Abstract
Paraquat (PQ) is an organic compound, which is commonly used as a herbicide in the agriculture sector, and it is also known to stimulate critical damages in the male reproductive system. Gossypetin (GPTN) is one of important members of the flavonoid family, which is an essential compound in flowers and calyx of Hibiscus sabdariffa with potential pharmacological properties. The current investigation was aimed to examine the ameliorative potential of GPTN against PQ-instigated testicular damages. Adult male Sprague–Dawley rats (n = 48) were distributed into four groups: control, PQ (5 mg/kg), PQ + GPTN (5 mg/kg + 30 mg/kg respectively), and GPTN (30 mg/kg). After 56 days of treatment, biochemical, spermatogenic indices, hormonal, steroidogenic, pro-or-anti-apoptotic, and histopathological parameters were estimated. PQ exposure disturbed the biochemical profile by reducing the activities of catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPx), and glutathione reductase (GSR), while it increased the concentration of reactive oxygen species (ROS) and malondialdehyde (MDA) level. Furthermore, PQ exposure decreased the sperm motility, viability, number of hypo-osmotic tail swelled spermatozoa, and epididymal sperm count; additionally, it increased sperm morphological (head mid-piece and tail) abnormalities. Moreover, PQ lessened the follicle-stimulating hormone (FSH), luteinizing hormone (LH), and plasma testosterone levels. Besides, PQ-intoxication downregulated the gene expression of steroidogenic enzymes (StAR, 3β-HSD, and 17β-HSD) and anti-apoptotic marker (Bcl-2), whereas upregulated the gene expression of apoptotic markers (Bax and Caspase-3). PQ exposure led to histopathological damages in testicular tissues as well. Nonetheless, GPTN inverted all the illustrated impairments in testes. Taken together, GPTN could potently ameliorate PQ-induced reproductive dysfunctions due to its antioxidant, androgenic, and anti-apoptotic potential.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Paraquat (PQ) is an organic compound widely used as a herbicide (Gramoxone, TM Syngenta) (Kim and Kim 2020). It is one of the well-known redox cycling compounds (reduced by nicotinamide adenine dinucleotide phosphate, (NADPH), before being oxidized by dioxygen to produce superoxide, a major reactive oxygen species), that is quick and non-selective in action, capable of inducing cellular damages by enhancing the generation of reactive oxygen species (ROS) and subsequent oxidative stress (OS) (Asghari et al. 2017). It adversely affects humans and farm animals by accumulating in their lungs, liver, kidneys, and heart (Ahmed 2010). It also causes neurological (Kamel 2013) as well as reproductive impairments (Nasibeh et al. 2015). In animals, mortality associated with PQ exposure can range between 60 and 80% (Kuan et al. 2016). It also acts as an anti-androgen that exerts its anti-fertility effect by its action, on the hypothalamus-pituitary–gonadal axis (HPGA) or via directly altering hormonal levels in the reproductive organs that lead to inhibition of spermatogenesis (Ofoego et al. 2018).
In animals, PQ exposure occurs through damaged skin or inhalation (Qian et al. 2019), which is then disseminated through the blood to all tissues and organs of the body following the absorbance from the respiratory and digestive tract (Fortenberry et al. 2016). Inside the cells, it conflicts with the endogenous anti-oxidant activities, which as feedback raises the level of ROS production which eventually leads to cell death as well as tissue damage (Meng et al. 2013; Blanco‐Ayala et al. 2014). The generation of free radicals in the male reproductive tract after PQ exposure has become a great concern due to its detrimental effects on sperm quality and function, such as significantly lower reproductive potential due to reduced numbers of Leydig cells (LCs), spermatocytes, and spermatids (Nasibeh et al. 2015; Li et al. 2019), and high rate of abnormal sperm production (Chen et al. 2017).
Gossypetin (GPTN; 3,5,7,8,3′,4′ hexa hydroxy flavone) is an important flavonoid, which is a vital compound in flowers and calyx of Hibiscus sabdariffa with diverse pharmacological activities that include anti-oxidant (Xie et al. 2019), anti-tumor (Salvamani et al. 2014), anti-rheumatoid arthritis (Patel and Patel 2021b), anti-atherosclerotic (Lin et al. 2021), anti-cancer (Lee et al. 2017), anti-inflammatory (Patel and Patel 2021b), anti-depressant (Gulsheen et al. 2019), anti-microbial (Pawar et al. 2017), and neuroprotective activities (Patel and Patel 2021a). Despite the potential therapeutic properties of GPTN, its remedial potential against reproductive dysfunctions was not examined until now. To fulfill this knowledge gap, the present investigation, for the first time, aimed to explore the shielding impacts of GPTN against PQ-induced reproductive dysfunctions in the testicular tissues of adult male Sprague–Dawley rats.
Materials and methods
Chemicals
All chemicals used in the present study were bought from Sigma-Aldrich, Germany.
Animals
In this study, adult male Sprague–Dawley rats (n = 48) weighing (250 ± 20 g) were used as experimental animals. They were kept in steel cages (56 × 30 × 22 cm) at standard temperature (22–25 °C), 12 h light or dark cycle, and humidity (45 ± 5%) in animal house, University of Agriculture, Faisalabad (UAF). Tap water was provided ad libitum. Rats were fed a commercial diet (Oxbow Essentials adult rat food, Oxbow Animal Health, Murdock, USA), comprising of 4% fat; 15% protein was used as standard rat feed. All experimental protocols were performed in compliance with the guidelines of the UAF animal protection and handling Committee.
