Abstract
Supervised field trail on dissipation of co-formulation with herbicides clodinafop-propargyl and oxyfluorfen in spring onion showed similar pattern of dissipation during two different seasons. Residues of clodinafop-propargyl reached ≤ limit of quantitation (LOQ, 0.05 mg kg−1) on 3rd day after application at both standard and double dose during both the seasons. Oxyfluorfen residues followed first-order kinetics in both the doses during first season with half-life of 0.81 to 3.14 days. The residues of clodinafop-propargyl were detected in soil at both the doses during first season. However, residues were ≤ LOQ (0.05 mg kg−1) during second season. The residues of oxyfluorfen were detected only in double dose during first season in soil. In all other cases and in onion bulb, residues were ≤ LOQ (0.05 mg kg−1) at the time of harvest. As the residues were either ≤ LOQ (0.05 mg kg−1) on 3rd day or have a half-life of 3.14 days, the co-formulation can be used safely, provided a pre harvest interval (PHI) of 3 days is followed. On the basis of maximum residue limits (MRLs) in other commodities and from the data of present study, a default MRL of 0.05 mg kg−1 is proposed for both the pesticides.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As short duration horticultural crop, onion (Allium cepa) (Brewster 2008) is grown for its edible bulb, since ancient times. Its flavor, aroma, unique taste, and medicinal properties are highly valued (Selvaraj 1976; Griffiths et al. 2002). It is used in curries with other vegetables or in salads, as a condiment, or cooked with other vegetables. Its other preparations such as pickles, powder, paste, and flakes are also consumed through out the world. Onion is known for its medicinal values and has been used in many indigenous cultures for its medicinal virtues which can be attributed to bioactive compounds present in onion. Its numerous pharmacological properties include antimicrobial, antioxidant, analgesic, anti-inflammatory, anti-diabetic, hypolipidemic, anti-hypertensive, and immunoprotective effects (Teshika et al. 2019).
Second only to tomato, onion is widely cultivated worldwide (FAO 2012) with total production of 74,250,809 tonnes from an area of 4,364,000 hectares in the whole world. In year 2021, worldwide production of onion was 968,016 tonnes (https://www.atlasbig.com/en-us/countries-onion-production, accessed 15th July 2021). Primary onion-growing countries are China and India (FAO 2012). In India, production of onion was 2,601,9000 tonnes from an area of 1,431,000 hectares during years 2019–2020 (Singh et al. 2021).
Different pests like onion thrips, maggot, and earwig (http://www.eagri.org/eagri50/ENTO331/lecture27/onion/001.html, accessed on 22nd June, 2022) lead to severe losses in onion production. Due to slow growing nature of onion, most serious threat to onion yield is posed by weeds, making it crucial to control weeds, so as to obtain marketable products and get high yield. Different regulations/legislations among countries make the selection of herbicides difficult for tackling the issue of weeds in onion crop. It is therefore essential to build an integrated weed management program, combining cultural practices and co-formulations of herbicides (https://www.ics-agri.com/onion-weed-control-management-program.html#:~:text=Onions%20do%20not%20compete%20well,between%20mechanical%20or%20chemical%20control, accessed on 22nd June 2022). Therefore, co-formulations of herbicides with different modes of action are being developed. One such formulation is combination product of clodinafop-propargyl and oxyfluorfen.
Clodinafop-propargyl is a carboxylic ester, a member of pyridines, and a propyzamide used as herbicide for the control of annual grass weeds in cereal crops. It acts as acetyl-CoA carboxylase inhibitor. Although it has low aqueous solubility and non-volatility, it tends not to be environmentally persistent and is not expected to leach to groundwater (http://sitem.herts.ac.uk/aeru/ppdb/en/Reports/165.htm, accessed on 22nd June 2022). Oxyfluorfen is a contact herbicide and causes membrane disruption through lipid peroxidation leading to necrosis of leaves and stems (https://wric.ucdavis.edu/PPTs/FENNIMORE_Contact_herbicides-Nov05.pdf, accessed on 22nd June 2022). Oxyfluorfen inhibits protoporphyrinogen oxidase, an enzyme involved in chlorophyll biosynthetic pathway, leading to accumulation of phototoxic chlorophyll precursors. These molecules, in the presence of light, produce activated oxygen species which rapidly disrupt cell membrane integrity. Oxyfluorfen must contact plant foliage to show its effects. Plants that are actively growing are most susceptible to oxyfluorfen. A chemical barrier by oxyfluorfen on the soil surface affects plants at emergence. Because of the long soil half-life of oxyfluorfen, this barrier may last up to 3 months (https://pubchem.ncbi.nlm.nih.gov/compound/Oxyfluorfen#section=General-Manufacturing-Information, accessed on 22nd June 2022).
