Abstract
Two anthelmintic macrocyclic lactones—ivermectin and moxidectin—have revolutionized parasite control in cattle. These drugs are only partly metabolized by livestock, and the main route of excretion is via feces. In seasonally inundated floodplains, cattle feces come into direct contact with surface water. Important differences in pharmacokinetics and pharmacodynamics between these drugs may bear on their ecotoxicology in aquatic ecosystems. Moxidectin strongly binds to organic matter and thereby may be consumed in aquatic food webs, but there is a scarcity of data on toxicity to freshwater invertebrates. The objectives of this work were to determine the effect of moxidectin spiked in cattle dung on survival and growth of three representative aquatic invertebrates: the zooplankton Ceriodaphnia dubia, the amphipod Hyalella curvispina, and the snail Pomacea canaliculata. Moxidectin-laced dung was added in microcosms and concentrations were measured in water, sediment + dung, roots of the aquatic plant Salvinia biloba, and the aforementioned invertebrates. The influence of moxidectin on nutrient concentrations was also evaluated. Dung was spiked with moxidectin to attain concentrations of 750, 375 and 250 µg kg−1 dung fresh weight, approximating those found in cattle dung at days 2, 3, and 5 following subcutaneous injection. Concentrations of moxidectin in dung during the first week of excretion were lethally toxic for the tested invertebrate taxa. The persistence of moxidectin in the sediment + dung and the uptake of the drug in roots of S. biloba increase its potential exposure to aquatic food webs. Moxidectin also reduced the rate of release of soluble reactive phosphorus to the water.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Veterinary pharmaceuticals are used in large amounts to treat cattle around the world (Tolls 2001). The anthelmintic macrocyclic lactones (MLs) are endectocides that provide the basis of modern parasite control. Since their development in the early 1980s, the success of the MLs has relied on their remarkable broad-spectrum activity, safety profile, and ease of administration (McKellar and Benchaoui 1996; Kita et al. 2007). Two widely used MLs are ivermectin (IVM) and moxidectin (MOX). These drugs have a common macrocyclic lactone ring that binds selectively and with high affinity on the ligand-gate chloride ion channel receptors of invertebrate nerve and muscle cells, causing irreversible opening of these channels (Rohrer and Arena 1995; Omura 2002).
Despite the similar modes of action of IVM and MOX, distinct physicochemical properties may account for differences in formulation flexibility, kinetic behaviour, pharmacodynamics, and toxicity to target and nontarget invertebrates (Prichard et al. 2012). Both MOX and IVM have high adsorption coefficients (Koc > 1000), indicating that they are not likely to be found in high concentrations in the water column (Lumaret et al. 2012), and are likely to become tightly bound to organic matter in soil and sediments, immobile in the environment (Cunningham et al. 2010).
In cattle, major differences exist between IVM and MOX with a larger volume of distribution and a faster (plasma) clearance for MOX (Lanusse et al. 1997; Bousquet-Mélou et al. 2004), presumably due to a more rapid partition into adipose tissue. The higher lipophilicity of MOX compared with IVM (log10 of the octanol/water partitioning coefficient for MOX = 6; IVM = 4.8: HyperChem 7.0, HyperCube Inc., Vellarkad et al. 1989) determines its higher proclivity to accumulate in the body fat of cattle, resulting in a longer elimination half-life (Lifschitz et al. 1999; Prichard et al. 2012).
Residues of MOX and IVM in feces following cattle injection show insecticidal activity, with effects on survival, reproduction, and development of nontarget terrestrial arthropods (Steel and Wardhaugh 2002). From many studies comparing the effects of both drugs on dung arthropods, MOX clearly appears ecologically safer than IVM (Herd 1995; Butters et al. 2012). In cattle, a dose rate of 0.2 mg kg−1 IVM is toxic to dung beetles feeding on the feces, whereas MOX had no adverse effects on adult dung beetles at the same dose (Fincher and Wang 1992). Fecal residues of both drugs presented a similar pattern of chemical degradation after environmental exposure of the dung, revealing that the differences in nontarget toxicity were not due to distinct drug degradation kinetics (Suarez 2002). The mechanism underlying these differences remains to be clarified. It may be linked to a lower affinity of MOX for the insect ligand-gated ion channel receptors or to IVM and MOX not targeting the same subset of receptors (Strong and Wall 1994).
Studies of the ecotoxicology of MOX and IVM in aquatic ecosystems have led to concerns that make them a high priority for further environmental study and monitoring (Lumaret et al. 2012). Due to the drugs’ tendencies to absorb strongly to soil and sediment organic matter, erosion of particulate matter containing these drugs and direct excretion by treated livestock into water bodies or into land that is subsequently flooded represents the most important routes of entry into the freshwater environment (Kövecses and Marcogliese 2005). Although aquatic invertebrates are more likely to be exposed to MOX and IVM by consumption of particulate matter rather than directly from water, only a few studies have addressed exposure of aquatic benthic invertebrates to IVM via sediment (Thain et al. 1997; Davies et al. 1998; Allen et al. 2007; Egeler et al. 2010) and only two of IVM via feces (Schweitzer et al. 2010; Mesa et al. 2017). Mesa et al. (2017) studied the toxicity of IVM spiked in fresh dung on invertebrates representative of the Paraná River floodplain system. Concentrations between 458 and 1150 µg kg−1 were lethal for Ceriodaphnia dubia and Hyalella sp., and high concentrations of this drug were detected in the apple snail Pomacea sp., the aquatic plant Salvinia sp. and in the sediment + dung. An apparent suppression of bacterial nitrification by IVM also was observed. In contrast to this work on IVM, no studies have assessed the toxicity of MOX spiked in dung to aquatic invertebrates and the uptake of this drug in biotic compartments such as plants and invertebrates.