Experimental design
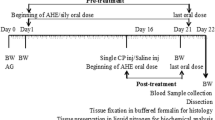
Adult male Sprague–Dawley rats (n = 48) were distributed into four separate groups, each containing n = 12 per group: Control group; PQ administered group (5 mg/kg. b. wt. orally); PQ + GPTN co-treated group (5 mg/kg b.wt. of PQ and 30 mg/kg. b.wt. of GPTN orally, respectively); and GPTN treated group (30 mg/kg.b.wt orally). After 56 days of the entire experiment, rats were given anesthesia by diethyl ether and then decapitated and blood was collected. To separate plasma, trunk blood was drawn into heparinized syringes. Blood centrifugation was performed for 10 min at 3000 revolutions per minute (rpm). After isolation, plasma was stored at – 20 °C for further analysis. For histopathological evaluation, the left testis was fixed in a 10% formaldehyde solution for about 48 h. The right testis was kept at –80 °C to evaluate the activities of biochemical enzymes. Testicular tissues were homogenized in sodium phosphate (Na3PO4) buffer at 12,000 rpm for 15 min and the temperature was maintained at 4 °C. This supernatant was ultimately used to assess multiple parameters.
Biochemical evaluation
Activity of catalase
In the testicular tissues, the activity of catalase (CAT) was determined according to the method described by Chance and Maehly (1955). Various chemicals, including 2.5 mL of 50 mM phosphate buffer (pH 5.0), 0.4 mL of 5.9 mM H2O2, and 0.1 mL enzyme extract were mixed to make reaction mixture. Absorbance changes in the mixture were noted at 240 nm. One unit of CAT activity was considered as an absorbance change of 0.01 as units/min.
Activity of superoxide dismutase
Superoxide dismutase (SOD) activity was estimated by following the method of Kakkar et al. (1984). Reaction mixture comprising of 1.2 mL of sodium pyrophosphate buffer (0.052 mM; pH 7.0) and 0.1 mL of phenazine methosulfate (186 mM). 0.3 mL of supernatant after centrifugation (1500 × g for 10 min followed by 10,000 × g for 15 min) of homogenate was added to the reaction mixture. Then, 0.2 mL of NADH (780 mM) was added to initiate an enzyme reaction, which was later on terminated by adding 1 mL of glacial acetic acid. Finally, the chromogen amount was assessed by noticing the change in color intensity (at 560 nm). The values of SOD activity were presented as unit/mg of protein.
Activity of glutathione peroxidase
Glutathione peroxidase (GPx) activity was evaluated according to the method of Lawrence and Burk (1976), in which testicular homogenates were centrifuged, and the supernatant was used to measure GPx activity in the presence of glutathione reductase, reduced glutathione, and cumene hydroperoxide, and enzymatic reaction was measured spectrophotometrically at 37 °C at a wavelength of 412 nm. GPx activity was measured as U/mg protein.
Activity of glutathione reductase
Glutathione reductase (GSR) activity was measured according to the method of Carlberg and Mannervik (1975) by estimating the nicotinamide adenine dinucleotide phosphate (NADPH) disappearance. The change in absorbance was estimated at 340 nm by using spectrophotometer (UV–Visible/NIR-UH5700). NADPH was used as a substrate. An extinction coefficient of 6.22 × 103 M−1 cm−1 was used for calculations. The values obtained were expressed as nM NADPH oxidized min−1 mg−1 tissue.
Reactive oxygen species concentration
Hayashi et al. (2007) protocol was employed to determine the concentration of reactive oxygen species (ROS). Homogenate (5 μL) and 0.1 M sodium acetate buffer (140 μL) with pH 4.8 were mixed and dispensed in 96-well plate. After incubating at 37 °C for 5 min, 100 μL of ferrous sulfate solution and N, N-diethyl-para-phenylenediamine were dispensed to each plate and then incubated at 37 °C for 1 min. At 505 nm, the absorbance was observed with the help of a microplate reader for 180 s with a 15-s interval. In the end, the standard curve was plotted. ROS concentration was recorded as unit g−1 tissues.
Malondialdehyde level
Malondialdehyde (MDA) level was used as the indicator of terminal phase of lipid peroxidation (LP), which was measured with the 2-thiobarbituric acid method of Placer et al. (1966) using 1, 1,3,3-tetraethoxypropane as standard. This reaction produces a colored product, the color intensity of which was measured at a wavelength of 532 nm. The results were expressed in nmol/mg protein.
Semen analysis
A hemocytometer was employed to count epididymal sperm. Firstly, the caudal piece of epididymis was isolated to take semen samples, which was after finely minced in physiological-saline (5 mL), incubated at 37 °C for about 30 min that allowed sperm to abscond from the epididymis. A solution containing 25 mg eosin/100 mL of distilled water (H2O), 1 mL formalin (35%), and 5 g sodium bicarbonate (NaHCO3) was utilized 1:100 to dilute the supernatant. Lastly, a 10-mL droplet of the above mixture was put in a sperm-counting chamber and examined (ten fields) microscopically at 400X (Yokoi et al. 2003). On the other hand, sperm motility (%) was recorded with the help of a phase-contrast microscope at 400X (Kenjale et al. 2008). Correspondingly, sperm viability was assessed by eosin or nigrosin staining, accompanied by a microscopic evaluation (Halvaei et al. 2012). Also, sperm morphological abnormalities (head, tail, and mid-piece) were ascertained via the protocol of Cao et al. (2017). The units of measurement are the percentage (%). The integrity of the sperm plasma membrane was ascertained by the hypo-osmotic swelling (HOS) test, according to Correa and Zavos (1994) method. The test was carried by placing 20 μL of semen in 180 μl of fructose solution, keeping the osmotic pressure at 80 mOsm/L for about 20 min. After subsequent incubation and processing, the sperms were stained with eosin or nigrosin. Finally, using a light microscope (400X), two hundred spermatozoa with swollen and non-swollen tails were estimated.
