Abstract
There is emerging evidence indicating that short-term exposure to air pollution is associated with the development and occurrence of allergic rhinitis (AR), but limited studies have been conducted in China, and their results were inconsistent. So, quasi-Poisson time series regressions with distributed lag non-linear models (DLNM) were applied to evaluate the lag association between six air pollutants and daily outpatient visits for AR in Lanzhou, China, from January 1, 2014, to December 31, 2019. Stratified analyses were further performed by gender, age, and season. Overall, we found that short-term exposure to air pollutants including PM2.5, PM10, SO2, NO2, O38h, and CO was significantly associated with an increased risk of AR outpatient visits. The strongest associations were observed at a lag of 0–7 days for PM2.5 (relative risk [RR] = 1.035, 95% confidence intervals [CI]: 1.019–1.052), PM10 (RR = 1.006, 95% CI: 1.002–1.011), at a lag of 0–2 days for SO2 (RR = 1.048, 95% CI: 1.017–1.081), NO2 (RR = 1.025, 95% CI: 1.010–1.041), at a lag of 0–6 days for O38h (RR = 1.028, 95% CI: 1.016–1.041), and at a lag of 0–7 days for CO (RR = 1.128, 95% CI: 1.054–1.206). Stratified analyses indicated that males and adults (15–59 years old) appeared to be more sensitive to PM2.5, SO2, NO2, O38h, and CO exposure than females and those in other age groups. The effect of CO exposure was statistically significant in all subgroups. Associations between PM2.5, PM10, NO2, and O38h and AR outpatients were more pronounced in the warm season than in the cold season. The influences of PM2.5, PM10, SO2, NO2, O38h, and CO were found to be significantly relevant to AR-associated outpatient. Different pollutants played different roles for different genders, ages, and seasons.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Allergic rhinitis (allergic rhinitis, AR) is a type I allergy that involves various inflammatory mediators and cytokines. The pathogenesis is attributed to the impact of environmental factors on the body, resulting in an IgE-mediated immune response. This in turn causes an imbalance between Th1 and Th2 immune responses, leading to an allergic response that is mainly characterized by Th2 immune responses in the nasal mucosa. Typical clinical symptoms include nasal itching, paroxysmal sneezing, excessive watery nasal discharge, and nasal congestion. AR has a significant impact on patients’ daily lives, as well as on their ability to sleep, work, and study, and it places a huge economic burden on both individuals and society (Wu et al. 2022). As the world continues to industrialize and people’s lifestyles change rapidly, coupled with the ever-changing ecological environment, the incidence of AR has been on an obvious upward trend. Epidemiological survey data indicates that the incidence of AR worldwide has increased from 10 to 15% in the middle of the twentieth century to 40% at present (Subspecialty Group of Rhinology et al. 2016). In China, studies have revealed that the incidence of AR is also on the rise, affecting approximately 4 to 31% of Chinese children and 4 to 38% of adults (Zhang and Zhang 2014). The pathogenesis of AR is complex, with both environmental and genetic factors playing a crucial role. Research on environmental factors that cause AR indicates that, of the many factors affecting the incidence of this condition, environmental factors may play a more critical role than genetic factors (Dunlop et al. 2016). With the increasing attention to the molecular biology and immunology of AR, people have a more profound understanding of AR; AR is the result of the complex interaction between genetic and environmental factors. The significant rise in the worldwide incidence of allergic rhinitis cannot be solely attributed to changes in human genetic susceptibility. The interaction of various environmental factors plays an extremely important role in the onset and progression of AR, rather than simply being an issue of allergens. Experts, both domestically and internationally, agree that air pollution is a significant contributing factor to the global increase in respiratory allergic diseases, including AR. The onset and severity of AR are linked to air quality. As a vital respiratory organ, the nasal cavity serves as the primary barrier to exposure to air pollution. Its regulatory and immune defense processes have critical physiological significance for the respiratory and entire immune systems. Numerous studies have proven that the onset and development of respiratory symptoms or diseases are closely related to air quality. When air pollution disturbs the nasal immune balance, the mucosal area delivers an inappropriate immune response, leading to tissue damage as well as chronic and