When these co-formulations are applied to the plants in field, the constituent pesticides may influence the activity of each other and their dissipation. Half-life may also be affected. Not much research has been conducted on dissipation of combination product of clodinafop-propargyl and oxyfluorfen. Therefore, the present study was undertaken to understand dissipation kinetics and half-life of constituents of the co-formulation of clodinafop-propargyl and oxyfluorfen.
Materials and methods
Chemicals, reagents, solvents, and certified reference materials (CRMs)
The chemicals, reagents, and solvents used in the present study were of pesticide or LC–MS/MS grade with highest purity. These were checked for any impurities and/or were glass distilled before putting into use. Only those chemicals, reagents, and solvents were used which showed no interfering peaks.
CRMs and formulations used in the study were provided by UPL Ltd., India. Purity of Clodinafop-propargyl was 99.54% and that of oxyfluorfen was 99.9%.
Stock, intermediate, and working solutions of standards
Primary stock (400 mg kg−1, after adjusting purity of CRM) solutions of the standards of approximately were prepared by weighing 10 mg of standard on an analytical balance. The volume was then made up to 25 mL in volumetric flask. Acetonitrile was used as solvent for preparing standard solutions. Serial dilutions were made to prepare intermediate and working standards. Working standard solutions were prepared by standard addition method in control matrix.
Method validation
Control sample (15 g) (free from any residue of pesticides) was weighed in 50-mL capacity centrifuge tubes. Target limit of quantitation (LOQ) (X) was 0.05 mg kg−1 for both clodinafop-propargyl and oxyfluorfen. The samples were fortified at X, 5X, and 10X concentrations. Samples were allowed to stand for 20 min followed by extraction and analysis as mentioned below.
Method validation was performed for all parameters like linearity/sensitivity, accuracy, precision, limit of quantitation, ruggedness, selectivity in accordance with the procedure, and acceptance criteria defined in SANTE 2019. Blank and control samples were injected and analyzed for any interfering peaks at the place of peak of interest. When no interfering peaks are observed, the method was established to be selective.
Calibration curves were plotted in the range of 0.001 to 0.5 mg kg−1 for clodinafop-propargyl and 0.025 to 1.0 mg kg−1 for oxyfluorfen in diluted cucumber matrix. Dilution of the cucumber matrix has been reported to compensate for the matrix effect in most of the cases (Chawla et al. 2017; Kwon et al. 2018).
Accuracy (as percent recovery) and precision (as relative standard deviation) were validated at target LOQ (X) 0.05 mg kg−1clodinafop-propargyl and oxyfluorfen and at 5X and 10X levels with 5 replicates at each spiking concentration. The lowest concentration at which all the method validation parameters were within the acceptance range as per SANTE 2019 was established as method LOQ. Measurement uncertainty (MU) was calculated as 2 times RSD (SANTE 2019).
Details of field trial, crop, and formulation application
Randomized supervised field trials were conducted for studying residues and dissipation of combination product of clodinafop-propargyl + oxyfluorfen in onion samples at Main Vegetable Research Station, Anand Agricultural University, Anand, Gujarat. The trials were conducted during two seasons in successive years as mentioned below.