Land use on the Middle Paraná River floodplain in Argentina has changed significantly in recent decades, as the expansion of upland soybean production has forced the relocation of cattle to marginal floodplain sites, where the stocking density has recently increased greatly (PROSAP 2009; Quintana et al. 2014). Injection of cattle herds with MOX and IVM has been a practical and accessible tool for parasite control in this region (Mesa et al. 2017). The availability of these drugs without a veterinarian prescription and the presence of several generic formulations in the pharmaceutical market have led to a high frequency of treatment of herds without requiring a parasitological diagnosis. In the Paraná River floodplain, cattle enter wetlands for grazing and drinking immediately following injection, depositing feces that may represent a threat to these aquatic ecosystems (Mesa et al. 2017).
The present study examined the uptake and effects of MOX in dung on three aquatic invertebrates and a macrophyte typical of the floodplain system. The purposes of this work were (1) to determine the effect of MOX spiked in dung on survival and growth (indicated by length) of the planktonic cladoceran Ceriodaphnia dubia, the pleustonic amphipod Hyalella curvispina, and the benthic snail Pomacea canaliculata; (2) to analyze the concentration of MOX in water, sediment + dung, roots of Salvinia biloba, and the aforementioned invertebrates; and (3) to evaluate the influence of MOX nutrient release to water. We hypothesized that MOX is less toxic to aquatic invertebrates than IVM and that uptake of this drug in aquatic organisms would be greater than we previously observed for IVM.
Materials and Methods
Test Organisms
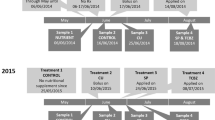
A four-species, water–sediment microcosm experiment was performed to simulate exposure of freshwater invertebrates to direct deposition of fresh cattle dung spiked with MOX into water bodies. The design of this microcosm was similar to that performed in a previous study with IVM (Mesa et al. 2017) for comparative purposes. Each microcosm consisted of a glass vessel containing water, sediment, a small floating aquatic fern (Salvinia biloba), and three invertebrates—the zooplanktonic microcrustacean Ceriodaphnia dubia (Crustacea: Branchiopoda), the benthic amphipod Hyalella curvispina (Crustacea: Amphipoda) and the apple snail Pomacea canaliculata (Mollusca: Gastropoda) (Fig. 1). These invertebrates were selected as representative of planktonic and benthic taxa of floodplain water bodies along the Middle Paraná River and of South American floodplains in general. S. biloba was included as a widely distributed macrophyte in wetlands of this floodplain system. All of these taxa were taken from our own stock cultures. Water temperatures averaged 25 ± 1 °C in these cultures. C. dubia were fed with Chlorella sp. ad libitum, H. curvispina with Tetramin® fish food, and snails with romaine lettuce every day before the initiation of the experiments.
Spiking Dung with MOX
Fresh cattle dung used in the experiments was collected near wetlands of the Middle Paraná River system where cattle congregate to sleep. Dung was collected from cattle that had not been injected with MLs (MOX and IVM) for at least 7 months before to ensure the minimum concentration or inexistence of these drugs in their bodies. Dung was kept refrigerated at 6 °C for 24–48 h until the initiation of the experiment. Moxidectin pure standard (Sigma-Aldrich, 97%) was used to prepare the stock solutions. Stock solutions and dilutions in cattle dung were prepared with acetone as solvent (Fig. 1). Twenty grams of fresh cattle dung were spiked with different concentrations of MOX. The applied nominal MOX concentrations were 750 (T3), 375 (T2), and 250 (T1) µg kg−1 dung fresh weight, corresponding to those found in dung at days 2, 3, and 5, respectively, in studies conducted following subcutaneous injection (200 µg kg−1) (Lifschitz et al. 1999; Suarez et al. 2003). The percentage of the administered dose excreted in dung is 44% (Lifschitz et al. 1999, 2000).
The MOX solutions were sprayed on the surface of the 20 g of fresh dung to attain the different nominal concentrations. A sufficient volume of acetone was used to ensure homogeneous application of MOX solution on all of the surface of the fresh dung. Special care was taken to allow a complete absorption of the solution into the dung. An identical methodology was used in Mesa et al. (2017) and confirmed analytically by high recovery rates compared with the nominal concentrations. Samples were left for 90 min after adding MOX solutions to allow evaporation of the acetone.
Microcosm Set-up
Six test vessels (glass vessels, 13-cm diameter, 14-cm height, 1.45-L volume) were prepared for each treatment (T1, T2, and T3), six for the control (C0), and six for the solvent control (Cs) (total = 30 test vessels) (Fig. 1). The artificial sediment based on OECD guideline 218 (OECD 2004) consisted of quartz sand (75%, 291 grams) kaolinite clay (20%, 29 g), and peat powder (5%, 0.83 grams). This sediment had a water content of 32% (1.25 L) and a pH of 6.8, which had been adjusted using CaCO3. Unchlorinated water was included in each vessel.
Individuals of C. dubia were placed inside two small vessels (6-cm height, 2-cm diameter, 30-mL volume, covered with a 50-µm mesh) to enable the rapid visual inspection of the individuals (Fig. 1). The experiment was carried out under a light regime of 12 h light/12 h dark at constant environmental conditions (continuously gently aerated water, water temperature 25 ± 1 °C), without food addition. During the course of the experiment, evaporated water was replaced by unchlorinated tap water.