Hormonal assay
For the evaluation of hormonal levels such as follicle-stimulating hormone (FSH) (Bio-Check Inc., USA Catalog No. BC-1029; sensitivity 0.0083 ng/mL), luteinizing hormone (LH) (Bio–Check Inc., USA Catalog No. BC-1031; sensitivity 0.0069 ng/mL), and plasma testosterone (Bio–Check Inc., USA Catalog No. BC-1115; sensitivity 0.07 ng/mL) enzyme-linked immunosorbent assay (ELISA) kits were employed as per the guidelines of the manufacturer (Bio–Check Inc., USA). The units of measurement are ng/mL.
Ribonucleic acid extraction and real-time quantitative reverse transcription-polymerase chain reaction
The steroidogenic expressions of the acute regulatory protein (StAR), 3β-hydroxysteroid dehydrogenase (3β-HSD), and 17β-hydroxysteroid dehydrogenase (17β-HSD), as well as the anti-or pro-apoptotic markers such as B-cell leukemia/lymphoma 2 (Bcl-2), Bcl-2 associated X, apoptosis regulator (Bax), and cysteine-aspartic acid protease-3 (Caspase-3), were estimated via real-time quantitative reverse transcription-polymerase chain reaction (RT-qPCR). By using TRIzol (Invitrogen) reagent (Life Technologies, New York, USA), total ribonucleic acid (tRNA) isolation was carried out, which was succeedingly converted into complementary deoxyribonucleic acid (cDNA) by reverse transcription. Alterations in the expression of these steroidogenic enzymes and apoptotic markers were analyzed via 2, −ΔΔCT admitting that β-actin is the interior regulator (Livak and Schmittgen 2001). β-actin and targeted genes primer sequences are presented in Table 1, as demonstrated earlier (Ijaz et al. 2021a).
Histopathology
For the evaluation of testicular histopathology, firstly, previously fixed testicular tissues were dehydrated in ascending grades of alcohol and inserted in paraffin wax. Then 5-μm-thick pieces were cut, stained via hematoxylin–eosin (H & E), and examined microscopically (Nikon, 187,842, Japan). Sequentially Leica-LB (Olympus Optical Co. LTD, Japan) and image-J2x software was employed to take and analyze the images of specimens.
Statistical analysis
Results were shown as mean ± standard error (SE). Data were interpreted with a one‐way analysis of variance (ANOVA), followed by Turkey’s multiple comparison test using Minitab Software. The p < 0.05 were set statistically meaningful.
Results
Effect of GPTN on PQ-disrupted testes biochemical markers
Table 2 displays the mean values of biochemical markers. In the present study, PQ-intoxication significantly (p < 0.05) lowered the CAT, SOD, GPx, or GSR activities, while increased the concentration of ROS and MDA levels in contrast to the control group. Nevertheless, GPTN supplementation in the PQ-cotreated group prompted significant (p < 0.05) increased in the activities of CAT, SOD, GPx, or GSR, and significantly (p < 0.05) decreased the concentration of ROS and MDA in contrast to the PQ group. Additionally, non-significant differences were observed between the GPTN only treated and control groups.
Effect of GPTN on PQ-disrupted sperm motility, viability, count, and HOS test of sperm
Table 3 presents the mean values of spermatogenic indices. PQ exposure significantly (p < 0.05) lessened the epididymal sperm or HOS coil-tailed sperm count, sperm motility, and viability, while morphological sperm anomalies (sperm head–tail and mid-piece) were elevated in the PO-intoxicated rats as compared to the control rats. Conversely, GPTN significantly (p < 0.05) inverted all these sperm indices to a normal state in the PQ-cotreated group in contrast to the PQ group. However, a non-significant deviation was seen within the mean values of GPTN alone treated and the control groups.
Effect of GPTN on PQ-disrupted hormones plasma concentration
Table 4 shows the mean values of the hormonal levels. Results of the hormonal assay reported that PQ-exposure significantly (p < 0.05) lowered the levels of FSH, LH, and plasma testosterone in PQ-intoxicated rats, as compared to the control rats. Nonetheless, GPTN treatment significantly (p < 0.05) recovered the hormonal levels in PQ co-treated group. GPTN alone treatment exhibited normal hormonal levels with no significant alterations as compared with the control group.
Effect of GPTN on PQ-disrupted genes expression of steroidogenic enzymes
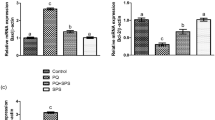
Figure 1 presents the comparative differences in the steroidogenic enzyme expressions. Outcomes of the investigation revealed that PQ-intoxication significantly (p < 0.05) reduced the StAR, 3β-HSD, and 17β-HSD gene expressions in PQ intoxicated rats as compared to control rats. Nevertheless, GPTN provision significantly (p < 0.05) recovered steroidogenic enzyme expressions in PQ co-treated group in comparison to the PQ group. However, non-significant differences were noted between GPTN only treated and control groups.
Representing the impact of paraquat and gossypetin on the expression of (a) 3b-HSD, (b) 17b-HSD, and (c) StAR. The bar graphs are composed based on mean ± SE values (n = 12/group). Different superscripts on the bars showing significant differences at p < 0.05. 3b-HSD, 3b-hydroxysteroid dehydrogenase; 17b-HSD, 17b-hydroxysteroid dehydrogenase
Effect of GPTN on PQ-disrupted genes expression of pro-apoptotic and anti-apoptotic markers
To ascertain the anti-apoptotic activity of GPTN, a property that provides its protective effects against PQ-instigated testicles dysfunction, we estimated the alterations in the gene expression of the pro-apoptotic-markers, particularly, Caspase-3 and Bax, and in the anti-apoptotic protein Bcl-2 (Fig. 2). Results revealed that PQ intoxication significantly (p < 0.05) downregulated the gene expression of Bcl-2, whereas upregulated the gene expression of Bax and Caspase-3 in the PQ group as compared to the control group. However, GPTN supplementation in the PQ co-treated group significantly (p < 0.05) recovered these anti-apoptotic and apoptotic gene expressions as compared to the PQ group. GPTN alone treatment presented insignificant differences in the anti-apoptotic and apoptotic gene expressions as compared with the control group.
