Abstract
Tetrabromobisphenol A (TBBPA) is the most abundant brominated flame retardant and bisphenol A (BPA) is often identified as the metabolic product of TBBPA. Both of them are highly bioconcentrated and show serious biological toxicity. In this study, an analytical method was optimized to simultaneously determine TBBPA and BPA in plant samples. Moreover, the uptake and metabolism of TBBPA in maize were investigated through hydroponic exposure experiment. The whole analysis procedure included ultrasonic extraction, lipid removal, purification by solid-phase extraction cartridge, derivatization, and detection by GC/MS. Optimizations were conducted for each pretreatment step above. After improvement, methyl tert-butyl ether (MTBE) was chosen as the extraction solvent; the lipid removal was conducted by repartition between organic solvent and alkaline solution. The best suitable pH condition is 2–2.5 for the inorganic solvent before used for further purification by HLB and silica column with the optimized elute solvent of acetone and mixtures of acetone and hexane (1:1), respectively. The recoveries of TBBPA and BPA spiked in maize samples were 69±4% and 66±4% with the relative standard deviation less than 5%, respectively, for the entire treatment procedure. Limits of detections were 4.10 ng/g and 0.13 ng/g for TBBPA and BPA in plant samples, respectively. In the hydroponic exposure experiment (100 μg/L, 15 d), the concentrations of TBBPA in maize cultivated in pH 5.8 and pH 7.0 Hoagland solutions were 1.45 and 0.89 μg/g in roots and 8.45 and 6.34 ng/g in stems, while they were all below the detection limit for leaves, respectively. The distribution of TBBPA in different tissues was as the following order: root>>stem>leaf, illustrating the accumulation in the root and the translocation to the stem. The uptake variations under different pH conditions were attributed to the change of TBBPA species, now that it shows greater hydrophobicity at lower pH condition as a kind of ionic organic contaminant. Monobromobisphenol A and dibromobisphenol A were identified as metabolisms products of TBBPA in maize. The efficiency and simplicity of the method that we proposed characterize its potential application as a screening tool for environmental monitoring and contribute to a comprehensive study of the environmental behavior of TBBPA.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tetrabromobisphenol A (TBBPA) is the most abundant brominated flame retardant (BFR) currently used in plastic polymers, electronic appliances, and entertainment equipments (Okeke et al. 2022; Xie et al. 2020). Bisphenol A (BPA) is a major industry product widely used in resins and poly-carbonate plastics (Staples et al. 1998; Xie et al. 2020). TBBPA may degrade to less brominated compounds and bisphenol A through pyrolysis, chemical reaction, microbial degradation, and biological metabolism (Liu et al. 2019; Arbeli et al. 2006; Liu et al. 2016a). Due to their wide application and higher persistence, TBBPA and BPA have been detected in various environmental matrices and biological samples including air, dust, surface water, wastewater, sediment, soil, sewage sludge, and aquatic organism (Han et al. 2013; Xie et al. 2020; Pan et al. 2022; Liu et al. 2016b); however, very few data are available for plant samples, relatively. TBBPA and BPA, as a kind of typical endocrine disruptors, can pose potential threat to human and wildlife even in environmental relevant dosage (Covaci et al. 2009; Pan et al. 2022; Liu et al. 2016c; Wu et al. 2021). They both show toxic effects on plants by inhibiting the seed germination and causing lipid peroxidation of plants (Dogan et al. 2010). Moreover, BPA exhibits more toxic effects than TBBPA as to seed germination and oxidative stress (Dogan et al. 2010).
Plants exhibit essential roles in the translocation and transformation of organic compounds. Plant uptake of organic contaminant is a significant process associated with its ecology risk, environmental fate, and the potential threat to the food chain (Collins et al. 2006; Wang et al. 2011a). The ability of plants to absorb organic pollutants is a central process in biogeochemical cycling, which increases the risk of human exposure to them through the food chain (Collins et al. 2006). Understanding the behaviors of TBBPA and its derivatives in intact plants will help to elucidate its environmental behaviors. The metabolism of persistent organic pollutants is an important factor in determining their bioaccumulation, fate, and potential toxicity (Hakk and Letcher 2003). TBBPA could be reductively dehalogenated to bisphenol A (BPA) in slurry of anaerobic sediment (Ronen and Abeliovich 2000; Arbeli and Ronen 2003). For biotic experiment, tribromobisphenol A was found in the rat liver and was excreted into the feces via bile when TBBPA was administered to adult female Wistar rats (Hakk and Letcher 2003). Dehalogenation was observed in plants for other brominated flame retardants, such as polybrominated diphenyl ethers (Zhao et al. 2012; Wang et al. 2011b; Huang et al. 2010). Previous studies have reported that uptake of TBBPA by plant root systems and the resulting translocation to the aboveground part by active transport through the vascular network are common in plants (Li et al. 2011). The previous study presented that derivatives of TBBPA including Tri-BBPA, DBBPA, MBBPA, and BPA are more toxic than TBBPA from the microtox and algal assay (Debenest et al. 2010). Meanwhile, the effects of molecular species in different pH conditions on the uptake of TBBPA on plant are still unknown. Therefore, it is interesting to investigate metabolism derivatives of TBBPA in plants in different pH conditions.