Thirty randomly selected individuals of each studied taxon were taken from cultures for length measurements before the initiation of the experiment (day 0). The exposure started when the 20 g of fresh cattle dung with the different nominal concentrations of MOX was placed into each vessel. To simulate direct dung deposition by cattle into water bodies, solid fresh dung was introduced into each vessel from the surface of the water, falling through the water column, and remaining on the sediment until the end of the experiment. Some of the added dung broke apart and became mixed with the sediment.
One day after addition of dung, ten C. dubia (5 in each small vessel), ten H. curvispina, and ten P. canaliculata were introduced to the vessels (Fig. 1). The number of individuals used in this experiment was the same that in Mesa et al. (2017) for comparative purposes. Juvenile individuals of each taxon were selected according to approximate length criteria: C. dubia 0.7 ± 0.1 mm, H. curvispina 3 ± 1 mm, and P. canaliculata 10 ± 1 mm (shell length). Eighteen grams (wet weight) of S. biloba was included in each test vessel, covering 70% of its surface (Fig. 1).
Responses of Invertebrate Taxa
Survival and length of C. dubia were recorded at day 7. Survival of H. curvispina and P. canaliculata were observed at day 17, whereas length was recorded at days 7 and 17. These days were the same as in the experiments of Mesa et al. (2017) to facilitate comparison. Each surviving C. dubia individual was observed under a microscope, and length was determined from the head just above the compound eye to the base of the tail spine. After 7 days of exposure, individuals of H. curvispina were collected from three replicates of the controls and treatments by passing the sediment and water through a strainer. Length was measured from high-resolution photographs of each individual under a stereoscopic microscope, digitized using the TPSdig2 program (Rohlf 2006). On the same day, five individuals of P. canaliculata were removed from each replicate (n = 30 for each treatment and control) and shell length was measured according to Boulding and Hay (1993). Mortality was determined when snails failed to maintain the operculum closed. At the end of the experiment (day 17), all individuals of H. curvispina and P. canaliculata were sieved from water and sediment + dung using a 200-µm mesh. Surviving individuals of P. canaliculata were placed in dechlorinated tap water for one day to flush undigested sediment out of the mantle cavity and gut, which would otherwise create an inaccurate assessment of the body burden (King and Davies 1987; Van Roon 2000). The snails were anesthetized using 1 g L−1 of benzocaine–methanol solution (Garr et al. 2012), and later the visceral mass was separated above the edge of the mantle for MOX analysis (Gomot-de Vaufleury and Pihan 2002).
Analysis of MOX Concentrations
Samples of each treatment were analyzed to determine MOX concentrations, and additional samples of fresh dung were prepared to corroborate the absence of MOX in control samples. Salvinia roots, sediment + dung, and water were taken from three test vessels of controls and treatments at the middle (day 7) and at the end of the experiment (day 17). Entire Salvinia plants were removed, washed, and their roots separated and homogenized for further analysis. Samples of sediment were collected with a spoon (4 g wet weight approximately). Particulates of dung with MOX that were incorporated within the sediment were included in these samples. Ten milliliters of water were obtained with a graduate pipette. For each control and treatment, surviving individuals of C. dubia and H. curvispina and visceral mass of P. canaliculata were separately pooled and preserved at − 20 °C for analysis of MOX concentrations.
The extraction from samples and analysis of MOX were carried out following Lifschitz et al. (1999). Concentrations were determined by high-pressure liquid chromatography (HPLC) with a Shimadzu 10 A fluorescence detector (Lifschitz et al. 1999; Mesa et al. 2017). The extraction of MOX from spiked and experimental samples was performed following the technique described by Lifschitz et al. (1999) and adapted by Mesa et al. (2017) for these matrixes. Samples were weighed, homogenized, and combined with the internal standard compound (abamectin). Briefly, 0.5 g of water, 0.5 g of sediment + dung, and 0.1 g of Pomacea and roots of Salvinia were combined with abamectin (Sigma-Aldrich) as the internal standard (10 ng). For Ceriodaphnia and Hyalella, the total amount of sample collected was used. One milliliter of acetonitrile was added, and the mixture was shaken (Multi Tube Vortexer, VWR Scientific Products, USA) for 20 min. After mixing the dung, visceral mass of Pomacea and roots of Salvinia samples were sonicated during 10 min (Transsonic 570/H, Lab Line Instruments Inc., USA). The solvent-sample mixture was centrifuged at 2000g for 15 min. The supernatant was manually transferred into a tube and the procedure repeated once for sediment + dung, snails, and root samples.