Effect of GPTN on histopathological profile against PQ induced oxidative damage by mediating free radical scavenging potential
The histopathological alterations following PQ and GPTN exposure are shown in Table 5 and Fig. 3. The results of the study revealed that PQ exposure significantly (p < 0.05) lessened the diameter as well as the epithelial height of seminiferous tubules, besides the thickness of tunica propria. Moreover, it increased the luminal diameter of tubules. PQ-treatment also significantly (p < 0.05) diminished the number of spermatogonia, primary and secondary spermatocytes along with spermatids, in the PQ-intoxicated rats as compared to the control rats. Nevertheless, GPTN supplementation in the co-treated group significantly (p < 0.05) recovered all these structural damages as well as germ cell count as compared with the PQ group. However, the insignificant differences were observed between the GPTN only treated group and the control group.
Micro-images of the adult Sprague–Dawley rats (n = 12/group) testicles (H&E, 400X): (A) Control group demonstrating impenetrable germinal epithelium including different stages of germ cells and the tapered luminal area containing spermatozoa; (B) Paraquat-intoxicated group demonstrating sloughing of the epithelial layer, vacant lumen, and degenerated area of interstitial spaces; (C) Paraquat + gossypetin cotreated group displaying restoration in epithelial part, and tubular lumen; filled with seminiferous tubules as well as retrieved the deteriorated interstitial spaces; (D) Gossypetin-treated group representing compacted seminiferous tubules with less interstitial spaces and luminal part filled with all stages of spermatogenic cells thus improved spermatogenesis. IS, interstitial spaces; TL, tubular lumen; SHE, seminiferous epithelial height
Discussion
The current investigation was aimed to elucidate the defensive role of GPTN against PQ‐prompted testicular dysfunctions. PQ exposure prompted the damage in the status of biochemical, spermatogenic, steroidogenic, hormonal, apoptotic, and histopathological parameters. As reported earlier, PQ is a widely applied agricultural pesticide globally (Sandström et al. 2017). Yet, the use of PQ comes at a high cost, as it is a potential threat to the environment and the other non-targeted organisms (Mussi 2010). Many studies on PQ have shown its harmful effects on the reproductive system of males, i.e., testes, accessory sex glands including the neuroendocrine system due to oxidative stress (OS) (Ofoego et al. 2018). However, co-treatment of rats with GPTN for 56 days significantly cured all the above PQ-induced toxic alterations in rat testes. The natural plant-derived flavonoid GPTN has several therapeutic properties. Earlier studies stated that GPTN is a powerful antioxidant compound that potently scavenges free radicals to mitigate lipid peroxidation (LP). Furthermore, it exhibits anti-inflammatory, nephroprotective (Patel and Patel 2020), and hepatoprotective properties (Khan et al. 2015). Hence, the current investigation mainly emphasized the antioxidant potential of GPTN against PQ-instigated testicle toxicities.
The endogenous antioxidant enzymes primarily include CAT, SOD, GPx, and GSH (Momtaz and Abdollahi 2012). Therefore, CAT, SOD, GPx, and GSR are known as the first lines of defense that protect biomolecules such as lipids, proteins, and DNA from OS by reducing ROS generation. Nitric oxide (NO), hydrogen peroxide (H2O2), superoxide dismutase (O2−), and hydroxyl radical (OH) are the most reactive nitrogen and oxygen species (Davalli et al. 2016). SOD catalyzes the dismutation of O2− to form H2O2 (Ighodaro and Akinloye 2018), whereas H2O2 is transformed into water by CAT and GPx (Aslani and Ghobadi 2016). On the other hand, reduced GSH functions as an electron donor in these reactions (Birben et al. 2012). The concentration of GSH is retained by GSR, which restores reduced GSH from GSSG (oxidized form) for the perpetual functioning of GPx (Ali et al. 2020). Thus, a reduction in the activities of antioxidant enzymes elevates the concentration of ROS. When concentration of ROS exceeds the body’s antioxidant-defense capacity, OS occurs (Huang et al. 2016). OS is a state in which a higher concentration of ROS harms cells, tissues, or organs (Barbosa et al. 2020). When the concentration of ROS in tissues is high, it damages polyunsaturated fatty acids (PUFAs) in the sperm plasma membrane and triggers a series of chemical reactions known as lipid peroxidation (LP) (Fois et al. 2018), and in this study, MDA is a marker of LP. LP, in turn, may lead to damages that affect membrane integrity as well as the fluidity and permeability. Our results are compatible with the previous investigation, in which PQ treatment decreased the antioxidant activities of CAT and SOD, which, as a result, significantly raised the level of LP in the lung tissues of PQ-treated animals (Rasooli et al. 2020). However, reduced activity of the antioxidant enzymes in the current study was increased by the co-treatment of rats by GPTN for 56 days. Co-treatment of GPTN alleviated harmful impacts of PQ by declining OS. The present study has shown that GPTN exposure significantly elevated the activities of CAT, SOD, GPx, or GSR while lowered the concentration of ROS and MDA levels in the testicular tissues presenting its potential antioxidant property. This free radical scavenging activity of GPTN was probably due to its ring structure (Khan et al. 2015).
