Abstract
Bisphenol analogues are compounds extensively used which have been potentially linked to adverse health effects. Nevertheless, few studies reported the analysis of compounds, other than bisphenol A, in environmental solid samples and none in soil samples. In this study, a rapid and sensitive analytical method is presented for the simultaneous determination of 13 bisphenols in soil samples. The method combines ultrasonic-assisted extraction of samples placed in small columns and GC-MS/MS analysis. Manual and on-line derivatizations were compared and results showed that significant higher chromatographic responses were achieved with on-line derivatization. Different parameters such as the quantity of derivatization agent, the extraction solvent, or the extraction time were assayed. The detection limits for all target bisphenols ranged from 0.04 to 0.27 ng g−1, for BPC and BPA, respectively. Analysis of spiked soil samples gave satisfactory recovery results, from 70 to 111%, for all the compounds. Finally, the validated method was applied to soil samples from several Spanish areas, and 3 of the 13 target bisphenols (BPAF, BPF, and BPA) were detected, although only BPF and BPA could be quantified with levels up to 127 ng g−1.

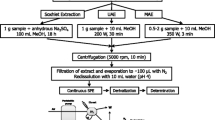
Schematic diagram of the developed method for the determination of bisphenol analogues
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bisphenol A (BPA) is one of the most widely used chemicals because it has many industrial and commercial applications, such as the production of polycarbonate plastics and epoxy resins. Both polymers are used in many consumer products such as food containers, paper, water pipes, toys, or medical devices, among others [1]. Different studies have reported the occurrence of BPA in human biological samples revealing a global exposure which can be harmful to humans. Numerous researches have documented effects of BPA as endocrine disruptor and have been potentially linked to adverse health effects as diabetes, obesity, or cancer [1, 2]. The concern over widespread human exposure to BPA and its potential health effects has led to regulations on the production and usage of BPA in the European Union (EU). Thus, the EU restricted the use of BPA in plastic infant feeding bottles since 2011 [3]. When the European Food Safety Authority (EFSA) evaluated the dietary exposure to BPA in 2006, very sparse data were available and the experts had to make several conservative assumptions about consumption and levels of BPA in food. However, in January 2015, new data and refined methodologies have led EFSA’s experts to reduce the safe level of BPA from 50 μg kg−1 of body weight per day to 4 μg kg−1 body weight per day as temporary tolerable daily intake. Due to better data and less conservative assumptions for the exposure calculations, dietary exposure is from 4 to 15 times lower than previously estimated by EFSA in 2006, depending on the age group considered. Thus, EFSA’s comprehensive reevaluation of BPA exposure and toxicity concludes that BPA poses no health risk to consumers of any age group from dietary exposure and low health concern from aggregated exposure [4]. Nevertheless, from 2010 to 2012, several EU Member States (Denmark, Austria, Belgium, France, Sweden) proposed bans on the use of BPA for food packaging intended for young children (0 to 3 years old) [4].
As a result of the public concern and governmental regulations on BPA, the development and production of alternative substances to replace BPA has been encouraged and compounds, structurally similar to BPA, are being used in the manufacture of polycarbonate plastics and epoxy resins. These chemicals have two hydroxyphenyl functionalities and are collectively referred to as bisphenol analogues. Recently, two reviews were published regarding bisphenols analogues which pointed that data concerning the levels of multiple bisphenols and derivatives in different samples (environment, consumer products, and foodstuffs) are still scarce [5, 6]. The review by Chen et al. [5] described the state of the art knowledge on the occurrence of bisphenol analogues in the environment, consumer products and foodstuffs, human exposure and biomonitoring, and toxicity, whereas the review by Caballero-Casero et al. [6] was mainly focused in the main analytical methods reported for the determination of mixtures of bisphenol analogues and/or derivatives in human and environmental exposure sources and biological fluids.