There is a lack of information concerning the analysis and concentration of TBBPA in plant sample relatively, which need strict extraction and purification procedure due to the complicate lipid components of the plant sample. There have been many methods reported to date for determining TBBPA and its metabolisms in various samples (Covaci et al. 2009; Okeke et al. 2022, Environmental Research), such as gas chromatography-mass spectrometry (GC/MS) and liquid chromatography-electrospray tandem mass spectrometry LC/MS(/MS) (Tolosa et al. 2021; Sánchez-Brunete et al. 2009; Okeke et al. 2022). Although LC/MS/MS exhibits higher sensitivity and better detection limits, GC/MS is more available with lower cost in the analysis laboratory. Moreover, the deficiency could be remedied by optimizing the cleanup procedures in reducing the matrices effects. Therefore, the objective of this study was to develop an analytical method for simultaneous determination of TBBPA and BPA in plant sample, including the improvement and optimization for the extraction and cleanup procedures. Then, the new developed method was applied to investigate the uptake and the effects of pH on the uptake of TBBPA in maize, which is regarded as a model plant to investigate the fates of persistent organic compounds because of its extensive cultivation. Here, hydroponic exposures were adopted to focus on the plant metabolism of TBBPA without parallel soil microorganism interactions. Finally, the possible metabolites of TBBPA in maize were identified by GC-MS. This study will be helpful to assess the ecological risk of TBBPA in food chain, analyze its environmental behaviors, and broaden the knowledge of its environmental fates comprehensively.
Materials and methods
Chemicals and compounds
The target compounds TBBPA and BPA together with the derivation reagent (BSTFA: TMCS 99:1) were purchased from Sigma-Aldrich Inc. (Sigma Inc., St. Louis, MO, USA). 13C-tetrabromobisphenol and 13C-PCB-208 were obtained from Cambridge Isotope Laboratories (CIL, Andover, MA, USA). The target compounds were dissolved in ethyl acetate and stored at −4°C in dark. The pure water was supplied by the Milli-Q system (Millipore Corporation). The organic solvents including methane, acetone, hexane, ethyl acetate, dichloromethane (DCM), and methyl tert-butyl ether (MTBE) were of chromatography grade and purchased from Fisher Scientific Inc. (Thermo Fisher, MA, USA).
Hydroponic culture
Maize was selected as a typical model terrestrial plant in this work (Huang et al. 2010; Wang et al. 2011a). Seeds of maize were obtained from the Chinese Academy of Agricultural Sciences, Beijing, China. Seeds were sterilized by soaking in 10% H2O2 for 20 min, followed by thoroughly washing with de-ionized water and subsequently germinating on filter paper in dark at 25°C. After sowing for 3 days, the maize was moved in Hoagland nutrition solution. Then, they were exposed to TBBPA (100 μg/L) at pH of 5.8 and 7.0 to investigate effects of pH on its plant uptake. The exposure solution was renewed once for 24 h. The entire exposure lasted for 15 d. Meanwhile, the blank group with TBBPA free was set with three replicates. The day/light temperature regime was 20–25 °C and the relative humidity was maintained at 60–70%. The photoperiod was set as 14 h/day at a light intensity of 132.50W/m2. The plants were harvested and washed with tap water at least for three times. Then, the plant samples were freeze-dried for 48 h and chopped finely before further pretreatment.
Sample extraction and lipid removal
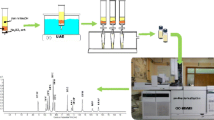
The whole method diagram is illustrated in Fig. 1 and each step was optimized. Firstly, the collected maize plant samples were washed by pure water; then, they were freeze-dried, following which they were cut and grinded homogeneously. 0.1 g dried plant sample was weighted and spiked with 60 ng 13C-TBBPA as a surrogate dissolved in 10 μL methane. The prepared plant sample was placed in dark conditions for 24 h until all methanol evaporated. Then, the plant was extracted with 8–10 mL different organic solvents in ultrasonic bath for 20 min. Four extracting solvents including ethyl acetate, dichloromethane, MTBE, and hexane which showed different polarities were tested and compared in this study. After the extraction, the organic solvent was collected after centrifugation at 3000 rad/min for 20 min. The above process was conducted for three times.