Onion plants (variety “GAWO-2”) were treated with single application of UPH 716 (Clodinafop-propargyl 12.25% + Oxyfluorfen 14.7% EC) at the time of 2–3 leaf stage of weeds as a foliar spray using knapsack sprayer fitted with flat fan nozzle within two lines in soil on weed. Spray volume was 500 L ha−1. Formulation was applied as standard dose of 125 + 150 g a.i ha−1 (gram of active ingredient per hectare) and double dose of 250 + 300 g a.i ha−1 (gram of active ingredient per hectare). Combination product of clodinafop-propargyl and oxyfluorfen is new. Therefore, the doses for the application are established based on the dose that effectively kills the most tolerant pest for which the product is recommended. Thus, the recommended rate is often one that is much higher than needed for effective management of some the more susceptible target species and is effective under a range of environmental conditions that can be expected in the settings for which the pesticide is recommended (Duke 2017). Control plots were sprayed with water. Three plots were taken for each application. Plot area was 2.0 × 20.0 m with 445 plants per plot. In total, three replicates were taken for each treatment. Average maximum and minimum temperature was 13.0 °C and 30.6 °C in first season and 11.9 °C and 29.7 °C in second season, respectively. Relative humidity ranged from 36.2 to 83.5% with average relative humidity 59.7% with rainfall recorded at 5.4 mm in first season. In second season, relative humidity was 33.6% and 81.5% with average relative humidity 57.6% with no rainfall recorded. Details of application and the sample collection during two seasons are as follows:
Application/sample collection | First season | Second season |
Date of transplantation | 21–11-2017 | 12–12-2018 |
Date of application of formulation | 29–01-2018 | 21–01-2019 |
Sample collection | Date (sample collected and analyzed) | |
0-day | 29–01-2018 (spring onion) | 21–01-2019 (spring onion) |
1-day | 30–01-2018 (spring onion) | 22–01-2019 (spring onion) |
3-day | 01–02-2018 (spring onion) | 24–01-2019 (spring onion) |
5-day | 03–02-2018 (spring onion) | 26–01-2019 (spring onion) |
7-day | 05–02-2018 (spring onion) | 28–01-2019 (spring onion) |
10-day | 08–02-2018 (spring onion) | 31–01-2019 (spring onion) |
15-day | 13–02-2018 (spring onion) | 05–02-2019 (spring onion) |
20-day | 18–02-2018 (spring onion) | 10–02-2019 (spring onion) |
30-day | 28–02-2018 (spring onion) | 20–02-2019 (spring onion) |
45-day | 15–03-2018 (spring onion) | 07–03-2019 (spring onion) |
57-day (harvest) | 27–03-2018 (spring onion, onion bulb, soil) | 22–03-2019 (spring onion, onion bulb, soil) |
Sample processing
Onion samples (2 kg) were collected (on different days as mentioned above), homogenized in/on high volume homogenizer (Robot coupe, Germany) at 3000 rpm for 2.0 min. For soil, 1 kg soil was collected, dried under shade, and ground to pass through 2 mm sieve and sub-sample was drawn for residue analysis. In all cases, three replicates were taken for each treatment.
Method of analysis (extraction and clean-up)
Spring onion, onion bulb
A representative sample (15 g) was weighed into QuEChERS tube (50 mL capacity). This was than extracted using 15 mL of 1% acetic acid in acetonitrile (Lehotay et al. 2005). Anhydrous magnesium sulfate (MgSO4) (6.0 g) and sodium acetate (1.5 g) were added to the sample in QuEChERS tube and shaken vigorously for 1.0 min followed by centrifugation at 3500 rpm for 2.0 min. An aliquot of 6.0 mL supernatant was drawn and transferred to QuEChERS tube (15 mL capacity) containing 0.9 g MgSO4 and 0.3 g primary secondary amine (PSA) and vortexed for 2.0 min for dispersive clean-up. The samples were then centrifuged at 2500 rpm for 2.0 min. Supernatant (0.2 mL) was taken and diluted to 2.0 mL with mobile phase (Solvent A: Solvent B, 20:80 v/v) and filtered through 0.22 µm syringe nylon filter and analyzed on LC–MS/MS and in GC–MS/MS for oxyfluorfen.
Soil
Soil samples were extracted using modified QuEChERS (Asensio-Ramos et al. 2010). Representative soil sample (10 g) was taken in 50-mL capacity centrifuge tube and 10 mL distilled water was added. To this, 20 mL of acetonitrile was added and vigorously shaken for 1 min followed by addition of 4.0 g MgSO4 and 1.0 g NaCl (sodium chloride). The tubes were than centrifuged for 2 min at 3500 rpm. From this, 10 mL supernatant was taken in 15-mL centrifuge tube already containing 1.5 g MgSO4 and 0.25 g PSA followed by centrifugation at 2500 rpm for 2 min. Supernatant (0.2 mL) was taken and diluted to1.0 mL with mobile phase and filtered through 0.22-µm syringe nylon filter and analyzed on LC–MS/MS and in GC–MS/MS for oxyfluorfen.
