Abstract
A modified version of the QuEChERS method has been developed for the determination of a group of ten organophosphorus pesticides (i.e. ethoprofos, dimethoate, diazinon, malaoxon, chlorpyrifos-methyl, fenitrothion, malathion, chlorpyrifos, fenamiphos and phosmet) and one thiadiazine pesticide (buprofezin) in three different types of soils (forestal, ornamental and agricultural). The method was validated through linearity, recovery, precision and accuracy studies, and also by carrying out a matrix-matched calibration for the three soils owing to the existence of a strong matrix effect. Acceptable recovery values were obtained (between 45 and 96%) for all the pesticides and soils, except for malathion and malaoxon in forestal and ornamental soils, from which they could not be quantitatively extracted. Limits of detection of the whole method ranged between 0.48 and 7.78 ng/g. The method was finally applied to the determination of chlorpyrifos concentration in a treated soil for cultivation of potatoes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the last few decades the application of chemical pesticides has become an important matter for discussion. These products are used in many situations, such as livestock farming, cropping, forestry, home gardening, horticulture, and roadsides. Once they have been applied, they should stay, in an ideal manner, in the area of interest until the desired effect is achieved and then they should degrade into harmless products. The truth is that this does not fully happen: they strongly tend to move off-site owing to the effects of wind drift, leaching, runoff, etc. depending on several parameters, such as the chemical properties and persistence of the pesticide, the technique and rate of application, the frequency and timing of rains and irrigation and last, but not least, soil properties, such as organic carbon content (K OC) [1].
The study of the capability of soil to retain, filter and degrade pesticides is important nowadays since the presence and bioavailability of pesticides in this medium can impact human and animal health in a detrimental manner and they can also move off-site, contaminating surface waters, and also groundwater, causing harm to aquatic ecosystems [2]. That is why it is also important to develop rugged and robust methods able to determine these compounds at very low concentrations in such complex matrices. Traditionally and in general terms, the analysis of pesticides in soils has required a liquid–solid extraction assisted by ultrasounds or mechanical shaking followed by a clean-up step consisting in most cases of a solid phase extraction procedure [3].
The QuEChERS method (named for quick, easy, cheap, effective, rugged and safe), firstly introduced by Anastassiades et al. [4] and developed for the extraction of pesticides from fruits and vegetables, consists of an acetonitrile extraction/partitioning and a dispersive solid phase extraction step as the clean-up. This method and several modified versions have been lately applied for the extraction of different types of pesticides from, above all, fruits and vegetables [4–13]. To a much lesser extent, it has also been applied to the extraction of pesticides from other kinds of foodstuff, for instance rice [14], baby food [15], olives [16], milk [13, 17], herbs [18] and honey [19]. Although this method has provided good results for the extraction of apolar, medium-polarity and polar pesticides in non-fatty foods, it is also interesting to fully state if it can be applied to the analysis of these compounds in other non-fatty matrices such as soils, in which pesticides tend to frequently occur. In this sense, to the best of our knowledge, the QuEChERS method has only been applied to the extraction of pesticides from soils in five occasions [11, 20–23] using in all cases mass spectrometry (MS) detection except in one case, in which an electron-capture detector was used [22]. However, in four of these works [13, 20–22], a very small number of pesticides were analysed. In particular, Chen et al. [11] developed a modified QuEChERS method for the analysis of only one pesticide, procymidone, in leeks and soils to study its dissipation, and obtained recovery values between 74.9 and 100.8% and limits of detection (LODs) in the range 20–175 ng/g. In the second of these works, by Drożdżyński et al. [20], only three pesticides, namely azadyrachtin, spinosad and rotenone, were determined in soil by QuEChERS-UPLC-MS with recovery values between 67 and 108% (depending on the matrix and the spiking level) and LODs between 1.8 and 3.6 ng/g. In the third work, Dong et al. [21] analysed metaflumizone by QuEChERS-UPLC-MS/MS and obtained recovery values between 78.1 and 84.7% (LOD of 1 ng/g). Finally, Shi et al. [22] also analysed only one pesticide, oxadiargyl, and obtained recoveries between 95.5 and 112.0% and a LOD of 5 ng/g. Only on one occasion [23] were a relatively higher number of pesticides (24) analysed [12 were analysed using gas chromatography (GC)–MS and the rest were analysed by high-performance liquid chromatography–MS/MS]. However, the aim of that work was to compare the extraction efficiency of a new ultrasound solvent extraction procedure with three extraction methods (including direct application of the QuEChERS method), not to study in depth the use of the application of the QuEChERS method. In fact, for some of the pesticides the need to study in further works possible matrix effects was even stated since recovery values higher than 120% were obtained in some cases.