Lipid removal optimization
In the lipid removal optimization, concentrated sulfuric acid (Liang et al. 2010), freezing-lipid filtration (Ahn et al. 2007), and alkaline were used. For the freezing-lipid filtration, the extract was exposed in −20°C conditions for 48 h to observe the separation efficiency of the lipid and solvent. The obtained extract was concentrated to about 5 mL through nitrogen flow and then 5 mL tested lipid removal solvents including concentrated sulfuric acid or NaOH (1 M) added individually. After mechanic shaking for 10 min and centrifugation (3000 rad/min, 10 min), the organic phase and inorganic solvents were separated. For the alkaline set, the organic solvent was abandoned while the alkaline solution was collected, whereas, for concentrated sulfuric acid set, the organic solvent was collected while the acid solution was abandoned. The above operations were done twice for each set, respectively.
pH conditions optimization
The pH of extraction solution containing the target compounds was adjusted to desired value by diluted HCl. The influence of different pH conditions on the recoveries was also studied in the range of 2–9.5 through adding the diluted HCl. Then, it was loaded onto HLB cartridge for cleanup. TBBPA and BPA (50 ng) were spiked into the unpolluted plant sample or extract beforehand to test the recovery for extraction efficiency and the effects of pH in the lipid removal.
HLB cartridge and silica gel column cleanup
The HLB cartridge (Oasis Corp., 6 cc, 200 mg) was activated with 10 mL methanol and 10 mL ultrapure water sequentially before it was used. The extracted sample was loaded with the flow rate of 1 mL/min. After washed with 10 mL 10% methanol in water (v/v) and 10 mL ultrapure water in sequence, the column was dried in vacuum condition for 2 h to remove the residual water in the cartridge. Several organic solvents (10 mL) with different polarities including dichloromethane, MTBE, ethyl acetate, and acetone were chosen to test the eluting efficiency.
The obtained eluent was dried in nitrogen flow and dissolved in 2 mL hexane. After the silica column (Waters Corp., 6 cc, 500 mg) was conditioned by 10 mL hexane, the sample was loaded and dropped naturally by gravity. Then, it was washed by 10 mL hexane and the sample was eluted with 10 mL tested organic solvents or mixtures. The tested six different eluents included dichloromethane, dichloromethane:hexane (3:1, v:v), dichloromethane:hexane (1:1, v:v), acetone, acetone:hexane (3:1, v:v), and acetone:hexane (1:1, v:v). The eluents were dried by the nitrogen flow and it was dissolved in ethyl acetate ready for derivation.
TBBPA and BPA (50 ng) were spiked into the unpolluted extract prior to test the recovery of the HLB cartridge and the silica column cleanup procedures to optimize the elute solvents and assure the recovery in the purification.
Derivatization
The standard compounds and purified plant samples dissolved in 200 μL ethyl acetate were added with 40 μL derivation chemical BSTFA:TMCS (99:1, v:v). Then, the reaction was controlled at 60 °C for an hour in sealed environment. After the samples were cooled to room temperature, they were detected immediately. 13C-PCB-208 was used as an internal standard prior to instrumental measurement.
Instrumental parameters
The measurement of target compounds was performed by gas chromatography-mass spectrometry (Agilent 7890A-5975C) with a DB-XLB column (30 m × 0.25 mm × 0.25 μm). The gas of helium with the flow rate of 1 mL/min was used. The volume of injection was 1 μL with the splitless mode. The temperature program was set as follows: oven temperature set at 75°C and held for 1 min, then increased to 300°C at the rate of 20°C/min and held for 10 min. The temperatures of oven and injection inlet were set as 300°C and 250°C, respectively. The temperatures of ion source, transfer line, and quadropole were 230°C, 280°C, and 150°, respectively. For mass spectrometry information, the characteristic ions of target compounds, surrogate compound, and internal compound are listed in Table 1. Moreover, the quantification ions and the retain time for each compound were also included. The chromatography information of target compounds is shown in Fig. 6.
Quality assurance and quality control
In order to obtain good quantitative accuracy, stable isotope techniques have also been introduced to analyze TBBPA. Here, 13C-TBBPA is applied for the recovery control of TBBPA and BPA during the total procedure. The sample analysis and the method optimizations for each step were set as triplicate. The known dosage of TBBPA and BPA (50 ng) were added to the unpolluted plant sample, which then experienced the entire optimized procedure of extraction, purification, and detection processes (Fig. 1) to test the recovery.
Data analysis
The software Origin Pro 9.1.1 (Origin, USA) was used to analyze the data.
Results and discussion
Extraction
Four different hydrophobic solvents were used to extract the target compounds. The extraction recoveries of different solvents were calculated. As shown in Fig. 2, ethyl acetate, MTBE, and dichloromethane exhibit excellent recoveries (>92%) for both compounds. The recovery of hexane extraction of BPA was only 55%, whereas the recovery of TBBPA extraction by hexane could reach more than 92%. The phenomena maybe attributed to the relative low polarity of hexane among all selected solvents. BPA show higher polarity than TBBPA with a pKa of 9.8–10.4, a logKow of 3.4, and a water solubility of 120 mg/L, whereas TBBPA has a pKa 6.79–7.06, a logKow of 5.9, and a water solubility of 0.08–4.16 mg/L (Han et al. 2013; Vasiljevic and Harner 2021). Solvents with higher polarity are helpful to recovery of a larger amount of polar compounds like BPA (Liu et al. 2019). Therefore, hexane was not suitable for simultaneous extraction of TBBPA and BPA.