The pooled supernatants were then placed on an Aspec XL autosampler (Gilson, Villiers Le Bell, France) for the automatic solid-phase extraction process. The methanol elution was collected and concentrated to dryness under a stream of nitrogen. The derivatization of MOX was done with 100 μl of a solution of N-methylimidazole (Sigma Chemical, St. Louis, MO) in acetonitrile (1:1) and 150 μl of trifluoroacetic anhydride (Sigma Chemical) solution in acetonitrile (1:2). After completion of the reaction (< 30 s), an aliquot (100 μl) of this solution was injected directly into the HPLC system. MOX concentrations were determined by HPLC using a Shimadzu 10 A HPLC system with autosampler (Shimadzu Corporation, Kyoto, Japan). HPLC analysis was undertaken using a reverse phase C18 column (Kromasil, Eka Chemicals, Bohus, Sweden, 5 μm, 4.6 mm × 250 mm) and an acetic acid 0.2% in water/methanol/acetonitrile (1.6/60/38.4) mobile phase at a flow rate of 1.5 mL/min at 30 °C. MOX was detected with a fluorescence detector (Shimadzu, RF-10 Spectrofluorometric detector, Kyoto, Japan), reading at 365 nm (excitation) and 475 nm (emission wavelength). Calibration curves were established using least squares linear regression over a range between 0.5 and 150 ng g−1; correlation coefficients were > 0.99. The limit of quantification was established by HPLC analysis of blanks spiked with the internal standard and by measurement of the baseline noise at the time of retention of the MOX peak. The mean baseline noise plus six (10) standard deviations was defined as the theoretical quantification limit. The quantification limits of the analytical technique were 0.5 ng g−1 (dung + sediment, Pomacea and Salvinia) and 0.2 ng g−1 (water, Ceriodaphnia and Hyalella).
Physicochemical Variables
Conductivity, pH (corrected to 25 °C), dissolved oxygen (all measured with a Hanna meter), and water temperature (standard thermometer) were determined daily during the experiment in all test vessels at the same time of the day. Ten milliliters of subsurface water were obtained with a pipette from the controls and treatments for nutrient analyses. Water samples were taken before the beginning of the experiment (day 0) and at days 1, 4, 7, 11, 13, and 16. Water was immediately filtered through Whatman GF/F glass-fiber filters and kept frozen until determination of dissolved components. Total ammonia (NH4+ + NH3) was determined by the indophenol blue method (Koroleff 1970), whereas unionized ammonia (NH3) was estimated from pH and water temperature according to Emerson et al. (1975). Nitrate + nitrite (NO3− + NO2−) was measured by reduction of NO3− with hydrazine sulfate and subsequent colorimetric determination of NO2− (Hilton and Rigg 1983) and soluble reactive phosphorus (SRP) by the ascorbic acid method (Murphy and Riley 1962).
Data Analyses
Survival and length data were transformed with arcsine and log (x + 1), respectively, and then were analyzed by one-way ANOVA followed by Dunnett’s test or, in case of variance heterogeneity, Dunnett’s T3 test. If no significant differences between control and solvent control were detected using the Mann–Whitney U test, treatments were compared with the pooled controls. In case of significant differences, they were compared with the solvent control. Data normality was tested with the Kolmogorov–Smirnov goodness-of-fit test, and homogeneity of variance was assessed with Cochran’s test. To determine if there was a significant effect of MOX on nutrient release to the water, the relationship between the concentration of each nutrient in water at day of peak concentration (dependent variable) and the nominal concentration of MOX in each treatment (independent variable) was assessed by Linear Regression. Only the value of each nutrient at its peak observed concentration was considered, because it corresponded to the maximal net release (accumulated over time) from dung (Hou et al. 2013). Statistical analyses were conducted with PAST software (version 2.17, Hammer et al. 2001).
Comparison of IVM and MOX Uptake
Uptake of MOX at day 17 in sediment + dung, roots of Salvinia, and visceral mass of Pomacea, expressed as the percent ratio between measured concentrations in each item and the nominal target concentration of MOX in the dung added to each treatment, were compared with the concentration based on data reported for IVM in Mesa et al. (2017) at the same day. Identical microcosm design, methods, and analytical methodologies between the IVM and MOX experiments facilitated this comparison. Concentrations of IVM reported in Mesa et al. (2017) in sediment + dung, roots of Salvinia, and visceral mass of Pomacea were transformed to uptake values as described earlier. The nominal concentrations of MOX and IVM in fresh dung in this study and in the Mesa et al. (2107) study were based on observed values during first week of excretion after cattle injection (MOX days 2 and 5, 750 and 250 µg kg−1; IVM days 3 and 7, 1150–457 µg kg−1). All tests were performed at the 5% level of significance using PAST software (version 2.17, Hammer et al. 2001).
Results
Effect of MOX on Invertebrates
There were no significant differences in survival and growth (indicated by length) of invertebrates between control and solvent control treatments (Mann–Whitney U test, P > 0.05). Survival of H. curvispina and P. canaliculata was significantly reduced at MOX concentrations between 250 and 750 µg kg−1 compared with the pooled control value (Table 1). MOX was highly toxic to H. curvispina, producing complete mortality of individuals by day 7 of the experiment in all treatments (250-750 µg kg−1 MOX). By day 17, survival of P. canaliculata was 63 and 80% at concentrations of MOX in dung of 375 and 250 µg kg−1, respectively, whereas 80% mortality was observed at a concentration of 750 µg kg−1. No significant effects on growth of the studied taxa were detected at the different nominal concentrations of MOX (ANOVA and Dunnett’s test, P > 0.05; Table 1).
Concentration of MOX in Water, Sediment + Dung, and Invertebrates
No MOX was detected in the additional control samples of fresh dung. For all treatment levels, measured concentrations in freshly prepared dung samples spiked with MOX were in good agreement with nominal concentrations. Recovery (measured concentrations of MOX as % of the nominal concentrations) ranged from 77 to 100%. Table 2 shows the concentrations of MOX measured in water, sediment, roots of S. biloba, and invertebrates at days 7 and 17 of the experiment. No MOX was detected in any of the water samples. Among invertebrates, MOX was only detected in P. canaliculata at all studied concentrations (250–750 µg kg−1). Concentrations of MOX in sediment + dung, roots of S. biloba, and P. canaliculata did not show an increase with time, being similar at days 7 and 17 (ANOVA and Dunnett’s test, P > 0.05; Table 2).
