Abstract
The current study was performed to examine the acute toxicity of mercuric chloride (HgCl2) on the silver carp (Hypophthalmichthys molitrix) larvae. Probit analysis was used to determine the median lethal concentration (LC50). The LC50 values of Hg2+ for the fish larvae at 24, 48, 72, and 96 h were 267.72, 252.97, 225.57, and 97.80 μg/L, respectively. The safe concentration of Hg was 9.78 μg/L for fish larvae. Based on the 96 h LC50, fish were exposed to four different groups including 0, 6.11, 12.23, and 24.45 μg/L for 96 h to assess the effects of different concentrations of Hg2+ on antioxidant capacity, energy metabolism parameters, and related gene expression. The findings revealed that there were no significant differences in the activities of superoxide dismutase (SOD) and total antioxidant capacity (T-AOC) in fish larvae among all the groups (P < 0.05). In the 12.23 μg/L group, fish larvae had a maximum in catalase (CAT) activity. The creatine kinase (CK) activities of fish larvae in control and 6.11 μg/L groups were significantly lower than those groups (P < 0.05). A high concentration of Hg2+ significantly upregulated the mRNA levels of heat shock protein 70 (HSP70) and metallothionein (MT) genes in fish larvae. Furthermore, the IBR index value showed the highest value in the 24.45 μg/L group. Overall, this study provides an increased understanding of the effects of Hg-acute toxicity on silver carp larvae.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mercury (Hg), as we all know, is a biologically, chemically, and geologically active element (Yu et al. 2020; Mao et al. 2021). Hg is usually present in aquatic ecosystems in both inorganic (e.g., elemental Hg (Hg0) and oxidized Hg (HgII)) and organic (e.g., methyl Hg (MeHg) and dimethyl Hg (DMeHg)) forms (Yu et al. 2020). Of these, inorganic mercury is the main form released into the environment and methyl mercury is the most toxic form to human health (Zupo et al. 2019). Because of its high toxicity, difficulty to degrade, easy migration, and enrichment characteristics, as well as its powerful harmful effect on aquatic organisms and humans, it has sparked widespread concern (Anatone et al. 2020).
Currently, Hg has been widely detected in the aquatic environment. For example, the mean concentrations of total Hg in the overlying water of the main stem and tributaries of the Yangtze River in China were 7.94 ng/L and 7.64 ng/L (Wang et al. 2019). The average concentrations of total Hg and MeHg in the seawater from the six sites in Laizhou Bay (North China) were 57.8 ng/L and 0.33 ng/L (Cao et al. 2020). Hg pollution in the aquatic environment has become a serious environmental problem that requires attention. Hg can be enriched in aquatic organisms through biomagnification in the food chain in the kidney, gill, spleen, liver, and gall bladder, causing permanent damage in the aquatic environment (Harley et al. 2015; Rua-Ibarz et al. 2019).
The effects of acute or chronic exposure to inorganic and organic mercury on the behavior, growth, reproduction, tissue structure, and physiology and biochemistry of fish have been widely reported (Crump and Trudeau 2009; Huang et al. 2010; Wang et al. 2013; Sfakianakis et al. 2015; Chen et al. 2017; Zheng et al. 2019). Most studies have focused on the more toxic organic mercury (MeHg), but the toxic effects of inorganic mercury on fish are poorly studied, especially in the early developmental stages of fish. Silver carp (Hypophthalmichthys molitrix) is one of the four important carp species in China, accounting for 12.34% of total cultured freshwater fish production in 2020 (Yearbook 2020). Several studies related to the acute toxicity, physiological effect, and bioconcentration of heavy metals in the aquatic environment on silver carp have been reported (Hedayati and Ghaffari 2013; Liu et al. 2021). However, there is still a lack of studies on the acute toxic effects of fish and their toxicity mechanisms, especially on the toxic effects of the early developmental stages of silver carp. Fish larvae are an ideal model for the toxicological testing of aquatic animals. They are more sensitive to environmental contaminants during the early stages of growth and development.
Therefore, the objective of this study was to calculate the median lethal concentration (LC50) of silver carp larvae at various periods under Hg stress. And this study also evaluated the potential acute toxicity (96 h) effects of silver carp larvae exposed to different concentrations of mercury chloride (HgCl2) from the perspective of the antioxidant capacity, energy metabolism, parameters, and related gene expression. These findings provide a reference for studying the mechanism of mercury toxicity to fish and a theoretical basis for ecological risk assessment.