During the repartition of target compounds between organic solvent and alkaline solution, 25% of BPA and 19% of TBBPA were found to dissolve into ethyl acetate due to its relative higher polarity. In contrast, nontarget compounds were found in other three solvents suggesting BPA and TBBPA were all distributed into alkaline solution. Meanwhile, the lipids in the plant were dissolved into organic phases. At last, MTBE was selected as the extraction solvent which was also selected in BPA extraction for animal meat, sludge, and sediment samples in other studies (Evenset 2009; Chu et al. 2005). Moreover, MTBE showed higher volatility than the other solvents tested and was benefit for the concentrated procedure. Therefore, MTBE was chosen as the extractant for the following analysis.
Lipid removal and comparison with other studies
Purification of the plant sample in the analysis is a key step and lipid removal is necessary for the pretreatment of organic contaminants in plant sample. The most common used lipid removal methods included concentrated sulfuric acid and hexane (Tang et al. 2015; Liang et al. 2010), freezing-lipid filtration (Ahn et al. 2007) and alkaline for lipid removal (Evenset 2009). Freezing-lipid filtration was efficiently to remove lipid in fish sample (Mita et al. 2011; Ahn et al. 2007). However, it was not practicable for lipid removal because the extract in acetonitrile cannot precipitate at −24°C for 2 h in the pre-experiment. Therefore, freezing-lipid filtration cannot be used in this study. This was possibly attributed to the differences of lipid components between plant and meat samples. Similarly, the procedure of freezing-lipid filtration was far from satisfactory for the purification of the plant sample (Niu et al. 2011). Plant extracts were purified with sulfuric acid to remove lipids (Li et al. 2019), where only TBBPA was analyzed. In another preliminary experiment here, the lipid was successfully eliminated by concentrated sulfuric acid followed by the re-extraction in hexane for target compounds. However, BPA was completely lost during this process. Although the recovery of TBBPA after this treatment was 98.8%, it was ignored since TBBPA and BPA could not be simultaneously obtained. Finally, the procedure of lipid removal by alkaline was conducted. The target compounds were dissolved in NaOH solution while the lipid was distributed into organic phase, and they were successfully separated. Therefore, NaOH solution (1M) was selected used in the lipid removal procedure at last by comparison. There are many plant species. Most of the previous studies were about aquatic plants or pumpkins (Hou et al. 2018; Jiang et al. 2020; Li et al. 2019). Here, maize is focused on as a kind of typical terrestrial plants. The mangrove plant samples were only purified and cleaned with ENVI-Carb SPE cartridges without lipid removal (Hou et al. 2018). The purification of lipid removal by alkaline used in this study is more suitable than other methods like freezing-lipid filtration and concentrated sulfuric acid in other reports.
Effects of solution pH on the recovery during HLB cartridge cleanup
The pH condition was optimized before the sample extract was loaded into the cartridge. In the range of pH 2.2 to 9.5, the recoveries of BPA were stable and they were all above 93%; therefore, the pH has little influence on this process as illustrated in Fig. 3. However, in the range of pH 2.2 to 6.0, the elution recovery of TBBPA decreased from 94 to 69% on average with the increase of pH. Furthermore, it was completely lost when the pH was higher than 6.0. A similar behavior was found for ionic pharmaceutical compounds including ibuprofen, naproxen, and ketoprofen (Rodriguez et al. 2004). The above results were attributed to the molecular properties of ionic organic compounds. The non-dissociated species of organic contaminants can bind to HLB cartridges more strongly (Liu et al. 2004). More TBBPA was washed through the aqueous phase during the process of hydrophilic-lipophilic balance because the ionic species, in contrast to the molecular form, was more hydrophilic (Buchholz and Pawliszyn 1994) and was harder to block by the adsorption materials in the HLB cartridge. Compared with TBBPA, BPA was less sensitive to pH owing to its higher pKa value (10.2) than the pKa value of TBBPA (7.5), so the fraction of BPA species mainly existed in protonated form in the pH range studied. Similar results were observed in the extraction of chlorophenols from aqueous samples; the acid condition showed greater recovery for phenols with lower pKa values (Buchholz and Pawliszyn 1994). Therefore, it was advised to adjust the pH of extraction solution to 2–2.5 in order to ensure that both recoveries of TBBPA and BPA were greater than 85% simultaneously in the subsequent experiment.
Influences of elution solvents on the recovery for HLB cartridge
The recovery of organic compounds during cartridge cleanup is highly dependent on the polarity of the elution solvents. Four different solvents including acetone, MTBE, dichloromethane, and ethyl acetate were selected to elute the target compounds for optimization. The results (Fig. 4) showed that BPA was poorly recovered by dichloromethane due to relatively polar characteristics of BPA. The recoveries for two compounds were both improved in the sequence of MTBE, ethyl acetate, and acetone, as the polarity of tested solvent increased. Consequently, acetone was chosen as the elution solvent for simultaneously efficient extraction of two target compounds.