Findings of the present investigation also showed that PQ exposure significantly reduced epididymal sperm as well as HOS coiled-tailed sperm count, motility, and viability, in addition to anomalies in the head–tail and mid-piece of sperm. PQ produces free radicals that have been documented to cause deleterious effects on reproductive parameters such as count, motility, and architecture of sperm (Ofoego et al. 2018). As reported previously, OS is a culprit behind deteriorated semen quality (Ijaz et al. 2021b). Spermatozoa, especially the mid-piece segment, is potentially vulnerable to the toxic effects of free radicals (Nair 2015). Additionally, PQ exposure decreases ATP production by damaging mitochondria (Qian et al. 2019). This ATP reduction in spermatozoa hampers the flagellar movement, causing immobility of sperm (Anam et al. 2019). These findings are compatible with the results of Chikere et al. (2020). However, the GPTN treatment potentially resettled all the spermatogenic impairments, which indicated its excellent free radical scavenging property.
The pituitary hormone FSH and androgens locally generated in response to LH are critically responsible for the development and maintenance of spermatogenic cells (O'Shaughnessy 2014). However, PQ administration reduced the production of gonadotropins (FSH and LH) and testosterone, possibly due to the disturbed hypothalamus-pituitary–gonadal (HPG) axis. The outcomes of our study are also consistent with the findings of Okorondu et al. (2019). PQ exposure stimulates the formation of free radicals that, as a result, triggers the hypothalamic–pituitary–adrenal (HPA) axis, which discharges corticosterone (in rodents) and cortisol (in humans). These stress hormones, through the cross-talk among the HPG and HPA axes, affect LH and FSH discharge from the anterior pituitary (O'Shaughnessy 2014). In males, FSH stimulates the proliferation of immature Sertoli cells (SCs) and spermatogonia, whereas, on the other hand, LH stimulates Leydig Cells (LCs) to produce testosterone (Ramaswamy and Weinbauer 2015). Besides, testosterone in alliance with FSH stimulates the growth of spermatids and the release of sperms (O’Shaughnessy et al. 2010). Hence, adequate levels of hormones are necessary for spermatogenesis. However, GPTN treatment significantly improved hormonal levels. GPTN may alleviate these deadly changes in hormonal levels due to withdrawal in regulating the hypothalamic-pituitary-testicles axis, which might as feedback restored these spermatogenic damages.
Leydig cells (LCs) are present in the testicular stroma and are responsible for testosterone production (Tremblay 2015). Testosterone is an important male hormone that regulates spermatogenesis process and secondary sexual characters development (Zirkin and Papadopoulos 2018). To explore the philosophy behind the reduced testosterone level following PQ intoxication, the expression of steroidogenic enzymes such as StAR, 3β-HSD, and 17β-HSD was estimated. The key enzymes, 3β-HSD, and 17β-HSD regulate the steroidogenic activities and play a central role in androgenesis. StAR also acts as a rate-limiting enzyme in steroidogenesis (Castillo et al. 2015) that controls the transport of cholesterol into the mitochondria for testosterone biosynthesis (Das et al. 2012). Thus, the transformation of cholesterol to testosterone occurs in a chain of reactions catalyzed by primary steroidogenic enzymes of the endoplasmic reticulum (ER), such as 3β-HSD or 17β-HSD (Ye et al. 2011). Outcomes of the investigation showed that PQ exposure significantly repressed the genes expression of StAR, 3β-HSD, or 17β-HSD. These outcomes are also compatible with the earlier study in which PQ impairs steroidogenesis (Li et al. 2019). Lower expression of these androgenic enzymes due to PQ-prompted OS appeared in a lower level of the male reproductive hormone testosterone. Conversely, GPTN significantly increased the repressed testosterone level via upregulating the expression of the steroidogenic enzymes may be due to its androgenic property.
In the current study, PQ exposure upregulates the apoptotic protein expressions such as Bax and caspase-3 while lowered the anti-apoptotic protein Bcl-2 expression. Apoptosis is regulated by pro-apoptotic and anti-apoptotic proteins via mitochondria-dependent and independent pathways (Venkatadri et al. 2016). The relative imbalance between these proteins results in apoptotic cell death (Zhao et al. 2019). Bax is an apoptotic marker, which encourages cell death, while Bcl-2 is an anti-apoptotic protein that impersonates a part in the inhibition of apoptosis (Ghasemi et al. 2019). On the other hand, caspase-3 belongs to the caspase family (protease enzyme), and its activation is an irreversible phase, which prompts apoptosis. Thus, reduction in Bcl-2 and elevation in Bax adversely alter the permeability of the mitochondrial membrane, leading to the liberation of cytochrome c within the cytosol (Gu et al. 2017). This augmented cytochrome c in cytosol ultimately activates the expression of caspase-3, which leads to apoptosis and cell death (Kaur et al. 2020). However, GPTN could protect the apoptotic damages by minimizing the expression of Bax and caspase-3, along with improving the expression of the anti-apoptotic protein, Bcl-2. Conclusively, GPTN showed its anti-apoptotic activity mainly by regulation Bcl-2/Bax ratio.
In the present investigation, PQ exposure has elicited severe histoarchitectural damage in testicles by causing a diminution in height and diameter of seminiferous tubules’ epithelium, as well as tunica propria height. Whereas, the diameter of the tubular lumen was increased. Moreover, PQ scaled down the number of spermatogonia, primary and secondary spermatocytes, in addition to spermatids. PQ administration induced retrogressive histopathological impairments in seminiferous tubules such as the reduced outer diameter of seminiferous tubules as well as germinal epithelial thickness and reduced spermatogenic cell, which is associated with spermatogenic failure (Ojha and Srivastava 2014). According to previous studies, PQ exposure revealed necrotic testicular tissue, with testicular atrophy, and loss of sperm numbers due to the upsurge of ROS (Ofoego et al. 2018). However, GPTN alleviated the adverse histological degeneration of testicular tissues and the number of germ cells in the male reproductive system owing to its androgenic, anti-apoptotic, and anti-oxidant potential.