Instrumentation
Clodinafop-propargyl was analyzed using liquid chromatography coupled to tandem mass spectrometry (LC–MS/MS) on API 3200 (ABSciex, USA) (Fig. 1),whereas oxyfluorfen was analyzed on gas chromatography coupled to ion trap mass spectrometry (GC–MS/MS-Thermo PolarisQ (Thermo Fischer, USA).
Parameters: liquid chromatography coupled to tandem mass spectrometry (API 3200)
HTC-18 column was used with dimensions 50 × 2.1 mm and pore size 1.8 μm for separation. Mobile phase consisted of solvent A (5mm ammonium formate in water) and solvent B (Acetonitrile) in gradient flow with total run time of 5 min as mentioned below:
Time (min) | Flow rate (mL) | % A | % B |
|---|---|---|---|
Initial | 0.30 | 65 | 35 |
1.0 | 0.30 | 10 | 90 |
4.0 | 0.30 | 10 | 90 |
5.0 | 0.30 | 65 | 35 |
Injection volume was 5.0 µL. Ionization mode for analysis on mass spectrometer was electrospray ionization. Ion spray voltage was 5500V, curtain gas (N2) flow was 20mL, and heater temperature was 550°C. Gas1 (GS1) and Gas2 (GS2) were zero air with flow of 50 psi and 55psi, respectively. Collision gas (CAD) (N2) at 6psi. The compound parameters for clodinafop-propargyl were as follows:
Precursor ion (Q1) | Declustering potential (DP) | Entrance potential (EP) | Collision cell entrance potential (CEP) | Product ion (Q3) | Collision energy (CE) | Collision cell exit potential (CXP) |
|---|---|---|---|---|---|---|
350 | 54 | 10 | 30 | 266 (quantitation) | 24 | 7 |
54 | 10 | 30 | 91 (confirmation) | 45 | 7 |
Parameters: gas chromatography-mass spectrometry (Thermo PolarisQ)
Oxyfluorfen was analyzed on column GS BP 5 ms, 30 m × 0.25 mm i.d. × 0.25 μm FT in ion trap mode. Injector temperature was 250 °C. Injection was performed in splitless mode with column flow of 1.0 mL min−1. Carrier gas was helium. Temperature of transfer line and detector was 290 °C and 250 °C, respectively. Analysis was performed in selective ion monitoring (SIM) with solvent cut time of 4.0 min. Column programming was as given below:
The analyte peak and ions were matched with the library provided with the mass spectrometer by the supplier.
Dissipation kinetics, half-life, and pre harvest interval (PHI)
There are two approaches to describe the dissipation kinetics and derive kinetic models:
-
(i)
To use best-fit kinetics
-
(ii)
Use first-order kinetics (Boesten et al. 2005)
Regulatory authorities apply first-order kinetics to the entire dissipation period so as to study the dissipation kinetics (Whitmyre et al. 2004; Sharma et al. 2021). Therefore, in the present study, we used first-order kinetics equation Ct = C0e−kt (where Ct = residues at time t, C0 = initial concentration, and k = rate constant) to study dissipation kinetics and calculate half-life and pre harvest intervals. Half-life was calculated as described by Hoskins (1961).
Result and discussion
Method validation
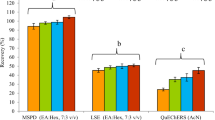
No interferences were found at the point of interest in the blank samples confirming specificity of the method. Linearity graphs for clodinafop-propargyl and oxyfluorfen showed that the correlation coefficient R2 was ≥ 0.999 (Fig. 2) (SANTE 2019). At the default LOQ of 0.05 mg kg−1, the recovery of clodinafop-propargyl was 87.5, 108.1, and 89.8% in spring onion, onion bulb, and soil, respectively (Table 1). For oxyfluorfen, the recovery was 96.4, 86.9, and 90%, respectively, at fortification level of 0.05 mg kg−1. At fortification levels of 0.25 and 0.5 mg kg−1, the recovery of clodinafop-propargyl ranged from 99.1 to 120.5% and that of oxyfluorfen ranged from 82.6 and 100.5%. All the recoveries were within the acceptance range of 70–120% with relative standard deviation ≤ 20% (SANTE 2019) (Table 1). As all the method validation parameters were within the acceptance range, 0.05 mg kg−1 is established as limit of quantitation (LOQ) for the method.
