phthalate esters (PAEs) in the water-suspended particulate matter (SPM) system of Lake Chaohu China. Sci Total Environ 661:285–293. https://doi.org/10.1016/j.scitotenv.2019.01.161
Hu R, Zhao H, Xu X, Wang Z, Yu K, Shu L, Yan Q, Wu B, Mo C, He Z (2021) Bacteria-driven phthalic acid ester biodegradation: current status and emerging opportunities. Environ Int 154:106560. https://doi.org/10.1016/j.envint.2021.106560
Ji Y, Wang F, Zhang L, Shan C, Bai Z, Sun Z, Liu L, Shen B (2014) A comprehensive assessment of human exposure to phthalates from environmental media and food in Tianjin China. J Hazard Mater 279:133–140. https://doi.org/10.1016/j.jhazmat.2014.06.055
Kaewlaoyoong A, Vu CT, Lin C, Liao CS, Chen JR (2018) Occurrence of phthalate esters around the major plastic industrial area in southern Taiwan. Environ Earth Sci 77:1–11. https://doi.org/10.1007/s12665-018-7655-4
Kõljalg U, Nilsson RH, Abarenkov K, Tedersoo L, Taylor AF, Bahram M, Bates ST, Bruns TD, Bengtsson-Palme J, Callaghan TM, Douglas B, Drenkhan T, Eberhardt U, Dueñas M, Grebenc T, Griffith GW, Hartmann M, Kirk PM, Kohout P et al (2013) Towards a unified paradigm for sequence-based identification of fungi. Mol Ecol 22:5271–5277. https://doi.org/10.1111/mec.12481
Koniecki D, Wang R, Moody RP, Zhu J (2011) Phthalates in cosmetic and personal care products: concentrations and possible dermal exposure. Environ Res 111:329–336. https://doi.org/10.1016/j.envres.2011.01.013
Lee YS, Lee S, Lim JE, Moon HB (2019) Occurrence and emission of phthalates and non-phthalate plasticizers in sludge from wastewater treatment plants in Korea. Sci Total Environ 692:354–360. https://doi.org/10.1016/j.scitotenv.2019.07.301
Leirós MC, Trasar-Cepeda C, Seoane S, Gil-Sotres F (1999) Dependence of mineralization of soil organic matter on temperature and moisture. Soil Biol Biochem 31:327–335. https://doi.org/10.1016/S0038-0717(98)00129-1
Li X, Wang Q, Jiang N, Lv H, Liang C, Yang H, Yao X, Wang J (2023) Occurrence, source, ecological risk, and mitigation of phthalates (PAEs) in agricultural soils and the environment: A review. Environ Res 220:115196. https://doi.org/10.1016/j.envres.2022.115196
Liu Z, Sun Y, Zeng Y, Guan Y, Huang Y, Chen Y, Li D, Mo L, Chen S, Mai B (2022) Semi-volatile organic compounds in fine particulate matter on a tropical island in the South China Sea. J Hazard Mater 426:128071. https://doi.org/10.1016/j.jhazmat.2021.128071
Lü H, Mo C, Zhao H, Xiang L, Katsoyiannis A, Li Y, Cai Q, Wong M (2018) Soil contamination and sources of phthalates and its health risk in China: a review. Environ Res 164:417–429. https://doi.org/10.1016/j.envres.2018.03.013
Ma B, Wang L, Tao W, Liu M, Zhang P, Zhang S, Li X, Lu X (2020) Phthalate esters in atmospheric PM2.5 and PM10 in the semi-arid city of Xi’an, Northwest China: Pollution characteristics, sources, health risks, and relationships with meteorological factors. Chemosphere 242:125226. https://doi.org/10.1016/j.chemosphere.2019.125226
Mathur SP (1974) Phthalate esters in the environment: pollutants or natural products? J Environ Qual 3:189–197. https://doi.org/10.2134/jeq1974.00472425000300030001x
Mi L, Xie Z, Zhao Z, Zhong M, Mi W, Ebinghaus R, Tang J (2019) Occurrence and spatial distribution of phthalate esters in sediments of the Bohai and Yellow seas. Sci Total Environ 653:792–800. https://doi.org/10.1016/j.scitotenv.2018.10.438
Mo C, Cai Q, Li Y, Zeng Q (2008) Occurrence of priority organic pollutants in the fertilizers China. J Hazard Mater 152:1208–1213. https://doi.org/10.1016/j.jhazmat.2007.07.105
Mu D, Gao F, Fan Z, Shen H, Peng H, Hu J (2015) Levels of phthalate metabolites in urine of pregnant women and risk of clinical pregnancy loss. Environ Sci Technol 49:10651–10657. https://doi.org/10.1021/acs.est.5b02617
New York State Department of Environmental Conservation (2010) CP-51/Soil Cleanup Guidance. http://www.dec.ny.gov/docs/remediation_hudson_pdf/cpsoil.pdf (accessed 6 June 2016)