Conclusion
In conclusion, the outcomes of the present investigation stated that PQ exposure significantly (p < 0.05) reduced the activities of antioxidant enzymes and increased the concentration of ROS and the level of MDA. Furthermore, PQ-intoxication promoted apoptosis, lowered the germ cell count, and led to structural impairment of testicles. PQ exposure also primarily posed harmful effects on the hormonal axis in rats that eventually led to reduced sperm count, motility, and viability. Moreover, it down-regulated the gene expression of steroidogenic enzymes. However, GPTN treatment recovered these testicular damages due to its remarkable antioxidant, androgenic, and anti-apoptotic potentials. The results of the current study show promising clinical prospects to address the infertility issues of filed workers exposed to pesticides.
Data availability
The datasets used/analyzed in this study are available from the corresponding author on reasonable request.
The study was approved by the Institutional Biosafety/Bioethics Committee (IBC) of the University of Agriculture, Faisalabad, in compliance with this (CEE Council 86/609) protocol.
References
Ahmed MM (2010) Radio and chemoprotective properties of hesperidin against genotoxicity induced by gamma radiation and/ or paraquat in rat bone marrow cells. J Radiol Sci Appl 23:233–244
Ali SS, Ahsan H, Zia MK et al (2020) Understanding oxidants and antioxidants: classical team with new players. J Food Biochem 44:13145. https://doi.org/10.1111/jfbc.13145
Anam C, Marhendra AP, Rahayu S (2019) Alcohol Intake Investigation of Adult Rats Based on Sperm Parameters. J Exp Life Sci 9, 128–132. https://doi.org/10.21776/ub.jels.2019.009.02.11
Asghari MH, Moloudizargari M, Bahadar H et al (2017) A review of the protective effect of melatonin in pesticide-induced toxicity. Expert Opin Drug Metab Toxicol 13:545–554. https://doi.org/10.1080/17425255.2016.1214712
Aslani BA, Ghobadi S (2016) Studies on oxidants and antioxidants with a brief glance at their relevance to the immune system. Life Sci 146:163–173. https://doi.org/10.1016/j.lfs.2016.01.014
Barbosa ML, de Meneses AAPM, de Aguiar RPS et al (2020) Oxidative stress, antioxidant defense and depressive disorders: a systematic review of biochemical and molecular markers. Neurol Psychiatry Brain Res 36:65–72. https://doi.org/10.1016/j.npbr.2020.02.006
Birben E, Sahiner UM, Sackesen C et al (2012) Oxidative stress and antioxidant defense. World Allergy Organ J 5:9–19. https://doi.org/10.1097/WOX.0b013e3182439613
Blanco-Ayala T, Anderica-Romero AC, Pedraza-Chaverri J (2014) New insights into antioxidant strategies against paraquat toxicity. Free Radic Res 48:623–640. https://doi.org/10.3109/10715762.2014.899694
Cao Z, Shao B, Xu F et al (2017) Protective effect of selenium on aflatoxin B1-induced testicular toxicity in mice. Biol Trace Elem Res 180:233–238. https://doi.org/10.1007/s12011-017-0997-z
Carlberg I, Mannervik B (1975) Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J Biol Chem 250:5475–5480. https://doi.org/10.1016/S0021-9258(19)41206-4
Castillo AF, Orlando U, Helfenberger KE et al (2015) The role of mitochondrial fusion and StAR phosphorylation in the regulation of StAR activity and steroidogenesis. Mol Cell Endocrinol 408:73–79. https://doi.org/10.1016/j.mce.2014.12.011
Chance B, Maehly A (1955) [136] assay of catalases and peroxidases. Methods Enzymol 2:764–775. https://doi.org/10.1016/S0076-6879(55)02300-8
Chen Z, Zuo X, Li H et al (2017) Effects of melatonin on maturation, histone acetylation, autophagy of porcine oocytes and subsequent embryonic development. Anim Sci J 88:1298–1310. https://doi.org/10.1111/asj.12779
Chikere OU, Uchechi EE, Ikenna IM et al (2020) Ameliorative effect of aframomum melegueta (Alligator Pepper) against paraquat induced testicular damage. World J Pharm Res 9:2105–2124. https://doi.org/10.20959/wjpr20205-17442
Correa JR, Zavos PM (1994) The hypoosmotic swelling test: its employment as an assay to evaluate the functional integrity of the frozen–thawed bovine sperm membrane. Theriogenology 42:351–360. https://doi.org/10.1016/0093-691X(94)90280-1
Das J, Ghosh J, Manna P et al (2012) Taurine protects rat testes against doxorubicin–induced oxidative stress as well as p53, Fas and caspase 12–mediated apoptosis. Amino Acids 42:1839–1855. https://doi.org/10.1007/s00726-011-0904-4
Davalli P, Mitic T, Caporali A et al (2016) ROS, cell senescence, and novel molecular mechanisms in aging and age-related diseases. Oxid Med Cell Longev 2016. https://doi.org/10.1155/2016/3565127
Fois AG, Paliogiannis P, Sotgia S et al (2018) Evaluation of oxidative stress biomarkers in idiopathic pulmonary fibrosis and therapeutic applications: a systematic review. Respir Res 19:51. https://doi.org/10.1186/s12931-018-0754-7
Fortenberry GZ, Beckman J, Schwartz A et al (2016) Magnitude and characteristics of acute paraquat-and diquat-related illnesses in the US: 1998–2013. Environ Res 146:191–199. https://doi.org/10.1016/j.envres.2016.01.003
Ghasemi Y, Tayebi-Khosroshahi H, Abedi B et al (2019) Tiny non-coding RNAs in body fluids, possible biomarkers for autosomal dominant polycystic kidney disease. Iran J Kidney Dis 13:151–164
