Abstract
Despite its wide production and several applications, veterinary antiparasitics from macrocyclic lactones and benzimidazole classes have not received much scientific attention concerning their environmental risks. Thus, we aimed to provide insights into the state of the environmental research on macrocyclic lactone and benzimidazole parasiticides, emphasizing their toxicity to non-target aquatic organisms. We searched for relevant information on these pharmaceutical classes on PubMed and Web of Science. Our search yielded a total of 45 research articles. Most articles corresponded to toxicity testing (n = 29), followed by environmental fate (n = 14) and other issues (n = 2) of selected parasiticides. Macrocyclic lactones were the most studied chemical group (65% of studies). Studies were conducted mainly with invertebrate taxa (70%), with crustaceans being the most predominant group (n = 27; 51%). Daphnia magna was the most used species (n = 8; 15%). Besides, it also proved to be the most sensitive organism, yielding the lowest toxicity measure (EC50 0.25 μg/L for decreased mobility after 48 h-abamectin exposure) reported. Moreover, most studies were performed in laboratory settings, tracking a limited number of endpoints (acute mortality, immobility, and community disturbance). We posit that macrocyclic lactones and benzimidazoles warrant coordinated action to understand their environmental risks.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As concerns over medicines in the environment grew, Boxall et al. (2003) discussed the role of veterinary pharmaceuticals, their potentially novel routes of contamination, and the significant gaps to be filled to identify and address their environmental risks. Veterinary pharmaceuticals reach aquatic environments through routes that depend on the production system in which they are used, as well as the host being treated and the route of administration. Examples of higher-risk activities are net pan enclosures at fish farming facilities and treatment of grass-fed cattle near water bodies, which are made greater when applied to large-scale herds (Kim et al. 2008a, b; Di Nica et al. 2015).
Within the broader category of veterinary pharmaceuticals, antiparasitics (also referred to as parasiticides) are drugs used to control parasites such as helminths and ticks. They present features that may characterize their environmental relevance. These chemicals reach the environment through novel routes described for veterinary pharmaceuticals at large, but in large volumes and causing detrimental effects comparable to antibiotics (Kools et al. 2008; Di Nica et al. 2015). The agricultural landscape is particularly important for the environmental risks posed by the large-scale use of veterinary antiparasitics (Kools et al. 2008; Di Nica et al. 2015). Plagues such as ticks, fleas, flies, and helminths are a continuous threat of financial loss in livestock production systems, so they must be closely controlled, leading to prophylactic and constant use of parasiticides (Kornele et al. 2014; French 2018). In the absence of specific limiting regulations, these drugs may be extensively and indiscriminately used, exerting high levels of xenobiotic-related stress on aquatic organisms in nearby ecosystems (Vieira et al. 2019).
Among the most relevant antiparasitic agents in agriculture, the classes of macrocyclic lactones (MLs) and benzimidazoles (BZs) stand out. MLs, including milbemycins and avermectins, are parasiticides and insecticides that are produced through fermentation by soil-dwelling microorganisms and have been used as insecticides and acaricides for crop protection or parasiticides for animal health. Ivermectin is the first ML that was released for use in both humans and animals and has shown both excellent efficacy and high tolerability in the treatment of parasite infestations. Other MLs, such as abamectin, emamectin, and moxidectin, were subsequently commercialized (Yang 2012). In turn, BZs are broad-spectrum nematocides, with some drugs also having activity against flukes and tapeworms. Fenbendazole is a well-known BZ that is effective against the protozoan parasite Giardia. Many BZs kill larval stages of nematodes as well as adults, but usually, larval efficacy is lower than for adults (Kaplan 2009).
Considering the large number of animals used in major cattle-producing countries, veterinary antiparasitics warrant attention. In 2018, flea and tick medications accounted for 29.4% of the US animal health market (Pham and Donovan 2018), while antiparasitics have been the most important therapeutic class in the Brazilian animal health market since 2015, having surpassed antibiotics in 2014 (SINDAN 2019). This is in line with the arguments used by Wardhaugh (2005) to draw attention to parasiticides used in livestock production, warning of the need for usage information and the potential risks to non-target organisms in dung and pasture. Since then, other such calls for research on the matter have appeared in the literature (Loeb 2018; Powell et al. 2018).
Parasiticides are often not fully metabolized and are excreted via urine and feces by treated animals. For example, ivermectin is only partially metabolized by the liver and then excreted mostly through the feces between 40 and nearly 80% (Halley et al. 1989; Chiu et al. 1990; Lifschitz et al. 2000). Many antiparasitics are also available in formulations for external use (e.g., pastes, sprays, aspersion, and baths) to treat ectoparasites such as fleas, ticks, and flies. In these cases, metabolization is irrelevant, as the compounds may reach nearby ecosystems via wash-off. For aquatic environments, common pollution routes include direct deposition by animals reared in the pasture (Boxall et al. 2003), lixiviation to nearby bodies of water, and incorrect waste disposal (Kim et al. 2008a, b). Drugs used in aquacultures such as emamectin benzoate and teflubenzuron are particularly concerning in the area around facilities, possibly reaching (Bloodworth et al. 2019).
Once they reach aquatic ecosystems through such routes, these drugs exert a several ecotoxicological effects. In an extensive review that aimed to assess the non-target effects of MLs in aquatic and terrestrial environments, Lumaret et al. (2012) discussed a tendency for these compounds to be more toxic to aquatic invertebrates, especially during early life stages. In addition, Carlsson et al. (2013) sought to assess the adverse effects of 15 veterinary pharmacists (10 antiparasitic and 5 antibiotics) on zebrafish embryos and observed several endpoints such as mortality, malformations, and other sublethal responses, suggesting that this high toxicity may extend to early developmental stages of fish. This may be a product of the evolutionary relationships bootstrapping the parasite, the host, and the non-target species (Brady et al. 2017), as many of these chemicals disrupt conserved structures present in target parasites (mainly arthropods and helminths), as well as in non-target organisms (Anadón et al. 2009; Akre 2016; Zhang et al. 2020).