prolonged inflammation. This would further cause nasal diseases and may even impact the health of the lower respiratory tract and the immune regulatory mechanism of the whole body. International studies have suggested that air pollution, including traffic-related pollutants, particulate matter, and organic compounds in indoor dust, impacts both the onset and severity of AR. Ambient particulate matter (particles with aerodynamic diameters less than 2.5 μm and less than 10 μm, PM2.5 and PM10), sulfur dioxide (SO2), and nitrogen dioxide (NO2) have been proven to be associated with an increased risk of AR. For example, a 2018 meta-analysis including 13 studies summarized that exposure to air pollution is probable to increase the prevalence of childhood AR, and the prevalence of AR will increase when exposed to NO2, SO2, PM10, and PM2.5 (Zou et al. 2018). A meta-analysis of 35 studies in 2022 demonstrates an association between air pollutants (PM2.5, PM10, NO2, SO2, and O3) and the prevalence of AR in people of all ages, and no significant association was observed between CO and AR (Li et al. 2022). It should be noted that the literature selected in the above two meta-analysis articles mainly used self- or guardian-reported in questionnaires or interviews to evaluate the relationship between air pollutants and allergic rhinitis, which may cause information bias in the study. In addition, limited researches have explored the acute effect of six air pollutants on AR with time-series methods (Teng et al. 2017; Wang et al. 2020a; Wu et al. 2022; Yang et al. 2022). The epidemiological evidence on the short-term effects of ambient air pollution on AR morbidity is still not conclusive to date. Some studies reported that air pollution could possibly lead to an increased risk of AR. A time-series study in Changchun indicated that AR morbidity was positively associated with a short-term increase in concentrations of PM2.5 (RR: 1.034, 95% CI: 1.022–1.047 per 10 μg/m3), PM10 (RR: 1.016, 95% CI: 1.007–1.026 per 10 μg/m3), SO2 (RR: 1.023, 95% CI: 0.992–1.059 per 10 μg/m3), NO2 (RR: 1.102, 95% CI: 1.055–1.151 per 10 μg/m3), and O3 (RR: 1.002, 95% CI: 0.991–1.012 per 10 μg/m3) (Teng et al. 2017). A study in Xinxiang, China, reported that a 10 ug/m3 increase in PM2.5, PM10, SO2, NO2, O3, and a 1 mg/m3 increase in CO resulted in relative risks of 1.007 (95% CI: 1.000–1.014), 1.008 (95% CI: 1.004–1.012), 1.034 (95% CI: 1.015–1.054), 1.045 (95% CI: 1.030–1.061), 1.010 (95% CI: 0.999–1.021), and 1.070 (95% CI: 1.020–1.120) for AR morbidity, respectively (Wang et al. 2020a). Another study conducted in Beijing, China, showed that each 10 μg/m3 increase of PM2.5, PM10, SO2, and NO2 was associated with a relative risk of 1.012 (95% CI: 1.007–1.018), 1.008 (95% CI: 1.005–1.012), 1.050 (95% CI: 1.012–1.090), and 1.031 (95% CI: 1.017–1.044), respectively, for outpatient AR (Wu et al. 2022). And in Chongqing, a time series study found that per 10 μg/m3 increase in PM2.5, PM10, NO2, SO2, and O3 and per 1 mg/m3 in CO concentrations were associated with a relative risk of 1.083 (95% CI: 1.011–1.160), 1.070 (95% CI: 0.996–1.149), 1.003 (95% CI: 0.857–1.175), 1.031 (95% CI: 0.988–1.075), 1.066 (95% CI: 1.008–1.127), and 0.997 (95% CI: 0.884–1.127), respectively, for outpatients with AR (Yang et al. 2022).
Overall, the studies mentioned above mainly used generalized additive models (GAM) to explore the association between short-term exposure to air pollution and outpatient visits for AR. There was only one study investigating the adverse effect of air pollutants on AR outpatients using the distributed lag non-linear model (DLNM) (Yang et al. 2022).
Compared with GAM, DLNM has a greater advantage in dealing with lagged effects (Gasparrini et al. 2010; Gasparrini 2011). GAM (generalized additive model) simply put the daily concentration of air pollutants or their lagged term into the model and could only describe the negative impact of the daily concentration of air pollutants or the moving average concentration of air pollutants defined by a certain rule on human health. It is limited in the ability to consider the delayed distribution and cumulative effect of air pollutant exposure at multiple time points at the same time. The distributed lag non-linear model (DLNM) takes into account the distribution of air pollutants and their lag terms and more realistically simulates the impact of air pollutants at adjacent time points by establishing a cross-basis, resulting in a more scientific evaluation of the effect (Gasparrini et al. 2010; Gasparrini 2011). Hence, we further used a distributed lag non-linear model combined with quasi-Poisson regression to evaluate the associations between various air pollutants and outpatient visits for AR.