Gu YP, Yang XM, Duan ZH et al (2017) Inhibition of chemotherapy-induced apoptosis of testicular cells by squid ink polysaccharide. Exp Ther Med 14:5889–5895. https://doi.org/10.3892/etm.2017.5342
Gulsheen, Kumar, A. and Sharma, A., 2019. Antianxiety and antidepressant activity guided isolation and characterization of gossypetin from Hibiscus sabdariffa Linn. calyces. J Biol Act Prod Nat 9: 205–214. https://doi.org/10.1080/22311866.2019.1615552
Halvaei I, Roodsari HRS, Harat ZN (2012) Acute effects of Ruta graveolens L. on sperm parameters and DNA integrity in rats. J Reprod Fertil 13:33–38
Hayashi I, Morishita Y, Imai K et al (2007) High-throughput spectrophotometric assay of reactive oxygen species in serum. Mutat Res Genet Toxicol Environ Mutagen 631:55–61. https://doi.org/10.1016/j.mrgentox.2007.04.006
Huang W, Quan C, Duan P et al (2016) Nonylphenol induced apoptosis and autophagy involving the Akt/mTOR pathway in prepubertal Sprague-Dawley male rats in vivo and in vitro. Toxicology 373:41–53. https://doi.org/10.1016/j.tox.2016.11.006
Ighodaro OM, Akinloye OA (2018) First line defence antioxidants–superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria J Med 54:287–293. https://doi.org/10.1016/j.ajme.2017.09.001
Ijaz MU, Tahir A, Samad A et al (2021a) Nobiletin ameliorates nonylphenol-induced testicular damage by improving biochemical, steroidogenic, hormonal, spermatogenic, apoptotic and histological profile. Hum Exp Toxicol 40:403–416. https://doi.org/10.1177/0960327120950007
Ijaz MU, Anwar H, Iqbal S et al (2021b) Protective effect of myricetin on nonylphenol-induced testicular toxicity: biochemical, steroidogenic, hormonal, spermatogenic, and histological-based evidences. Environ Sci Pollut Res 28:22742–22757. https://doi.org/10.1007/s11356-020-12296-5
Kakkar P, Das B, Viswanathan PN (1984) A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys 21:130–132
Khan A, Manna K, Das DK et al (2015a) Gossypetin ameliorates ionizing radiation-induced oxidative stress in mice liver—a molecular approach. Free Radic Res 49:1173–1186. https://doi.org/10.3109/10715762.2015.1053878
Kamel F (2013) Paths from pesticides to Parkinson’s. Sciences 341: 722 –723. https://doi.org/10.1126/science.1243619
Kaur P, Dhandayuthapani S, Venkatesan T et al (2020) Molecular mechanism of C-phycocyanin induced apoptosis in LNCaP cells. Bioorg Med Chem 28:115272. https://doi.org/10.1016/j.bmc.2019.115272
Kenjale R, Shah R, Sathaye S (2008) Effects of Chlorophytum borivilianum on sexual behaviour and sperm count in male rats. Phytotherapy Research: An International Journal Devoted to Pharmacological and Toxicological Evaluation of Natural Product Derivatives 22: 796–801. https://doi.org/10.1002/ptr.2369
Kim JW, Kim DS (2020) Paraquat: toxicology and impacts of its ban on human health and agriculture. Weed Sci 68:208–213. https://doi.org/10.1017/wsc.2019.70
Kuan CM, Lin ST, Yen TH et al (2016) Paper-based diagnostic devices for clinical paraquat poisoning diagnosis. Biomicrofluidics 10:034118. https://doi.org/10.1063/1.4953257
Lawrence RA, Burk RF (1976) Glutathione peroxidase activity in selenium–deficient rat liver. Biochem Biophys Res Commun 71:952–958. https://doi.org/10.1016/0006-291X(76)90747-6
Lee MS, Tsai CW, Wang CP et al (2017) Anti-prostate cancer potential of gossypetin via inducing apoptotic and autophagic cell death. Mol Carcinog 56:2578–2592. https://doi.org/10.1002/mc.22702
Li H, Hong T, Zhu Q et al (2019) Paraquat exposure delays late-stage Leydig cell differentiation in rats during puberty. Environ Pollut 255:113316. https://doi.org/10.1016/j.envpol.2019.113316
Lin HH, Hsieh MC, Wang CP et al (2021) Anti-atherosclerotic effect of gossypetin on abnormal vascular smooth muscle cell proliferation and migration. Antioxidants 10:1357. https://doi.org/10.3390/antiox10091357
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Meng XX, Wang RL, Gao O (2013) Effect of alinastation on Paraquat induced oxidative stress in humans type II alveolar epithelial cells. World J Emerg Med 4:133–137. https://doi.org/10.5847/wjem.j.issn.1920-8642.2013.02.009
Momtaz S, Abdollahi M (2012) A comprehensive review of biochemical and molecular evidences from animal and human studies on the role of oxidative stress in aging: an epiphenomenon or the cause. Asian J Anim Vet Adv. https://doi.org/10.3923/ajava.2012
Mussi MA (2010) Calcaterra NB (2010) Paraquat-induced oxidative stress response during amphibian early embryonic development. Compara Biochem Physiol Part C 151:240–247. https://doi.org/10.1016/j.cbpc.2009.11.003
Nair N (2015) Dose-dependent short–term study of di-n-butyl phthalate on the testicular antioxidant system of Wistar rats. Environ Sci Pollut Res 22:2196–2204. https://doi.org/10.1007/s11356-014-3457-8
Nasibeh F, Mohammad H, Morteza H et al (2015) Effects of paraquat on testicular histomorphometry of male rats. Biol Forum 7:573–575
O’Shaughnessy PJ, Monteiro A, Verhoeven G et al (2010) Effect of FSH on testicular morphology and spermatogenesis in gonadotropin-deficient hypogonadal mice lacking androgen receptors. Reproduction 139:177–184. https://doi.org/10.1530/REP-09-0377