With the aim of studying in depth and extending the application of the QuEChERS method to the analysis of pesticides in soils, as well as to study possible matrix effects, in this work we investigated the application of the QuEChERS method for the extraction of a group of ten organophosphorus pesticides (i.e., ethoprofos, dimethoate, diazinon, malaoxon, chlorpyrifos–methyl, fenitrothion, malathion, chlorpyrifos, fenamiphos and phosmet) and one thiadiazine pesticide (buprofezin) in three types of soils (forestal, ornamental and agricultural) with fairly different physicochemical properties. For this purpose, GC with nitrogen–phosphorus detection (NPD) was used, and it was shown that this highly selective detection system can be used for detection purposes when this method is used for the analysis of this type of matrix. In addition, the application of the method developed was also tested to provide a scheme for the concentration of chlorpyrifos in a treated soil dedicated to the cultivation of potatoes. Confirmation of the presence of chlorpyrifos by GC-MS was also developed in this last case. The selection of these pesticides was based on the fact that they have been widely used for many years in the Canary Islands for the treatment of banana cultivars [8], which are the most important crop in terms of economic production together with grapes and tomatoes, up to the point of the Canary Islands being the leading banana producer among EU production regions. To the best of our knowledge, this is the first time that this group of pesticides (except chlorpyrifos and chlorpyrifos-methyl) has been extracted from soils employing a QuEChERS approach and also the first time that the matrix effect has been assessed for this method and these samples. It also represents the first work in which a complete validation of the QuEChERS method has been carried out for samples of this type.
Materials and methods
Chemicals
Pesticide analytical standards of ethoprophos (O-ethyl S,S-dipropyl phosphorodithioate), dimethoate (2-dimethoxyphosphinothioylthio-N-methylacetamide), diazinon (O,O-diethyl O-2-isopropyl-6-methylpyrimidin-4-yl phosphorothioate), malaoxon [2-(dimethoxyphosphorylthio) butanedioic acid diethyl ester], chlorpyrifos-methyl (O,O-dimethyl O-3,5,6-trichloro-2-pyridyl phosphorothioate), fenitrothion (O,O-dimethyl O-4-nitro-m-tolyl phosphorothioate), malathion [diethyl (dimethoxyphosphinothioylthio)succinate], chlorpyrifos (O,O-diethyl O-3,5,6-trichloro-2-pyridyl phosphorothioate), fenamiphos [(RS)-(ethyl 4-methylthio-m-tolyl isopropylphosphoramidate)], buprofezin (2-tert-butylimino-3-isopropyl-5-phenyl-1,3,5-thiadiazinan-4-one), phosmet (O,O-dimethyl S-phthalimidomethyl phosphorodithioate) and triphenylphosphate (internal standard) from Sigma-Aldrich Chemie (Madrid, Spain) were used without further purification (purity higher than 93.1%). Stock solutions of each pesticide of approximately 150 mg/L were prepared in cyclohexane and stored in darkness at 4 °C. Table S1 shows some of the characteristics of these pesticides.
All chemicals were of analytical reagent grade and used as received. Distilled water was deionized by using a Milli-Q system from Millipore (Bedford, MA, USA). Acetonitrile was from Scharlau Chemie (Barcelona, Spain) and cyclohexane, potassium chloride and anhydrous Na2SO4 were from Merck (Darmstadt, Germany). Sodium chloride, MgSO4·H2O, sodium citrate tribasic dihydrate and sodium hydrogencitrate sesquihydrate were from Sigma-Aldrich Chemie. Bondesil-PSA (40 µm) was from Varian (Harbor City, CA, USA).