The composition and concentration of air pollution differ in different countries or cities. Although some previous studies have examined the association between individual pollutants and daily outpatient visits for AR in some areas, few studies have focused on allergic rhinitis in Lanzhou, China. Lanzhou is a city surrounded by mountains to the north and south, and the Yellow River flows through the city from west to east, lending it the characteristics of a banded basin city. The city has a typical temperate continental monsoon climate with four distinct seasons and a large temperature range throughout the year. Additionally, during the winter and spring seasons, affected by the complex topography of the river valley basin, the city has lower wind speeds and frequent temperature inversions, which prevent the dispersion of air pollutants and contribute to the accumulation of pollutants in the atmosphere, leading to serious air pollution, considering that air pollutants can aggravate the symptoms of patients with allergic diseases and worsen the condition. Thus, the generalized additive model (GAM) and the distributed lag non-linear model (DLNM) were used to evaluate the associations of six ambient air pollutants on outpatient visits for AR in Lanzhou from 2014 to 2019. Moreover, we performed subgroup analysis to investigate the potential modifying effects of gender, age, and season. This study can provide reasonable prevention suggestions for individuals who are sensitive to AR, as well as provide a scientific basis for the government to formulate air pollution control and other related policies.
Materials and methods
Study area
Lanzhou is the capital city of Gansu Province and comprises four urban districts with a total area of 1100 km2. The city has a population of 3.84 million. In addition, Lanzhou has an arid continental climate with four distinctive seasons. Winter is very dry and cold, and summer is sunny and hot.
Data collection
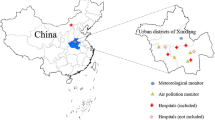
The daily outpatient visits for AR from 2014 to 2019 were obtained from four large general hospitals. These four hospitals are distributed in the central districts of Lanzhou (Fig. 1) and are surrounded by densely populated areas, with rapid and convenient transportation around the hospital. In addition, these hospitals are well-equipped, and the doctors are highly qualified. These hospitals are famous for their good medical treatment of respiratory diseases. We selected these four hospitals as data sources. The information on cases included gender, age, dates of hospital visits, disease diagnoses, and the International Classification of Diseases (ICD, 10th revision) codes. We diagnosed allergic rhinitis according to medical history, related symptoms, and clinical examination results (skin prick test and serum allergen-specific IgE assay). Data on all outpatient visits for AR were extracted and identified using the ICD-10 codes (J30).
In this study, we collected hourly concentrations of PM2.5, PM10, SO2, NO2, O3, and CO from 3 monitoring stations in Lanzhou over the same period. Then, daily PM2.5, PM10, SO2, NO2, and CO were calculated using the data recorded from these three sites. For O3, maximum daily 8-h ozone concentrations (O38h) were calculated based on hourly O3 concentrations. Data on meteorological factors in our study were obtained from the Meteorological Administration of Gansu Province, including daily mean temperature and relative humidity. The locations of the air pollution and meteorological monitoring stations are shown in Fig. 1.
Statistical analysis
For the whole population of Lanzhou, daily AR hospital outpatients were relatively less, belonging to small probability events, which approximately followed the Poisson distribution, and some previous studies have suggested that there are both non-linear and delayed associations of air pollution with health outcomes. Hence, a quasi-Poisson generalized additive model combined with the distributed lag non-linear model (DLNM) was used to assess the association between ambient air pollution (PM2.5, PM10, SO2, NO2, O38h, and CO) and daily hospital outpatients for AR.
The model is as follows:
Log(μt) = α + βXt, l + ns(Temt, df) + ns(rht, df) + ns(Timet, df) + factor(Dow) + factor(Holiday) In the model above, t represents the day of observation, μt denotes the number of outpatient visits for AR on day t, α is the constant term, Xt,l refers to the cross-basis matrix obtained by applying the DLNM to six air pollutants, β refers to the vector of coefficients for Xt,l, and l is the lag days, and we defined the matrix about the linear and lagged of air pollutants using the linear function and the natural cubic spline function. ns denotes the natural cubic spline function. Temt and rht are the daily mean temperature and relative humidity, respectively. Timet is the time variable, which is used to adjust for seasonality and long-term trends. DOW refers to day of week (1, 2, 3, …,7), and Holiday is a dummy variable for holidays. In our model, we chose the degrees of freedom (df) for each variable according to the minimized value of quasi Akaike information criterion (Q-AIC). In the final model, the df for the lag of air pollutant was specified to be 3. The df of temperature is 3, the df of humidity is 3, and a natural cubic spline with 7° of freedom per year was used for time (Çapraz et al. 2017; Jin et al. 2023).