Impact of elution solvents on the recovery for silica column
The selection of favorable washing solvent is important for the silica column purification because the weaker polar solvent cannot elute the target compounds completely, whereas the stronger polar solvent may elute more unexpected co-pollutants in sample analyzed. Dichloromethane and acetone mixed in hexane with different volume ratios were used as the eluting solvents for improvement. The results as shown Fig. 5 indicated that TBBPA could be easily eluted with excellent recovery by all organic solvents studied. However, BPA was only eluted completely with an acetone-hexane mixture and less than 12% was recovered with a dichloromethane-hexane mixture. This was attributed to the higher polarity of BPA, which facilitated the adsorption on the silica gel material in the cartridge, so only strong polar solvent was effective to elute this compound. Finally, a mixture of acetone and hexane (1:1, v:v, 10 mL in total) was selected as the elute solvent. The loss caused by filtration through the membrane (0.22μm, nylon material) for TBBPA and BPA was less than 2.5%. Thus, it is an ideal membrane used before injection into analytical instrument.
Quality control of the method
The results showed that recoveries of BPA and TBBPA spiked in unpolluted plant sample were 66±4% and 69±4%, respectively, for the whole treatment procedure as illustrated in Fig. 1 with the relative standard error less than 5% with excellent reproducibility. The lowest detection limit of the measurement instrument was determined according to the signal-to-noise ratio S/N=3 and they were 1.64 μg/L and 0.05 μg/L for TBBPA and BPA, respectively. The lowest detection limit for the plant sample was 4.10 and 0.13 ng/g for TBBPA and BPA, respectively. For the lowest limit of quantification, they were 4.92 μg/L and 0.15 μg/L for TBBPA and BPA, respectively.
The SIM chromatogram, retention time, and characteristic ions of target compounds are shown in Fig. 6 and Table 1. The chromatogram showed that the separation effect of the target compound is very good, and the method exhibited good selectivity. The linear range of GC/MS for the determination of BPA and TBBPA was 10–1000 μg/L and 10–500 μg/L with a fixed amount (20 μg/L) of internal standard, respectively. Table 2 shows the equations fitted by concentration (x) and response abundance ratio of target compound to that of internal standard (y). The correlation coefficients r2 more than 0.997 with good linearity were also included as shown in Table 1. The results illustrated the robustness of this newly developed and optimized analysis method.
Uptake and metabolite of TBBPA in maize
The optimized extraction, purification, and detection method above was used to analyze the pollutants in the plant of maize exposed in the TBBPA and BPA solutions in microcosm environment for 2 days. The results show that concentration of TBBPA in all tissues of maize from the control set with no TBBPA exposure was all below the detection limit. However, for the exposed samples, the content of TBBPA in different maize tissues decreased as the following order: root >> stem > leaf. It is similar to the findings of other studies of PCBs and PBDEs (Zhang et al. 2015), The contaminants in the solutions may enter the roots and pass up the xylem to the remainder of the plants (Collins et al. 2006). The concentrations of TBBPA in root of maize cultivated in pH 5.8 and pH 7.0 solutions were 1.45 μg/g and 0.89 μg/g, respectively. The concentrations of TBBPA in stem were 8.45 and 6.34 ng/g, respectively, while it was below the detection limit for leaves in both pH conditions. Similarly, the previous studies also showed higher concentrations of TBBPA accumulate in roots of radish, cabbage, and mangrove species rather than in the shoots and other tissues (Li et al. 2011; Sun et al. 2014). This indicated that a large amount of TBBPA accumulated in the root and then translocated to stem. Concentration of TBBPA in maize cultivated at pH 5.8 increased by 63±4% compared with that at pH 7.0 although the pH had no impact on the biomass of maize. The phenomena may be caused by the changes of the contaminant speciation under different pH conditions. More protonated species of TBBPA were available at lower pH with higher value of Kow coefficients and they can be more easily partitioned into the plant than dissipated species. Similar research results were observed in aquatic plant to assimilate 2,4,5-trichlorophenol where the uptake rates positively correlated with molecular fractions and mass transfer into the plant was linearly dependent on amount of the protonated species (Tront and Saunders 2006).
Full scan model with the m/z range from 200 to 700 was used to tentatively identify the possible metabolic products of TBBPA in maize root. Compared with the control group, the characteristic ions of monobromobisphenol A and dibromobisphenol derivatives were found for the exposed sample. However, none of other debrominated products including bisphenol A was found. The debromination was also found in plant exposed in other brominated flame retardants (BFRs) like HBCD and PBDE (Wang et al. 2011b; Huang et al. 2010). The nitrate reductase and glutathione S-transferase in the maize root play the key role in promoting the debromination (Huang et al. 2013). The debromination processes of TBBPA were also found on other organisms, like microalgae (Peng et al. 2014) and anaerobic microorganisms in the contaminated soil (Chang et al. 2012). The results implied that the debromination of TBBPA in maize species was a significant transformation mechanism.
