Abstract
Oxidative stress (OS) is a phenomenon induced by excessive production and accumulation of reactive oxygen species (ROS) in living cells. These increased ROS productions connected, coupled with many neurological and physiological diseases. Several antioxidants were utilized recently to combat OS, and lactic acid bacteria have a potent radical-scavenging activity to minimize OS. The present work was designed to find out the protective effects of Lactobacillus brevis MG000874 (L. brevis MG000874) against oxidative injuries induced by D-galactose (D-gal) in vivo and to explore the gene expression of OS-related gene mice. Sixty male mice were randomly split into six groups. The first four groups were different control groups as no treatment (N), positive (G), probiotic (B), and ascorbic acid (A); the remaining two groups were treatment groups such as probiotic treatment (BG) and ascorbic acid treatment (AG). L. brevis MG000874 (0.2 ml of 1010 CFU/ml) and ascorbic acid (0.2 ml of 25 mg/ml) were administered orally daily for 5 weeks. It was revealed that these significantly affect the weight of treated mice: 40.22 ± 1.5 and 33.0 ± 0.57 g on days 0 and 36, respectively. D-gal induction in mice declined the levels of SOD and CAT determined by spectrophotometer. Administration of L. brevis MG000874 improved the antioxidant status of the stress mice and recovered the antioxidant activities of SOD and CAT enzymes. In addition, L. brevis MG000874–altered gene expression of OS marker at the messenger RNA (mRNA) levels was determined by RT-PCR in the mouse model. L. brevis MG000874 significantly improved the GST, GPX, SOD, CAT, and ß-actin levels in the kidney and the liver of the D-gal-induced mice (p < 0.05). Moreover, the histological investigation indicated that L. brevis MG000874 mitigated damage to the kidney and liver effectively in mice induced by D-gal. Therefore, it could be concluded from the current results that L. brevis MG000874 may act as a powerful antioxidant agent, and this study can provide the baseline data for drug development against OS-linked diseases.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Oxidative stress (OS) is an imbalance between the production and the accumulation of free radical oxygen species (FROS). These FROS thus adversely alter the properties of lipids, proteins, and DNA, which in turn triggered many diseases (Ghazi et al. 2022). FROS is one of the most common action mechanisms in aging as well (Stroustrup 2018; Rehman et al. 2022). The stability of FROS and antioxidants becomes unstable in the case of the OS. The living systems always seek to maintain the level of FROS for their normal functions. Although, elevated levels of FROS directly affected the activity of the antioxidant enzyme defense system (AEDS) and non-enzymatic proteins (GSH), which influenced the homeostasis of the whole biological defense system (Liguori et al. 2018; Arya et al. 2021).
It has been suggested that the OS might be regulated by the configuration of gut microbiota (Wells et al. 2020). The sources of these gut microbiota are different via food and probiotic supplements. Probiotics may activate, modify, and adjust the host’s immune status by prompting the stimulation of particular genes of confined host cells (El-Sayed et al. 2021). Probiotics comprising Lactobacillus and Bifidobacterium have appealed considerable attention due to their vital role in health promotion. The important purpose of isolating probiotics from all over the world is to scale up the beneficial global food system by the production of healthy organisms, particularly with prolonged existence (Fanzo et al. 2020). The antioxidant capability of lactic acid bacteria (LAB) and their constituents (e.g., Proteins and exopolysaccharides) are normally specified and considered as standard for the assessment of probiotic functions (Li et al. 2015). Assessment of antioxidant properties of LAB that comprises in vitro oxidation reaction system (free radical scavenging, metal ion chelation, and lipid peroxidation inhibition) and in vivo animal confirmation of the antioxidant role of natural additives may be supported by Yadav et al. (2019), Unban et al. (2021) Asan et al. (2022), and Li et al. (2022). Hence, the LAB having the quenching ability of FROS becomes more significant in investigating the mechanism of antioxidants. The literature revealed that a high concentration of D-galactose (D-gal) helped in the generation of FROS by the accumulation of advanced glycation end products, as a result of the OS and cellular damage produced in the tissues (Shwe et al. 2018). In the current era, several reports about the antioxidant evaluation of LAB have been published. However, a systematic evaluation of the antioxidant mechanism from the gene expression level of mice has been reported rarely. In our previous study, L. brevis MG000874 was extracted from the camel and used to investigate its antioxidant ability against D-gal (Noureen et al. 2019). The current study was therefore to explore the antioxidant ability of L. brevis MG000874 by investigating the gene expression of OS-related gene markers in mice.