Historically, greater attention has been paid to the ecotoxicological risk of antibiotics (i.e., chemicals active against bacteria) rather than antiparasitic agents. Both have similarities in terms of mechanisms of combating target organisms, such as how they can affect cell integrity. However, antibiotics and antiparasitics differ with regard to the mechanisms of action. For antibiotics, the mechanisms can be classified according to the target site and the structural alterations promoted (Lorian 1999). Among the main ones are: inhibition of cell wall synthesis or nucleic acid synthesis, inhibition of ribosomal or cell membrane function, and inhibition of folate metabolism (Dowling et al. 2017). Antiparasitics such as the BZ class act by preventing the dimerization of α-tubulin, consequently preventing the polymerization of microtubules, which generates loss of function in various parts of the cell (Alves and Barbosa 2018). In turn, MLs act on the central nervous system, causing hyperpolarization of neurons and inhibition of the passage of nervous stimuli, resulting in flaccid paralysis (Spinosa et al. 2008; Rosa et al. 2016).
Despite the large volume of use of MLs and BZs and their potential ecotoxicological risks, studies on these antiparasitic drugs are still scarce, especially those considering their effects on aquatic organisms. Accordingly, the current study aimed to provide a preliminary picture of the role of MLs and BZs as environmental toxicants in aquatic environments. We use a systematic review as a stepping stone to examine these chemical groups as separate chemical entities regarding their risks to aquatic wildlife. Data concerning the bibliometric parameters (i.e., number of articles, geographical distribution, number of citations, and impact factor), exposure conditions, species, effects on non-target organisms, toxicity parameters, type of samples taken in environmental fate studies, and detection methods were summarized and discussed. Also, articles were arranged into three categories according to their objectives and information provided, namely: (i) toxicity testing, (ii) environmental fate, and (iii) others. Furthermore, several research gaps and recommendations for future research are also presented.
Materials and methods
Using PubMed (https://pubmed.ncbi.nlm.nih.gov/) and Web of Science (https://www.webofscience.com), we gathered the scientific articles retrieved between August 2021 and November 2022 by the following 24 keyword combinations (see Table S1). The combinations were as follows: (i) “veterinary antiparasitic” and “aquatic environment”; (ii) “veterinary parasiticide” and “aquatic environment”; (iii) “veterinary antiparasitic,” “aquatic,” and “non-target”; (iv) the name of each antiparasitic of the classes under study with “aquatic,” “non-target,” and/or “aquatic environment.” The articles retrieved were curated by the following inclusion and exclusion criteria:
-
i)
Inclusion criteria: studies on compounds used to treat parasites in animals, toxicity to aquatic organisms, risk assessment contributions, studies on environmental samples (e.g., biological tissue, sediment, and water), and peer-reviewed literature.
-
ii)
Exclusion criteria: studies on other classes of antiparasitics (e.g., organophosphates), studies on other pharmaceutical groups (e.g., antibiotics), efficacy studies, clinical cases, analysis of products for human consumption, review papers, technical reports, protocols, studies in languages other than English, and non aquatic organisms.
The included papers were then scanned for relevant information, which included their objective, chemicals and organisms studied, exposure conditions, effects observed, toxicity metrics (LC50, EC50, and NOEC), type of samples taken in environmental fate studies, and detection method. The geographical location of study was identified from the mailing address of the corresponding author. To quantitatively record the data retrieved from toxicity studies, we used an entry system in which one entry equals the study of one chemical in a single organism. In this manner, one paper may correspond to one or more entries, depending on how many chemicals and how many organisms it investigated.
Results and discussion
Overview
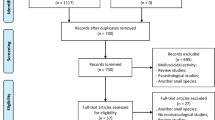
The combinations of keywords in Pubmed yielded a total of 150 unique results, of which only 40 were left once the inclusion/exclusion criteria were applied. While on the Web of Science, 26 articles were found with the combinations, leaving only 9 after the inclusion/exclusion criteria. After that, it was possible to observe 4 articles that were in the two databases, with 45 articles remaining in total. These articles were arranged into three categories according to their objectives and information provided, namely: toxicity testing (Table 1), environmental fate (Table 2), and others (Table 3). This third category was necessary to accommodate relevant information that did not fit into toxicity or environmental fate studies. It comprised one paper about the uptake and depuration kinetics of selected veterinary pharmaceuticals in blue mussels (Brooks et al. 2019) and one related to environmental risk assessment (Liebig et al. 2010).
These results provide a robust framework for discussion since they encompass a highly diverse set of methods, test organisms, and chemical compounds. Prominently, the high number of test organisms (representing a few major biological groups) and the diversity of chemical classes allowed for discussions of taxa-specific toxicity.
Bibliometrics
The first study involving at least one of the classes of parasiticides targeted by this review was published in 2005 by Löffler and collaborators, covering the environmental fate of several pharmaceuticals, including ivermectin. Since then, few works were published in the following years until 2010 (Fig. 1A). From this year onwards, there has been an increase in the number of published works. The beginning of studies with antiparasitics coincides with the increasingly constant reports on the presence of emerging contaminants in water reservoirs in the USA (Halden 2010). This provided a strong stimulus for research on the effects of pesticides, pharmaceuticals, and other micropollutants on aquatic organisms.
Studies were carried out by research groups from 19 countries, mainly by Germany (n = 7; 16%) and China (n = 6; 13%), followed by Brazil, Poland, and Belgium (n = 4; 9% each) (Fig. 1B). Similarly, Saiki et al. (2021) showed that Germany stands out in studies concerning the sediment toxicity assessment using zebrafish (Danio rerio). Besides, it is to be expected that in countries like Brazil, where agriculture is a relevant economic activity, there is a greater number of research groups interested in investigating the impacts of products derived from these activities on the environment.
Articles in this area have received great attention. This is revealed in a large number of citations (1.082), highlighting the period between 2008 and 2017 (770) (Fig. 1C). In addition, these works have gained space in the most prestigious journals in Environmental Chemistry, Toxicology and Risk Assessment, with most works (n = 34; 76%) having been published in vehicles with an impact factor ≥ 5.0 (Fig. 1D and E).
Macrocyclic lactones and benzimidazoles in numbers
A total of 29 articles on toxicity to non-target organisms were included in this study (Table 1). To provide a more concise analysis, we registered one entry per compound studied in each paper, then we categorized them by chemical group (Figs. 2A and 3). MLs accounted for 68% (61 entries) of the individual entries in toxicity papers, while for BZs were 32% (28 entries) (Fig. 2A and Table 1). As for specific compounds, the most investigated of the MLs was emamectin (15% of entries), while fenbendazole (57% of entries) was the most studied BZ (Table 1).