Materials and methods
Test chemicals and experimental fish
HgCl2 (purity > 99%, AR) was purchased from Sigma-Aldrich (St Louis, MO, USA). Deionized water was prepared into a 1-g/L HgCl2 stock solution. The solution was then stored in brown bottles at room temperature and shielded from light before getting diluted to the mass concentration required for the experimental design.
Silver carp larvae were purchased from the Genetic Breeding Center, Yaowan Experimental Farm, Yangtze River Fisheries Research Institute (Jingzhou, Hubei, China). Fertilized eggs were obtained by artificial insemination through injection of domperidone and luteinizing hormone-releasing hormone A2 drugs. The fertilized eggs were incubated in the incubation loop in running water at a temperature of 25.00 ± 1.00 °C during incubation. Fish larvae were fed with newly hatched brine shrimp (Artemia nauplii) twice a day (10:00 a.m.; 6:00 p.m.). Starvation treatment of fish larvae 24 h before the experiment, and healthy, uniformly sized 10-day-old fish were collected for relevant experiments. Dechlorinated tap water was used for the testing process. The water quality parameters were photoperiod 16 h:8 h light/dark, temperature 25.00 ± 1.00 °C, and pH 7.4 ± 0.2. Fish larvae were not fed during the exposure test. All procedures and animal handling comply with the guidelines approved by the Animal Ethics Committee of Yangtze University.
Experimental design
Acute toxicity test
A static acute toxicological test was performed to determine the LC50 of Hg2+ concentration in silver carp larvae after 24 h, 48 h, 72 h, and 96 h. Based on the results of the pre-experiment to determine the maximum concentration of all lethal for 24 h, seven groups (0, 40, 65, 104, 167, 270, and 435 μg/L) with equal logarithmic spacing were set. In the experiment, silver carp larvae were set up in seven groups, three replicates in each group. Each tank contained 10 fish. The reaction and swimming characteristics of silver carp larvae under mercury stress were observed and recorded within 8 h after the experiment started. At 24, 48, 72, and 96 h, fish larval mortality was recorded. The dead fish were later recovered. Fish larvae mortality was determined by the lack of response to the touch of sterile forceps on the fish body, and feeding was stopped during the whole test period. Regression probit analysis was performed using SPSS software with Hg2+ concentration as the natural logarithm.
Exposure experiment and sampling
Fish larvae (average initial weight 0.027 ± 0.004 g; average body length 1.25 ± 0.06 cm; (mean ± SEM)) were randomly assigned to 12 tanks (40 × 23 × 25 cm). Four groups were formed with three parallel settings for each group. Each tank contained 20 L tap water and 60 fish larvae. The subsequent exposure concentrations were set according to 1/16, 1/8, and 1/4 of the obtained 96 h LC50. Each group has a different concentration of 0, 6.11, 12.23, and 24.45 μg/L (named T0, T1, T2, and T3) . All solution samples were analyzed, and the measured Hg2+ concentration (0, 5.89 ± 0.60, 10.97 ± 1.85, and 22.04 ± 2.27 μg/L, corresponding to T0, T1, T2, and T3 groups, respectively) was within 20% of the nominal concentration during the exposure period. The experimental conditions were a water temperature of 25.00 ± 1.00 °C, a photoperiod of 16 h:8 h light/dark, and a pH of 7.4 ± 0.2. The exposure experiment lasted 96 h.
The mucus on the body surface of the fish larvae was rinsed off with sterile distilled water at the end of the experiment. After absorbing water from the surface of the fish with absorbent paper, the whole fish larvae were quickly placed in liquid nitrogen and then transferred to a − 80 °C refrigerator until further analysis. Due to the small size of the fish larvae, all samples for biochemical analysis and gene expression were taken from whole fish larvae.