In general, mixtures of bisphenol analogues from two to eight compounds (BPA, BPAF, BPAP, BPB, BPE, BPF, BPP, BPS, BPZ; see Table 1 for compound name) were analyzed on different environmental compartments, food, consumer products, and human biological samples [7]. In environmental samples, BPA was evaluated in all samples mainly in combination with BPF, followed by mixtures of BPA with BPB, BPAF, or BFS and, in less studies, with BPP, BPZ, and BPAP [6]. BPA was still the main analogue found in environmental monitoring studies; nevertheless, high concentrations of BPAF, BPF, and BPS have been reported in the environment and human urine from some regions [5]. These three bisphenol analogues are among the main substitutes of BPA in the manufacturing of polycarbonate plastics and epoxy resins, although a total of 16 bisphenol analogues have been documented for industrial applications [5]. The list of bisphenol analogues currently in use is long and increasing, but the studies about the presence of bisphenol analogues in environmental samples are scarce in the available literature and they are mainly focused in sediments, sludge, and water [8,9,10,11].
The determination of mixtures of bisphenol analogues in environmental samples have been done by liquid chromatography-tandem mass spectrometry (LC-MS/MS) [6], because many of them were liquid samples. Nevertheless, the analysis by gas chromatography-mass spectrometry (GC-MS) or tandem mass spectrometry (GC-MS/MS) have been successfully used to the determination of BPA and analogues in sediments, packed food, and human breast milk [6, 7, 11]. Due to the high polarity of these compounds, a derivatization step is necessary to increase their volatility when they were analyzed by GC. Silylation and acetylation were the main derivative process for these compounds. The derivatization reagent most frequently used in the silylation process is N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA), which leads to the formation of trimethylsilyl derivatives [12, 13], whereas the acetylation process is carried out with acetic anhydride [6, 11]. In general, silylation needs temperature control, a time period to complete the process, and an anhydrous medium while acetylation needs an aqueous medium and can be carried out at room temperature, but the extract has to be in an organic solvent before its chromatographic analysis. Derivatization using BSTFA have been successfully applied in our laboratory for the determination of BPA in soil samples, [13]. Recently, Wang et al. [11] reported the analysis of BPA and six analogues in sediments after derivatization with acetic anhydride. To carry out this acetylation process, the extract was previously evaporated to dryness and dissolved with 2 mL of a buffer solution (pH = 11.3) followed by extraction with hexane.
The main objective of this study was to develop a quick, selective, sensitive, and efficient analytical method for quantitative simultaneous determination of BPA and twelve BPA analogues in soil samples (see Table 1) by GC-MS/MS with on-line derivatization in the GC system. The extraction method developed was based on ultrasound-assisted extraction (UAE) in small columns, due to its main advantages as miniaturization of the extraction procedure, short processing time, and use of conventional laboratory equipment. GC-MS/MS was selected as a technique able to determine a high number of bisphenol compounds in a single run with a high selectivity making unnecessary a clean-up step before the chromatographic analysis. Additionally, on-line derivatization in the GC injector is an attractive alternative because it avoids preparative steps, accelerates reaction rates, and reduces evaporative losses and operator exposure. Finally, the validated method was used to monitor these contaminants in agricultural and industrial soil samples. As indicated above, few studies have reported the analysis of mixtures of a wide set of bisphenol analogues in environmental samples and, to the best of our knowledge, this is the first work reporting simultaneous determination of these compounds in soil.
Experimental
Chemical and reagents
Acetonitrile (ACN), ethyl acetate (EtAc), and methanol (MeOH), for GC residue analysis, were supplied by Aldrich (Steinheim, Germany). Anhydrous sodium sulfate was obtained from Probous (Barcelona, Spain). Standards of BPA, BFAF, BPAP, BPBP, BPC, BPB, BPE, BPF, BPG, BPM, BPP, BPS, and BPZ (purity ≥98%) were purchased from Aldrich (Steinheim, Germany). 13C12-BPA (purity >99%, 50 μg mL−1 in MeOH) was supplied by Cambridge Isotope Laboratories (Andover, MA, USA). Compound names, chemical structures, and physical-chemical properties of the target analytes are given in Table 1. A mixture of N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA) and trimethylchorosilane (TMCS) (99:1 v/v), obtained from Aldrich (Steinheim, Germany), was used as reagent in the silylation process.