Frequency (%) of macrocyclic lactones and benzimidazoles tested for toxicity to non-target organisms (a); of biological groups used in toxicity assays with veterinary antiparasitics (b); of invertebrates within those biological groups (c). In (a) each entry represents a study of one compound within the chemical class on a research article. In (b) and (c) each entry represents a study of the effects of an individual chemical on a single test organism within a research article
In the category of environmental fate studies, we had a total count of 14 articles and 35 entries, with MLs appearing in 60% of entries and BZs in 40% (Table 2). Some studies investigated more than one compound: one with fenbendazole and flubendazole (BZs), one with emamectin benzoate (ML), one using three MLs, and another using eight BZs. The studies used a range of analytical chemistry methods to study the properties or presence of the compounds in fish, soil, sediment, water, and/or muscle. The samples were obtained in their respective countries, namely: Argentina, Belgium, Brazil, China, France, Germany, Greece, Morocco, Norway, Poland, and Scotland.
The interest of researchers in the study of MLs and BZs falls in line with the wide use of these major classes of pharmaceuticals. The ML ivermectin is a major parasiticide worldwide, used to treat various diseases such as worm infections in animals and river blindness in humans (Molento 2020). The BZs are also prominent drugs used worldwide to treat parasitic and fungi-related illnesses in humans and animals (Brauer et al. 2019; Porto et al. 2020).
Effects on non-target organisms
While the number of individual studies on toxicity to non-target organisms was limited (29 papers), only 12 of them were single-species investigations. In addition, 2 articles included toxicity data on 9 organisms or more, which resulted in a relatively large number of entries for non-target organisms tested (75 entries in total) (Fig. 2B). Due to the diversity of test organisms in the included studies, we divided them into approximate biological groups rather than taxonomically solid categories (Table S2). This diverse set of test organisms also yielded a variety of outcomes that ranged from the individual level to the population level (from liver gene expression to community abundance). The level of identification of organisms also varied, with some studies distinguishing between individual species in each genus while others provided only order and phylum level information about the studied organisms (e.g., Nematoda, Cladocera, and Tardigrada). Where provided, we also comprehensively include metrics such as EC50 values to compare toxicity between biological groups (Tables S3 and S4).
Invertebrates were the most studied group of organisms, accounting for 70.6% of the total number of entries (Fig. 2B). This category included all animals outside the phylum Chordata. Among them, crustaceans were the most predominant (n = 27; 51%), with daphnids appearing most frequently (n = 9; 17%), and Daphnia magna occupying the position of most studied individual species (n = 8; 15%). D. magna also proved to be a sensitive test organism, being the most sensitive organism to five of the compounds as well as yielding some of the lowest mean effect concentrations (EC50) in the studies included (Tables S3 and S4).
Overall, the trend of higher toxicity to invertebrates reflects the fact that antiparasitics are intended to target this group. Many active ingredients in antiparasitics, such as pyrethroids and organophosphates, are commonly found in insecticide and pesticide formulations (Akre 2016). Other active ingredients in veterinary antiparasitics include pyrethrins and carbamates (Anadón et al. 2009; Akre 2016), which illustrates how these pharmaceuticals intersect with pesticides in terms of vulnerable non-target organisms. Most importantly, parasiticides, agricultural pesticides, and insecticides are designed to maximize toxicity to specific invertebrate taxa (mostly arthropods and nematodes) and in many cases optimized for conserved structures to allow a broader spectrum of activity (Powell et al. 2018). This “toxicity by design” could be why Kools et al. (2008) and Di Nica et al. (2015) found that antibiotics and parasiticides typically ranked high in their risk-based assessments of veterinary pharmaceuticals.
Accordingly, the retrieved EC50 data indicate a trend of higher toxicity toward invertebrates, especially microcrustaceans such as D. magna, which was the most sensitive species to four out of the seven compounds (ivermectin, fenbendazole, abamectin, and doramectin) tested in more than one species. In fact, the ubiquity of D. magna as a test organism, combined with its low EC50, frequently placed it as the most sensitive species in multi-species studies (Tables 1 and 3). This trend is in line with previous calls for research on veterinary antiparasitics that were primarily concerned with declines in populations of insects and small riverine invertebrates (Powell et al. 2018).
We favored EC50 to compare toxicities among non-target organisms because substantially more articles disclosed EC50s than LC50s or NOECs. We also consider that EC50 values represent a more sensitive metric compared to LC50, as many chemicals still produce detrimental effects that manifest at the population level without causing mortality. Additionally, only specific effects are required by many standardized protocols, such as the OECD Test No. 211, which tests the reproductive output of D. magna in response to chemical exposure (OECD 2018). Despite not being lethal, this is an effect that leads to a reduction in population size.
Prominent examples of this phenomenon of trickling up to detrimental populational outcomes are the disruption of developmental and neuronal processes. Benzimidazoles, for instance, are beta-tubulin inhibitors that interfere with cell division during early development of zebrafish, causing skeletal deformities and adversely impacting movement (Zhang et al. 2020), which ultimately reduces fitness. This type of sub-lethal drop-in fitness can also be expected for chemicals with neurotoxic effects, as previously reported for MLs (Spinosa et al. 2008; Rosa et al. 2016). Avermectins (ivermectin, abamectin, doramectin) and milbemycins (moxidectin and nemamectin) are two of the main groups of macrocyclic lactones. The main difference between these groups is the presence of the bisoleandrosyloxy substituent at C-13 of avermectins, which is not found in milbemycins. Furthermore, avermectins have a glycosylated lactone backbone, while milbemycins do not. Despite chemical differences, the molecular structures of both groups are superimposable and exert neurotoxic effects on targets, mainly interfering with neurotransmission via GABA (Lumaret et al. 2012).
Other classes of veterinary antiparasitics, such as pyrethroids, pyrethrins, organophosphates, and carbamates, also exert neurotoxic effects from the inhibition of acetylcholinesterase (AChE), which has been documented in several aquatic organisms, including fish, crustaceans, and clams (Toumi et al. 2016; Arora et al. 2017; Singh et al. 2018; Li et al. 2018). At the population level, neurotoxicity at large may lead to behavioral changes that increase predation or disrupt social behaviors (Sandoval-Herrera et al. 2019; Armstrong et al. 2019; Bedrossiantz et al. 2020; Faria et al. 2020). An aggravating factor for these classes is that they commonly appear as pour-on treatments, which provide a direct track for the compounds to reach nearby ecosystems unchanged (Loeb 2018).