Materials and methods
Hydrogen peroxide (H2O2) (Daejung, Siheung-si, Korea), MRS broth (Oxide, UK), sodium chloride (Merck, Germany), phosphate-buffered saline, tablet (sigma), (pyrogallol (sigma), D-gal, (Germany), ascorbic acid (Germany), RNA extraction kit, Syber green master mix, and cDNA extraction kit were procured from a local dealer.
Bacterial culture
The bacterial strain (L. brevis MG000874) was isolated from the camel intestinal tract as the method used in our previous article by Noureen et al. (2019). The isolated strains of L. brevis MG000874 were incubated at 37 °C by culturing in MRS broth (pH 6.6 ± 02) overnight. For further use in treatments, these isolates were separated by centrifugation at 3000 rpm and suspended in a PBS buffer.
In vivo evaluation of antioxidant prospective
Sixty days old male albino mice (Mus musculus, weight: 40.0 ± 2.0 g, n = 60) were set aside in cages under schematized circumstances (temperature 22 ± 2 °C, dampness 45 ± 5%, 12-h light/dark cycles). During the experiments, open access was provided for water and food. The mice were indiscriminately divided into six groups after 1 week of acclimatization. These groups were negative control group (N)—no treatment, probiotic cells, and treatment group with L. brevis MG000874 (B)—and positive control group (G)D-gal treatment group (300 mg/BW), ascorbic acid treatment group (A), D-gal and L. brevis MG000874 treatment group (BG), and D-gal and ascorbic acid treatment group (AG).
Dose setting
L. brevis MG000874 (MG000874) and ascorbic acid were given via gastric gavage. D-gal was injected at the dose of 300 mg/kg BW/day through a subcutaneous route for 5 weeks, on behalf of many pilot studies for a quick aging process (data not included). The animals were anesthetized after 5 weeks of treatment. Organ index, kidney, and liver tissue homogenization were prepared according to the Noureen et al. (2019) method.
Estimation of antioxidants
The antioxidant level in the liver and kidney was estimated through SOD and CAT. Briefly, in the SOD assay, the reaction mixture was incubated at 25 °C for 20 min comprising 2.8 ml Tris HCl buffer (0.01 mmol/l, pH 85) and 0.1 ml sample. Absorbance was noted at 412 nm for 3 min by the addition of 0.1 ml pyrogallol of 8 mmol/l in the mixture.
Furthermore, in catalase, the addition of 0.1 ml of H2O2 (0.1 mmol/l) in the reaction mixture initiated the reaction which consisted of 190 ml PBS of 1.0 mmol/l (pH 6.8) and 0.1 ml sample. At 240 nm, the variation in absorbance was noted for 3 min. Catalase and SOD activities in tissue were calculated according to Noureen et al. (2019).
Histopathological studies
For the histopathological study, tissues of liver and kidney samples (nearly 1 cm2 thick) were fixed in a 10% formalin solution and transferred to different grades of absolute alcohol dehydration. Then, the samples were fixed in paraffin and 3–4-µm-thick slices. These slices were transferred to glass slides and rehydrated and then stained with hematoxylin and eosin staining. Prepared slides were observed under the light microscope having a fitted camera (Labomed, USA) for histopathological examination (Qiu et al. 2017; Iqbal et al. 2022).
Extraction of RNA and preparation of cDNA
RNA was extricated from tissues following the TRIzol method (Brown et al. 2018). The purity and quantity of attaining RNA (ribonucleic acid) were estimated through ND-1000 (Nanodrop, Thermo Fisher Scientific). cDNA (complementary DNA) was made through Synthesis Kit (First-Strand cDNA kit).
RT-PCR
RNA expression of SOD, CAT, GST, GPX, and ß-actin was investigated using RT-PCR. Each primer sequence’s detail is provided in Table 1. Concisely, the qPCR reaction mixture (20 µl) contained CDNA (0.1 µl), primer (1.4 µl), SYBR Green master mix (12.5 µl), and nuclease-free water (9.5 µl). Forty cycles of qPCR were done on an RT-PCR system (Bio-Rad CFX): 3 min denaturation at 95 °C, 10 s annealing at 54–59 °C, and 30 s elongation at 72 °C. The expression of mRNA was determined by average quantification cycle (Cq) values. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a reference gene (Eissa et al. 2016). The expression was calculated by the following:
Statistical analysis
One-way ANOVA was performed for group comparison (p-values ˂ 0.05, significant: Tukey’s test) by using IBM SPSS 21.0 (Inc., Chicago, IL). Tests were done in triplicate, and their outcomes were displayed as the standard error of means (SEM) (Lin et al. 2018).
Results
Organ index
Table 2 reveals that the indexes of the liver and kidney in the stress group (G) were significantly lesser than those in the normal group (N: p < 0.05), presenting that induction of the D-gal was the cause of organ stress or aging. However, the liver and kidney index of treated groups BG and AG was significantly higher than those in the G group and showed more observable effects on inhibiting organ aging.