Niu L, Xu Y, Xu C, Yun L, Liu W (2014) Status of phthalate esters contamination in agricultural soils across China and associated health risks. Environ Pollut 195:16–23. https://doi.org/10.1016/j.envpol.2014.08.014
Petersen JH, Jensen LK (2010) Phthalates and food-contact materials: enforcing the 2008 European Union plastics legislation. Food Addit Contam 27:1608–1616. https://doi.org/10.1080/19440049.2010.501825
PlasticsEurope (2020) Plastics-the facts 2020. An analysis of European plastics production, demand and waste data. https://plasticseurope.org/knowledge-hub/plastics-the-facts-2020/ (accessed 29 Nov 2020)
Routti H, Harju M, Lühmann K, Aars J, Ask A, Goksøyr A, Kovacs KM, Lydersen C (2021) Concentrations and endocrine disruptive potential of phthalates in marine mammals from the Norwegian Arctic. Environ Int 152:106458. https://doi.org/10.1016/j.envint.2021.106458
Saiya-Cork KR, Sinsabaugh RL, Zak DR (2002) The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol Biochem 34:1309–1315. https://doi.org/10.1016/S0038-0717(02)00074-3
Seeley ME, Song B, Passie R, Hale RC (2020) Microplastics affect sedimentary microbial communities and nitrogen cycling. Nat Commun 11:1–10. https://doi.org/10.1038/s41467-020-16235-3
Shen Z, Postnikoff S, Tyler JK (2019) Is Gcn4-induced autophagy the ultimate downstream mechanism by which hormesis extends yeast replicative lifespan? Curr Genet 65:717–720. https://doi.org/10.1007/s00294-019-00936-4
Shetty SA, Lahti L (2019) Microbiome data science. J Biosci 44:1–6. https://doi.org/10.1007/s12038-019-9930-2
Staples CA, Peterson DR, Parkerton TF, Adams WJ (1997) The environmental fate of phthalate esters: a literature review. Chemosphere 35:667–749. https://doi.org/10.1016/S0045-6535(97)00195-1
Stoppa F, Schiazza M, Pellegrini J, Ambrosio FA, Rosatelli G, D’Orsogna MR (2017) Phthalates, heavy metals and PAHs in an overpopulated coastal region: inferences from Abruzzo, central Italy. Mar Pollut Bull 125:501–512. https://doi.org/10.1016/j.marpolbul.2017.08.008
Sun J, Pan L, Li Z, Zeng Q, Wang L, Zhu L (2018) Comparison of greenhouse and open field cultivations across China: soil characteristics, contamination and microbial diversity. Environ Pollut 243:1509–1516. https://doi.org/10.1016/j.envpol.2018.09.112
Tan W, Yu H, Huang C, Li D, Zhang H, Zhao X, Li R, Wang G, Zhang Y, He X, Xi B (2018) Intercropping wheat and maize increases the uptake of phthalic acid esters by plant roots from soils. J Hazard Mater 359:9–18. https://doi.org/10.1016/j.jhazmat.2018.07.026
Tang J, Rong X, Jin D, Gu C, Chen A, Luo S (2020) Biodegradation of phthalate esters in four agricultural soils: main influencing factors and mechanisms. Int Biodeterior Biodegradation 147:104867. https://doi.org/10.1016/j.ibiod.2019.104867
Tang X, Zhou M, Fan C, Zeng G, Gong R, Xu Q, Song B, Yang Z, Yang Y, Zhou C, Ren X, Wang W (2021) Benzyl butyl phthalate activates prophage, threatening the stable operation of waste activated sludge anaerobic digestion. Sci Total Environ 768:144470. https://doi.org/10.1016/j.scitotenv.2020.144470
Tao Y, Feng C, Xu J, Shen L, Qu J, Ju H, Yan L, Chen W, Zhang Y (2022) Di(2-ethylhexyl) phthalate and dibutyl phthalate have a negative competitive effect on the nitrification of black soil. Chemosphere 293:133554. https://doi.org/10.1016/j.chemosphere.2022.133554
Tran H, Lin C, Bui X, Nguyen MK, Cao NDT, Mukhtar H, Hoang HG, Varjani S, Ngo HH, Nghiem LD (2022) Phthalates in the environment: characteristics, fate and transport, and advanced wastewater treatment technologies. Bioresour Technol 344:126249. https://doi.org/10.1016/j.biortech.2021.126249
Tyler C, Jobling S, Sumpter JP (1998) Endocrine disruption in wildlife: a critical review of the evidence. Crit Rev Toxicol 28:319–361. https://doi.org/10.1080/10408449891344236
USEPA (2013) Mid atlantic risk assessment. Regional screening level (RSL) summary table. United States Environmental Protection Agency, Washington DC, USA. https://www.epa.gov/risk/regional-screening-levels-rsls-generic-tables-may-2016. Accessed 16 Mar 2022