Endpoints and testing conditions
Mode of action (MoA) and toxicity to the target species are not the only variables affecting the ultimate toxicity endpoints observed in a given species. Other factors such as time and length of exposure, chemical properties, experimental conditions, toxicokinetics and toxicodynamics, formation of derivates in the environment or by biotransformation, and specific biological peculiarities can cause disagreements in the effects between related species and chemicals. In a study with five benzimidazole-based antihelminthics, Oh et al. (2006) found various degrees of toxicity toward D. magna. Given that the compounds are structurally similar, the authors argue that the differences can be explained by the octanol–water partition coefficient (Kow) of the chemicals, which is a lipophilicity parameter.
The span and endpoints investigated in individual toxicity investigations also varied greatly, but mostly focused on standardized acute toxicity assays, which is another aspect of the diverse test organisms. For example, the OECD Protocol No. 201 requires a 72-h exposure to assess growth inhibition in alga and cyanobacteria over several generations, while the guidelines for D. magna acute toxicity (OECD 2004) require a 48-h exposure. The most reported endpoints for D. magna were “immobility and toxicity” (Tisler et al. 2006; Bundschuh et al. 2016). Another common toxicity test was with Vibrio fischeri, focusing on the “luminescence inhibition” endpoint (Tisler and Kozuh Erzen 2006; Wagil et al. 2015a, b). Longer-living organisms such as zebrafish require a 96-h exposure to assess acute toxicity toward embryos and 21 days to assess certain endocrine disturbances in sexually mature individuals (OECD 2009, 2013). Endpoints such as “toxicity” and “swimming activity” were widely reported in tests with zebrafish (Tisler and Kozuh Erzen 2006; Santos et al. 2023).
However, for the results to translate into ERA-relevant information, these hypotheses need to be investigated at several levels of biological organization, with tiered methodological approaches. The predominance of strictly laboratory-based tests in accordance with standardized guidelines (e.g., OECD ISO), compared to a smaller number of field and microcosm studies, is consistent with the single ERA paper in our pool (Liebig et al. 2010), which sook to establish a case-study, multi-tiered ERA for ivermectin. As noted by the authors, even though strictly laboratory-based assays provide useful data on non-target toxicity and chemical properties, more information and standardized protocols at higher-tier levels are imperative. Additionally, Di Nica et al. (2015) have documented the lack of chronic toxicity data as another potential source of hinderance.
Environmental fate
Although studies on the environmental fate of emerging contaminants have become increasingly common in recent years, our research data showed that this type of study with MLs and BZs is still proportionately less performed than toxicity tests. Toxicity assessments and the environmental fate of contaminants must go hand in hand in order to provide the construction of an environmental risk assessment that delineates potential health risks and supports decision-making processes.
Among the MLs, ivermectin was the most detected substance. Ivermectin showed a rapid loss in the system, moving rapidly from the water compartment into the sediment. This rapid and extensive sorption of ivermectin onto the sediment is mainly attributable to its lipophilicity (Löffler et al. 2005; Rath et al. 2016; Wang et al. 2019). This same pattern was reported for doramectin, eprinomectin, moxidectin, and abamectin by Chen et al. (2021). Eprinomectin and emamectin benzoate remain in the sediments for a considerable period of time (Litskas et al. 2013; Langford et al. 2014), while ivermectin and abamectin are rapidly degraded (Dionisio and Rath 2016; Rath et al. 2016).
Flubendazole and fenbendazole were the most common BZs. Both were frequently detected in water samples (De Steene et al. 2010; Van De Steene and Lambert 2011; Wagil et al. 2015a, b; Goessens et al. 2020; Chen et al. 2021), highlighting the high solubility of flubendazole in water and its ability to bind to sediment (Goessens et al. 2020). In addition to these two BZs, Chen et al. (2021) detected in water samples from the Tuojiang River (Sichuan, China) significant amounts of five other antiparasitic agents of the same class. If we put together the high solubility of BZs in water and the constant supply of these chemicals in aquatic environments, it is possible to delimit the potential risk of these chemicals to non-target organisms, especially adverse effects resulting from chronic exposure.
Conclusions
We provide an informative analysis of the toxicity of macrocyclic lactones and benzimidazoles to aquatic wildlife. The results also supported the speculated trend of toxicity toward invertebrates based on EC50 and NOEC values. Likewise, the high frequency of invertebrate entries indicates a preference by the authors to use them as test organisms, demonstrating the importance of this biological group for the toxicity testing of antiparasitics. Additionally, these classes of parasiticides have been frequently found in environmental samples, highlighting their high solubility in water and, for some specific compounds, high stability to degradation.
Therefore, our results provided a basis for a discussion covering the toxicity of antiparasitics of major importance to a large variety of test organisms. Given the importance of antiparasitic drugs in animal production systems worldwide, we posit that they warrant coordinated efforts to expand the literature about their environmental impacts. For this purpose, a range of methodological approaches may be necessary to inform prioritization and mitigation efforts. The data collected suggests that major priority should be given to quantifying compounds in environmental samples that can inform the significance of EC50 values. Higher-tier studies, chronic exposures, multispecies exposures, micro- and mesocosms, and transgenerational exposures may also provide realistic exposure scenarios that integrate variables related to both fate and toxicity. Additionally, toxicity assessments that include mechanistic and biochemical information (e.g., biomarker assays, bioaccumulation, biomagnification, and trophic transfer) may be valuable in refining the current information about the odds, routes, and impact of these chemicals.
Data availability
Not applicable.