Although LC/MS/MS exhibits higher sensitivity and better detection limits, GC/MS is more available with lower cost in the analysis laboratory. Moreover, an excellent purification pretreatment was optimized here. The best lipid removal method and the excellent elute dosage and conditions were selected by comparisons. For the limitations and uncertainty, the treatment of extraction and purification should be simplified in future study. Moreover, this analytical method should be used to test other plant species except the maize sample studied in this paper.
Conclusions
A method was developed for simultaneous determination of TBBPA and BPA in plant samples. MTBE was used during the ultrasonic extraction. The lipid in the plant and the target compounds were successfully separated by NaOH solution. Two different solid-phase extraction cartridges (HLB and silica columns) were used for further purification and the conditions applied were also improved. The measurement was conducted by GC/MS followed by derivation. The recoveries of TBBPA and BPA for the whole method were 69±4% and 66±4%, with the method detection limits of 4.10 ng/g and 0.13 ng/g, respectively. The distribution of TBBPA in different tissues of maize was followed the order: root>>stem>leaf. The contents of TBBPA in maize exposed in pH 5.8 Hoagland solutions were higher than those for pH 7.0 because of the strong partition affinity of molecular species of TBBPA. The debromination occurred for TBBPA in plant with the metabolite products of monobromobisphenol A and dibromobisphenol A was identified.
Data availability
The data and materials used during the current study are available from the corresponding author on reasonable request.
References
Ahn YG, Shin JH, Kim HY, Khim J, Lee MK, Hong J (2007) Application of solid-phase extraction coupled with freezing-lipid filtration clean-up for the determination of endocrine-disrupting phenols in fish. Anal Chim Acta 603:67–75. https://doi.org/10.1016/j.aca.2007.09.045
Arbeli Z, Ronen Z (2003) Enrichment of a microbial culture capable of reductive debromination of the flame retardant tetrabromobisphenol-A, and identification of the intermediate metabolites produced in the process. Biodegradation 14:385–395. https://doi.org/10.1023/a:1027304222436
Arbeli Z, Ronen Z, Díaz-Báez MC (2006) Reductive dehalogenation of tetrabromobisphenol-A by sediment from a contaminated ephemeral streambed and an enrichment culture. Chemosphere 64:1472–1478. https://doi.org/10.1016/j.chemosphere.2005.12.069
Buchholz KD, Pawliszyn J (1994) Optimization of solid-phase microextraction conditions for determination of phenols. Anal Chem 66:160–167. https://doi.org/10.1021/ac00073a027
Chang BV, Yuan SY, Ren YL (2012) Aerobic degradation of tetrabromobisphenol-A by microbes in river sediment. Chemosphere 87:535–541. https://doi.org/10.1016/j.chemosphere.2011.12.057
Chu S, Haffner GD, Letcher RJ (2005) Simultaneous determination of tetrabromobisphenol A, tetrachlorobisphenol A, bisphenol A and other halogenated analogues in sediment and sludge by high performance liquid chromatography-electrospray tandem mass spectrometry. J Chromatogr A 1097(1-2):25–32. https://doi.org/10.1016/j.chroma.2005.08.007
Collins C, Fryer M, Grosso A (2006) Plant uptake of non-ionic organic chemicals. Environ Sci Technol 40:45–52. https://doi.org/10.1021/es0508166
Covaci A, Voorspoels S, Abdallah MAE, Geens T, Harrad S, Law RJ (2009) Analytical and environmental aspects of the flame retardant tetrabromobisphenol-A and its derivatives. J Chromatogr A 1216:346–363. https://doi.org/10.1016/j.chroma.2008.08.035
Debenest T, Gagne F, Petit AN, Andre C, Kohli M, Blaise C (2010) Ecotoxicity of a brominated flame retardant (tetrabromobisphenol A) and its derivatives to aquatic organisms. CBP Part C: Toxicol Pharmacol 152:407–412. https://doi.org/10.1016/j.cbpc.2010.06.009
Dogan M, Yumrutas O, Saygideger SD, Korkunc MM, Gulnaz O, Sokmen A (2010) Effects of bisphenol A and tetrabromobisphenol A on chickpea roots in germination stage. Am –Eur J Agric Environ Sci 9(2):186–192
Evenset, A. Silver, Platinum, Sucralose, Bisphenol A, Tetrabrombisphenol A, Siloxanes,Phtalates and Phosphororganic flame retardants. 1-43 (Norwegian Pollution Control Authority, 2009).