Soil sample selection
Three types of soils (forestal, ornamental and agricultural) were collected in different places of Tenerife (Canary Islands, Spain). The forestal soil from between 0 and 68 cm deep in the ground in the forest of Las Mercedes, La Laguna, was placed directly in suitable plastic bags (1 kg). The ornamental soil was bought from a garden center (25 kg), whereas the agricultural soil from between 0 and 10 cm deep in the ground from an agricultural location dedicated to potato growing was collected in appropriate plastic bags (1 kg). Before use, soil samples were homogenized, sieved (2-mm mesh) and air-dried at room temperature. Table 1 shows the physicochemical characteristics of the three soils studied. For forestal and agricultural soils, organic matter (Walkley and Black method), texture (Boyoucos method), cation-exchange capacity and nitrogen content (Kjeldahl) were determined according to the standard methods described by Page et al. [24]. The pH of the soils in distilled water, the pH in KCl and the conductivity were measured in our laboratory in the same way as previously reported [25]. The forestal soil was a medium acid soil (pH 5.99) with good solubility of iron and a very high porosity. The ornamental soil, however, corresponded to a strongly acid soil (pH 5.25) with an excess of minerals such as Co, Cu, Fe, Mn and Zn and low amounts of Ca, K, N, Mg, Mo, P and S. Finally, the agricultural soil was extremely acidic (pH 4.20), which indicates a high amount of Al and Mn that may be toxic for some kinds of plants.
Ten grams of soil were weighed in a 50-mL centrifuge tube and spiked at several concentrations (as indicated in “Results and discussion”) with standards dissolved in cyclohexane. The tube cap was fitted and the soil was slightly shaken inside the tube to ensure a homogeneous mixture of the pesticides with the whole quantity of the soil. Samples were air-dried at room temperature and kept away from light for 1 day and then they were analysed. Extractions of blank samples were done in parallel to extractions of the spiked ones.
Apparatus and software
GC-NPD analyses were performed using a Varian 3800 (Walnut Creek, CA, USA) gas chromatographic system, equipped with a NPD and a Varian 8200 autosampler with the Varian Star Chromatography Workstation version 6.41 software program. Separation was developed in an poly(5% diphenyl/95% dimethylsiloxane)-bonded column (EquityTM-5; 30 m × 0.25 mm, 0.25-μm film). Nitrogen was used as the carrier gas (1.0 mL/min) and also as the make-up gas (30 mL/min). Two microlitres of a standard or sample solution were injected in the splitless mode at 280 °C using the following programme: 120 °C (for 3 min), then 7 °C/min ramp to 220 °C, held for 5 min and finally 5 °C/min ramp to 300 °C, held for 5 min. The NPD was maintained at 320 °C and the total run time was 43.29 min. The pH values were measured with a Crison GLP 22 pH meter (Barcelona, Spain), and conductivity values were measured with a Crison CM 35 portable conductimeter with temperature measurement capability.
GC-MS measurements were carried out to confirm the presence of chlorpyrifos in the samples. Analyses were performed with an Agilent 6890 N (Agilent Technologies, CA, USA) gas chromatographic system interfaced to a VG Autospec (VG Analytical, Micromass Instruments, Manchester, UK) mass spectrometer operating in electron impact ionization mode. The ion source temperature was kept at 230 °C and the ionization occurred with a kinetic energy of the impacting electrons of 70 eV. Separations were carried out in a J&W Scientific DB-5MS column (Agilent Technologies, CA, USA; \( {3}0{\hbox{m}} \times 0.{\hbox{25mm}} \times 0.{25} - \mu {\hbox{m}} \) film) with the same temperature programme as for the GC-NPD method. Version 2.0 of the NIST Mass Spectral Search Program for the NIST/EPA/NIH Spectral Library, with more than 150,000 mass spectra for standard compounds, was used.
Statgraphics Plus from Statistical Graphics (Rockville, MD, USA) was used for data processing.