References
Akre C (2016) The use of pyrethroids, carbamates, organophosphates, and other pesticides in veterinary medicine. Chemical Analysis of Non-antimicrobial Veterinary Drug Residues in Food. John Wiley Sons, Ltd, pp 383–426. https://doi.org/10.1002/9781118696781.ch7
Alak G, Yeltekin AÇ, Tas I, et al. (2017) Investigation of 8-OHdG, CYP1A, HSP70 and transcriptional analyses of antioxidant defence system in liver tissues of rainbow trout exposed to eprinomectin. Fish Camp; Shellfish Immunol 65:136–144. https://doi.org/10.1016/j.fsi.2017.04.004
Alves MS, Barbosa TN (2018) Resistência parasitária. In: Bezerra A, Silva MD, Silva C (eds) Fitoterapia e a Ovinocaprinocultura: uma associação promissora. EdUFERSA, Mossoró, pp 49–76. https://doi.org/10.7476/9786587108643.0005
Anadón A, Martínez-Larrañaga MR, Martínez MA (2009) Use and abuse of pyrethrins and synthetic pyrethroids in veterinary medicine. Vet J 182:7–20. https://doi.org/10.1016/j.tvjl.2008.04.008
Armstrong T, Khursigara AJ, Killen SS et al (2019) Oil exposure alters social group cohesion in fish. Sci Rep 9:13520. https://doi.org/10.1038/s41598-019-49994-1
Arora S, Balotra S, Pandey G, Kumar A (2017) Binary combinations of organophosphorus and synthetic pyrethroids are more potent acetylcholinesterase inhibitors than organophosphorus and carbamate mixtures: an in vitro assessment. Toxicol Lett 268:8–16. https://doi.org/10.1016/j.toxlet.2016.12.009
Babić S, Pavlović DM, Biošić M, Ašperger D, Škorić I, Runje M (2018) Fate of febantel in the aquatic environment—the role of abiotic elimination processes. Environ Sci Pollut Res 25(29):28917–28927. https://doi.org/10.1007/s11356-018-2935-9
Bedrossiantz J, Martínez-Jerónimo F, Bellot M et al (2020) A high-throughput assay for screening environmental pollutants and drugs impairing predator avoidance in Daphnia magna. Sci Total Environ 740:140045. https://doi.org/10.1016/j.scitotenv.2020.140045
Bloodworth JW, Baptie MC, Preedy KF, Best J (2019) Negative effects of the sea lice therapeutant emamectin benzoate at low concentrations on benthic communities around Scottish fish farms. Sci Total Environ 669:91–102. https://doi.org/10.1016/j.scitotenv.2019.02.430
Boonstra H, Reichman EP, den Brink PJ (2011) Effects of the veterinary pharmaceutical ivermectin in indoor aquatic microcosms. Arch Environ Contam Toxicol 60(1):77–89. https://doi.org/10.1007/s00244-010-9526-1
Boxall ABA, Kolpin DW, Halling-Sørensen B, Tolls J (2003) Are veterinary medicines causing environmental risks? Environ Sci Technol 37:286A-294A. https://doi.org/10.1021/es032519b
Brady SP, Monosson E, Matson CW, Bickham JW (2017) Evolutionary toxicology: toward a unified understanding of life’s response to toxic chemicals. Evol Appl 10:745–751. https://doi.org/10.1111/eva.12519
Brauer VS, Rezende CP, Pessoni AM et al (2019) Antifungal agents in agriculture: friends and foes of public health. Biomolecules 9:521. https://doi.org/10.3390/biom9100521
Brinke M, Höss S, Fink G, Ternes T et al (2010) Assessing effects of the pharmaceutical ivermectin on meiobenthic communities using freshwater microcosms. Aquat Toxicol (amsterdam, Netherlands) 99(2):126–137. https://doi.org/10.1016/j.aquatox.2010.04.008
Brooks SJ, Ruus A, Rundberget JT et al (2019) Bioaccumulation of selected veterinary medicinal products (VMPs) in the blue mussel (Mytilus edulis). Sci Total Environ 655:1409–1419. https://doi.org/10.1016/j.scitotenv.2018.11.212
Bundschuh M, Hahn T, Ehrlich B et al (2016) Acute toxicity and environmental risks of five veterinary pharmaceuticals for aquatic macroinvertebrates. Bull Environ Contam Toxicol 96:139–143. https://doi.org/10.1007/s00128-015-1656-8
Carlsson G, Patring J, Kreuger J et al (2013) Toxicity of 15 veterinary pharmaceuticals in zebrafish (Danio rerio) embryos. Aquat Toxicol 126:30–41. https://doi.org/10.1016/j.aquatox.2012.10.008
Carlsson G, Blomberg M, Pohl J, Örn S (2018) Swimming activity in zebrafish larvae exposed to veterinary antiparasitic pharmaceuticals. Environ Toxicol Pharmacol 63:74–77. https://doi.org/10.1016/j.etap.2018.08.015
Charuaud L, Jardé E, Jaffrézic A, Liotaud M, Goyat Q, Mercier F, Le Bot B (2019) Veterinary pharmaceutical residues in water resources and tap water in an intensive husbandry area in France. Sci Total Environ 664:605–615. https://doi.org/10.1016/j.scitotenv.2019.01.303
Chen S, Gan Z, Li Z, Li Y, Ma X, Chen M, Qu B, Ding S, Su S (2021) Occurrence and risk assessment of anthelmintics in Tuojiang River in Sichuan China. Ecotoxicol Environ Saf 220:112360. https://doi.org/10.1016/j.ecoenv.2021.112360
Cheng B, Van Smeden J, Deneer J, Belgers D, Foekema E, Roessink I, Matser A, Van den Brink PJ (2020) The chronic toxicity of emamectin benzoate to three marine benthic species using microcosms. Ecotoxicol Environ Saf 194:110452. https://doi.org/10.1016/j.ecoenv.2020.110452
Chiu SHL, Green ML, Baylis FP et al (1990) Absorption, tissue distribution, and excretion of tritium-labeled ivermectin in cattle, sheep, and rat. J Agric Food Chem 38:2072–2078. https://doi.org/10.1021/jf00101a015
Di Nica V, Menaballi L, Azimonti G, Finizio A (2015) RANKVET: a new ranking method for comparing and prioritizing the environmental risk of veterinary pharmaceuticals. Ecol Ind 52:270–276. https://doi.org/10.1016/j.ecolind.2014.12.021
Dionisio AC, Rath S (2016) Abamectin in soils: analytical methods, kinetics, sorption and dissipation. Chemosphere 151:17–29. https://doi.org/10.1016/j.chemosphere.2016.02.058
Dowling A, Odwyer J, Adley C (2017) Antibiotics: mode of action and mechanisms of resistance. Antimicrobial Res: Novel Bioknowl Educ Prog 1:536–545
Falkenberg LJ, Wrange A-L, Kinnby A, Havenhand JN, Lockyer A, Styan CA (2017) Low sensitivity of reproductive life-stages in the Pacific oyster ( Crassostrea gigas ) to abamectin. Chemosphere 182:665–671. https://doi.org/10.1016/j.chemosphere.2017.05.085
Faria M, Wu X, Luja-Mondragón M et al (2020) Screening anti-predator behaviour in fish larvae exposed to environmental pollutants. Sci Total Environ 714:136759. https://doi.org/10.1016/j.scitotenv.2020.136759
French KE (2018) Plant-based solutions to global livestock anthelmintic resistance. Ethnobiol Lett 9:110–123. https://doi.org/10.2307/26607680
Garric J, Vollat B, Duis K, Péry A, Junker T, Ramil M, Ternes TA (2007) Effects of the parasiticide ivermectin on the cladoceran Daphnia magna and the green alga Pseudokirchneriella subcapitata. Chemosphere 69:903–910. https://doi.org/10.1016/j.chemosphere.2007.05.070