Effects of MOX on Nutrient Dynamics
Water temperature, pH, conductivity, and dissolved oxygen remained relatively constant throughout the study across all treatments, with values of 24 ± 1 °C, 7.5 ± 0.5, 540 ± 10 µS cm−1, and 7.8 ± 0.5 mg L−1 respectively. At the beginning of the experiment, values of pH were lower in treatments 2 and 3 (T2, T3) than in treatment 1 (T1) and controls (Cs and C0) (Fig. 2a). Concentrations of NH4+ + NH3 showed similar trends in the controls and the MOX treatments and were significantly higher at day 1 compared with the later days of the experiment (ANOVA and Dunnett’s test, P < 0.001; Fig. 2b). Concentrations of unionized ammonia in controls (Cs and C0) and T1 peaked at day 1, whereas in T2 and T3, the highest concentrations occurred at days 7 and 11 of the experiment, respectively (ANOVA and Dunnett’s post hoc comparison, P < 0.001; Fig. 2c). Concentrations of NO3− +NO2− were significantly higher on the first day of the experiment in the solvent control and all treatments (ANOVA, P < 0.01; Fig. 2d). Concentrations of SRP progressively increased from the beginning of the experiment, reaching the highest values at days 11 and 13 (ANOVA, P < 0.05; Fig. 2e).
Mean values (n = 3) of pH (a) and concentrations (mg N L−1) of ammonium + ammonia (b), unionized ammonia (c), nitrate + nitrite (d), and soluble reactive phosphorus (e) in the control (C0), solvent control (Cs), and treatments (T1, T2, T3: see Fig. 1) during the experiment. Vertical bars are standard errors based on three replicates. Peak concentrations are marked with arrows
Peak concentrations of SRP showed a significant negative relationship with nominal concentrations of MOX in dung (r2 = 0.55, P < 0.05), whereas the inorganic nitrogen forms (NH4+ + NH3, NH3, NO3− + NO2−) did not show a significant relationship with nominal concentrations (r2 < 0.15, P > 0.05).
Comparison of MOX and IVM Uptake
Uptake of MOX and IVM in sediment + dung was not proportional to the nominal concentrations of these drugs in dung, with considerably higher ratios at the higher dung concentrations (Table 3). In contrast, uptake of MOX and IVM for S. biloba and P. canaliculata were lower than expected at the higher nominal concentration of these drugs in dung.
Discussion
The results of this work showed that MOX in dung is accumulated by and toxic to aquatic invertebrates and that this drug accumulates and persists in sediments containing dung as well as in a representative aquatic macrophytes (Tables 1, 3). Concentrations of MOX commonly found in dung during the first week of excretion of cattle following injection reduced the survival of C. dubia, H. curvispina, and P. canaliculata and significantly altered nutrient release from dung to the water column (Fig. 2).
Based on many studies comparing the effects of both drugs on terrestrial arthropods (Diptera and Coleoptera), it was concluded that MOX is ecologically safer than IVM (Herd 1995; Fincher and Wang 1992; Hempel et al. 2006). In this study, MOX and IVM in dung showed different effects on survival of aquatic invertebrates. In accordance with the hypothesis of this work, toxicity of MOX for C. dubia was lower than that we previously observed with IVM in a similar microcosm experiment (Mesa et al. 2017); survival of this taxon was greater than 50% when it was exposed to dung with MOX at concentrations between 250 and 750 µg kg−1, whereas IVM caused complete mortality of C. dubia at similar concentrations (458–1150 µg kg−1). In addition, both MOX and IVM in dung were lethal for H. curvispina; a complete mortality of this taxon was observed at concentrations of both drugs found in cattle dung during the first week of excretion. Hyalella could be exposed to these drugs either from sediment or water or through their feeding habits (Saigo et al. 2009; Mesa et al. 2017). In contrast with our hypothesis, toxic effects of MOX on P. canaliculata were greater than previously observed for IVM, producing mortality > 70% at concentrations in dung of 750 µg kg−1. All snails survived upon exposure to IVM in dung in a similar experiment (Mesa et al. 2017).
MOX in dung did not show a significant effect on growth of the aquatic taxa. This result agrees with Mesa et al. (2017), where IVM did not show an effect on growth of the same taxa at concentrations between 22 and 1150 µg kg−1. In contrast, Schweitzer et al. (2010) found that 1314 µg kg−1 of IVM in dung had a significant effect on larval growth of chironomids. Differences in toxicological effects of MOX and IVM on invertebrates may be explained by the distinct pharmacodynamic and pharmacokinetic characteristics of these drugs. Although the mechanisms are not well known, the specificity of MOX and IVM for different glutamate-gated chloride channels, different affinities of these drugs for the invertebrate ligand-gated ion channel receptors, and distinct targeting to the same subset of receptors could explain their different effects on the survival and growth of aquatic invertebrates.
In contrast with our hypothesis, uptake of MOX and IVM was observed only for P. canaliculata, and uptake values were lower than expected at higher nominal concentrations of this drug in dung (Table 3). Detoxification mechanisms in snails (Mackay and Fraser 2000; Ruamthum et al. 2010; Lingpeng et al. 2011) may limit the uptake and increase elimination of these drugs. The observation that S. biloba took up MOX approximately in proportion to nominal values in dung (Table 3) indicates that it is suitable for monitoring of MOX pollution in freshwater ecosystems.