Biochemical analysis
After thawing the whole fish larvae, three fish larvae in each group were homogenized to form a 10% solution using 0.86% sterile saline solution at a ratio of m (tissue, g):v (saline solution, mL) 1 to 9 and centrifuged at 3500 rpm for 10 min at 4 °C. The supernatant was immediately extracted and analyzed for biochemical parameters using a microplate reader (Bio Tek Epoch, USA). The superoxide dismutase (SOD) assay kit (WST-1 method; No. A001-3-2), total antioxidant capacity (T-AOC) assay kit (ABTS method; No. A015-2-1), catalase (CAT) assay kit (Visible light; No. A007-1-1), creatine kinase (CK) assay kit (Colorimetric method; No. A032-1-1), pyruvate kinase (PK) assay kit (Ultraviolet colorimetry method; No. A076-1-1), phosphofructokinase (PFK) test kit (Ultraviolet colorimetry method; No. A129-1-1), and total protein quantitative assay kit (Bradford method; No. A045-2-2) were measured using commercial assay kits (Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s instructions.
RNA extraction, synthesis of cDNA, and real-time PCR analysis
Total RNA was extracted from the nine samples of whole fish larvae in each group using the Trizol Reagent (AG, Hunan, China). The concentration and quality of total RNA were analyzed by micro-spectrophotometer. First-strand cDNAs were synthesized using the SweScript RT I First Strand cDNA Synthesis Kit (AG, Hunan, China) for real-time quantitative PCR analysis (qPCR). The qPCR was performed using a Roche LightCycle 96® real-time PCR instrument (Roche, Switzerland) to investigate the mRNA expression of CK, metallothionein (MT), and heat shock protein 70 (HSP70). The 18S RNA was used as the internal reference gene. Gene names, accession numbers, and forward and reverse primer sequences are shown in Table 1. The qPCR was performed in a 20-μL reaction volume, including 10-μL SYBR qPCR Master Mix, 2 μL cDNA template, 1.5 μL each of 2.5 μmol/L forward and reverse primers, and 5 μL sterile water. The program for the qPCR reaction was 95 °C for 30 s, 45 cycles at 95 °C, for 10 s, and 60 °C for 30 s. Gene fold change was analyzed according to the 2−∆∆Ct method (Livak and Schmittgen 2001).
Integrated biomarker response
IBR values were assessed for selected biomarkers (antioxidant enzyme parameters, energy metabolism parameters). The IBR calculation method was described in detail in our previous study (Chen et al. 2022).
Statistical analysis
All values were presented as mean ± standard errors (mean ± SEM). Statistical analysis was conducted using the SPSS software (version 25, SPSS Inc., Chicago, IL, USA). All data were checked for homogeneity of variance using the Levene’s equal variance test and normal distribution using the Shapiro-Wilk test. Statistical significance was determined using one-way analysis of variance (ANOVA) followed by Tukey’s HSD multiple range tests if the data were normally distributed and homogeneity of variance was met. A value of P < 0.05 was considered statistically significant. In addition, the Spearman correlation coefficient was calculated to determine the relationships between all parameters. The calculation and visualization of the matrix were performed using the Origin software (version 9.8, OriginLab Corporation, Massachusetts, USA).
Results
The poisoning symptoms of fish larvae
The poisoning symptoms of fish larvae under different concentrations of Hg2+ were as follows: There were no abnormalities in the fish larvae in the treatment groups at the beginning of the experiment. The movement area of fish larvae was mainly concentrated at the bottom of the water, which was similar to the control group. The fish larvae in the high concentration (435 μg/L) group showed excitement phenomenon after 3 h, concentrated at the water surface, and swam rapidly, with a significantly faster swimming speed than the control group. After 6 h, some fish larvae turned sideways in the beaker container or had abnormal swimming posture, activity speed gradually slowed down, slow response to external stimuli, and head floating phenomenon. After 8 h, some fish larvae died and white secretions appeared on their bodies and sank to the bottom. After death, the fish showed a series of typical symptoms of poisoning, including tail flexion, spine flexion, and bleeding from the gills and tail.
Acute toxicity test
The results of acute toxicity tests on silver carp are shown in Table 2. The number of dead fish increases with the increase in Hg2+ concentration. The results of the probit analysis revealed that the LC50 for 24 h, 48 h, 72 h, and 96 h were 267.72, 252.97, 225.57, and 97.80 μg/L, respectively. The safe concentration of Hg was 9.78 μg/L for silver carp larvae (Table 2).