Goessens T, Baere SD, Troyer ND, Deknock A, Goethals P, Lens L, Pasmans F, Croubels S (2020) Highly sensitive multi-residue analysis of veterinary drugs including coccidiostats and anthelmintics in pond water using UHPLC-MS/MS: application to freshwater ponds in Flanders, Belgium. Environ Sci: Process Impacts 22(10):2117–2131. https://doi.org/10.1039/D0EM00215A
Halden R (2010) Introduction to contaminants of emerging concern in the environment: ecological and human health considerations. In Contaminants of emerging concern in the environment: ecological and human health considerations (pp. 1–6). ACS Symposium Series; Vol. 1048. American Chemical Society. https://doi.org/10.1021/bk-2010-1048.ch001
Halley BA, Jacob TA, Lu AYH (1989) The environmental impact of the use of ivermectin: environmental effects and fate. Chemosphere 18:1543–1563. https://doi.org/10.1016/0045-6535(89)90045-3
Heinrich AP, Zöltzer T, Böhm L et al (2021) Sorption of selected antiparasitics in soils and sediments. Environ Sci Eur 33(1):77. https://doi.org/10.1186/s12302-021-00513-y
Huang Y, Hong Y, Huang Z, He H (2020) Cytotoxicity induced by abamectin exposure in haemocytes of Chinese mitten crab, Eriocheir sinensis. Environ Toxicol Pharmacol 77:103384. https://doi.org/10.1016/j.etap.2020.103384
Kaplan RM (2009) Anthelmintic treatment in the era of resistance. In: Anderson D, Rings M (eds) Food animal practice 5th ed. pp 470–478. https://doi.org/10.1016/B978-141603591-6.10097-1
Kim Y, Jung J, Kim M et al (2008a) Prioritizing veterinary pharmaceuticals for aquatic environment in Korea. Environ Toxicol Pharmacol 26:167–176. https://doi.org/10.1016/j.etap.2008.03.006
Kim Y, Jung J, Kim M, Park J, Boxall ABA, Choi K (2008b) Prioritizing veterinary pharmaceuticals for aquatic environment in Korea. Environ Toxicol Pharmacol 26(2):167–176. https://doi.org/10.1016/j.etap.2008.03.006
Kołodziejska M, Maszkowska J, Białk-Bielińska A et al (2013) Aquatic toxicity of four veterinary drugs commonly applied in fish farming and animal husbandry. Chemosphere 92:1253–1259. https://doi.org/10.1016/j.chemosphere.2013.04.057
Kools SAE, Boxall A, Moltmann JF et al (2008) A ranking of European veterinary medicines based on environmental risks. Integr Environ Assess Manag 4:399–408. https://doi.org/10.1897/IEAM_2008-002.1
Kornele ML, McLean MJ, O’Brien AE, Phillippi-Taylor AM (2014) Antiparasitic resistance and grazing livestock in the United States. J Am Vet Med Assoc 244:1020–1022. https://doi.org/10.2460/javma.244.9.1020
Langford KH, Øxnevad S, Schøyen M, Thomas KV (2014) Do antiparasitic medicines used in aquaculture pose a risk to the Norwegian aquatic environment? Environ Sci Technol 48(14):7774–7780. https://doi.org/10.1021/es5005329
Li D, Wang P, Wang C et al (2018) Combined toxicity of organophosphate flame retardants and cadmium to Corbicula fluminea in aquatic sediments. Environ Pollut 243:645–653. https://doi.org/10.1016/j.envpol.2018.08.076
Liebig M, Fernandez AA, Blübaum-Gronau E et al (2010) Environmental risk assessment of ivermectin: a case study. Integr Environ Assess Manag 6(Suppl):567–587. https://doi.org/10.1002/ieam.96
Lifschitz A, Virkel G, Sallovitz J et al (2000) Comparative distribution of ivermectin and doramectin to parasite location tissues in cattle. Vet Parasitol 87:327–338. https://doi.org/10.1016/s0304-4017(99)00175-2
Litskas VD, Karamanlis XN, Batzias GC, Tsiouris SE (2013) Are the parasiticidal avermectins resistant to dissipation in the environment? The case of eprinomectin. Environ Int 60:48–55. https://doi.org/10.1016/j.envint.2013.07.017
Loeb J (2018) Are spot-ons damaging the environment? Veterinary Record 183:490–490. https://doi.org/10.1136/vr.k4501
Löffler D, Römbke J, Meller M, Ternes TA (2005) Environmental fate of pharmaceuticals in water/sediment systems. Environ Sci Technol 39(14):5209–5218. https://doi.org/10.1021/es0484146
Lorian V (1999) Modes of Action of Antibiotics and Bacterial Structure. Handbook of Animal Models of Infection 105–115. https://doi.org/10.1016/b978-012775390-4/50151-2
Lozano IE, Piazza YG, Babay P, Sager E, de la Torre FR, Lo Nostro FL (2021) Ivermectin: a multilevel approach to evaluate effects in Prochilodus lineatus (Valenciennes, 1836) (Characiformes, Prochilodontidae), an inland fishery species. Sci Total Environ 800:149515. https://doi.org/10.1016/j.scitotenv.2021.149515
Lumaret J-P, Errouissi F, Floate K et al (2012) A review on the toxicity and non- target effects of macrocyclic lactones in terrestrial and aquatic environments. Curr Pharm Biotechnol 13:1004–1060. https://doi.org/10.2174/138920112800399257
Mayor DJ, Solan M, Martinez I, Murray L, McMillan H, Paton GI, Killham K (2008) Acute toxicity of some treatments commonly used by the salmonid aquaculture industry to Corophium volutator and Hediste diversicolor: whole sediment bioassay tests. Aquaculture 285(1–4):102–108. https://doi.org/10.1016/j.aquaculture.2008.08.008
Mesa LM, Hörler J, Lindt I et al (2018) Effects of the antiparasitic drug moxidectin in cattle dung on zooplankton and benthic invertebrates and its accumulation in a water-sediment system. Arch Environ Contam Toxicol 75(2):316–326. https://doi.org/10.1007/s00244-018-0539-5
Mesa L, Gutiérrez MF, Montalto L, Perez V, Lifschitz A (2020) Concentration and environmental fate of ivermectin in floodplain wetlands: na ecosystem approach. Sci Total Environ 706:135692. https://doi.org/10.1016/j.scitotenv.2019.135692
Molento MB (2020) COVID-19 and the rush for self-medication and self-dosing with ivermectin: a word of caution. One Health 10:100148. https://doi.org/10.1016/j.onehlt.2020.100148
Muniz MS et al (2021) Moxidectin toxicity to zebrafish embryos: bioaccumulation and biomarker responses. Environ Pollut 283:117096. https://doi.org/10.1016/j.envpol.2021.117096
Nunes B, Pinheiro D, Gomes A (2021) Effect of sublethal concentrations of the antiparasitic ivermectin on the polychaeta species Hediste diversicolor: biochemical and behavioral responses. Ecotoxicology 30(9):1841–1853. https://doi.org/10.1007/s10646-021-02444-z
OECD (2004) Test no. 202: Daphnia sp. acute immobilisation test. In: OECD Guidelines for the Testing of Chemicals, Section 2, OECD Publishing, Paris. https://doi.org/10.1787/9789264069947-en.