The lack of detectable MOX in water and its high concentration in sediment + dung are because this drug is highly hydrophobic (Prichard et al. 2012). As discussed for IVM by Mesa et al. (2017), MOX and IVM are highly lipophilic, binding tightly to soil and sediments, and therefore are classified as immobile (Park et al. 2013). Concentrations of MOX and IVM in sediment + dung were 8 and 5 times higher at the highest nominal concentrations compared with the lowest concentration at day 17 of the experiment (Table 3 and Mesa et al. 2017). Uptake and persistence of these drugs in sediment also have been observed in other studies of aquatic ecosystems (Boxall et al. 2004; Sanderson et al. 2007), including experimental additions of IVM to sediment (Davies et al. 1998; Egeler et al. 2010) and dung (Schweitzer et al. 2010; Mesa et al. 2017). Contamination of aquatic ecosystems with these drugs presents a potential risk for the aquatic biota due to strong binding to sediment and uptake by macrophytes and the persistence in these compartments over time.
Regarding nutrient cycling, MOX showed a negative effect on SRP release, suggesting that this drug could favor the retention of this nutrient in sediment + dung. Exchange of phosphorus between the sediment and the water column is generally considered as an abiotic process driven by changes in pH and redox potential (Maine et al. 1992), but microorganisms have a significant role through mineralization of organic P compounds (Gächter and Meyer 1993; Hupfer and Lewandowski 2008). Neither antibacterial nor antifungal properties of MOX have been previously observed (Lumaret et al. 2012), but this topic has been poorly studied (Koops and Pommerening-Röser 2001).
On the other hand, the kaolinite clay present in the artificial sediment could have contributed to the adsorption of the phosphate released from dung (Charfi et al. 2013). The sorption of phosphate on kaolinite is maximum around pH 4.0 and decreases with increasing pH (Chen et al. 1973). MOX may be considered a weak acid due to its pKa, which has been calculated as 12.8 ± 1.0 (Awasthi 2012). Therefore, lower values of pH may have occurred in the interstitial water of dung + sediment with higher MOX content, even though the pH of water in the vessels did not change. In addition, an initial slight decrease in pH was observed in water of treatments with higher MOX content, which also would decrease the percentage of unionized NH3 (Emerson 1975). The effect of MOX on phosphate release from dung as well as the previous observation of a significant impact of IVM on nitrogen transformations (Mesa et al. 2017) point to the need for more study of how these drugs may affect nutrient cycling in aquatic ecosystems.
The significant increase in cattle densities in the Middle Paraná River floodplain in recent decades and the accompanying massive usage of antiparasitic drugs pose a potential threat to aquatic ecosystems that demands further study. This topic should be investigated under more realistic conditions using higher tier test systems, such as larger microcosm or preferably outdoor mesocosm systems, in which longer-term effects and population recovery are monitored (Campbell et al. 1983; Van den Brink et al. 2006; OECD 2006). Further investigations in the field are required to address possible effects on aquatic invertebrates that may be caused by direct excretion of dung containing MOX and IVM into surface water. In the floodplain systems of Middle Paraná River, cattle are typically injected with IVM at a dose of 200 µg kg−1 and after 15 days they receive a second injection with the same concentration. During summer-spring, herds of approximately 150 cows enter floodplain wetlands in groups of 20 and remain approximately 2 h for drinking, grazing, and thermoregulation. During this time, cattle defecate, each animal eliminating between 2 and 8 kg of dung with IVM directly into water.
This study provides a better understanding of the effects of MOX on invertebrates typical of floodplains and many other freshwater ecosystems. To our knowledge, this is the first study to show persistence of MOX in freshwater sediments and uptake of this drug in macrophytes and invertebrates. The potential transfer of these compounds into higher trophic levels (including fish, birds and mammals) and their toxic effects (including possible risk for humans) merits urgent study. Risk mitigation measures may be necessary to avoid the introduction of anthelmintic compounds into aquatic environments. It may be appropriate, for example, to recommend that producers keep treated cattle away from waterbodies for at least a week following treatment to reduce the potential for contamination of the aquatic ecosystem.