Antioxidant capacity
There was no significant difference in the activities of SOD and T-AOC among all the groups (P > 0.05; Fig. 1A and B). The fish larvae of the T2 and T3 groups had lower activity of CAT than the T1 group, but no significant difference in the T1 group compared to the T0 group (P < 0.05; Fig. 1C). Detailed information about the statistical results of the data in Fig. 1 is shown in Table 3.
Energy metabolism parameters
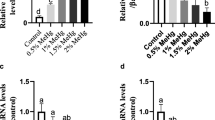
The activity of energy metabolism parameters of fish larvae in the different concentrations of Hg2+ is shown in Figs. 2 and 3. The activity of CK in fish larvae was significantly lower in the T0 and T1 groups than in the T2 and T3 groups. (P < 0.05; Fig. 2A). There was no significant difference in PK and PEK activities among all the groups. (P > 0.05; Fig. 2B and C). Detailed information about the statistical results of the data in Fig. 2 is shown in Table 4. The relative expression of the CK gene in the fish larvae was significantly downregulated in the T0 and T1 groups compared to the T2 and T3 groups (P < 0.05; Fig. 3A). Compared with the control, the highest values of HSP70 were found in fish larvae exposed to T2 and T3 groups (P < 0.05; Fig. 3B). The highest values of MT were found in fish larvae exposed to the T3 group than the other groups (P < 0.05; Fig. 3C). Detailed information about the statistical results of the data in Fig. 3 is shown in Table 5.
Relative mRNA expression levels of the silver carp larvae in response to different concentrations of Hg2+ stress. CK mRNA expression (A); HSP70 mRNA expression (B); MT mRNA expression (C). Vertical bars indicate the mean ± SE (n = 3). Data with different letters represent significant differences between all groups (P < 0.05)
Spearman correlation coefficients among the parameters
The visualization results are shown in Fig. 4. MT gene expression and HSP70 gene expression were significantly positively correlated with CK activity (P < 0.05). MT gene expression was significantly negatively correlated with CK gene expression and HSP70 gene expression (P < 0.05). T-AOC activity was significantly negatively correlated with CAT activity (P < 0.05).
A heatmap depicts the correlations between the various metrics examined. A color scale ranging from 1 to 1 represents the correlation value, with saffron yellow indicating a positive correlation and dark cyan indicating a negative correlation. “Asterisks” represent a significant difference (P < 0.05)
Integrated biomarker response analysis
The responses of fish larvae in different groups to antioxidant capacity (SOD, T-AOC, and CAT activity) and energy metabolism parameters (CK, PK, and PFK activity; CK, HSP70, and MT gene) biomarkers were standardized, and radar plots were shown in Fig. 5. The IBR index values in the various groups revealed that biomarkers responded differently. The T3 group had the highest IBR value (12.62), followed by the T0 (9.39), T1 (8.84), and T2 groups (7.67).
Discussion
As a short-term toxicological test, the acute toxicity test is important in environmental risk assessment and hazard classification. The relative toxicity of various toxic substances in different species is assessed by testing the main effects of toxic substances in aquatic species during the short term of their life cycle (Ebrahimpour et al. 2010). LC50 was an important parameter for measuring the toxicity of toxicants present in water to aquatic animals and is widely used in aquatic environmental toxicology studies (Hoffman et al. 2002). In an acute toxicity test of Hg2+ and its safety concentration evaluation study on Xiphophorus helleri, the LC50 values at 24, 48, and 96 h were 1710, 1350, and 840 μg/L, respectively (Chunfeng and Zhanqiang 2005). The 96 h LC50 of Hg2+ for the juveniles Prochilodus argenteus was 339 μg/L (da Silva et al. 2021). In this study, the mortality of silver carp larvae increased with the increase of Hg2+ concentration. Based on probit analysis, the LC50 values at 24, 48, 72, and 96 h were 267.72, 252.97, 225.57, and 97.80 μg/L, respectively. The LC50 decreased relatively gently at 24, 48, and 72 h, but sharply at 96 h. These results indicate that with the increase of time and concentration, the accumulation of Hg in silver carp larvae increases and exceeds the tolerance range of the body, which eventually leads to the death of a large number of fish larvae. In addition, acute toxicity exposure to Hg compared to other species showed that different species have different sensitivity to Hg toxicity.