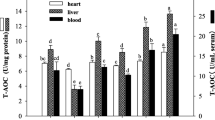
Enzyme activities of SOD and CAT in liver and kidney tissues
The antioxidant activity of the enzyme can exactly reveal oxidative injury. So, we used the two indicators of OS SOD and CAT. The enzyme activities of SOD and CAT in the liver and kidney of the G group were noticeably the lowest (Fig. 1). But the levels of these enzymes were significantly improved in the treatment of BG or AG. Particularly, the levels of the SOD and CAT enzymes in the B or A group were very close to the N group, illustrating that L. brevis MG000784 and ascorbic acid were better at delaying the stress factor.
Effects of L. brevis MG000874 and ascorbic acid on D-Gal induced changes in SOD and CAT of liver and kidney. N: control; G: d-gal; B: L. brevis MG000874; A: Ascorbic Acid; BG: group getting both D-Gal and L. brevis MG000874; AG: Group getting Ascorbic acid and D-Gal. Data represent Mean ± SEM; means with different superscript letter (a, b, c, d) exhibits significant differences (𝑃 < 0.05)
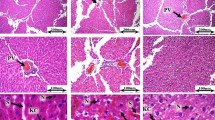
Histological examination of mouse liver and kidney
The histological alteration in the liver of treated mice is given in Fig. 2. It was evident from the results that the morphology of the hepatocytes in the N group was consistent with no change (Fig. 2a). However, notable alterations were recorded in the positive control (G) including messy hepatocytes (MH), cell inflammation (CI), necrosis (N), and rough shape of the central vein (RSCv) (Fig. 2c). The groups B and A (Fig. 2b and d) exhibit the normal histology as in group N except for some degree of inflammation in hepatocytes. The treatment groups BG and AG (Fig. 2 f and g), therefore, showed the protective effects of L. brevis MG000874 and ascorbic acid, respectively, against D-gal-induced toxicity or OS. The kidney photomicrograph were shown ( Fig 3) that the Bowman‘s capsule of N and B group had normal distal convoluted tubules, vascular pole, renal parenchyma, inner cellular layer of podocytes and outer squamous capsular cells.
Illustration of histological alterations in kidney tissues (H&E; X400) (a) normal kidney tissues, (b) L. brevis MG000874 group, (c) B: G: D-gal group, (d) A: Ascorbic Acid group, (e) BG: D-Gal + L. brevis MG000874, (f) AG: Ascorbic acid + D-Gal., showed hepatocytes (H), central vein (Cv), messy hepatocytes (MH), cell inflammation (CI), uneven cell margin (UCM), rough shape of central vein (USCv) and medium messy cells (MMC)
Illustration of histological alterations in liver tissues (H&E; X400) (a) normal kidney tissues, (b) L. brevis MG000874 group, (c) B: G: D-gal group, (d) A: Ascorbic Acid group, (e) BG: D-Gal + L. brevis MG000874, (f) AG: Ascorbic acid + D-Gal., showed glomerulus (G), tubules (T), degeneration of renal paranchyma (DRP), cellular necrosis of tubular epithelium (CNTE) and pykonotic nuclei (PN)
Liver gene expression content
The overall changes in the mRNA expressions of antioxidant enzymes of all groups in liver tissue are summarized in Fig. 4. These gene expressions consisted of SOD, CAT, GST, GPX, and B-actin, and they were determined by RT-PCR. The expression levels of SOD, CAT, GST, GPX, and B-actin in group N were the highest. However, in the D-gal group (G), the mRNA expression levels of the above genes were downregulated. Raised SOD and CAT mRNA levels were also noted in mice treated with L. brevis MG000874 (B) or ascorbic acid (A) as compared with N. These results were in agreement with the corresponding enzyme activity results (Fig. 1). In treatment groups, BG and AG, the expressions of SOD, CAT, GST, GPX, and B-actin were significantly improved (p < 0.05).
Effects of L. brevis MG000874 and ascorbic acid on D-Gal induced changes on the gene expression level of SOD, CAT, GST, GPX and B- actin in kidney. N: control; G: d-gal; B: L. brevis MG000874; A: Ascorbic Acid; BG: group getting both D-Gal and L. brevis MG000874; AG: Group getting Ascorbic acid and D-Gal. Data represent Mean ± SEM; means with different superscript letter (a, b, c, d) exhibits significant differences (𝑃 < 0.05).