Some studies have reported that the lagged effects of exposure to air pollutants on AR are relatively short, which is usually about a week or even only a few days (Yang et al. 2022). So, we chose the longest lag period of 7 days. In the current study, we described the potential lag effect of PM2.5, PM10, SO2, NO2, O38h, and CO on outpatient visits for AR by introducing single-day lags (from lag0 to lag7) and multiple-day lags (from lag01 to lag07). Then, we also explored the potential effect modification by gender (male and female), age (0–14, 15–59, and ≥ 60 years), and season (warm season: April to September; cold season: October to March).
Finally, we conducted some sensitivity analysis to evaluate the robustness of the results as follows: (1) double-pollutant models were fitted to check the effects after employing co-pollutants. Considering the correlation coefficient for PM2.5 and PM10 (0.86) is higher, in the following two-pollutant models for PM2.5, PM10 was excluded. (2) We change the degrees of freedom of the time trends (6–10 dfs/year).
We present the associations with the relative risks (RRs) and 95% confidence intervals (CIs) per 10 μg/m3 increase in the PM2.5, PM10, SO2, NO2, and O3 concentrations and per 1 mg/m3 increase in the level of CO. All the calculations were done using R software version 3.6.3 (R Core Team, 2014) with its “dlnm” package.
Results
Table 1 shows the characteristics of daily hospital outpatient visits for AR, meteorological conditions, and air pollutant levels. During the 6-year study period, a total of 60,964 cases of AR outpatient visits were included. On average, 28 outpatients were admitted per day. Of all patients, 30,957 (50.8%) were males, and 35,579 (58.3%) were patients aged 15–59 years. The daily average concentrations of PM2.5, PM10, SO2, NO2, O38h, and CO were 48.92, 114.84, 21.12, 47.34, 88.24 μg/m3, and 1.24 mg/m3, respectively. Meanwhile, the daily mean temperature and relative humidity were 11.34 °C and 51.03%, respectively.
Table 2 shows the Spearman correlation between air pollutant levels and meteorological factors during the study period. The levels of PM2.5, PM10, SO2, NO2, and CO were positively correlated with each other pollutant (correlation coefficient = 0.46–0.86) and negatively correlated with temperature (correlation coefficient = −0.57 to −0.22). In contrast, O38h was negatively correlated with other air pollutants (except NO2).
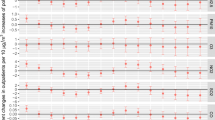
In Table 3, the associations between air pollution and daily outpatient visits for AR in single pollutant models were shown. In single pollutant models, the positive association was evidenced at lag0, lag6, lag7, and lag03–lag07 for PM2.5, at lag0, lag6, lag7, and lag06–lag07 for PM10, at lag01–lag03 for SO2 and NO2, and at lag0 and all the multiple-day lags for O38h and CO. The highest RR values associated with AR outpatient visits per 10 μg/m3 increase in PM2.5 concentrations at lag07, PM10 at lag07, SO2 at lag02, NO2 at lag02, and O38h at lag06 were 1.035 (95% CI: 1.019, 1.052), 1.006 (95% CI: 1.002, 1.011), 1.048 (95% CI: 1.017, 1.081), 1.025 (95% CI: 1.010, 1.041), and 1.028 (95% CI: 1.016, 1.041), respectively. For a 1 mg/m3 increase in CO levels in lag07, the highest RR was 1.128 (95% CI: 1.054, 1.206).
Figure 2 presents the results of gender-specific analyses. In single pollutant models for PM2.5, the most significant RR in both male and female groups was at lag07 (RR = 1.035, 95% CI: 1.018–1.052; 1.032, 95% CI: 1.014–1.051, respectively). In single-day lag models for PM10, the most significant RR in groups of males and females was also at lag07 (RR = 1.006, 95% CI: 1.001–1.010; 1.007, 95% CI: 1.002–1.011, respectively). The associations between SO2 exposure and AR outpatient visits reached a maximum at lag02 for both genders (RR = 1.051, 95% CI: 1.019–1.085 for males; RR = 1.042, 95% CI: 1.008–1.078 for females). As for NO2, the strong associations were significant on lag02 for males and females, with RRs of 1.029 (95% CI: 1.013, 1.045) and 1.019 (95% CI: 1.002, 1.036), respectively. These associations between gaseous air pollutants (O38h and CO) and AR morbidity were persisted for both males and females; a significantly positive association was observed at lag0 and all the multiple-day lags. CO had the greatest impact on each subgroup analysis. Males appeared to be more vulnerable to all pollutants except for PM10.