Reactive oxygen species (ROS) are commonly used to describe the many reactive molecules and free radicals formed during normal mitochondrial oxidative metabolism. The production and elimination of ROS are in a dynamic equilibrium, and large amounts of ROS are produced when fish are chronically stressed by pollutants in the water column (Hamed et al. 2021). Antioxidant defenses are very sensitive to reveal pro-oxidant conditions and are often used as effective biomarkers of oxidative stress in fish (Ahmad et al. 2006). The antioxidant defense system involves a series of antioxidant enzymes (SOD, T-AOC, and CAT), of which the SOD-CAT system is often considered the first line of defense in the production of ROS under environmental stress (Wen et al. 2018). Changes in SOD activity can reflect the degree of damage to the organism. SOD can catalyze the conversion of superoxide anion radicals (O2−) into O2 and H2O2, which plays an important role in maintaining the balance of ROS (Zhang et al. 2004). CAT is mainly responsible for the reduction of hydrogen peroxide produced by the metabolism of long-chain fatty acids in the peroxisome, converting it into H2O and oxygen molecules (Ni et al. 2019). The current study found that the enzymatic activities of antioxidant enzymes (SOD and T-AOC) of fish larvae in the different Hg concentration groups did not show significant differences. This may be attributed to the fact that the current concentration of Hg2+ did not change the dynamic equilibrium of ROS in silver carp larvae. T-AOC is a comprehensive expression of the body’s antioxidant capacity, reflecting the strength of its antioxidant defense system (Kong et al. 2021). In our study, CAT activity in the T1 group was significantly elevated, which demonstrated that such a low concentration can enhance antioxidant capacity. However, high concentrations of Hg2+ (T2 and T3 groups) did not show significant differences compared to the control group. The reason for this effect could be that low concentrations of Hg stress may have produced excitotoxic effects. The systemic enzymes and non-enzymatic systems were mobilized to remove excessive ROS from the fish larvae, thus reducing the damage caused by excessive ROS to the organism. However, it should be pointed out that the mechanism of the antioxidant system response to Hg toxic exposure in silver carp larvae remains unclear.
Fish under stress (e.g., exogenous contaminants) can increase the demand for energy substances and interfere with the normal path of glucose metabolism in the organism. CK is a crucial enzyme in the energy metabolism of living creatures. It catalyzes the creation of creatine phosphate and adenosine diphosphate (ADP) in the body from creatine and adenosine triphosphate (ATP). CK is commonly found in the cytoplasm and mitochondria, and it provides energy primarily for muscle contraction and vital organ functions (O'Brien et al. 2014). In a previous study of Oreochromis niloticus exposed to cadmium, a significant increase in activity with CK activity in red muscle was observed (Almeida et al. 2002). The results of this study showed that the CK activity, as well as the relative expression of the CK gene, gradually increased with increasing Hg2+ concentration. We speculate that the glycolytic capacity of the fish may be accelerated under Hg stress, thus providing energy to the organism. PFK and PK are key enzymes in the process of glycolysis, of which changes in activity can reflect energy metabolism in tissue cells (Serafini et al. 2019; Moniruzzaman et al. 2020). In the present study, the PFK activity in fish larvae of the T2 and T3 groups showed an increasing trend after 96 h of Hg exposure. It may be due to the accelerated process of glycolysis and protein turnover metabolism to meet the body’s demand for energy after exposure to Hg2+. In contrast, PK activity did not show significant changes, probably due to the insensitivity of PK to Hg2+.
Heat shock proteins are a protein family that is highly conserved and plays a major role in cell and tissue physiology. HSP70 is one of them, which improves cellular resistance and acts as a stress protector when the organism is subjected to extreme stress (Moniruzzaman et al. 2020). In this study, the relative expression of the HSP70 gene was up-regulated in the high Hg2+ concentration. This may be due to the response to the metal-contaminated external environment by increasing the expression of HSP70. MT is a cysteine-rich and detoxifying low molecular protein that can reduce the toxic effects of heavy metals on organisms and rapidly scavenge free radicals such as -OH and O2− from organisms to enhance the body’s resistance to stress (Cretì et al. 2010). Heavy metals in aquatic environments have been shown to easily induce MT synthesis in organisms, and MT may be a sensitive biomarker of metal toxicity resistance in aquatic animals (Kim and Kang 2016). In this study, the relative expression of the MT gene gradually increased with increasing Hg2+ concentration. It may be due to the increased synthesis of MT under Hg2+ stress to reduce the toxic effects of heavy metals on fish larvae.