Hakk H, Letcher RJ (2003) Metabolism in the toxicokinetics and fate of brominated flame retardants—a review. Environ Int 29:801–828. https://doi.org/10.1016/S0160-4120(03)00109-0
Han W, Wang S, Huang H, Luo L, Zhang S (2013) Simultaneous determination of brominated phenols in soils. J Environ Sci 25(11):2306–2312. https://doi.org/10.1016/S1001-0742(12)60298-8
Hou X, Yu M, Liu A, Li Y, Ruan T, Liu J, Schnoor JL, Jiang G (2018) Biotransformation of tetrabromobisphenol A dimethyl ether back to tetrabromobisphenol A in whole pumpkin plants. Environ Pollut 241:331–338. https://doi.org/10.1016/j.envpol.2018.05.075
Huang H, Zhang S, Christie P, Wang S, Xie M (2010) Behavior of decabromodiphenyl ether (BDE-209) in the soil−plant system: uptake, translocation, and metabolism in plants and dissipation in soil. Environ Sci Technol 44:663–667. https://doi.org/10.1021/es901860r
Huang HL, Zhang SZ, Wang S, Lv JT (2013) In vitro biotransformation of PBDEs by root crude enzyme extracts: potential role of nitrate reductase (NaR) and glutathione S-transferase (GST) in their debromination. Chemosphere 90(6):1885–1892. https://doi.org/10.1016/j.chemosphere.2012.10.013
Jiang Y, Lu H, Wang Y, Hong H, Wang Q, Liu J, Yan C (2020) Uptake, biotransformation and physiological response of TBBPA in mangrove plants after hydroponics exposure. Mar Pollut Bull 151:110832. https://doi.org/10.1016/j.marpolbul.2019.110832
Li Y, Zhou Q, Wang Y, Xie X (2011) Fate of tetrabromobisphenol A and hexabromocyclododecane brominated flame retardants in soil and uptake by plants. Chemosphere 82:204–209. https://doi.org/10.1016/j.chemosphere.2010.10.021
Li H, Hu Y, Sun Y et al (2019) Bioaccumulation and translocation of tetrabromobisphenol A and hexabromocyclododecanes in mangrove plants from a national nature reserve of Shenzhen City, South China. Environ Int 129:239–246. https://doi.org/10.1016/j.envint.2019.05.034
Liang X, Zhu S, Chen P, Zhu L (2010) Bioaccumulation and bioavailability of polybrominated diphynel ethers (PBDEs) in soil. Environ Pollut 158:2387–2392. https://doi.org/10.1016/j.envpol.2010.04.008
Liu R, Zhou JL, Wilding A (2004) Simultaneous determination of endocrine disrupting phenolic compounds and steroids in water by solid-phase extraction–gas chromatography–mass spectrometry. J Chromatogr A 1022:179–189. https://doi.org/10.1016/j.chroma.2003.09.035
Liu A, Qu G, Yu M, Liu Y, Shi J, Jiang G (2016a) Tetrabromobisphenol-A/S and nine novel analogs in biological samples from the Chinese Bohai Sea: implications for trophic transfer. Environ Sci Technol 50:4203–4211. https://doi.org/10.1021/acs.est.5b06378
Liu C, Niu X, Song X (2016b) A simulation research on the natural degradation process of tetrabromobisphenol A in soil under the atmospheric different environments. Environ Sci Pollut Res 23:16406–16416. https://doi.org/10.1007/s11356-016-6767-1
Liu K, Li J, Yan S, Zhang W, Li Y, Han D (2016c) A review of status of tetrabromobisphenol A (TBBPA) in China. Chemosphere 148:8–20. https://doi.org/10.1016/j.chemosphere.2016.01.023
Liu A, Zhao Z, Qu G, Liang X, Shi J, Jiang G (2019) Identification of transformation/degradation products of tetrabromobisphenol A and its derivatives. Trends Anal Chem 111:85–99. https://doi.org/10.1016/j.trac.2018.12.003
Mita L, Bianco M, Viggiano E, Zollo F, Bencivenga U, Sica V, Monaco G, Portaccio M, Diano N, Colonna A, Lepore M, Canciglia P, Mita DG (2011) Bisphenol A content in fish caught in two different sites of the Tyrrhenian Sea (Italy). Chemosphere 82(3):405–410. https://doi.org/10.1016/j.chemosphere.2010.09.071
Niu Y, Zhang J, Wu Y, Shao B (2011) Simultaneous determination of bisphenol A and alkylphenol in plant oil by gel permeation chromatography and isotopic dilution liquid chromatography–tandem mass spectrometry. J Chromatogr A 1218:5248–5253. https://doi.org/10.1016/j.chroma.2011.06.005
Okeke ES, Huang B, Mao G, Chen Y, Zhengjia Z, Qian X, Wu X, Feng W (2022) Review of the environmental occurrence, analytical techniques, degradation and toxicity of TBBPA and its derivatives. Environ Res 206:112594–112629. https://doi.org/10.1016/j.envres.2021.112594
Pan YF, Liu S, Tian F, Chen HG, Xu XR (2022) Tetrabromobisphenol A and hexabromocyclododecanes in sediments from fishing ports along the coast of South China: Occurrence, distribution and ecological risk. Chemosphere 302:134872–134881. https://doi.org/10.1016/j.chemosphere.2022.134872
Peng F, Ying G, Yang B, Liu Y, Lai H, Zhou G, Chen J, Zhao J (2014) Biotransformation of the flame retardant tetrabromobisphenol-A (TBBPA) by freshwater microalgae. Environ Toxicol Chem 33:1705–1711. https://doi.org/10.1002/etc.2589