Stock solutions (10 μg mL−1) of each bisphenol analogue were prepared by dissolving the commercial products in ACN. A working mixture solution containing all the compounds (500 ng mL−1), used to spike samples, was prepared by dilution of the stock solution in ACN. All the standard solutions were kept at −20 °C in the dark prior to use.
Equipment
GC-MS/MS analysis was performed on an Agilent 7890A (Waldbronn, Germany) gas chromatograph coupled to an Agilent 7000 triple quadrupole mass spectrometer equipped with an automatic injector model HP 7683. A fused silica capillary column ZB-5MS, 5% phenyl-arylene, and 95% dimethylpolysiloxane, as nonpolar stationary phase (30 m × 0.25 mm i.d. and 0.25 μm film thickness), from Phenomenex (Torrance, CA, USA) was used. The helium (purity 99.995%), as carrier gas, was maintained at a constant flow of 1 mL min−1. Two-layer sandwich injections in the GC injector at 300 °C, drawing 1 μL of sample and 0.8 μL of the silylation reagent (BSTFA:TMCS), were carried out in pulsed splitless mode (pulsed pressure 45 psi for 0.5 min and flow rate of 100 mL min−1) with the purge valve activated 0.5 min after sample injection. The column temperature was initially set at 80 °C for 0.5 min, then increased 20 °C min−1 until 300 °C and held for 5 min. The total analysis time was 16.5 min with a solvent delay of 6 min. First, retention time and mass spectra were acquired in the full scan mode, working with a mass range from 50 to 500 m/z, with a scan time of 150 ms. The mass spectrometric detector (MSD) was operated in electron impact ionization mode with an ionizing energy of 70 eV. Ion source and line transfer temperatures were 230 and 280 °C, respectively. Precursor ions for each compound were set in relation to a high ion m/z and abundance. The product ion spectra were obtained by the dissociation of the precursor ions at collision energies ranging from 5 to 50 eV. Multiple reaction monitoring (MRM) was employed for quantitative analysis, using one quantifier and one qualifier transition to identify each target analyte. For positive confirmation, quantifier-qualifier ratios must range within 20% and retention time must be within ±0.2 min of the expected time. Table 2 shows GC-MS/MS parameters for the analysis of 13 bisphenol analogues selected for this study. To estimate the total recovery and quantification of analytes, matrix-matched calibration was used. Quantification of the compounds was based on their response factor relative to five external standards mixtures prepared fortifying blank soil extracts in the range of 1 to 30 ng g−1. All reported concentrations of the bisphenol analogues are expressed as ng g−1.
Samples
For this study, soil samples were collected from several agricultural fields (horticultural and forested) located in different Spanish regions and from industrial soils in the area of Bilbao, an important industrial region of Spain. Soil used in the recovery assays was collected from an experimental plot located in the region of Madrid (Spain) with the following characteristics: pH 7.7, sand content 44%, silt 37%, clay 18%, and organic matter 1%. In all cases, the samples were taken from the upper layer (0–10 cm), air dried, 2 mm-sieved, and stored frozen (−18 °C) in glass containers until their evaluation.
Extraction procedure
The extraction method is based on the previous ones developed in our laboratory [13] with some modifications. Anhydrous sodium sulfate (2 g) was transferred to a glass column (20 mL) 10 cm × 20 mm i.d. (Normax, Portugal) containing two filter paper circles of 2 cm diameter at the end. Sieved soil (5 g ± 0.001) was weighed into a 10 mL weighing funnel and placed in the glass column. The luer tips of the columns were closed with one-way stopcocks. For recovery studies, soil samples were previously spiked with a working standard solution containing 500 ng mL−1 of the 13 bisphenols studied to reach final concentrations of 2, 10, and 30 ng g−1. Fortified soil was left at room temperature for 24 h to allow complete solvent evaporation.