Dissipation, dissipation kinetics, and half-life
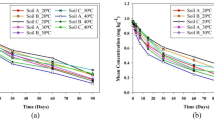
Initial accumulation (0 day) of clodinafop-propargyl in spring onion was 0.04 and 0.08 mg kg−1 in the first season for standard and double dose respectively. The residues increased to 0.12 and 0.19 mg kg−1 on 1st day after application in standard and double dose respectively and there after decreased to ≤ LOQ (0.05 mg kg−1) levels from 3rd day onward in the first season (Table 2). In second season, the initial accumulation of clodinafop-propargyl was 0.06 and 0.1 mg kg−1 in standard and double dose respectively. The residues then decreased to ≤ LOQ levels in standard dose from 1st day onwards (Table 2). In double dose, the residues decreased marginally to 0.09 mg kg−1 on 1st day and thereafter reached ≤ LOQ levels from 3rd day onwards in spring onion. The application of the combination product was in the soil on the weeds in between the lines of the onion crop. The application in the soil might have led to translocation of the clodinafop-propargyl in the spring onion leading to increase in the residue level on 1st day after application. The residues of clodinafop-propargyl were ≤ LOQ in onion bulb and soil at the time of harvest. As the residues of clodinafop-propargyl were below LOQ from 3rd day onwards, it was not possible to study dissipation kinetics.
Initial residues of oxyfluorfen were 0.81 and 0.86 mg kg−1 in first season for standard and double dose respectively. The residues in standard dose showed decreasing trend from 1st day after application and reached ≤ LOQ (0.05 mg kg−1) level on 7th day (onward). In double dose, the residues increased slightly on 1st day reaching to 1.59 mg kg−1. The residues decreased by 47% and 69% on 3rd and 5th day respectively. On 7th day, the residues were at LOQ and thereafter remained ≤ LOQ levels in spring onion (Table 2). The residues were ≤ LOQ in all the harvest samples (Table 2).
The dissipation kinetics study for oxyfluorfen in both the doses during first season and in double dose during second season showed that the r2 values range from 0.909 to 0.976 when log of residues was plotted against time (Fig. 2). To establish the dissipation kinetics model of any pesticide, the value of regression coefficient must be 0.85 to 1.0 with r2 near to 0.99 (Boesten et al. 2005). Thus, the dissipation kinetic model followed by the residues in the present study was first-order kinetics. Most of the pesticides have been reported to follow first-order kinetics (Sharma et al. 2021, 2014; Mojsak et al. 2018; Raj et al. 2012; Chawla et al. 2018).
The dissipation kinetics reveals that the residues of clodinafop-propargyl were ≤ LOQ of 0.05 mg kg−1 on 3rd day onwards in both the doses during both seasons suggesting that clodinafop-propargyl may not pose significant health hazard in humans and can be used on/in spring onion provided a pre harvest interval of 3 days is followed along with good agricultural practices (GAP). As the residues were ≤ LOQ of 0.05 mg kg−1 on 3rd day onwards, the half-life for clodinafop-propargyl could not be calculated. Clodinafop-propargyl has been registered in India in wheat as a single product and is also registered as combination product with metsulfuron, metribuzin in wheat, and with sodium acifluorfen on soybean (CIB&RC 2022). The MRL (maximum residue limit) for clodinafop-propargyl in soybean is 0.05 and in wheat is 0.1 mg kg−1 (FSSAI 2020). Based on the results from the present study and the MRLs of clodinafop-propargyl in soybean, a default MRL of 0.05 mg kg−1 (LOQ) can be proposed in/on onion/spring onion till enough data is available to calculate proper MRLs for clodinafop-propargyl, when applied as co-formulation.