Rodriguez I, Carpinteiro J, Quintana JB, Carro AM, Lorenzo RA, Cela R (2004) Solid-phase microextraction with on-fiber derivatization for the analysis of anti-inflammatory drugs in water samples. J Chromatogr A 1024:1–8. https://doi.org/10.1016/j.chroma.2003.10.049
Ronen Z, Abeliovich A (2000) Anaerobic-aerobic process for microbial degradation of tetrabromobisphenol A. Appl Environ Microbiol 66:2372–2377. https://doi.org/10.1128/AEM.66.6.2372-2377.2000
Sánchez-Brunete C, Miguel E, Tadeo JL (2009) Determination of tetrabromobisphenol-A, tetrachlorobisphenol-A and bisphenol-A in soil by ultrasonic assisted extraction and gas chromatography-mass spectrometry. J Chromatogr A 1216(29):5497–5503. https://doi.org/10.1016/j.chroma.2009.05.065
Staples CA, Dome PB, Klecka GM, Oblock ST, Harris LR (1998) A review of the environmental fate, effects, and exposures of bisphenol A. Chemosphere 36:2149–2173. https://doi.org/10.1016/s0045-6535(97)10133-3
Sun F, Kolvenbach BA, Nastold P, Jiang B, Ji R, Corvini PFX (2014) Degradation and metabolism of tetrabromobisphenol A (TBBPA) in submerged soil and soil–plant systems. Environ Sci Technol 48:14291–14299. https://doi.org/10.1021/es503383h
Tang B, Zeng YH, Luo XJ, Zheng XB, Mai BX (2015) Bioaccumulative characteristics of tetrabromobisphenol A and hexabromocyclododecanes in multi-tissues of prey and predator fish from an e-waste site, South China. Environ Sci Pollut Res 22:12011–12017. https://doi.org/10.1007/s11356-015-4463-1
Tolosa I, Huertas D, Choyke S, Sander S, Aminot Y (2021) A comprehensive evaluation of two sample treatment procedures for the determination of emerging and historical halogenated flame retardants in biota. Environ Sci Pollut Res 28:59345–59357. https://doi.org/10.1007/s11356-020-10966-y
Tront JM, Saunders FM (2006) Role of plant activity and contaminant speciation in aquatic plant assimilation of 2,4,5-trichlorophenol. Chemosphere 64:400–407. https://doi.org/10.1016/j.chemosphere.2005.12.025
Vasiljevic T, Harner T (2021) Bisphenol A and its analogues in outdoor and indoor air: Properties, sources and global levels. Sci Total Environ 789:148013. https://doi.org/10.1016/j.scitotenv.2021.148013
Wang S, Wu T, Huang HL, Ping H, Lu AX, Zhang SZ (2011a) Analysis of hydroxylated polybrominated diphenyl ethers in plant samples using ultra performance liquid chromatography-mass spectrometry. Sci China Chem 54:1782–1788. https://doi.org/10.1007/s11426-011-4383-y
Wang S, Zhang S, Huang H, Zhao M, Lv J (2011b) Uptake, translocation and metabolism of polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) in maize (Zea mays L.). Chemosphere 85:379–385. https://doi.org/10.1016/j.chemosphere.2011.07.002
Wu H, Wang J, Xiang Y, Li L, Qie H, Ren M, Lin A, Qi F (2021) Effects of tetrabromobisphenol A (TBBPA) on the reproductive health of male rodents: A systematic review and meta-analysis. Sci Total Environ 781:146745. https://doi.org/10.1016/j.scitotenv.2021.146745
Xie Q, Cao J, Sun D, Lu H, Xia M, Hou B, Li D, Jia L (2020) Determination of aqueous bisphenol A and tetrabromobisphenol A using molecular-complex-based liquid-liquid microextraction. J Mol Liq 303:112501–112506. https://doi.org/10.1016/j.molliq.2020.112501
Zhang Y, Luo X, Mo L (2015) Bioaccumulation and translocation of polyhalogenated compounds in rice ( Oryza sativa L.) planted in paddy soil collected from an electronic waste recycling site South China. Chemosphere 137:25–32. https://doi.org/10.1016/j.chemosphere.2015.04.029
Zhao M, Zhang S, Wang S, Huang H (2012) Uptake, translocation, and debromination of polybrominated diphenyl ethers in maize. J Environ Sci 24:402–409. https://doi.org/10.1016/S1001-0742(11)60748-1
Funding
This work was funded by Research Foundation for High-level Talents of Inner Mongolia Agricultural University (NDYB2016-07).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study design. The first draft of the manuscript was written by Xuehui Xu. Wei Han revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to participate
Not applicable
Consent for publication
All authors agreed to publish this article in Environmental Science and Pollution Research.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Ester Heath
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOC 61 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, X., Han, W. Analysis of tetrabromobisphenol A and bisphenol A in plant sample—method optimization and identification of the derivatives. Environ Sci Pollut Res 30, 82770–82779 (2023). https://doi.org/10.1007/s11356-023-28241-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-28241-1