Kidney gene expression content
The gene expression level of Kidney tissue is presented in Fig. 5. The mRNA expression of SOD, CAT, GST, GPX, and B-actin of the normal group (N) in comparison to the D-gal group (G) was the lowest, and these results were in agreement with Fig. 1. In the treatment groups, SOD, CAT, GPX, GST, and B-actin mRNA expressions and B-actin were increased. The expression of L. brevis MG000874 alone in the treatment group (B) was highest in comparison to the N group, which was stronger than that of ascorbic acid.
Effects of L. brevis MG000874 and ascorbic acid on D-Gal induced changes on the gene expression level of SOD, CAT, GST, GPX and B- actin in liver. N: control; G: d-gal; B: L. brevis MG000874; A: Ascorbic Acid; BG: group getting both D-Gal and L. brevis MG000874; AG: Group getting Ascorbic acid and D-Gal. Data represent Mean ± SEM; means with different superscript letter (a, b, c, d) exhibits significant differences (𝑃 < 0.05)
Discussion
LABs are abundant and ubiquitous, found in nutrient-rich habitats, including animal feed, humans, plants, and soil. They possess specific enzymes and produce many chemical molecules during fermentation. They have a great influence on human life and are commonly utilized in many industries, like poultry, livestock, and food production (Landete et al. 2017; Ramos et al. 2020). LAB strains isolated from different sources like plants and animals, compost, fermented substances, the gastrointestinal tract of animals, and silage may have antioxidant activity (Rezaei et al. 2020; Unban et al. 2021). This trait is strain and species-specific. Therefore, the study aimed to investigate the antioxidant potential of the L. brevis MG000874 strain of LABs isolated from the animal (Asan et al. 2022), in the D-gal-stressed mice.
The constant administration of D-gal injections simulates and induces the OS by increasing the production of the FROS and depletion of the antioxidant activity (Li et al. 2015). The stress-affiliated mechanisms may be damaged by the unusual production of ROS (Ge et al. 2021); thus, the current research designated the D-gal-induced OS in mice to find the effects of the L. brevis MG000874 as an antioxidant against stress-related gene expression in the liver and kidney (Li et al. 2020). In our previous study, we injected 150 mg/kg/body weight D-gal in mice for inducing OS at 8 weeks (Noureen et al. 2019). In the current study, we selected the high dose of D-gal 300 mg/kg/BW for a quicker result. At this dose, the aging sign and symptoms appeared within 4 weeks in the mouse model. Important and basic indicators in biomedical research for observing the stress effects are the organ index in mice. In the stressed body, atrophy of the liver is more noticeable (Xu et al. 2016). An immune organ of an animal is the liver and its atrophy may be a source of immune deficiency (Chen et al. 2020). The literature revealed that the liver index of OS mice was considerably decreased as compared to the normal mouse liver (Sang et al. 2017). The metabolic organ is the kidney, and a decrease in kidney index has a great influence on metabolic ability (Xu et al. 2018). Thus, alterations in the organization of mouse organs can be noted in the organ index and were significantly important for evaluating the successful induction of OS in mice (Sang et al. 2017; Shen et al. 2011). The outcomes of this study presented that D-gal caused the atrophy of organs and induction of OS in mice (Woo et al. 2014; Qiu et al. 2017). OS processing was efficiently postponed by treating with L. brevis MG000874 (109 CFU/kg), proposing the noticeable anti-stress effect of L. brevis MG000874.
Histopathological inspection is the microscopic study of the cells, tissues, or organ morphology to verify the body changes (Di Meo et al. 2016; Qian et al. 2018). D-gal-induced OS mice revealed that some changes in liver and kidney tissues in comparison to the normal group were noticed. In the liver, corrupted hepatocytes with nucleus and nucleolus, high vascular degeneration in sinusoidal spaces with a hazy appearance, and uneven cell margin with messy cells were noticed as well. While in treatment groups, mice revealed a normal appearance of hepatocytes with a nucleus, clear radiating hepatic cells, stable interlobular bile duct, regular portal triad, and usual endothelial cells (Fig. 2e). In the treatment of L. brevis MG000874 (B) or ascorbic acid (A), the morphological abnormalities of the liver and kidney tissues were considerably upgraded, and the upgrading impact of L. brevis MG000874 was the most noticeable in the liver. The liver tissue morphology of the L. brevis MG000874–treated group was very close to that of group N. In the kidney, Bowman’s capsule of N and B groups had regular inner podocyte cellular layer, outer squamous capsular cells, normal renal parenchyma, vascular pole, and proximal–distal convoluted tubules (Fig. 3c). Renal parenchyma of the D-gal group deteriorated, and tubular epithelial cells of the pyknotic nuclei were observed. These histopathological changes supported the results of antioxidant enzyme activity.