References
Allen YT, Thain JE, Haworth S, Barry J (2007) Development and application of long-term sublethal whole sediment tests with Arenicola marina and Corophium volutator using ivermectin as the test compound. Environ Poll 146:92–99
Awasthi A (2012) Stability evaluation of selected anthelmintic macrocyclic lactone compounds and formulations. Doctoral dissertation. University of Auckland
Boulding EG, Hay TK (1993) Quantitative genetics of shell form of an intertidal snail: constraints on short-term response to selection. Evolution 47:576–592
Bousquet-Mélou A, Mercadier S, Alvinerie M, Toutain PL (2004) Endectocide exchanges between grazing cattle after pour-on administration of doramectin, ivermectin and moxidectin. Int J Parasitol 34:1299–1307
Boxall ABA, Fogg LA, Kay P, Blackwell PA, Pemberton EJ, Croxford A (2004) Veterinary medicines in the environment. Rev Environ Contam Toxicol 180:1–91
Butters MP, Kobylinski KC, Deus KM, Da Silva IM, Gray M, Sylla M, Foy BD (2012) Comparative evaluation of systemic drugs for their effects against Anopheles gambiae. Acta Trop 121:34–43
Campbell WC, Fisher MH, Stapley EO, Albers-Schonberg G, Jacob TA (1983) Ivermectin: a potent new antiparasitic agent. Science 221:823–828
Charfi A, Sahnoun RD, Bouaziz J (2013) Characterization and mechanical properties of phosphate-kaolin clay. Powder Technol 235:633–639
Chen YS, Butler JN, Stumm W (1973) Kinetic study of phosphate reaction with aluminum oxide and kaolinite. Environ Sci Technol 7:327–332
Cunningham FM, Elliott J, Lees P (2010) Comparative and veterinary pharmacology. Springer, Berlin
Davies IM, Gillibrand PA, McHenery JG, Rae GH (1998) Environmental risk of ivermectin to sediment dwelling organisms. Aquaculture 163:29–46
Egeler P, Gilberg D, Fink G, Duis K (2010) Chronic toxicity of ivermectin to the benthic invertebrates Chironomus riparius and Lumbriculus variegates. J Soils Sediments 10:368–376
Emerson K, Russo RC, Lund RE, Thurston RV (1975) Aqueous ammonia equilibrium calculations: effect of pH and temperature. J Fish Board Canada 32:2379–2383
Fincher GT, Wang GT (1992) Injectable moxidectin for cattle: effects on two species of dung-burying beetles. Southwest Entomol (USA)
Gächter R, Meyer JS (1993) The role of microorganisms in mobilization and fixation of phosphorus in sediments. In: Proceedings of the third international workshop on phosphorus in sediments. Springer, Dordrecht, pp. 103–121
Garr AL, Posch H, McQuillan M, Davis M (2012) Development of a captive breeding program for the Florida apple snail. Pomacea paludosa: relaxation and sex ratio recommendations. Aquaculture 370:166–171
Gomot-de Vaufleury AGD, Pihan F (2002) Methods for toxicity assessment of contaminated soil by oral or dermal uptake in land snails: metal bioavailability and bioaccumulation. Environ Toxicol Chem 21:820–827
Hammer UT, Harper D, Ryan P (2001) PAST: paleontological statistics software package for education and data analysis. Palaeontol Electron 4:1–9
Hempel H, Scheffczyk A, Schallnaß HJ, Lumaret JP, Alvinerie M, Römbke J (2006) Toxicity of four veterinary parasiticides on larvae of the dung beetle Aphodius constans in the laboratory. Environ Toxicol Chem 25:3155–3163
Herd R (1995) Endectocidal drugs: ecological risks and counter-measures. Int J Parasitol 25:875–885
Hilton J, Rigg E (1983) Determination of nitrate in lake water by the adaptation of the hydrazine-copper reduction method for use on a discrete analyser: performance statistics and an instrument- induced difference from segmented flow conditions. Analyst 108:1026–1028
Hou D, He J, Lü C, Sun Y, Zhang F, Otgonbayar K (2013) Effects of environmental factors on nutrients release at sediment-water interface and assessment of trophic status for a typical shallow lake, Northwest China. The Sci World J 2013:1–16
Hupfer M, Lewandowski J (2008) Oxygen controls the phosphorus release from lake sediments a long lasting paradigm in limnology. Int Rev Hydrobiol 93:415–432
King DG, Davies IM (1987) Laboratory and field studies of the accumulation of inorganic mercury by the mussel Mytilus edulis (L). Mar Pollut Bulletin 18:40–45
Kita K, Shiomi K, Ömura S (2007) Advances in drug discovery and biochemical studies. Trends Parasitol 23:223–229
Koops HP, Pommerening-Röser A (2001) Distribution and ecophysiology of the nitrifying bacteria emphasizing cultured species. FEMS Microbiol Ecol 37:1–9
Koroleff F (1970) The above paper revised. Int Con Explor Sea. Information on techniques and methods for sea water analysis. Interlab Rep 3:19–22
Kövecses J, Marcogliese DJ (2005) Avermectins: potential environmental risks and impacts on freshwater ecosystems in Quebec Scientific and technical report ST-233E Environment Canada. St Lawrence Centre, Montreal
Lifschitz A, Virkel G, Imperiale F, Sutra JF, Galtier P, Lanusse C, Alvinerie M (1999) Moxidectin in cattle: correlation between plasma and target tissues disposition. J Vet Pharmacol Therapeutics 22:266–273
Lifschitz A, Virkel G, Sallovitz J, Sutra JF, Galtier P, Alvinerie M, Lanusse C (2000) Comparative distribution of ivermectin and doramectin to parasite location tissues in cattle. Vet Parasitol 87:327–338
Lingpeng D, Wanxian W, Xinjiao D, Renyong H, Xuyang N (2011) Molluscicidal activity of cardiac glycosides from Nerium indicum against Pomacea canaliculata and its implications for the mechanisms of toxicity. Environ Toxicol Pharmacol 32:226–232
Lumaret JP, Errouissi F, Floate K, Römbke J, Wardhaugh K (2012) A Review on the toxicity and non-target effects of macrocyclic lactones in terrestrial and aquatic environments. Curr Pharm Biotechnol 13:1004–1060