Exploring the correlations between different biomarkers will facilitate the assessment of ecotoxicity. This will enable the screening of suitable biomarkers to elucidate the interactions that have occurred between contaminants and organisms. In the current study, correlations of biomarkers (measured biochemical parameters) were evaluated. The results showed that there were not many relationships with significant correlations between parameters. It was not intuitive to use to assess the overall health status of fish. Therefore, we quoted the calculation of the IBR index, as in previous toxicological studies. The IBR index has been used to evaluate the toxicological effects of environmental contaminants, and it employs a multi-biomarker response to evaluate fish sensitivity to toxicants (Kim et al. 2014; Paul et al. 2020). Previous studies have shown that the IBR index provides a comprehensive assessment of the stress status of fish due to exposure to contaminants (Li et al. 2011; Chen et al. 2022). Thus, the overall health status of the fish could be assessed. The IBR index showed a maximum in the T3 group, indicating high stress under high Hg2+ concentration stress. However, the control group did not show the lowest value, which may be due to the excitatory toxicity under low-concentration stress. It is important to note that when using this index, the selection of biomarkers is critical in the calculation of the final IBR index value (Biswal et al. 2021).
Conclusions
Hg-induced toxicity causes various behavioral changes in silver carp larvae. Probit analysis revealed that the LC50 values for 24, 48, 72, and 96 h were 267.72, 252.97, 225.57, and 97.80 μg/L, respectively. The safe concentration of Hg was 9.78 μg/L for silver carp larvae. After 96 h of exposure, the effects of different concentrations of Hg on the antioxidant capacity of fish larvae were generally not significant. The energy metabolic parameters of fish larvae were disturbed under high concentrations of Hg (T2 and T3 group) stress. Different concentrations of Hg2+ influenced fish larvae relative expression of HSP70 and MT, with the T3 group having the largest values. Furthermore, the IBR index values revealed that the high Hg2+ concentration (T3 group) increases the overall pressure of toxicity in fish larvae. Our results increased our understanding of the Hg-induced acute toxicity to silver carp.
Data availability
The data and materials that support the findings of this study are available from the corresponding author upon reasonable request.
Code availability
Not applicable.
References
Ahmad I, Pacheco M, Santos MA (2006) Anguilla L. oxidative stress biomarkers: an in situ study of freshwater wetland ecosystem (Pateira de Fermentelos, Portugal). Chemosphere 65:952–962
Almeida J, Diniz Y, Marques S, Faine L, Ribas B, Burneiko R, Novelli E (2002) The use of the oxidative stress responses as biomarkers in Nile tilapia (Oreochromis niloticus) exposed to in vivo cadmium contamination. Environ Int 27:673–679
Anatone K, Baumann Z, Mason RP, Hansen G, Chernoff B (2020) Century-old mercury pollution: evaluating the impacts on local fish from the eastern United States. Chemosphere 259:127484
Biswal A, Srivastava PP, Pal P, Gupta S, Varghese T, Jayant M (2021) A multi-biomarker approach to evaluate the effect of sodium chloride in alleviating the long-term transportation stress of Labeo rohita fingerlings. Aquaculture 531:735979
Cao L, Liu J, Dou S, Huang W (2020) Biomagnification of methylmercury in a marine food web in Laizhou Bay (North China) and associated potential risks to public health. Mar Pollut Bull 150:110762
Chen Q-L, Sun Y-L, Liu Z-H, Li Y-W (2017) Sex-dependent effects of subacute mercuric chloride exposure on histology, antioxidant status and immune-related gene expression in the liver of adult zebrafish (Danio rerio). Chemosphere 188:1–9