The results of the age stratification are shown in Fig. 3. PM2.5 and O38h were significantly associated with the outpatient visits with AR in all age groups at lag0. In terms of age-specific results, adults (15–59 years old) seemed to be more susceptible to air pollutants than the other two age groups.
The seasonal stratification results are shown in Table 4. PM2.5, PM10, NO2, and O38h were significantly associated with daily outpatient visits for AR in the warm season (Apr–Sep). In contrast, a significant association between SO2 and CO and hospital outpatient visits for AR was observed in the cold season (Oct–Mar). Moreover, SO2 showed no obvious seasonal susceptibility differences in their associations.
The lag day with the most considerable RR value in the single-pollutant model was chosen as the lag date of the exposure–response curves and sensitivity analyses. Figure 4 depicts the exposure-response (ER) curves for the associations between each air pollutant and outpatient AR visits, and the dose-response curves of air pollutants were approximately linear, with no evident thresholds. We found that the RR on AR-related morbidity increased as the concentration of air pollutants increased.
Table S1 shows the results of two-pollutant models using the lag day with the maximum effect estimates. The associations of all six pollutants were stable when adjusting for another pollutant. In addition, the effect estimates remained stable after changing the df for the smoothness of time (see Supplementary Table S2).
Discussion
In the current study, we found that short-term exposure to air pollution was positively associated with outpatient visits for AR in Lanzhou, and the association was higher with gaseous pollutants than with air particulate matter (PM2.5 and PM10). Subgroup analysis suggested that the associations of PM2.5, SO2, NO2, O38h, and CO were stronger in males than in females, where the adverse effects of ambient air pollutants were more pronounced in adults (15–59 years old) than in other age groups. In season analyses, the associations of PM2.5, PM10, NO2, and O38h appeared to be more significant in warm seasons than in cold seasons.
Our study showed that an increase of 10 μg/m3 air pollutant could cause an RR of 1.035 (95% CI: 1.019, 1.052) for PM2.5 and 1.006 (95% CI: 1.002, 1.011) for PM10, which were similar to several previous studies. For example, a study in Changchun, China, reported an increase of 10 μg/m3 in PM2.5, and PM10 was associated with an increased risk of AR morbidity in the single pollutant model at 1.034 (95% CI: 1.022, 1.047) and 1.016 (95% CI: 1.007, 1.026), respectively (Teng et al. 2017). In another study conducted in Xinxiang, China, each 10 μg/m3 increase in PM2.5 and PM10 increased the risk of AR morbidity with RR 1.007 (95% CI: 1.000–1.014) and RR 1.008 (95% CI: 1.004–1.012), respectively (Wang et al. 2020a). An epidemiologic study in Chongqing, China, found that exposure to PM2.5 and PM10 was associated with an increase in hospital visits for AR, with RRs of 1.083 (95% CI: 1.011–1.160) and 1.070 (95% CI: 0.996–1.149), respectively (Yang et al. 2022). Overall, both our study and literature reports found that the estimated effects of PM2.5 and PM10 were both associated with the increased daily numbers of AR, but the effect size values reported by different studies are different, and Yang et al. also did not find a positive association between PM10 and AR risk in Chongqing, China (Yang et al. 2022). The discrepancy in findings across studies might be due to the differences in air pollution levels, population susceptibility, study design, sample size, and setting of model parameters.
In this study, we also observed that an elevation of SO2 and NO2 concentrations at lag01 to lag03 was significantly associated with a higher risk of AR visits, suggesting the potential instant effects of these two gaseous pollutants on AR exacerbation. The findings are consistent with three studies on the associations between gaseous pollutants and AR onset (Wang et al. 2020a; Wu et al. 2022). For example, Wu et al. (2022) showed that short-term exposures to SO2 and NO2 could be positively associated with increased outpatient visits for a time-series analysis in Beijing, China (Wu et al. 2022). Furthermore, another time-series study conducted in Xinxiang, China, also identified that the risk of outpatient visits for AR peaked on lag0 for SO2 and NO2 (Wang et al. 2020a). SO2 and NO2 have an auxiliary effect on airway allergy and may enhance allergic inflammation and induce the development of allergic immunity. Due to the complex etiology of AR, the impact of air pollution on the pathogenesis of AR needs to be further explored.
In China, research on the harmful effects of O3 is rare and still controversial. For example, a study conducted in Chongqing, China, found that O38h was significantly and positively associated with the prevalence of AR (Yang et al. 2022). However, other two researches from Xinxiang and Changchun, China, observed a weak and insignificant association between O38h and the acute onset of AR (Wang et al. 2020a; Teng et al. 2017). Variations in the study population, design, air pollutant levels, and weather conditions partially explain the inconsistencies between these results.