Dissipation kinetics analysis of oxyfluorfen suggests first-order dissipation kinetic model in/on spring onion (Fig. 3) with half-life ranging from 0.81 (in double dose, second season) to 3.14 (in standard dose, first season). The residues of oxyfluorfen were ≤ LOQ on 3rd day after application; therefore, it was not possible to apply kinetic models. Oxyfluorfen is registered in rice, tea, onion, potato, groundnut, and mentha as single product. As co-formulation with glyphosate, it is registered in tea, propaquizafop, and quizalofop-ethyl in onion (CIB&RC 2022). The MRL of oxyfluorfen in rice, groundnut, and mentha is 0.05 mg kg−1, in tea 0.2 mg kg−1, and in potato it is 0.01 mg kg−1 (FSSAI 2020). Thus, the present study shows that the combination product of clodinafop-propargyl and oxyfluorfen can be applied, provided a PHI of 3 days is followed for spring onion/onion and GAP is followed.
The residues of both clodinafop-propargyl and oxyfluorfen were ≤ LOQ in onion bulb and soil at harvest (57th day after application) suggesting that the co-formulation got dissipated by this time and does not pose and significant health hazard.
Conclusion
The method validated for analysis of clodinafop-propargyl and oxyfluorfen was suitable for analysis of these compounds in onion/spring onion as all the method validation parameters were within the acceptable range as per European Union Commission guidelines (SANTE 2019). Less than LOQ (0.05 mg kg−1) levels on and after 3rd day of application in most of the cases suggest that the use of combination product of clodinafop-propargyl and oxyfluorfen ay not pose significant health hazard for human. Therefore, the co-formulation can be used provided a PHI of 3 days is used. On the basis of MRLs in other commodities and from the data of the present study, a default MRL of 0.05 mg kg−1 is proposed for clodinafop-propargyl in/on spring onion/onion and for both clodinafop-propargyl and oxyfluorfen as co-formulation in/on onion.
Data availability
All the data is available with the laboratory.
References
Asensio-Ramos A, Hernandez-Borges J, Randos-Perez LM, Rodriguez-Olegao M (2010) Evaluation of modified QuEChERS method for extraction of pesticides from ornamental and forestral soils. Anal Bioanal Chem 396:2307–2310
Boesten JJTI, Aden K, Beigel C, Beulke S, Dust M, Dyson JS, Fomsgaard IS, Jones RL, Karlsson S, van der Linden AMA, Richter O, Magrans JO, Soulas G (2005) Sanco/10058/2005, version 2.0, June 2006 Guidance document on estimating persistence and degradation kinetics from environmental fate studies on pesticides in EU registration. The Final Report of the Work Group on Degradation Kinetics. https://esdac.jrc.ec.europa.eu/public_path/projects_data/focus/dk/docs/finalreportFOCDegKinetics.pdf of FOCUS (FOrum for the Co-ordination of pesticide fate models and their USe)
Brewster JL (2008) Onions and other vegetable alliums, 1st edn. CAB International, Wallingford, UK, p 5
Chawla S, Patel HK, Gor HN, Vaghela KM, Solanki PP, Shah PG (2017) Evaluation of matrix effects in multiresidue analysis of pesticide residues in vegetables and spices by LC-MS/MS. J AOAC Int 100(3):616–623
Chawla S, Shah PG, Patel AR, Patel HK, Vaghela KM, Solanki PP (2018) Residue determination of β-cyfluthrin and imidacloprid as mix formulation in/on chickpea (Cicer arietinum) pods and soil and its risk assessment. Food Quality and Safety 2:75–81
CIB&RC (Central Insecticide Board and Research Committee) (2022) Major uses of insecticides (herbicides) as 0n 31.05.2022. https://doi.org/10.1093/fqsafe/fyy007
Duke SO (2017) Pesticide dose: effects on the environment and target and non-target organisms ACS Symposium Series. American Chemical Society, Washington, DC, p 2017
FAO (2012) World onion production. Food and Agriculture Organization of the United Nations. http://faostat.fao.org. Accessed 27 Feb 2017