Ofoego UC, Ekwujuru EU, Nwakanma A et al (2018) Protective and ameliorating effects of methanolic seed extract of Mucuna Pruriens on paraquat induced testicular damage. Adv Life Sc Technol 53:8–16
Ojha A, Srivastava N (2014) In vitro studies on organophosphate pesticides induced oxidative DNA damage in rat lymphocytes. Mutat Res Genet Toxicol Environ Mutagen 761:10–17. https://doi.org/10.1016/j.mrgentox.2014.01.007
Okorondu M, Okorondu S, Alisi C et al (2019) Ameliorative effect of psidium guajava leaf extract on paraquat induced renal and reproductive hormone toxicity. Sci Res J 7:2201–2796. https://doi.org/10.31364/SCIRJ/v7.i4.2019.P0419633
O’Shaughnessy PJ (2014) Hormonal control of germ cell development and spermatogenesis. Semin Cell Dev Biol 29:55–65. https://doi.org/10.1016/j.semcdb.2014.02.010
Patel DK, Patel K (2020) P0174 Nephroprotective activity of gossypetin through inhibitory effect on xanthine oxidase, nuclear factor kappa B (NF-KB) and soluble epoxide hydrolase (SEH): in-vitro and in-silico experiment. Nephrol Dial Transplant 35:142–174. https://doi.org/10.1093/ndt/gfaa142.P0174
Patel DK, Patel K (2021a) P-MD005. Neuroprotective effects of gossypetin in Alzheimer’s disease: therapeutic approaches to evaluate the acetylcholinesterase and butyl cholinesterase inhibitory potential. Clin Neurophysiol 132:97–98. https://doi.org/10.1016/j.clinph.2021.02.225
Patel K, Patel DK (2021) Therapeutic benefit and pharmacological activities of gossypetin: biological importance in the medicine through scientific research data analysis. Metab Clin Exp 116:154550. https://doi.org/10.1016/j.metabol.2020
Pawar JS, Mustafa S, Ghosh I (2017) Gossypetin inhibits ROS generation in HeLa and HepG2 cell lines. Res Rev J Toxicol 7:25–31
Placer ZA, Cushman L, Johnson BC (1966) Estimation of products of lipid peroxidation (malonyldialdehyde) in biochemical systems. Anal Biochem 16:359–364. https://doi.org/10.1016/0003-2697(66)90167-9
Qian JY, Deng P, Yu ZP et al (2019) 8-Formylophiopogonanone B antagonizes paraquat-induced hepatotoxicity by suppressing oxidative stress. Front Pharmacol 10:1283. https://doi.org/10.3389/fphar.2019.01283
Ramaswamy S, Weinbauer GF (2015) Endocrine control of spermatogenesis: Role of FSH and LH/ testosterone. Spermatogenesis 4: 996025. https://doi.org/10.1080/21565562.2014.996025
Rasooli R, Kamali Y, Mandegary A (2020) Effects of pirfenidone, vitamin E, and pirfenidone–vitamin E combination in paraquat-induced pulmonary fibrosis. Comp Clin Path 29:667–673
Salvamani S, Gunasekaran B, Shaharuddin NA et al (2014) Antiartherosclerotic effects of plant flavonoids. Biomed Res Int: 2014: 480258. https://doi.org/10.1155/2014/480258
Sandström J, Broyer A, Zoia D et al (2017) Potential mechanisms of development-dependent adverse effects of the herbicide paraquat in 3D rat brain cell cultures. Neurotoxicology 60:116–124. https://doi.org/10.1016/j.neuro.2017.04.010
Tremblay JJ (2015) Molecular regulation of steroidogenesis in endocrine Leydig cells. Steroids 103:3–10. https://doi.org/10.1016/j.steroids.2015.08.001
Venkatadri R, Muni T, Iyer AKV et al (2016) Role of apoptosis-related miRNAs in resveratrol-induced breast cancer cell death. Cell Death Dis 7:2104. https://doi.org/10.1038/cddis.2016.6
Xie X, Liu K, Liu F et al (2019) Gossypetin is a novel MKK3 and MKK6 inhibitor that suppresses esophageal cancer growth in vitro and in vivo. Cancer Lett 442:126–136. https://doi.org/10.1016/j.canlet.2018.10.016
Ye L, Su Z, Ge R (2011) Inhibitors of testosterone biosynthetic and metabolic activation enzymes. Molecules 16:9983–10001. https://doi.org/10.3390/molecules16129983
Yokoi K, Uthus EO, Nielsen FH (2003) Nickel deficiency diminishes sperm quantity and movement in rats. Biol Trace Elem Res 93:141–154. https://doi.org/10.1385/BTER:93:1-3:141
Zhao M, Gu L, Li Y et al (2019) Chitooligosaccharides display anti-tumor effects against human cervical cancer cells via the apoptotic and autophagic pathways. Carbohydr Polym 224:115171. https://doi.org/10.1016/j.carbpol.2019.115171
Zirkin BR, Papadopoulos V (2018) Leydig cells: formation, function, and regulation. Biol Reprod 99:101–111. https://doi.org/10.1093/biolre/ioy059
Funding
The authors did not receive any financial support for this research.
Author information
Authors and Affiliations
Contributions
SM and MUI conceived the idea and designed the study. MUI, SM, HA1, and SI performed the experiments. HA2 and SI helped in statistical analysis. MUI, SM, and QA wrote the manuscript. All the authors read and approved the final version of manuscript.
Corresponding author
Ethics declarations
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Mohamed M. Abdel-Daim
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mustafa, S., Anwar, H., Ain, Q.u. et al. Therapeutic effect of gossypetin against paraquat-induced testicular damage in male rats: a histological and biochemical study. Environ Sci Pollut Res 30, 62237–62248 (2023). https://doi.org/10.1007/s11356-023-26469-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-26469-5