Moreover, significant associations with AR were found for ambient CO level in our study. Until now, the evidence of the effect of CO exposure on AR morbidity is currently limited and inconsistent. For example, Wang et al.’s study conducted in Xinxiang City has shown that the incidence rate of AR was positively correlated with the CO concentration in the air (Wang et al. 2020a). In contrast, two other studies discovered no significant correlation between CO and AR outpatient visits (Teng et al. 2017; Yang et al. 2022). There are no apparent plausible mechanisms that underlie the associations between CO and AR. There are many environmental factors, such as geographical location, climatic type and air quality, population, and allergen exposure (time, dose, and number), that need to be considered in future investigations.
In the gender-specific analyses, the findings showed that PM2.5, SO2, NO2, O38h, and CO were significantly associated with outpatient visits for AR among males and females, and higher associations in males than those in females, which were in line with previous studies (Wang et al. 2020a; Wu et al. 2022). The possible reason for these differences between males and females was still unclear and needed further investigation. One possible explanation was the differences in occupational factors and temporal activity patterns between genders, as males spend more time outdoors because of their jobs, resulting in exposure to more accumulative concentrations of air pollutants. In terms of age, the association between air pollution and AR outpatients appeared to be bigger in adults (15–59 years) than in those under 15 years and ≥ 60 years of age, which was inconsistent with previous findings. In some early studies, they reported that children had significantly higher risks of outpatient visits than adults with respect to air pollution exposure because they have smaller airways and less developed lung and immune defense functions. There may be more outdoor activities in adults, and persons in this age group may engage in more outdoor work, which could explain why air pollution has more severe negative impacts on AR and causes higher cumulative exposures to air pollution.
Results of our research also indicated that the associations between PM2.5, PM10, NO2, and O38h and outpatient visits for AR were higher in the warm season when compared with the cold one, which was consistent with previous studies (Chu et al. 2019; Wang et al. 2020b; Zhang et al. 2013). We could not find an apparent plausible explanation for this in the literature, but tentatively, we attribute this to the pollen season in Lanzhou. In the spring, summer, and autumn of Lanzhou, the concentration of highly allergen-producing pollens in the air is relatively high due to the flourishing vegetation. Air pollutants can be pollen carriers or change the chemical composition and external morphology of airborne pollen to enhance the sensitization of pollen, resulting in the aggravated impact of plants that trigger allergies on the human body. Eventually, it can induce and exacerbate the immune response of patients with AR. It remains to be further explored with respect to the impacts of pollen and air pollutants in Lanzhou on the number of outpatient visits for AR by collecting pollen and meteorological data in the same period.
Currently, there are few studies on the combined effects of multiple air pollutants on the volume of outpatient visits for AR in China (Wang et al. 2020a; Wu et al. 2022; Yang et al. 2022). The method for evaluating the robustness of the single-pollutant model results is to examine whether the results would change after adjusting the influence of other pollutants using a dual-pollutant model. The results of the two-pollutant model fit in this study showed that the associations of all six pollutants were stable when adjusting for another pollutant, which is consistent with previous studies (Yang et al. 2022). As shown in a study by Yang et al. in Chongqing, China, they found that the results of fitting the two-pollutant model showed that the results were unchanged (Yang et al. 2022). However, it has also been found that in a two-pollutant model, it is uncertain whether adjusting for other pollutants will change the single-pollutant model results, with different pollutants acting in combination. For example, a study by Song et al. in Xinxiang, China, showed that the associations of PM10, NO2, and O3 with AR outpatients remained robust after adjusting for co-pollutants. However, when adjusting for SO2, a non-significant association was identified for PM2.5. For O3, all estimates were significant in the two pollutant models. Meanwhile, the estimated effects of CO were insignificant when adjusting for PM10, NO2, and SO2 (Wang et al. 2020a). Another study from Beijing showed that the associations remained significant for PM2.5, PM10, and NO2 when adjusted by SO2, while there was no significance for SO2 when adjusting for PM and NO2, indicating the vital role of PM and NO2 in the risk of AR outpatients (Wu et al. 2022). However, it is worth noting that although this study and the literature mentioned above have considered and controlled for collinearity between air pollutants, the complex relationship between air pollutants in space and time, as well as the different demographic characteristics and economic levels in different regions, may result in biased results. Therefore, it is still worth investigating whether the combined effects of air pollutants increase health risks for the population, and more studies are needed to clarify the underlying mechanisms involved.