Mackay D, Fraser A (2000) Bioaccumulation of persistent organic chemicals: mechanisms and models. Environ Pollut 110:375–391
Maine MA, Hammerly JA, Leguizamon MS, Pizarro MJ (1992) Influence of the pH and redox potential on phosphate activity in the Parana Medio system. Hydrobiology 228:83–90
McKellar QA, Benchaoui HA (1996) Avermectins and milbemycins. J Vet Pharmacol Ther 19:331–351
Mesa LM, Lindt I, Negro L, Gutiérrez MF, Mayora G, Montalto L, Ballent M, Lifschitz A (2017) Aquatic toxicity of ivermectin in cattle dung assessed using microcosms. Ecotoxicol Environ Saf 144:422–429
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chem Acta 27:31–36
OECD (2004) Guidelines for the testing of chemical-218. Sediment-water Chironomid Toxicity Test Using Spiked Sediment, Organisation for Economic Co-operation and development
OECD (2006) Guidance Document on Simulated Freshwater Lentic Field Tests (Outdoor Microcosms and Mesocosms). OECD Series on Testing and Assessment, No 53. Organisation for Economic Co-operation and Development, Paris
Omura S (2002) Macrolide antibiotics: chemistry, biology, and practice. Academic, Tokyo
Park JH, Choi JH, Abd El-Aty AM, Park JS, Kim BM, Na TW, Park KH, Yang A, Rahman MM, Shim JH (2013) Development of an extraction method for the determination of avermectins in soil using supercritical CO2 modified with ethanol and liquid chromatography-tandem mass spectrometry. J Sep Sci 36:148–155
Prichard R, Ménez C, Lespine A (2012) Moxidectin and the avermectins: consanguinity but not identity. Int J Parasitol: Drugs and Drug Resistance 2:134–153
PROSAP (2009) Estrategia provincial para el sector agroalimentario Provincia de Entre Ríos Ministerio de Agricultura. Ganadería y Pesca de la Nación [en línea] www.desarrolloentrerrianowordpress.com
Quintana RD, Vicari R, Magnano A, Madanes N (2014) Resiliencia de humedales frente al cambio climático En Pascale. In: Zubillaga CM, Taboada N (eds) Los suelos. la producción agropecuaria y el cambio climático: avances en la Argentina Ministerio de Agricultura. Ganadería y Pesca (Argentina)
Rohlf FJ (2006) TpsDig version 210. Department of Ecology and Evolution, State University of New York, Stony Brook
Rohrer SP, Arena JP (1995) Ivermectin interactions with invertebrate ion channels. In: Molecular action of insecticides on ion channels. ACS Symposium Series, Rahway, pp 264–283
Ruamthum W, Visetson S, Milne JR, Bullangpoti V (2010) Toxicity of botanical insecticides on golden apple snail (Pomacea canaliculata). Commun Agric Appl Biol Sci 75:191–197
Saigo M, Marchese M, Montalto L (2009) Hábitos alimentarios de Hyalella curvispina Shoemaker. 1942 (Amphipoda: Gammaridea) en ambientes leníticos de la llanura aluvial del río Paraná Medio. Nat Neotropicalis 1:43–59
Sanderson H, Laird B, Pope L, Brain R, Wilson C, Johnson D, Solomon K (2007) Assessment of the environmental fate and effects of ivermectin in aquatic mesocosms. Aquat Toxicol 85:229–240
Schweitzer N, Fink G, Ternes TA, Duis K (2010) Effects of ivermectin-spiked cattle dung on a water–sediment system with the aquatic invertebrates Daphnia magna and Chironomus riparius. Aquat Toxicol 97:304–313
Steel JW, Wardhaugh KG (2002) Ecological impact of macrocyclic lactones on dung fauna. In: Vercruysse J, Rew RS (eds) Macrocyclic lactones and antiparasitic therapy. CAB International, Wallingford, pp 141–162
Strong L, Wall R (1994) Effects of ivermectin and moxidectin on the insects of cattle dung. Bull Entomol Res 84:403–409
Suarez VH (2002) Helminthic control on grazing ruminants and environmental risks in South America. Vet Res 33:563–573
Suarez VH, Lifschitz AL, Sallovitz JM, Lanusse CE (2003) Effects of ivermectin and doramectin faecal residues on the invertebrate colonization of cattle dung. J Appl Entomol 127:481–488
Thain JE, Davies IM, Rae GH, Allen YT (1997) Acute toxicity of ivermectin to the lug worm Arenicola marina. Aquaculture 159:47–52
Tolls J (2001) Sorption of veterinary pharmaceuticals in soils: a review. Environ Sci Technol 35:3397–3406
Van den Brink PJ, Blake N, Brock TC, Maltby L (2006) Predictive value of species sensitivity distributions for effects of herbicides in freshwater ecosystems. Hum Ecol Risk Assess 12:645–674
Van Roon MR (2000) The suitability of the gastropod snail Amphibola creneta as a bio-monitor of heavy metals in estuaries Working Paper 00-3 Planning Department. University of Auckland, New Zealand
Vellarkad VN, Ghose AK, Revankar GR, Robins RK (1989) Atomic physicochemical parameters for three dimensional structure directed quantitative structure-activity relationships. Additional parameters for hydrophobic and dispersive interactions and their application for an automated superposition of certain naturally occurring nucleoside antibiotics. J Chem Inf Comp Sci 29:163–172
Acknowledgements
The authors thank Dr. Stephen Hamilton for critical reading of the manuscript and constructive suggestions. This research was supported by the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) and ASaCTeI (Project code 2010-087-16).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mesa, L.M., Hörler, J., Lindt, I. et al. Effects of the Antiparasitic Drug Moxidectin in Cattle Dung on Zooplankton and Benthic Invertebrates and its Accumulation in a Water–Sediment System. Arch Environ Contam Toxicol 75, 316–326 (2018). https://doi.org/10.1007/s00244-018-0539-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-018-0539-5