During the process of bio-oxidation, a huge quantity of ROS is generated in the body. The stability of ROS is attained by numerous antioxidant defense systems, containing, SOD, CAT, GST, GSH, and vitamins (Claiborne 2018; Germoush et al. 2022). Their synergistic influence transforms excessive ROS into O2 and H2O2 molecules (Motataianu et al. 2022). LABs have a specific antioxidant ability for quenching free radicals, which can support the antioxidant enzymes. Antioxidant activity is a strain-specific feature. And the body’s metabolic process, LABs prevent the oxidation process by discharging antioxidant enzymes such as SOD (Feng et al. 2016). In this study, the L. brevis MG000874 significantly elevated the SOD activity and CAT activity in the kidney and liver tissue of the treated mice. The experimental outcomes confirmed that the induction of D-gal causes cellular OS and results in decreased SOD and CAT activity in liver and kidney tissues. Ascorbic acid or L. brevis MG000874 was good in the antioxidant effect during the measurement of SOD and CAT stress indicators in tissues (Di Meo et al. 2016).
To further enlighten the antioxidants, this research has observed the changes in SOD, CAT, GST, GPX, and B-actin expression levels. In animals, SOD is universally expressed as a mitochondrial antioxidant enzyme and helps in controlling free radicals’ production by keeping healthy (Zhao et al. 2018). CAT functions as scavenging O-free radicals and stimulating the disintegration of H2O2 by preventing oxidation damage (Selvaratnam and Robaire 2016). The literature revealed that OS causes tissue atrophy by dropping SOD and CAT expression (Hart et al. 2015; Hassani et al. 2018). The outcomes of this study revealed that mRNA expression levels of SOD and CAT in the liver and kidney tissues of the D-gal control group were significantly reduced (p < 0.05), representing that D-gal subcutaneous injection in mice may cause OS. Though, mRNA expression levels were significantly improved in treatment with L. brevis MG000874 (p < 0.05). GST is a broad-spectrum antioxidant. GPx may be used to regulate the intracellular hydroperoxide level in the gastrointestinal tract (Sharma et al. 2021). ß-actin is the main structural protein of the cytoskeleton, which plays a significant part in retaining the shape of the cell (El-Sayed et al. 2021). We found that GST, GPX, and ß-actin expression levels were upregulated positively by L. brevis MG000874 in D-gal-induced model mice as compared to the G group. These reports indicated that L. brevis MG000874 could perform as an antioxidant, which may directly hunt ROS.
Finally, histological investigation verified that cellular inflammation and apoptosis of hepatocytes were greater in the D-gal alone group, and treatment with L. brevis MG000874 showed normal hepatocytes with congested sinusoids in the liver. While in the kidney, the disintegration of renal parenchyma, some cellular necrosis, and the vanishing of tubules spaces were identified in the stress group as compared to group BG. L. brevis MG000874 (probiotic) possessed a renal, hepatic protective influence through raising activities of antioxidant enzymes (i.e., SOD, CAT) on D-gal-inducted stress (BG) and maintaining the cellular structure. These consequences were also connected to the stimulation of the antioxidant defence system by boosting SOD, CAT, GST, GPX, and ß-actin controlled gene expression. Our findings further proposed that L. brevis MG000874 could maintain the intracellular redox balance in the livers and kidneys of D-gal-treated mice by renewing the activities of antioxidant enzymes.
Conclusion