Allergic rhinitis is caused by the involvement of IgE-mediated mediators, such as histamine, leukotriene, bradykinin, a variety of immune cells, and cytokines, following contact with allergens by atopic individuals. This leads to telangiectasis, increased vascular permeability, smooth muscle contraction, and gland secretion, resulting in typical symptoms such as nasal congestion, nasal itching, runny nose, and sneezing. Therefore, enhanced allergen sensitization can induce an allergic reaction in the body and aggravate clinical symptoms. Air pollutants may enhance allergen sensitization through various mechanisms, including carrying allergens into the airway and chemically modifying allergens to increase their antigenicity through mechanisms such as epithelial cells, oxidative stress, and inflammation. Due to the complex composition of air pollutants, the mechanism by which they promote the onset of AR has not been fully clarified, but they have been proven to be able to cause immune dysfunction, directly or indirectly damage the defense function of the respiratory tract, cause damage to respiratory epithelial cells or the release of allergen-related inflammatory mediators, and initiate local allergic reactions in the respiratory tract (Bayram 2017; Bloemsma et al. 2016; Chen et al. 2018; Heinrich and Schikowski 2018). To sum up, air pollution is a major co-factor or trigger factor in the pathogenesis of AR.
Conclusion
In conclusion, this study indicated that the air pollutants had lag effects on the daily number of outpatients for AR in Lanzhou from 2014 to 2019, and stronger associations in males and adults were observed. Besides, the adverse effects of PM2.5, PM10, NO2, and O38h on AR in the warm season were higher than those in the cold season. This analysis provides evidence for the association between air pollution and the risk of AR outpatients; further studies about the effects of air pollutants on allergic diseases (e.g., AR) may be warranted.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available due to confidentiality agreements but are available from the corresponding author on reasonable request.
Abbreviations
- AR:
-
allergic rhinitis
- GAM:
-
generalized additive model
- DLNM:
-
distributed lag non-linear models
- RR:
-
relative risk
- Q-AIC:
-
quasi Akaike information criterion
References
Bayram H (2017) Impact of air pollution on COPD; underlying mechanisms. Tanaffos 16(Suppl 1):S10
Bloemsma LD, Hoek G, Smit LAM (2016) Panel studies of air pollution in patients with COPD: systematic review and meta-analysis. Environ Res 151:458–468. https://doi.org/10.1016/j.envres.2016.08.018
Çapraz Ö, Deniz A, Doğan N (2017) Effects of air pollution on respiratory hospital admissions in İstanbul, Turkey, 2013 to 2015. Chemosphere 181:544–550. https://doi.org/10.1016/j.chemosphere.2017.04.105
Chen FE, Lin ZJ, Chen RJ, Norback D, Liu C, Kan HD, Deng QH, Huang C, Hu Y, Zou ZJ, Liu W, Wang J, Lu C, Qian H, Yang X, Zhang X, Qu F, Sundell J, Zhang YP et al (2018) The effects of PM2.5 on asthmatic and allergic diseases or symptoms in preschool children of six Chinese cities, based on China, children, homes and health (CCHH) project. Environ Pollut 232:329–337. https://doi.org/10.1016/j.envpol.2017.08.072
Chu HY, Xin JY, Yuan Q, Wang ML, Cheng L, Zhang ZD, Lu MP (2019) The effects of particulate matters on allergic rhinitis in Nanjing, China. Environ Sci Pollut Res 26(11):11452–11457. https://doi.org/10.1007/s11356-019-04593-5
Dunlop J, Matsui E, Sharma HP (2016) Allergic rhinitis: environmental determinants. Immunol Allergy Clin North Am 36(2):367–377. https://doi.org/10.1016/j.iac.2015.12.012
Gasparrini A (2011) Distributed lag linear and non-Linear models in R: the package dlnm. J Stat Softw 43(8):1–20
Gasparrini A, Armstrong B, Kenward MG (2010) Distributed lag non-linear models. Stat Med 29(21):2224–2234. https://doi.org/10.1002/sim.3940
Heinrich J, Schikowski T (2018) COPD patients as vulnerable subpopulation for exposure to ambient air pollution. Curr Environ Health Rep 5(1):70–76. https://doi.org/10.1007/s40572-018-0178-z
Jin LM, Zhou T, Fang SY, Zhou XW, Han BS, Bai YN (2023) Association of air pollutants and hospital admissions for respiratory diseases in Lanzhou, China, 2014-2019. Environ Geochem Health 45(3):941–959. https://doi.org/10.1007/s10653-022-01256-2