The extraction was carried out twice with an ultrasonic water bath (Raypa, Barcelona, Spain) using 5 mL of MeOH as the extraction solvent. Columns were closed with stopcocks and placed upright in an ultrasonic water bath at room temperature (15 min), with the water level adjusted to equal the level of solvent inside the columns. After each sonication cycle, the columns were placed on a multiport vacuum manifold (Supelco, Visiprep, Madrid, Spain) and the solvent was collected in graduate tubes. Samples were washed with 1 mL of additional solvent. The combined extracts were concentrated to dryness, reconstituted with 0.5 mL of acetonitrile and transferred to a micro-insert for their chromatographic analysis.
Quality assurance/quality control
The quality assurance and quality control criteria used for this method included the analysis of reagent and sample blanks. No analytes were detected in reagent blanks but some compounds were found in sample blanks, particularly BPA. To check potential contamination from the preparative steps and to demonstrate laboratory background levels, one reagent blank was run with each set of samples. In order to avoid memory effects, the liner was changed frequently before injecting a set of samples. Precision of the method was estimated from the standard deviation of six replicates at one concentration analyzed during the same day (intra-day precision) and three replicates across 3 days (inter-day precision).
The developed method was validated in terms of linearity in the range from 10 to 300 ng mL−1, precision intra- and inter-day, accuracy, and detection limits.
Statistical analysis
The data analyses were performed using the statistical package Statgraphics Plus, release 5.0 (Manugistics, Maryland, USA).
Results and discussions
Derivatization and gas chromatographic determination
Derivatization efficiency mainly depends on the reagent amount and the reaction time. Thus, the optimization of the quantity of derivatization agent and the reaction time was firstly evaluated to obtain the best chromatographic response of the bisphenol analogues. The responses obtained for nine different mixtures (200 μL) of standards and BSTFA + 1% TMCS increasing the volume of the derivatization reagent (from 10 to 100 μL) were compared. In general, a proportion 4:1 v/v showed good chromatographic response and there was not a clear increase in the response when reagent volumes from 40 to 90 μL were used; although a sharp decrease in the signals were observed with 100 μL of BSTFA + 1% TMCS. Thus, a 40 μL reagent volume was selected for further assays. The effect of the reaction time (from 10 to 80 min) was also assayed. Best results were obtained after 45 min of reaction time and longer derivatization times did not improve the derivatization efficiency. Therefore, the best conditions were 45 min at 60 °C, with a standard:reagent volume ratio of 4:1.
In order to improve the chromatographic response and, at the same time, reduce the analysis time, the possibility of performing the derivatization process in the injector of the chromatograph was evaluated and compared with the benchtop derivatization. The automated liquid sampler is capable of making multilayer (sandwich) injections of the silylation reagent and the sample, allowing the derivatization to take place in the GC inlet.
The proportion of the sample to the derivatization reagent in the injection was evaluated. The chromatographic response of different sample:derivatization reagent ratios was assessed using volumes from 0.1 to 1.3 μL of BSTFA + 1% TMCS. An important increase in the chromatographic response were achieved with up to 0.8 μL of reagent; however, with higher reagent’s ratio, split peaks were observed, probably due to the injection of an excess of derivatization reagent. Therefore, for the on-line reaction, a two-layer sandwich injection drawing 1 μL of sample and 0.8 μL of BSTFA + 1% TMCS were used in further assays.