The current investigation found that L. brevis MG000874 has an antioxidant effect. Induction of D-gal-initiated OS and reduced the antioxidant activity by down-regulating the mRNA expression of different antioxidant enzymes. This down-regulation depends on the variability and different tissue responses. Supplementation of L. brevis MG000874 or ascorbic acid resulted in body weight gain, and organ index restored the antioxidant defence system by improving the antioxidant enzyme status of SOD and CAT and up-regulating the gene expression of SOD, CAT, GST, GPX, and ß-actin. As a whole, these findings verified that L. brevis MG000874 promoted the antioxidative gene expression and cellular antioxidative responses in vivo. Therefore, L. brevis MG000874 would have the potential to be further explored as an antioxidant functional food in the prevention of more stress-related diseases.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Almaghrabi OA (2015) Molecular and biochemical investigations on the effect of quercetin on oxidative stress induced by cisplatin in rat kidney. Saudi J Biol Sci 22:227–231. https://doi.org/10.1016/j.sjbs.2014.12.008
Arya A, Chahal R, Rao R, Rahman MH, Kaushik D, Akhtar MF, Saleem A, Khalifa SM, El-Seedi HR, Kamel M, Mittal V (2021) Acetylcholinesterase inhibitory potential of various sesquiterpene analogues for Alzheimer’s disease therapy. Biomolecules 11:350. https://doi.org/10.3390/biom11030350
Asan Ö, Meltem, Gunyakti A (2022). Investigation of a new Lactobacillus delbrueckii strain from human milk as a probiotic candidate. J Food Saf Food Qual-Archiv fur lebensmittelhygiene 73(2). https://doi.org/10.2376/0003-925X-73-58
Brown RA, Epis MR, Horsham JL, Kabir TD, Richardson KL, Leedman PJ (2018) Total RNA extraction from tissues for microRNA and target gene expression analysis: not all kits are created equal. BMC Biotechnol 18:1–11. https://doi.org/10.1186/s12896-018-0421-6
Chen Z, Xiao J, Liu H, Yao K, Hou X, Cao Y, Liu X (2020) Astaxanthin attenuates oxidative stress and immune impairment in D-galactose-induced aging in rats by activating the Nrf2/Keap1 pathway and suppressing the NF-κB pathway. Food Funct 11:8099–8111. https://doi.org/10.1039/D0FO01663B
Claiborne AL (2018) Catalase activity. In: Greenwald RA (ed) CRC handbook of methods for oxygen radical research. CRC Press, Boca Raton, FL, pp 283–284
Di Meo S, Reed TT, Venditti P, Victor VM (2016) Role of ROS and RNS sources in physiological and pathological conditions. Oxid Med Cell Longev 2016:1–44. https://doi.org/10.1155/2016/1245049
Eissa N, Hussein H, Wang H, Rabbi MF, Bernstein CN, Ghia JE (2016) Stability of reference genes for messenger RNA quantification by real-time PCR in mouse dextran sodium sulfate experimental colitis. PloS One 11:e0156289. https://doi.org/10.1371/journal.pone.0156289
El-Sayed A, Aleya L, Kamel M (2021) Microbiota’s role in health and diseases. Environ Sci Pollut Res 28:36967–36983. https://doi.org/10.1007/s11356-021-14593-z
Fanzo J, Covic N, Dobermann A, Henson S, Herrero M, Pingali P, Staal S (2020) A research vision for food systems in the 2020s: defying the status quo. Glob food Sec 26:100397. https://doi.org/10.1016/j.gfs.2020.100397
Feng Y, Yu YH, Wang ST, Ren J, Camer D, Hua YZ, Zhang Q, Huang J, Xue DL, Zhang XF, Huang XF (2016) Chlorogenic acid protects D-galactose-induced liver and kidney injury via antioxidation and anti-inflammation effects in mice. Pharm Biol 54:1027–1034. https://doi.org/10.3109/13880209.2015.1093510
Ge Q, Yang B, Liu R, Jiang D, Yu H, Wu M, Zhang W (2021) Antioxidant activity of Lactobacillus plantarum NJAU-01 in an animal model of aging. BMC Microbiol 21:1–9. https://doi.org/10.1186/s12866-021-02248-5
Germoush MO, Fouda M, Kamel M, Abdel-Daim MM (2022) Spirulina platensis protects against microcystin-LR-induced toxicity in rats. Environ Sci Pollut Res 29:11320–11331. https://doi.org/10.1007/s11356-021-16481-y
Ghazi S, Diab AM, Khalafalla MM, Mohamed RA (2022) Synergistic effects of selenium and zinc oxide nanoparticles on growth performance, hemato-biochemical profile, immune and oxidative stress responses, and intestinal morphometry of Nile tilapia (Oreochromis niloticus). Biol Trace Elem Res 200(1):364–374
Hart P, Mao M, de Abreu A, Fricano K, Ekoue D, Minshall R, Diamond A, Bonini M (2015) MnSOD/SOD2 upregulation sustains the Warburg effect via mitochondrial ROS and AMPK-dependent signaling in cancer. The FASEB J 29:884–962. https://doi.org/10.1038/ncomms7053
Hassani S, Maqbool F, Salek-Maghsoudi A, Rahmani S, Shadboorestan A, Nili-Ahmadabadi A, Amini M, Norouzi P, Abdollahi M (2018) Alteration of hepatocellular antioxidant gene expression pattern and biomarkers of oxidative damage in diazinon-induced acute toxicity in Wistar rat: a time-course mechanistic study. EXCLI J 17:57. https://doi.org/10.17179/excli2017-760