Van Wezel AP, Van Vlaardingen P, Posthumus R, Crommentuijn GH, Sijm DTHM (2000) Environmental risk limits for two phthalates, with special emphasis on endocrine disruptive properties. Ecotoxicol Environ Saf 46:305–321. https://doi.org/10.1006/eesa.2000.1930
Wang D, Xi Y, Shi X, Zhong Y, Guo C, Han Y, Li F (2021) Effect of plastic film mulching and film residues on phthalate esters concentrations in soil and plants, and its risk assessment. Environ Pollut 286:117546. https://doi.org/10.1016/j.envpol.2021.117546
Wang H, Liang H, Gao DW (2017) Occurrence and risk assessment of phthalate esters (PAEs) in agricultural soils of the Sanjiang Plain, northeast China. Environ Sci Pollut Res 24:19723–19732. https://doi.org/10.1007/s11356-017-9646-5
Wang J, Luo Y, Teng Y, Ma W, Christie P, Li Z (2013a) Soil contamination by phthalate esters in Chinese intensive vegetable production systems with different modes of use of plastic film. Environ Pollut 180:265–273. https://doi.org/10.1016/j.envpol.2013.05.036
Wang L, Liu M, Tao W, Zhang W, Wang L, Shi X, Lu X, Li X (2018) Pollution characteristics and health risk assessment of phthalate esters in urban soil in the typical semi-arid city of Xi'an, Northwest China. Chemosphere 191:467–476. https://doi.org/10.1016/j.envpol.2013.05.036
Wang L, Zhang X, Li Y, Sun R, Lin Y, Yu H, Xue Y, Zhou X, Liu W, Yan L, Zhang Y (2019) The drivers of bacterial community underlying biogeographical pattern in Mollisol area of China. Ecotoxicol Environ Saf 177:93–99. https://doi.org/10.1016/j.ecoenv.2019.03.114
Wang X, Lin Q, Wang J, Lu X, Wang G (2013b) Effect of wetland reclamation and tillage conversion on accumulation and distribution of phthalate esters residues in soils. Ecol Eng 51:10–15. https://doi.org/10.1016/j.ecoleng.2012.12.079
Wei L, Li Z, Sun J, Zhu L (2020) Pollution characteristics and health risk assessment of phthalate esters in agricultural soil and vegetables in the Yangtze River Delta of China. Sci Total Environ 726:137978. https://doi.org/10.1016/j.scitotenv.2020.137978
Wu J, Lai Y, Zhu H, Yang X, Ye X, Zhang A, Sun J (2023) Phthalate esters and their metabolites in paired soil-crop systems from farmland in major provinces of eastern China: pollution characteristics and implications for human exposure. Sci Total Environ 882:163645. https://doi.org/10.1016/j.scitotenv.2023.163645
Wu X, Wang Y, Liang R, Dai Q, Jin D, Chao W (2011) Biodegradation of an endocrine-disrupting chemical di-n-butyl phthalate by newly isolated Agrobacterium sp. and the biochemical pathway. Process Biochem 46:1090–1094. https://doi.org/10.1016/j.procbio.2011.01.031
Wu Y, Chen X, Zhu T, Li X, Chen X, Mo C, Li Y, Cai Q, Wong M (2018) Variation in accumulation, transport, and distribution of phthalic acid esters (PAEs) in soil columns grown with low-and high-PAE accumulating rice cultivars. Environ Sci Pollut Res 2518:17768–17780. https://doi.org/10.1007/s11356-018-1938-x
Xu Y, Jia W, Hu A, Wang J, Huang Y, Xu J, He Y, Lu Z (2022) Co-occurrence of light microplastics and phthalate esters in soils of China. Sci Total Environ 852:158384. https://doi.org/10.1016/j.scitotenv.2022.158384
Xue C, Peng L, Tang J, Lei M, Chen A, Shao J, Luo S, Mu Y (2020) Screening the main factors affecting phthalate esters adsorption on soils, humic acid, and clay organo-mineral complexes. Ecotoxicol Environ Saf 190:109143. https://doi.org/10.1016/j.ecoenv.2019.04.004
Yang F, Wang M, Wang Z (2013) Sorption behavior of 17 phthalic acid esters on three soils: effects of pH and dissolved organic matter, sorption coefficient measurement and QSPR study. Chemosphere 93(1):82–89. https://doi.org/10.1016/j.chemosphere.2013.04.081
Ye H, Zhao B, Zhou Y, Du J, Huang M (2021) Recent advances in adsorbents for the removal of phthalate esters from water: material, modification, and application. Chem Eng J 409:128127. https://doi.org/10.1016/j.cej.2020.128127