For comparative purpose, the same concentration of bisphenols mixture was derivatized manually and in the GC inlet. Results showed that significant higher chromatographic responses were achieved with on-line derivatization. This may be probably explained due to the decrease of the exposure of the extract to environmental moisture during the on-line derivatization step and because the high temperature in injection port assists evaporation of water (the silylation process is sensitive to moisture). In addition, a temperature in the injection port between 250 and 300 °C was high enough to accomplish the reaction fast and completely [14].
Figure 1 shows representative chromatograms when on-line or benchtop derivatization takes place. According to these results, on-line derivatization was selected for the analysis of bisphenol analogues in soils, as it provides higher chromatographic response, reduces the amount of reagent used, and requires less manipulation compared to manual derivatization. These advantages imply a lower cost of the analysis.
The chromatographic response of target analytes may be affected by the presence of matrix components; therefore, matrix effect was evaluated preparing a set of standard solutions in acetonitrile and another set spiking blank soil extracts in the same concentration range. The slopes obtained by plotting concentration at five levels against peak area, following linear regression analysis, were compared. Although in gas chromatography matrix effects frequently result in an increase of the response, in this case, a significant decrease of the chromatographic response was observed for all the compounds probably due to matrix interference in the derivatization process. To overcome matrix effects, matrix-matched standards are often used in spite of its main drawback, which is the lack of appropriate blank matrix. An alternative to matrix-matched calibration is the use of isotopically labeled standards, which avoids the dependence of the results obtained from the sample matrix, although their use is limited by being generally very expensive and because sometimes they are not available, as happens with the majority of the bisphenol analogues evaluated in this study. Therefore, the quantification was done by matrix-matched calibration, using fortified blank extracts as standards instead of standard solutions in solvent, in order to overcome matrix effects and to achieve a better analysis of the bisphenol analogues.
Extraction procedure
The selective extraction of analytes from environmental complex matrices, such as soils, is a very complicated task, because these matrices contain a large variety of compounds that may hinder their analysis. UAE on small columns was selected, based on our experience with this technique in the analysis of soil contaminants.
The extraction solvent is usually one of the main parameters to optimize in UAE methods. EtAc has been reported as the best solvent for the extraction of tetrabromobisphenol A (TBBPA), tetrachlorobisphenol A (TCBPA), and BPA in soil [13], and in the analysis of BPA and four chlorinated BPA derivatives in sludge samples using different extraction techniques [15]. In the determination of 40 organic contaminants in soil by pressurized liquid extraction (PLE), BPA recoveries were <80% using EtAc as extraction solvent, but extraction yields were enhanced when the polarity of the extraction solvent was increased and EtAc:MeOH (80:20 v/v) was selected [16]. In other works, MeOH or mixtures of this solvent with water or acetone were used as extraction solvent for the analysis of bisphenol analogues in sediment or sludge [8,9,10,11]. Therefore, EtAc and MeOH were selected as extraction solvents. Response surface methodology has proven to be a reliable statistical tool in research to optimize parameters with a minimal number of experiments. Taking into account that the extraction solvent and the extraction time are the two main factors that affect UAE methods, a statistical analysis was done to improve the efficiency of the procedure and simultaneously estimate the influence of the extraction time and different EtAc:MeOH ratios using a multilevel experimental factorial design. These parameters were optimized applying a 32 experimental design with three replicates of soil spiked at 18 ng g−1. In the mixture MeOH:EtAc, the proportion of MeOH ranged from 0 to 100% and two sonication cycles ranged from 5 to 25 min. In general, MeOH percentage significantly affected the extraction efficiency for 9 of the 13 bisphenols, whereas the extraction time did not show significant differences, except for BPG and BPF. Nevertheless, the responses depended on the analyte; thus, BPG showed a negative significant dependence with extraction time (optimal value 5 min), whereas this dependence was positive for BPF (optimal value 25 min) and no dependence was observed for the other bisphenol analogues. The statistical analysis showed that 100% MeOH was the optimum extraction solvent. Considering these results, an extraction time of 15 min was selected, as a compromise for all bisphenols evaluated. The first-order interactions obtained in this study for two representative compounds (BPA and BPG) are summarized in the Pareto charts and the response surfaces shown in Fig. 2.