Iqbal N, Zubair HM, Almutairi MH, Abbas M, Akhtar MF, Aleya L, Kamel M, Saleem A, Jabeen Q, Noreen S, Abdel-Daim MM (2022) Hepatoprotective effect of Cordia rothii extract against CCl4-induced oxidative stress via Nrf2–NFκB pathways. Biomed Pharmacother 156:113840. https://doi.org/10.1016/j.biopha.2022.113840
Landete JM, Gaya P, Rodríguez E, Langa S, Peirotén Á, Medina M, Arqués JL (2017) Probiotic bacteria for healthier aging: immunomodulation and metabolism of phytoestrogens. BioMed Res Int 2017:1–14. https://doi.org/10.1155/2017/5939818
Li JJ, Zhu Q, Lu YP, Zhao P, Feng ZB, Qian ZM, Zhu L (2015) Ligustilide prevents cognitive impairment and attenuates neurotoxicity in D-galactose induced aging mice brain. Brain Res 1595:19–28. https://doi.org/10.1016/j.brainres.2014.10.012
Li F, Huang G, Tan F, Yi R, Zhou X, Mu J, Zhao X (2020) Lactobacillus plantarum KSFY06 on d-galactose-induced oxidation and aging in Kunming mice. Food Sci Nutr 8:379–389. https://doi.org/10.1002/fsn3.1318
Li W, Huang W, Ma Y, Muhammad I, Hanif A, Ding Z, Guo X (2022) Antioxidant properties of lactic acid bacteria isolated from traditional fermented yak milk and their probiotic effects on the oxidative senescence of Caenorhabditis elegans. Food Funct 13:3690–3703. https://doi.org/10.1039/D1FO03538J
Liguori I, Russo G, Curcio F, Bulli G, Aran L, Della-Morte D, Gargiulo G, Testa G, Cacciatore F, Bonaduce D, Abete P (2018) Oxidative stress, aging, and diseases. Clin Interv Aging 13:757. https://doi.org/10.2147/CIA.S158513
Lin X, Xia Y, Wang G, Xiong Z, Zhang H, Lai F, Ai L (2018) Lactobacillus plantarum AR501 alleviates the oxidative stress of D-galactose-induced aging mice liver by upregulation of Nrf2-mediated antioxidant enzyme expression. J Food Sci 83:1990–1998. https://doi.org/10.1111/1750-3841.14200
Motataianu A, Serban G, Barcutean L, Balasa R (2022) Oxidative stress in amyotrophic lateral sclerosis: synergy of genetic and environmental factors. Int J Mol Sci 23:9339
Noureen S, Riaz A, Arshad M, Arshad N (2019) In vitro selection and in vivo confirmation of the antioxidant ability of Lactobacillus brevis MG 000874. J Appl Microbio 126:1221–1232. https://doi.org/10.1111/jam.14189
Qian YU, Zhang J, Zhou X, Yi R, Mu J, Long X, Pan Y, Zhao X, Liu W (2018) Lactobacillus plantarum CQPC11 isolated from sichuan pickled cabbages antagonizes d-galactose-induced oxidation and aging in mice. Molecules 23(11):3026. https://doi.org/10.3390/molecules23113026
Qiu Y, Ai PF, Song JJ, Liu C, Li ZW (2017) Total flavonoid extract from Abelmoschus manihot (L.) medic flowers attenuates d-galactose-induced oxidative stress in mouse liver through the Nrf2 pathway. J Med Food 20:557–567. https://doi.org/10.1089/jmf.2016.3870
Ramos OY, Basualdo M, Libonatti C, Vega MF (2020) Current status and application of lactic acid bacteria in animal production systems with a focus on bacteria from honey bee colonies. J Appl Microbiol 128:248–1260. https://doi.org/10.1111/jam.14469
Rehman FU, Farid A, Shah SU, Dar MJ, Rehman AU, Ahmed N, ... Shah KU (2022) Self-emulsifying drug delivery systems (SEDDS): measuring energy dynamics to determine thermodynamic and kinetic stability. Pharmaceuticals 15(9):1064. https://doi.org/10.3390/ph15091064
Rezaei M, Noori N, Shariatifar N, Gandomi H, Basti AA, Khaneghah AM (2020) Isolation of lactic acid probiotic strains from Iranian camel milk: technological and antioxidant properties. LWT 132:109823–109831. https://doi.org/10.1016/j.lwt.2020.109823
Sang Y, Zhang F, Wang H, Yao J, Chen R, Zhou Z, Yang K, Xie Y, Wan T, Ding H (2017) Apigenin exhibits protective effects in a mouse model of d-galactose-induced aging via activating the Nrf2 pathway. Food Funct 8:2331–2340. https://doi.org/10.1016/j.jff.2020.103957
Selvaratnam JS, Robaire B (2016) Effects of aging and oxidative stress on spermatozoa of superoxide-dismutase 1-and catalase-null mice. Biol Reprod 95:60–61. https://doi.org/10.1095/biolreprod.116.141671
Sharma VK, Singh TG, Garg N, Dhiman S, Gupta S, Rahman MH, ... Abdel-Daim MM (2021) Dysbiosis and Alzheimer’s disease: a role for chronic stress?. Biomolecules 11(05):678. https://doi.org/10.3390/biom11050678