Yu G, Cheng S, Fang H, Tian J, Xu M, Yu G, He S, Geng J, Cao Z (2018) Responses of soil nitrous oxide flux to soil environmental factors in a subtropical coniferous plantation: a boundary line analysis. Eur J Soil Biol 86:16–25. https://doi.org/10.1016/j.ejsobi.2018.02.002
Zhang J, Choudhury M (2017) The plasticizer BBP selectively inhibits epigenetic regulator sirtuin during differentiation of C3H10T1/2 stem cell line. Toxicol In Vitro 39:75–83. https://doi.org/10.1016/j.tiv.2016.11.016
Zhang Y, Huang F, Wang L, Guan R, Yu H, Qu J, Gao M, Du S (2021) Facilitating effect of heavy metals on di (2-ethylhexyl) phthalate adsorption in soil: new evidence from adsorption experiment data and quantum chemical simulation. Sci Total Environ 772:144980. https://doi.org/10.1016/j.scitotenv.2021.144980
Zhang Y, Li X, Zhang H, Liu W, Liu Y, Guo C, Xu J, Wu F (2022) Distribution, source apportionment and health risk assessment of phthalate esters in outdoor dust samples on Tibetan Plateau. China Sci Total Environ 834:155103. https://doi.org/10.1016/j.scitotenv.2022.155103
Zhang Y, Wang P, Wang L, Sun G, Zhao J, Zhang H, Du N (2015) The influence of facility agriculture production on phthalate esters distribution in black soils of northeast China. Sci Total Environ 506-507:118–125. https://doi.org/10.1016/j.scitotenv.2014.10.075
Zhang Z, Zhang J, Zhang H, Shi X, Zou Y, Yang G (2020) Pollution characteristics, spatial variation, and potential risks of phthalate esters in the water–sediment system of the Yangtze River estuary and its adjacent East China Sea. Environ Pollut 265:114913. https://doi.org/10.1016/j.envpol.2020.114913
Zhou B, Zhao L, Wang Y, Sun Y, Li X, Xu H, Weng L, Pan Z, Yang S, Chang X, Li Y (2020) Spatial distribution of phthalate esters and the associated response of enzyme activities and microbial community composition in typical plastic-shed vegetable soils in China. Ecotoxicol Environ Saf 195:110495. https://doi.org/10.1016/j.ecoenv.2020.110495
Zhou Y, He G, Jiang X, Yao L, Ouyang L, Liu X, Liu W, Liu Y (2021) Microplastic contamination is ubiquitous in riparian soils and strongly related to elevation, precipitation and population density. J Hazard Mater 411:125178. https://doi.org/10.1016/j.jhazmat.2021.125178
Zhu F, Zhu C, Doyle E, Liu H, Zhou D, Gao J (2018) Fate of di (2‑ethylhexyl) phthalate in different soils and associated bacterial community changes. Sci Total Environ 637:460–469. https://doi.org/10.1016/j.scitotenv.2018.05.055
Zhu F, Yan Y, Doyle E, Zhu C, Jin X, Chen Z, Wang C, He H, Zhou D, Gu C (2022) Microplastics altered soil microbiome and nitrogen cycling: the role of phthalate plasticizer. J Hazard Mater 427:127944. https://doi.org/10.1016/j.jhazmat.2021.127944
Funding
This research was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (No. XDA28130100), the Second Tibetan Plateau Scientific Expedition and Research Program (No. 2019QZKK1003), National Natural Science Foundation of China (Nos. 41977041, 31770558, 32101301), and the “Unveiling the List of Hanging” and Technology Project of Jinggangshan Agricultural High-tech Industrial Demonstration Zone (No. 20222-051244).
Author information
Authors and Affiliations
Contributions
Yuna Li: investigation, formal analysis, and writing—original draft. Shulan Cheng: investigation, supervision, and formal analysis. Huajun Fang: conceptualization, supervision, funding acquisition, and writing—review and editing. Yan Yang: specifically visualization. Yifan Guo: methodology investigation. Yi Zhou: investigation. Fangying Shi: investigation.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Thomas D. Bucheli
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 1088 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Y., Cheng, S., Fang, H. et al. Composition, distribution, health risks, and drivers of phthalates in typical red paddy soils. Environ Sci Pollut Res 30, 94814–94826 (2023). https://doi.org/10.1007/s11356-023-28815-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-28815-z