Chen C-Z, Li P, Wang W-B, Li Z-H (2022) Response of growth performance, serum biochemical parameters, antioxidant capacity, and digestive enzyme activity to different feeding strategies in common carp (Cyprinus carpio) under high-temperature stress. Aquaculture 548:737636
Chunfeng FZ, Zhanqiang F (2005) Evaluation on acute toxicity and safety concentration of mercury and selenium to swordtail Fish, Xiphophorus helleri. Environ Sci Technol 28:32–34
Cretì P, Trinchella F, Scudiero R (2010) Heavy metal bioaccumulation and metallothionein content in tissues of the sea bream Sparus aurata from three different fish farming systems. Environ Monit Assess 165:321–329
Crump KL, Trudeau VL (2009) Mercury-induced reproductive impairment in fish. Environ Toxicol Chem 28:895–907
da Silva ETL, Pedreira MM, Dias MLF, Gomes MVT, Soares MA, Pedreira RSF, Schorer M (2021) Mercury chloride toxicity in juveniles Prochilodus argenteus a species from southeastern Brazil. Environ Sci Pollut Res:1–8
Ebrahimpour M, Mosavisefat M, Mohabbati R (2010) Acute toxicity bioassay of mercuric chloride: an alien fish from a river. Toxicol Environ Chem 92:169–173
Hamed HS, Ismal SM, Faggio C (2021) Effect of allicin on antioxidant defense system, and immune response after carbofuran exposure in Nile tilapia, Oreochromis niloticus. Comp Biochem Physiol C: Toxicol Pharmacol 240:108919
Harley J, Lieske C, Bhojwani S, Castellini JM, López JA, O’Hara TM (2015) Mercury and methylmercury distribution in tissues of sculpins from the Bering Sea. Polar Biol 38:1535–1543
Hedayati A, Ghaffari Z (2013) Effect of mercuric chloride on some hematological, biochemical parameters in silver carp ( Hypophthalmichthys molitrix ). Int J Vet Med: Research & Reports 2013:1–11
Hoffman DJ, Rattner BA, Burton GA Jr, Cairns J Jr (2002) Handbook of ecotoxicology. CRC Press
Huang W, Cao L, Ye Z, Yin X, Dou S (2010) Antioxidative responses and bioaccumulation in Japanese flounder larvae and juveniles under chronic mercury exposure. Comp Biochem Physiol C: Toxicol Pharmacol 152:99–106
Kim J-H, Kang J-C (2016) Oxidative stress, neurotoxicity, and metallothionein (MT) gene expression in juvenile rock fish Sebastes schlegelii under the different levels of dietary chromium (Cr6+) exposure. Ecotoxicol Environ Saf 125:78–84
Kim W-K, Lee S-K, Park J-W, Choi K, Cargo J, Schlenk D, Jung J (2014) Integration of multi-level biomarker responses to cadmium and benzo [k] fluoranthene in the pale chub (Zacco platypus). Ecotoxicol Environ Saf 110:121–128
Kong Y, Li M, Shan X, Wang G, Han G (2021) Effects of deltamethrin subacute exposure in snakehead fish, Channa argus: biochemicals, antioxidants and immune responses. Ecotoxicol Environ Saf 209:111821
Li Z-H, Velisek J, Zlabek V, Grabic R, Machova J, Kolarova J, Li P, Randak T (2011) Chronic toxicity of verapamil on juvenile rainbow trout (Oncorhynchus mykiss): effects on morphological indices, hematological parameters and antioxidant responses. J Hazard Mater 185:870–880
Liu H, Zhang H, Zhang S, Wang A, Fu S (2021) Lead exposure induces structural damage, digestive stress, immune response and microbiota dysbiosis in intestine of silver carp (Hypophthalmichthys Molitrix)
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408
Mao L, Liu X, Wang Z, Wang B, Lin C, Xin M, Zhang B-T, Wu T, He M, Ouyang W (2021) Trophic transfer and dietary exposure risk of mercury in aquatic organisms from urbanized coastal ecosystems. Chemosphere 281:130836
Moniruzzaman M, Kumar S, Das D, Sarbajna A, Chakraborty SB (2020) Enzymatic, non enzymatic antioxidants and glucose metabolism enzymes response differently against metal stress in muscles of three fish species depending on different feeding niche. Ecotoxicol Environ Saf 202:110954
Ni H, Peng L, Gao X, Ji H, Ma J, Li Y, Jiang S (2019) Effects of maduramicin on adult zebrafish (Danio rerio): acute toxicity, tissue damage and oxidative stress. Ecotoxicol Environ Saf 168:249–259
O’Brien K, Mueller I, Orczewska J, Dullen K, Ortego M (2014) Hearts of some Antarctic fishes lack mitochondrial creatine kinase. Comp Biochem Physiol A: Mol Integr Physiol 178:30–36