Soil sample extraction and clean-up procedure
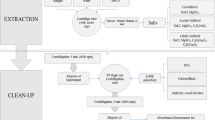
A 10-g portion of dried sieved soil was weighed in a 50-mL centrifuge tube and the internal standard solution was added and evaporated with a gentle stream of nitrogen. Then, 20 mL of acetonitrile was added and the sample was energetically shaken for 1 min. Next, 4 g of MgSO4·H2O was added as well as 1 g of NaCl, 1 g of sodium citrate tribasic dihydrate and 0.5 g of sodium hydrogencitrate sesquihydrate. The mixture was then vigorously shaken for 10 s, sonicated for 5 min in an Ultrasons ultrasonic bath working at 50/60 Hz and 100 W from Selecta (Barcelona, Spain) and centrifuged for 8 min at 4,000 rpm in a 5702 centrifuge from Eppendorf (Hamburg, Germany). Then, a clean-up dispersive solid phase extraction step was carried out by adding the supernatant volume (approximately 10 mL) to a 15-mL centrifuge tube that contained 1.5 g of MgSO4·H2O and 0.250 g of primary–secondary amine (PSA), shaking for a few seconds and then sonicating for 1 min. Afterwards, centrifugation was carried out at 4,400 rpm for 10 min and the supernatant-cleaned extract was evaporated to dryness at 40 °C and 200 mbar using a Rotavapor R-200 equipped with a V-800 vacuum controller, both from Büchi Labortechnik (Flawil, Switzerland). The dry residue was then redissolved in 1 mL of cyclohexane, a very small amount of anhydrous sodium sulphate was added and the supernatant was filtered through polytetrafluoroethylene filters (Chromafil® O-20/25 REF 729007 from Macherey-Nagel) and analysed by GC-NPD.
Results and discussion
Application of the QuEChERS method
The original QuEChERS approach includes the extraction of 10 g of homogenized fruit with 10 mL of acetonitrile, addition of 4 g of MgSO4, 1 g of NaCl and the use of a citrate-buffered medium to obtain high recovery values (1 g of sodium citrate dihydrate and 0.5 g of disodium hydrogencitrate sesquihydrate). After centrifugation, 5 mL of the supernatant is treated with 125 mg of PSA and 750 mg of MgSO4. When this procedure was applied to the extraction of 10 g of soil, it was found that no volume of the supernatant could be taken. With the aim of maintaining the same amount of sample and thus to achieve lower LODs, we increased the amount of acetonitrile, increasing at the same time in a suitable proportion the amounts of PSA and MgSO4. It was found that when 20 mL of acetonitrile was used and the supernatant (10 mL approximately) was treated with 250 mg of PSA and 1.5 g of MgSO4, the recovery values were high for all the pesticides selected in the three types of soils (between 45 and 96% for all the pesticides, except for malathion and its metabolite, malaoxon, which could not be quantitatively extracted from the forestal and the ornamental soils, probably owing the inherent properties of the soil). The employment of higher volumes of supernatant required the use of higher quantities of PSA and MgSO4. When lower amounts of PSA and MgSO4 were used, an important loss of chlorpyrifos, chlorpyrifos-methyl, fenamiphos, malathion and malaoxon occurred, owing to insufficient cleaning of the samples. That is why the previously indicated values were selected for subsequent validation experiments.
Validation of the method
Matrix-matched calibration
Owing to the complexity of soils, effects of the sample matrix on the quantification of the target analytes (effect on the chromatographic response) are very likely to occur, as several authors have demonstrated [25–29]. One of the ways in which the matrix may affect the determination of organic pollutants in soils is the presence of high amounts of matrix components that could protect the analyte from adsorption or degradation during evaporation of the sample in the inlet of the gas chromatograph, compared with solutions prepared in pure solvent [28]. For that reason and taking into account that the vast majority of the pesticides included in this study are included among the typical compounds known to be susceptible to matrix-induced enhancement [26], a statistical comparison between the calibration equations obtained from standards dissolved in pure solvent (cyclohexane in this case) and the ones obtained with the standards dissolved in the final sample extracts (also cyclohexane in this case) is highly recommended. If there is a change in the values of the intercepts or the slopes of the regression curves for each pesticide, then there is a matrix effect for the compound under study. If this were the case, it would be necessary to develop a calibration in the sample matrix to get truthful results.