As summary, the extraction process was carried out in 30 min with MeOH and derivatization was done in the GC inlet. It is worth to mention that this method is clearly faster, in comparison with previous works, where the extraction of seven bisphenol analogues from sediment was at least 120 min long, the extract was cleaned-up using a SPE cartridge, and another 20 min were needed in the derivatization step [11]. In comparison with our previous work on the determination of BPA, TBBPA, and TCBPA by GC-MS [13], the method developed in the present work allowed the selective determination of 13 bisphenols by GC-MS/MS without needing a clean-up of extracts. Additionally, a preparative step was not necessary in the on-line derivatization and therefore the analysis time was reduced.
Method performance
After optimization, the method was evaluated in terms of linearity, precision, accuracy, and detection limits. The linearity of the method was evaluated injecting six spiked soil blanks in the range from 10 to 300 ng mL−1 for all the studied compounds. A good linearity was obtained, with correlation coefficients equal or higher than 0.996 for all the compounds studied.
For recovery studies, soil samples were spiked with a mixture of bisphenols at final concentrations of 2, 10, and 30 ng g−1. Extraction efficiency obtained by UAE at these three different fortification levels ranged from 70 to 111% with standard deviations <13% (results are shown in Table 3).
The repeatability intra-day or precision was determined by analyzing on the same day six replicates of soil samples spiked at 2 ng g−1. The precision, expressed as relative standard deviations (RSDs), showed values lower than 9% for all compounds. Furthermore, the inter-day precision was evaluated doing three replicates in three different days. The inter-day precision was found to be lower than 10% for all the compounds, expressed as RSD. The limits of detection (LODs) and limits of quantification (LOQs) of the developed method were calculated analyzing six replicates of soil spiked at 2 ng g−1. These values were obtained following the t 99sLLMV approach developed by EPA [17]. As shown in Table 3, The LOQs values ranged from 0.12 to 0.89 ng g−1.
Real samples
In order to assess the suitability for the analysis of real samples, the developed method was applied to analyze agricultural and industrial soils collected in different areas of Spain. Reagent blanks were prepared together with the soil samples analyzed to check for contamination and bisphenol presence. The concentration of the studied bisphenols found in the soils analyzed is summarized in Table 4. Figure 3 depicts the MRM chromatograms of the target compounds found in an agricultural soil sample.
GC-MS/MS chromatograms of the bisphenol analogues in agricultural soil sample number 6 (see Table 4 for levels)
Only three compounds, BPAF, BPF, and BPA, were found in soil samples. BPA was found in all the soils with concentrations ranging from 1.1 to 17.9 ng g−1 in the agricultural soils, except for sample 6, irrigated with effluents from a wastewater treatment plant which showed 55.9 ng g−1. Values from 17.2 to 126.2 ng g−1 were found in the industrial soils. In general, these values of BPA are similar to those previously found for different soil types [13], although in soils irrigated with recycled water, or located in industrial areas, BPA concentrations could be higher. Ten out of the 13 bisphenol analogues studied were not detected in any of the samples. This fact could be explained by the still low consumption of these analogues; however, the presence of different bisphenol analogues with potential adverse health effect in different matrices have increased, whereas the concentration of BPA gradually declines [6, 9].
A comparison of the levels of bisphenol analogues and the frequency of detection obtained in our work with those reported in sediment and sewage sludge is shown in Table 5. BPF was a compound found in industrial soil samples with concentrations from 5 to 127 ng g−1. This compound only has been found in the agricultural soil number 6 which correspond to a soil irrigated with recycled water. No information on levels of BPF in soils has been found in the scientific literature. However, levels up to 9650 and 242 ng g−1 have been reported in sediments and sewage sludge, respectively [6]. BPF is the second most frequently found bisphenol analogue in sediment and sewage sludge samples (see Table 5).