Paul T, Kumar S, Shukla S, Pal P, Kumar K, Poojary N, Biswal A, Mishra A (2020) A multi-biomarker approach using integrated biomarker response to assess the effect of pH on triclosan toxicity in Pangasianodon hypophthalmus (Sauvage, 1878). Environ Pollut 260:114001
Rua-Ibarz A, Bolea-Fernandez E, Maage A, Frantzen S, Sanden M, Vanhaecke F (2019) Tracing mercury pollution along the Norwegian coast via elemental, speciation, and isotopic analysis of liver and muscle tissue of deep-water marine fish (Brosme brosme). Environ Sci Technol 53:1776–1785
Serafini S, de Freitas Souza C, Baldissera MD, Baldisserotto B, Picoli F, Segat JC, Baretta D, da Silva AS (2019) Fish exposed to eprinomectin show hepatic oxidative stress and impairment in enzymes of the phosphotransfer network. Aquaculture 508:199–205
Sfakianakis D, Renieri E, Kentouri M, Tsatsakis A (2015) Effect of heavy metals on fish larvae deformities: a review. Environ Res 137:246–255
Wang M, Wang Y, Zhang L, Wang J, Hong H, Wang D (2013) Quantitative proteomic analysis reveals the mode-of-action for chronic mercury hepatotoxicity to marine medaka (Oryzias melastigma). Aquat Toxicol 130:123–131
Wang Y, Yin D, Xiang Y, Xu Q, Zhang C, Xie Q, Wang D (2019) A review of studies on the biogeochemical behaviors of mercury in the Three Gorges Reservoir, China. Bull Environ Contam Toxicol 102:686–694
Wen B, Jin S-R, Chen Z-Z, Gao J-Z, Liu Y-N, Liu J-H, Feng X-S (2018) Single and combined effects of microplastics and cadmium on the cadmium accumulation, antioxidant defence and innate immunity of the discus fish (Symphysodon aequifasciatus). Environ Pollut 243:462–471
Yearbook CFS (2020) China fishery statistical yearbook. China Agriculture Press, Beijing, China 2020:24–34
Yu X, Khan S, Khan A, Tang Y, Nunes LM, Yan J, Ye X, Li G (2020) Methyl mercury concentrations in seafood collected from Zhoushan Islands, Zhejiang, China, and their potential health risk for the fishing community: capsule: methyl mercury in seafood causes potential health risk. Environ Int 137:105420
Zhang J, Shen H, Wang X, Wu J, Xue Y (2004) Effects of chronic exposure of 2, 4-dichlorophenol on the antioxidant system in liver of freshwater fish Carassius auratus. Chemosphere 55:167–174
Zheng N, Wang S, Dong W, Hua X, Li Y, Song X, Chu Q, Hou S, Li Y (2019) The toxicological effects of mercury exposure in marine fish. Bull Environ Contam Toxicol 102:714–720
Zupo V, Graber G, Kamel S, Plichta V, Granitzer S, Gundacker C, Wittmann KJ (2019) Mercury accumulation in freshwater and marine fish from the wild and from aquaculture ponds. Environ Pollut 255:112975
Funding
This work was financially supported by the National Key Research and Development Program of China (2018YFD0900902, 2018YFD0900905) and the Engineering Research Center of Ecology and Agricultural Use of Wetland, Ministry of Education (Yangtze University) (KFT202006).
Author information
Authors and Affiliations
Contributions
Cheng-Zhuang Chen: writing—original draft preparation; Ya-Jun Wang: sampling and index measurement; Ping Li: writing—reviewing and editing; Ling Liu: data analysis; Yi Chai: culture and sampling; Zhi-Hua Li: conceptualization and overall guidance.
Corresponding author
Ethics declarations
Ethics approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. Approval of Animal Ethics Committee of Yangtze University was taken.
Consent to participate
Not applicable.
Consent for publication
All the authors are in agreement with the publishment.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Bruno Nunes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, CZ., Chai, Y., Wang, YJ. et al. Physiological and molecular responses in the silver carp (Hypophthalmichthys molitrix) larvae after acute mercury exposure. Environ Sci Pollut Res 30, 49760–49770 (2023). https://doi.org/10.1007/s11356-023-25842-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-25842-8