Table 2 shows the calibration data of the standards dissolved in cyclohexane and in each type of soil extract (n = 6). To obtain the matrix-matched calibration curves, the three soils studied were firstly extracted to verify the presence or absence of pesticides. In none of the three types of soils were residues of the selected pesticides observed. Figure 1 shows the chromatograms of a matrix-matched calibration standard (forestal soil) and the blank samples for the three soils (forestal, ornamental and agricultural). Some peaks from the sample matrix were found for the three types of soils, but in none of the cases did this result in an overlapping between them and the peaks of interest. As can be seen in Table 2, the response of the detector was linear for each pesticide in the range tested, with correlation coefficients (R) higher than 0.998. Statistical comparison between the calibration in cyclohexane and in each type of soil extract was carried out using a statistical program (Statgraphics Plus) that calculates F and p values to compare the slopes and the intercepts (see “Apparatus and software”). In the case of buprofezin the p values were higher than 0.1 for both the slope and the intercept, whereas for the rest of the pesticides both values were lower than 0.01. As a result, and as can be seen in Table 2, all the pesticides show an important matrix effect in the three types of soils with respect to the standards in cyclohexane, except buprofezin, whose calibration curves in cyclohexane and in the ornamental soil extract are comparable. On the other hand, when the calibration curves of each soil were statistically compared, no significant differences were observed for the equations obtained for fenamiphos in the three types of soils. Nevertheless, between the forestal and the agricultural soil, there existed significant differences between both curves, except for ethoprofos, malaoxon, chlorpyrifos-methyl and malathion. For the rest of the curves obtained for each pesticide in every soil, a matrix effect existed. Therefore, quantification of the samples was carried out using the calibration curves obtained in each extracted soil sample to take into account this evident effect.
Gas chromatography (GC)–NPD chromatograms of A a spiked sample of the forestal soil (concentration approximately 100 ng/g for each pesticide) and B, C and D a non-spiked sample of the agricultural, ornamental and forestal soils (blank samples), respectively, after the application of the modified QuEChERS procedure. Column: EquityTM-5 30 m × 0.25-µm film. Flows of nitrogen as the carrier and make-up gases: 1.0 and 30 mL/min, respectively. Injection of 2 µL of a standard solution in the splitless mode at 280 °C using the following programme: 120 °C held for 3 min, then 7 °C/min ramp to 220 °C, held for 5 min, followed by 5 °C/min ramp to 300 °C, held for another 5 min. The NPD was maintained at 320 °C. Peak identification: 1 ethoprophos, 2 dimethoate, 3 diazinon, 4 malaoxon, 5 chlorpyrifos-methyl, 6 fenitrothion, 7 malathion, 8 chlorpyrifos, 9 fenamiphos, 10 buprofezin, IS triphenylphosphate and 11 phosmet. IS internal standard
Instrumental LODs calculated as the lowest concentration that yielded a signal-to-noise ratio of 3 ranged between 2.52 and 27.9 µg/L for the standards dissolved in cyclohexane. On the other hand, for the standards dissolved in the extracts of the three types of soils, they were similar among them and ranged between 4.87 and 58.5 µg/L for the forestal soil, between 5.58 and 67.2 µg/L for the ornamental soil and between 4.16 and 42.3 µg/L for the agricultural soil. Verification of these LODs was done experimentally. Table 2 shows these LODs as well as the limits of quantification (LOQs), which were calculated as the lowest concentration that yielded a signal-to-noise ratio of 10 (these values were also checked experimentally).