OECD (2009) Test no. 230: 21-day fish assay: a short-term screening for oestrogenic and androgenic activity, and aromatase inhibition. In: OECD Guidelines for the Testing of Chemicals, Section 2, OECD Publishing, Paris. https://doi.org/10.1787/9789264076228-en.
OECD (2013) Test no. 236: fish embryo acute toxicity (FET) test. In: OECD Guidelines for the Testing of Chemicals, Section 2, OECD Publishing, Paris. https://doi.org/10.1787/9789264203709-en.
OECD (2018) Daphnia magna reproduction test (OECD TG 211). In: Revised Guidance Document 150 on Standardised Test Guidelines for Evaluating Chemicals for Endocrine Disruption, OECD Publishing, Paris. https://doi.org/10.1787/9789264304741-12-en.
Oh SJ, Park J, Lee MJ et al (2006) Ecological hazard assessment of major veterinary benzimidazoles: acute and chronic toxicities to aquatic microbes and invertebrates. Environ Toxicol Chem 25:2221–2226. https://doi.org/10.1897/05-493R.1
Park K, Bang HW, Park J, Kwak IS (2009) Ecotoxicological multilevel-evaluation of the effects of fenbendazole exposure to Chironomus riparius larvae. Chemosphere 77(3):359–367. https://doi.org/10.1016/j.chemosphere.2009.07.019
Pham N, Donovan M (2018) The economic and social contributions of the animal health industry. Ndp Analytics Report, Washington, DC, p. 3
Porto RS, Pinheiro RSB, Rath S (2020) Leaching of benzimidazole antiparasitics in soil columns and in soil columns amended with sheep excreta. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-020-08389-w
Powell K, Foster C, Evans S (2018) Environmental dangers of veterinary antiparasitic agents. Veterinary Record 183:599–600. https://doi.org/10.1136/vr.k4690
Puckowski A, Stolte S, Wagil M, Markiewicz M, Łukaszewicz P, Stepnowski P, Białk-Bielińska A (2017) Mixture toxicity of flubendazole and fenbendazole to Daphnia magna. Int J Hyg Environ Health 220(3):575–582. https://doi.org/10.1016/j.ijheh.2017.01.011
Rain-Franco A, Rojas C, Fernandez C (2018) Potential effect of pesticides currently used in salmon farming on photo and chemoautotrophic carbon uptake in Central – Southern Chile. Aquaculture 486:271–284. https://doi.org/10.1016/j.aquaculture.2017.12.048
Rath S, Pereira LA, Bosco SM, Maniero MG, Fostier AH, Guimarães JR (2016) Fate of ivermectin in the terrestrial and aquatic environment: mobility, degradation, and toxicity towards Daphnia similis. Environ Sci Pollut Res Int 23(6):5654–5666. https://doi.org/10.1007/s11356-015-5787-6
Reinwald H, Alvincz J, Salinas G, Schäfers C, Hollert H, Eilebrecht S (2022) Toxicogenomic profiling after sublethal exposure to nerve- and muscle-targeting insecticides reveals cardiac and neuronal developmental effects in zebrafish embryos. Chemosphere 291:132746. https://doi.org/10.1016/j.chemosphere.2021.132746
Rosa PB, Neis VB, Ribeiro CM, Moretti M, Rodrigues ALS (2016) Antidepressant-like effects of ascorbic acid and ketamine involve modulation of GABAA and GABAB receptors. Pharmacol Rep 68(5):996–1001. https://doi.org/10.1016/j.pharep.2016.05.010
Sandoval-Herrera N, Mena F, Espinoza M, Romero A (2019) Neurotoxicity of organophosphate pesticides could reduce the ability of fish to escape predation under low doses of exposure. Sci Rep 9:10530. https://doi.org/10.1038/s41598-019-46804-6
Santos KPED, Ferreira Silva I, Mano-Sousa BJ, Duarte-Almeida JM, Castro WV, Azambuja Ribeiro RIM, Santos HB, Thomé RG (2023) Abamectin promotes behavior changes and liver injury in zebrafish. Chemosphere 311(Pt 1):136941. https://doi.org/10.1016/j.chemosphere.2022.136941
SINDAN (2019) Anuário da indústria de produtos para saúde animal. http://www.sindan.org.br/anuario2018/
Singh S, Tiwari RK, Pandey RS (2018) Evaluation of acute toxicity of triazophos and deltamethrin and their inhibitory effect on AChE activity in Channa punctatus. Toxicol Rep 5:85–89. https://doi.org/10.1016/j.toxrep.2017.12.006
Song Y, Rundberget JT, Evenseth LM, Xie L, Gomes T, Høgåsen T, Iguchi T, Tollefsen KE (2016) Whole-organism transcriptomic analysis provides mechanistic insight into the acute toxicity of emamectin benzoate in Daphnia magna. Environ Sci Technol 50(21):11994–12003. https://doi.org/10.1021/acs.est.6b03456
Spinosa HS, Xavier FG, Maruo VM (2008) Toxicology of drugs. In: Spinosa HS, Górniak SL, Neto JP (ed) Toxicology applied to veterinary medicine. Barueri: Manole, chap. 6:117–189
Tisler T, Kozuh Erzen N (2006) Abamectin in the aquatic environment. Ecotoxicology (london, England) 15(6):495–502. https://doi.org/10.1007/s10646-006-0085-1
Toumi H, Bejaoui M, Touaylia S et al (2016) Effect of carbaryl (carbamate insecticide) on acetylcholinesterase activity of two strains of Daphnia magna (Crustacea, Cladocera). J Environ Sci Health B 51:777–780. https://doi.org/10.1080/03601234.2016.1198645
Udebuani AC, Pereao O, Akharame MO, Fatoki OS, Opeolu BO (2021) Acute toxicity of piggery effluent and veterinary pharmaceutical cocktail on freshwater organisms. Environ Monit Assess 193(5):293. https://doi.org/10.1007/s10661-021-09085-z