FSSAI (2020) Food safety and standards (contaminants, toxins and residues) regulations, 2011 version –v (19.08.2020). https://www.fssai.gov.in/upload/uploadfiles/files/Compendium_Contaminants_Regulations_20_08_2020.pdf
Griffiths G, Trueman L, Crowther T, Thomas B, Smith B (2002) Onions: a global benefit to health. Phytother Res 16(7):603–615
Hoskins WM (1961) Mathematical treatment of the rate of loss of pesticide residues. FAO Plant Protect Bull 9:163–168
Kwon H-Y, Anastassiades M, Dörk D, Hong S-M, Moon B-C (2018) Compensation for matrix effects in GC analysis of pesticides by using cucumber extract. Anal Bioanal Chem 410:1–9. https://doi.org/10.1007/s00216-018-1197-1
Lehotay SJ, Kok A, Hiemstra M, Bodegraven PV (2005) Validation of a fast and easy method for the determination of residues from 229 pesticides in fruits and vegetables using gas and liquid chromatography and mass spectrometric detection. J AOAC Int 88(2):595–614
Mojsak P, Hrynko I, Rutkowska E, Szabuńko J, Łozowicka B, Kaczyński P (2018) Behavior of imidacloprid contamination in fruiting vegetables and their impact to human health. Desalin Water Treat 117(2018):32–41
Raj MF, Solanki PP, Singh S, Vaghela KM, Shah PG, Patel AR, Diwan KD (2012) Dissipation of spiromesifen in/on okra under Middle Gujarat conditions. Pestic Res J 24(1):25–27
SANTE (2019) Analytical quality control and method validation procedures for pesticide 442 residues analysis in food and feed. Document Nº SANTE/12682/2019
Selvaraj S (1976) Onion: queen of the kitchen. Kisan World 3(12):32–34
Sharma KK, Mukherjee I, Singh B, Mandal K, Sahoo SK, Banerjee H, Banerjee T, Roy S, Shah PG, Patel HK, Patel AR, Beevi SN, George T, Mathew TB, Singh G, Devi S (2014) Persistence and risk assessment of spiromesifen on tomato in India: a multilocational study. Environ Monit Assess 186:8453–8461. https://doi.org/10.1007/s10661-014-4016-y
Sharma KK, Tripathy V, Mohapatra S, Matadha NY, Pathan ARK, Sharma BN, Dubey JK, Katna S, George T, Tayade A, Sharma K, Gupta R, Walia S (2021) Disspation kinetics and consumer risk assessment of novaluron+lambda-cyhalothrin co-formulation in cabbage. Ecotoxicol Environ Saf 208:111494
Singh AK, Sankaran M, Murthy BNS (2021) Horticulture for food, nutritional and socio-economic security in India, Indian Horticulture. https://icar.org.in/sites/default/files/IH-Sep-Oct%202021.pdf. Accessed 22 June 2022
Teshika JD, Zakariyyah AM, Zaynab T, Zengin G, Rengasamy KRR, Pandian SK, Fawzi MM (2019) Traditional and modern uses of onion bulb (Allium cepa L.): a systematic review. Crit Rev Food Sci Nutr 59(sup1):S39–S70
Whitmyre GK, Ross JH, Lunchick C, Volger B, Singer S (2004) Biphasic dissipation kinetics for disloggable foliar residues in estimating post application occupational exposures to endosulfan. Arch Environ ContamToxicol 46:17–23. https://doi.org/10.1007/s00244-003-2166-v
Funding
Financial support and facilities for conducting the study from “ICAR- All India Network Project on Pesticide Residues” is acknowledged.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, performing the experiment: Ambalal V Parmar, Pratik V Raj. Analysis: Nitesh S Litoriya, Nirmal R Chauhan, Ravi L Kalasariya, Kaushik D Parmar, Paresh Shah. Over all supervision and resources: Paresh Shah. First draft of the manuscript, editing: Suchi Chawla. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All authors have given their consent for publication.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Gangrong Shi
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Litoriya, N.S., Chauhan, N.R., Kalasariya, R.L. et al. Dissipation kinetics of co-formulation with two herbicides, clodinafop-propargyl and oxyfluorfen, in/on onion (Allium cepa) samples. Environ Sci Pollut Res 30, 50225–50233 (2023). https://doi.org/10.1007/s11356-023-25785-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-25785-0