In our study, BFAF was detected in all the agricultural soils, although below or near the LOQ. However, this compound was not found in the industrial soil samples. Although the presence of BFAF in soil has not been previously described, the frequency of detection of this compound in sediment and sewage sludge was low (4.1 and 46% respectively), and there is scarce information about the presence of this compound in water samples.
Different studies focused in the determination of bisphenols in water samples from lakes, rivers, or effluents from wastewater treatment plants have reported levels of BPA (0.1–224.9 ng l−1), BPS (0.3–19.0 ng l−1), BPF (up to 31.8 ng l−1), BPAF (0.9–249.7 ng l−1), and BPE (20.3 ng l−1 in one sample) [8, 18,19,20,21]. As well as other organic pollutants, their capacity to be adsorbed to soil, the use of reclaimed waters for irrigation or the application of sewage sludge as soil amendment, make soil one important reservoir of bisphenol analogues that needs to receive more attention. These results demonstrate that different bisphenols are found in soil samples. although only the presence of BPA has been previously reported and methods for the determination of bisphenol analogues in environmental samples are scarce and focused in a few bisphenol compounds.
Conclusions
In this work, a quick, simple, selective, and sensitive method based on the UAE of samples placed in small columns and GC-MS/MS analysis, with on-line derivatization, was developed for the analysis of 13 bisphenols in soil. An important advantage of this method is the ease of use and the wide range of bisphenols that can be analyzed simultaneously. Another significant advantage of the described procedure is the reduction in the consumption of organic solvents and the sample preparation time versus other methodologies.
Satisfactory results were obtained for all the compounds studied in terms of extraction efficiency, reproducibility, and sensitivity with LODs ranging from 0.04 to 0.24 ng g−1. The proposed method was applied to agricultural and industrial soils from several Spanish areas, and 3 out of the 13 target bisphenols (BPAF, BPF, and BPA) were detected in the samples analyzed, although only the levels of BPF and BPA could be quantified. BPF was found in all the industrial soil samples, and in only one agricultural soil sample, with concentrations similar or slightly lower than BPA. BPA was found in all soil samples with higher concentrations in the industrial soils. The use of reclaimed waters for irrigation of agricultural fields was related with the presence of bisphenol analogues in the soil.
References
Muhamad MS, Salim MR, Lau WJ, Yusop Z. A review on bisphenol A occurrences, health effects and treatment process via membrane technology for drinking water. Environ Sci Pollut Res. 2016;23:11549–67.
Cano-Nicolau J, Valliant C, Pellegrini E, Charlier TD, Kah O, Coumailleau P. Estrogenic effects of several BPA analogs in the developing zebrafish brain. Front Neurosci. 2016;10:112.
The European Commission. Commission directive 2011/8/EU of 28 January 2011 amending directive 2002/72/EC as regards the restriction of use of bisphenol A in plastic infant feeding bottles, Official Journal of the European Union.
EFSA. Scientific opinion of European Food Safety Authority (EFSA) on the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs: executive summary. EFSA J. 2015;13(1):3978.
Chen D, Kannan K, Tan H, Zheng Z, Feng L, Wu Y, Widelka M. Bisphenol analogues other than BPA: environmental occurrence, human exposure, and toxicity—a review. Environ Sci Technol. 2016;50:5438–53.
Caballero-Casero N, Lunar L, Rubio S. Analytical methods for the determination of mixtures of bisphenols and derivatives in human and environmental exposure sources and biological fluids. A review. Anal Chim Acta. 2016;908:22–53.
Deceuninck Y, Bichon E, Marchand P, Boquien C-Y, Legrand A, Boscher C, Antignac JP, Le Bizec B. Determination of bisphenol A and related substitutes/analogues in human breast milk using gas chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2015;407:2485–97.