Li S, Wu W, Wang G, Zhang XY, Guo Q, Wang BB, Cao SZ, Yan ML, Pan XC, Xue T, Gong JC, Duan XL (2022) Association between exposure to air pollution and risk of allergic rhinitis: a systematic review and meta-analysis. Environ Res 205:112472. https://doi.org/10.1016/j.envres.2021.112472
R Core Team (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Subspecialty Group of Rhinology, Editorial Board of Chinese Journal of Otorhinolaryngology Head and Neck Surgery, Subspecialty Group of Rhinology, Society of Otorhinolaryngology Head and Neck Surgery, Chinese Medical Association (2016) Guidelines for diagnosis and treatment of allergic rhinitis (2015. Tianjin). Chin J Otorhinolaryngol Head Neck Surg 51(1):6–24. https://doi.org/10.3760/cma.j.issn.1673-0860.2016.01.004
Teng B, Zhang XL, Yi CH, Zhang Y, Ye SF, Wang YF, Tong DQ, Lu BF (2017) The association between ambient air pollution and allergic rhinitis: further epidemiological evidence from Changchun, Northeastern China. Int J Environ Res Public Health 14(3):226. https://doi.org/10.3390/ijerph14030226
Wang JY, Lu MX, An Z, Jiang J, Li J, Wang YB, Du S, Zhang XX, Zhou HF, Cui J, Wu WD, Liu Y, Song J (2020a) Associations between air pollution and outpatient visits for allergic rhinitis in Xinxiang, China. Environ Sci Pollut Res 27(19):23565–23574. https://doi.org/10.1007/s11356-020-08709-0
Wang MY, Wang SY, Wang XW, Tian YH, Wu Y, Cao YY, Song J, Wu T, Hu YH (2020b) The association between PM2.5 exposure and daily outpatient visits for allergic rhinitis: evidence from a seriously air-polluted environment. Int J Biometeorol 64(1):139–144. https://doi.org/10.1007/s00484-019-01804-z
Wu RS, Guo Q, Fan JP, Guo CS, Wang G, Wu W, Xu J (2022) Association between air pollution and outpatient visits for allergic rhinitis: effect modification by ambient temperature and relative humidity. Sci Total Environ 821:152960. https://doi.org/10.1016/j.scitotenv.2022.152960
Yang D, Yan YJ, Pu KX (2022) The association between air pollutants and daily outpatient visits for allergic rhinitis: a time-series analysis based on distribution lag nonlinear model in Chongqing, China. Risk Manag Healthc Policy 15:1501–1515. https://doi.org/10.2147/RMHP.S373085
Zhang FY, Krafft T, Ye BX, Zhang FS, Zhang JH, Luo HJ, Li MS, Zhang YJ, Zhou L, Xu J, Wang WY (2013) The lag effects and seasonal differences of air pollutants on allergic rhinitis in Beijing. Sci Total Environ 442:172–176. https://doi.org/10.1016/j.scitotenv.2012.09.039
Zhang Y, Zhang L (2014) Prevalence of allergic rhinitis in China. Allergy Asthma Immunol Res 6(2):105–113. https://doi.org/10.4168/aair.2014.6.2.105
Zou QY, Shen Y, Ke X, Hong SL, Kang HY (2018) Exposure to air pollution and risk of prevalence of childhood allergic rhinitis: a meta-analysis. Int J Pediatr Otorhinolaryngol 112:82–90. https://doi.org/10.1016/j.ijporl.2018.06.039
Acknowledgements
The authors acknowledge the dedication and cooperation of all the participants in this study.
Funding
This study was supported by the Natural Science Foundation of Gansu Province, China (Grant No. 21JR7RA864) and Hospital Fund of The First Hospital of Lanzhou University (ldyyyn2020-57).
Author information
Authors and Affiliations
Contributions
Jiyuan Dong contributed to the conception or design of the work. Jie Ji, Hushan Yu, and Yanxia Zhang contributed to the acquisition, analysis, or interpretation of data for the work. Jie Jia and Kangbing Chen drafted the manuscript. Jiyuan Dong critically revised the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The present study is considered exempt from institutional review board approval since the data used was collected for administrative purposes without any personal identifiers.
Consent for publication
Not applicable
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Lotfi Aleya
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 36.9 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ji, J., Chen, K., Dong, J. et al. Associations between air pollution and outpatient visits for allergic rhinitis in Lanzhou, China. Environ Sci Pollut Res 30, 91453–91465 (2023). https://doi.org/10.1007/s11356-023-28457-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-28457-1