Van De Steene JC, Lambert WE (2011) Determination of four basic pharmaceuticals and one pesticide in surface water with UPLC-ESI-MS/MS. Int J Environ Anal Chem 91(12):1218–1226. https://doi.org/10.1080/03067311003778664
Van De Steene JC, Stove CP, Lambert WE (2010) A field study on 8 pharmaceuticals and 1 pesticide in Belgium: removal rates in waste water treatment plants and occurrence in surface water. Sci Total Environ 408(16):3448–3453. https://doi.org/10.1016/j.scitotenv.2010.04.037
Veldhoen N, Ikonomou MG, Buday C, Jordan J, Rehaume V, Cabecinha M, Dubetz C, Chamberlain J, Pittroff S, Vallée K, van Aggelen G, Helbing CC (2012) Biological effects of the anti-parasitic chemotherapeutant emamectin benzoate on a non-target crustacean, the spot prawn (Pandalus platyceros Brandt, 1851) under laboratory conditions. Aquat Toxicol 108:94–105. https://doi.org/10.1016/j.aquatox.2011.10.015
Vieira CED, Costa PG, Caldas SS et al (2019) An integrated approach in subtropical agro-ecosystems: active biomonitoring, environmental contaminants, bioaccumulation, and multiple biomarkers in fish. Sci Total Environ 666:508–524. https://doi.org/10.1016/j.scitotenv.2019.02.209
Wagil M, Białk-Bielińska A, Puckowski A et al (2015a) Toxicity of anthelmintic drugs (fenbendazole and flubendazole) to aquatic organisms. Environ Sci Pollut Res 22:2566–2573. https://doi.org/10.1007/s11356-014-3497-0
Wagil M, Maszkowska J, Białk-Bielińska A, Stepnowski P, Kumirska J (2015b) A comprehensive approach to the determination of two benzimidazoles in environmental samples. Chemosphere 119(Suppl):S35–S41. https://doi.org/10.1016/j.chemosphere.2014.04.106
Wang D, Han B, Li S, Cao Y, Du X, Lu T (2019) Environmental fate of the anti-parasitic ivermectin in an aquatic micro-ecological system after a single oral administration. PeerJ 7:e7805. https://doi.org/10.7717/peerj.7805
Wardhaugh KG (2005) Insecticidal activity of synthetic pyrethroids, organophosphates, insect growth regulators, and other livestock parasiticides: an Australian perspective. Environ Toxicol Chem 24:789–796. https://doi.org/10.1897/03-588.1
Weichert FG, Floeter C, Meza Artmann AS, Kammann U (2017) Assessing the ecotoxicity of potentially neurotoxic substances – evaluation of a behavioural parameter in the embryogenesis of Danio rerio. Chemosphere 186:43–50. https://doi.org/10.1016/j.chemosphere.2017.07.136
Yang C-C (2012) Acute human toxicity of macrocyclic lactones. Curr Pharm Biotechnol 13(6):999–1003. https://doi.org/10.2174/138920112800399059
Zhang X, Zhang P, Perez-Rodriguez V et al (2020) Assessing the toxicity of the benzamide fungicide zoxamide in zebrafish (Danio rerio): towards an adverse outcome pathway for beta-tubulin inhibitors. Environ Toxicol Pharmacol 78:103405. https://doi.org/10.1016/j.etap.2020.103405
Zhang T, Dong Z, Liu F, Pan E, He N, Ma F, Wang G, Wang Y, Dong J (2022) Avermectin induces carp neurotoxicity by mediating blood-brain barrier dysfunction, oxidative stress, inflammation, and apoptosis through PI3K/Akt and NF-κB pathways. Ecotoxicol Environ Saf 243:113961. https://doi.org/10.1016/j.ecoenv.2022.113961
Zhang T, Dong Z, Liu F, Pan E, He N, Ma F, Wu X, Wang Y, Dong J (2022) Non-target toxic effects of avermectin on carp spleen involve oxidative stress, inflammation, and apoptosis. Pesticide Biochem Physiol 187:105190. https://doi.org/10.1016/j.pestbp.2022.105190
Acknowledgements
We thank the Universidade Federal da Paraíba (UFPB, Brazil), Fundação de Apoio à Pesquisa do Estado da Paraíba (FAPESQ, Brazil), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil) for supporting this research with grants and scholarships.
Funding
This research was funded by Public Call n. 03 Produtividade em Pesquisa PROPESQ/PRPG/UFPB, grant number PVA13245-2020, Public Call Demanda Universal FAPESQ, grant number 3045/2021, and CNPq Productivity Scholarship for T.LR., grant number 306329/2020–4.
Author information
Authors and Affiliations
Contributions
M.S.M, M.E.S.M, I.C.A.A., R.X.M., T.L.R., and D.F. designed and conducted the literature review and wrote the manuscript. All authors approved the submitted version.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All listed authors have approved the manuscript before submission, including the names and order of authors.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Ester Heath
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Most articles corresponded to toxicity testing.
• The macrocyclic lactone emamectin was the most studied parasiticide.
• Invertebrates were the most used group of organisms.
• Daphnia magna was the most used organism, in addition to being considered the most sensitive.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Muniz, M.S., Maia, M.E.S., Araruna, I.C.A. et al. A review on the ecotoxicity of macrocyclic lactones and benzimidazoles on aquatic organisms. Environ Sci Pollut Res 30, 54257–54279 (2023). https://doi.org/10.1007/s11356-023-26354-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-26354-1