Yang Y, Lu L, Zhang J, Yang Y, Wu Y, Shao B. Simultaneous determination of seven bisphenols in environmental water and solid samples by liquid chromatography-electrospray tandem mass spectrometry. J Chromatogr A. 2014;1328:26–34.
Liao CY, Liu F, Moon HB, Yamashita N, Yun SH, Kannan K. Bisphenol analogues in sediments from industrialized areas in the United States, Japan, and Korea: spatial and temporal distributions. Environ Sci Technol. 2012;46:11558–65.
Lee S, Liao C, Song G-J, Ra K, Kannan K, Moon HB. Emission of bisphenol analogues including bisphenol A and bisphenol F from wastewater treatment plants in Korea. Chemosphere. 2015;119:1000–6.
Wang Q, Zhu L, Chen M, Ma X, Wang X, Xia J. Simultaneously determination of bisphenol A and its alternatives in sediment by ultrasound-assisted and solid phase extractions followed by derivatization using GC-MS. Chemosphere. 2017;169:709–15.
Viñas P, Campillo N, Martinez-Castillo N, Hernandez-Cordoba M. Comparison of two derivatization-based methods for solid-phase microextraction-gas chromatography-mass spectrometric determination of bisphenol A, bisphenol S and biphenol migrated from food cans. Anal Bioanal Chem. 2010;397:115–25.
Sanchez-Brunete C, Miguel E, Tadeo JL. Determination of tetrabromobisphenol-A, tetrachlorobisphenol-A and bisphenol-A in soil by ultrasonic assisted extraction and gas chromatography-mass spectrometry. J Chromatogr A. 2009;1216:5497–503.
Wang Q, Ma L, Yin C, Xu L. Developments in injection port derivatization. J Chromatogr A. 2013;1296:25–35.
Dorival-Garcia N, Zafra-Gomez A, Navalon A, Vilchez JL. Analysis of bisphenol A and its chlorinated derivatives in sewage sludge samples. Comparison of the efficiency of three extraction techniques. J Chromatogr A. 2009;1253:1–10.
Albero B, Sanchez-Brunete C, Miguel E, Perez RA, Tadeo JL. Determination of selected organic contaminants in soil by pressurized liquid extraction and gas chromatography tandem mass spectrometry with in situ derivatization. J Chromatogr A. 2012;1248:9–17.
Bernal E. Limit of detection and limit of quantification determination in gas chromatography. In: Guo X, editor. Advances in Gas Chromatography. InTech; 2014. p. 57–81.
Shan XM, Shen DH, Wang BS, Lu BB, Huang FY. Simultaneous determination of bisphenols and Alkylphenols in water by solid phase extraction and ultra performance liquid chromatography-tandem mass spectrometry. Biomed Environ Sci. 2014;27:471–4.
Yin JF, Meng ZH, Zhu YS, Song MY, Wang HL. Dummy molecularly imprinted polymer for selective screening of trace bisphenols in river water. Anal Methods. 2011;3:173–80.
Ballesteros-Gomez A, Ruiz FJ, Rubio S, Perez-Bendito D. Determination of bisphenols A and F and their diglycidyl ethers in wastewater and river water by coacervative extraction and liquid chromatography-fluorimetry. Anal Chim Acta. 2007;603:51–9.
Moral A, Sicilia MD, Rubio S, Perez-Bendito D. Determination of bisphenols in sewage based on supramolecular solid-phase extraction/liquid chromatography/fluorimetry. J Chromatogr A. 2005;1100:8–14.
Acknowledgements
Authors wish to thank the Spanish Ministry of Economy, Industry and Competitiveness for financial support, project (RTA2014-00012C03), and for the contract of M. Ferriz.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Pérez, R.A., Albero, B., Férriz, M. et al. Rapid multiresidue determination of bisphenol analogues in soil with on-line derivatization. Anal Bioanal Chem 409, 4571–4580 (2017). https://doi.org/10.1007/s00216-017-0399-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-017-0399-2