Shen Q, Shang N, Li P (2011) In vitro and in vivo antioxidant activity of Bifidobacterium animalis 01 isolated from centenarians. Curr Microbiol 62:1097–1103. https://doi.org/10.1007/s00284-010-9827-7
Shen M, Zhao DK, Qiao Q, Liu L, Wang JL, Cao GH, ... Zhao ZW (2015) Identification of glutathione S-transferase (GST) genes from a dark septate endophytic fungus (Exophiala pisciphila) and their expression patterns under varied metals stress. PloS one 10(4):e0123418. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0123418
Shwe T, Pratchayasakul W, Chattipakorn N, Chattipakorn SC (2018) Role of D-galactose-induced brain aging and its potential used for therapeutic interventions. Exp Gerontol 101:13–36. https://doi.org/10.1016/j.exger.2017.10.029
Stroustrup N (2018) Measuring and modeling interventions in aging. Curr Opin Cell Biol 55:129–138. https://doi.org/10.1016/j.ceb.2018.07.004
Unban K, Chaichana W, Baipong S, Abdullahi AD, Kanpiengjai A, Shetty K, Khanongnuch C (2021) Probiotic and antioxidant properties of lactic acid bacteria isolated from indigenous fermented tea leaves (Miang) of north thailand and promising application in synbiotic formulation. Fermentation 7:195. https://doi.org/10.3390/fermentation7030195
Wells JC, Sawaya AL, Wibaek R, Mwangome M, Poullas MS, Yajnik CS, Demaio A (2020) The double burden of malnutrition: aetiological pathways and consequences for health. The Lancet 395:75–88. https://doi.org/10.1016/S0140-6736(19)32472-9
Woo JY, Gu W, Kim KA, Jang SE, Han MJ, Kim DH (2014) Lactobacillus pentosus var. plantarum C29 ameliorates memory impairment and inflammaging in a D-galactose-induced accelerated aging mouse model. Anaerobe 27:22–26. https://doi.org/10.1016/j.anaerobe.2014.03.003
Xu LQ, Xie YL, Gui SH, Zhang X, Mo ZZ, Sun CY, Li CL, Luo DD, Zhang ZB, Su ZR, Xie JH (2016) Polydatin attenuates d-galactose-induced liver and brain damage through its anti-oxidative, anti-inflammatory and anti-apoptotic effects in mice. Food Funct 7:4545–4555. https://doi.org/10.1039/c6fo01057a
Xu C, Li E, Suo Y, Su Y, Lu M, Zhao Q, Qin JG, Chen L (2018) Histological and transcriptomic responses of two immune organs, the spleen and head kidney, in Nile tilapia (Oreochromis niloticus) to long-term hypersaline stress. Fish Shellfish Immunol 76:48–57. https://doi.org/10.1016/j.fsi.2018.02.041
Yadav R, Khan SH, Mada SB, Meena S, Kapila R, Kapila S (2019) Consumption of probiotic Lactobacillus fermentum MTCC: 5898-fermented milk attenuates dyslipidemia, oxidative stress, and inflammation in male rats fed on cholesterol-enriched diet. Probiotics Antimicrob Prot 11:509–518. https://doi.org/10.1007/s12602-018-9429-4
Zhao J, Tian F, Yan S, Zhai Q, Zhang H, Chen W (2018) Lactobacillus plantarum CCFM10 alleviating oxidative stress and restoring the gut microbiota in d-galactose-induced aging mice. Food Funct 1:917–924. https://doi.org/10.1039/C7FO01574G
Zheng G, Xu X, Zheng J, Liu A (2016) Protective effect of seleno-β-lactoglobulin (Se-β-lg) against oxidative stress in D-galactose-induced aging mice. J Funct Foods 27:310–318. https://doi.org/10.1016/j.jff.2016.09.015
Funding
This research was funded by the NAHE Higher Education Commission of Pakistan through grant no. 532/IPFP-II (Batch-I) /SRGP/NAHE/HEC/2020/130.
Author information
Authors and Affiliations
Contributions
S. N: conceptualization, methodology, analysis, data curation, and writing — original draft; T. H: Analysis; A. N: reviewing and editing; A. E. A: reviewing and editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The protocol for these experiments was approved by the Animal Ethics Committee of the Virtual University of Pakistan.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Mohamed M. Abdel-Daim
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Noureen, S., Hussain, T., Noureen, A. et al. Effect of Lactobacillus brevis (MG000874) on antioxidant-related gene expression of the liver and kidney in D-galactose-induced oxidative stress mice model. Environ Sci Pollut Res 30, 84099–84109 (2023). https://doi.org/10.1007/s11356-023-28203-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-28203-7