Recovery, precision and accuracy study
The repeatability of the method was evaluated by the development of a recovery study at two concentrations for the three types of soils and by repeating all extractions five times (n = 5) at each level. Table 3 shows the results of the recovery study. Comparison of the pesticide peak area to internal standard peak area ratio with the ratios obtained for matrix-matched standards was performed to calculate the mean recovery values, which ranged between 60 and 96% for the forestal soil (except for malathion and malaoxon, which could not be extracted), between 45 and 87% for the ornamental soil (except for malathion and malaoxon, which were in the range 9–29%) and between 62 and 96% for the agricultural soil. In general and judging from the different works previously published, recovery values are also highly dependent on the type of soil analysed [23]. As can be deduced from the results, the recovery percentages for the ornamental soil were observed to be, to some extent, lower than for the other two kinds of soils. This fact was also observed in a previous work by our group in which a different extraction method (using carbon nanotubes as solid phase extraction materials) was employed to determine seven organophosphorus pesticides and a thiadiazine [25] also in soil samples. The reason might be the relatively high amount of organic matter that this soil contains compared with the rest of them. The ornamental soil was a mixture of a fertile soil and a large quantity of peat (accumulation of partially decayed vegetation matter) and, as a result, it contained a high percentage of organic components (fulvic and humic acids), which can affect the extraction efficiency of the pesticides under study (for most pesticides, the organic matter content is the most important soil property affecting the degree of adsorption; the higher the organic matter content, the stronger the adsorption of pesticides [3]).
At this point, it is important to mention the situation for malathion and malaoxon (malaoxon is a breakdown product of malathion) in the ornamental and the forestal soils. In the first of them, it was observed that low recovery percentages were obtained (between 9 and 29%). On the other hand, for the forestal soil, the results were inconsistent because considerably different percentages were obtained for the two concentrations. Of the pesticides studied, malathion has the shortest soil half life (an average of 0.17 days, or 4 h). Degradation of pesticides in soils is highly dependent on the characteristics of the soil and it may be possible that degradation of malathion in the forestal soil occurs in an unrepeatable manner.
The method LODs were between 0.54 and 6.54 ng/g for the forestal soil (excluding malaoxon and malathion), between 0.68 and 12.5 ng/g for the ornamental soil and between 0.48 and 5.71 ng/g for the agricultural soil (LOQs in the ranges 1.79–21.8, 2.27–41.6 and 1.61–19.0 ng/g, respectively). These LODs and LOQs (which were also checked experimentally) are in the same order of magnitude as or even lower than the ones obtained for similar pesticides in previous works [3, 23, 25, 30, 31]
To further demonstrate the potential of the method developed and with the aim of completing the validation, an accuracy study was carried out. For this, each soil sample was fortified with the 11 pesticides at two different concentrations (see Table 4) and was analysed with the proposed modified QuEChERS method in quintuplicate (n = 5). A one-sample test (Student’s t test) was used to compare the concentration found for each pesticide with the spiked concentration. The results obtained are shown in Table 4. In all cases, the t values were lower than the tabulated ones (for \( n = {5},{t_4} = {2}.{78} \), P = 0.05), which means that the null hypothesis might be valid, or in other words there are no significant statistical differences between the spiked values and the values obtained. The relative recovery percentages were between 93 and 113%, values that clearly demonstrate the suitability of the proposed method. Values corresponding to the extraction of malathion and malaoxon from the forestal and ornamental soils were not calculated because of their very low recoveries.
Application to real sample analysis
Once the method had been validated, it was used to study the concentration of chlorpyrifos in a soil that was being treated with this pesticide, devoted to the cultivation of potatoes. The plot where the potatoes were planted was divided into four equal parts that were perfectly separated: two pieces of irrigated land and two pieces of dry land. Periodically, a representative portion of soil from each of the four pieces of land was collected, and each of them was analysed in duplicate by GC-NPD and confirmed by GC-MS (see “Apparatus and software” for details on the GC-MS analysis). Figure 2 shows the GC-NPD chromatogram of one these samples, whereas Fig. 3 shows the GC-MS chromatogram of one of the soil samples containing chlorpyrifos, as well as the MS spectrum of the peak in full-scan mode. The full-scan spectrum of the samples gave ions at m/z 314, 286, 258, 199 and 198, which are the same as those given by a standard of chlorpyrifos by electron impact ionization at 70 eV as previously reported [32]. The presence of chlopyrifos in the soil samples was confirmed by the NIST library [33]. Figure 4 shows the results of the GC-NPD analyses considering an average value of the two samples from the irrigated land and an average value of the other two samples from the dry land, with the corresponding error bars representing the uncertainty in the determination. As can be seen in Fig. 4, the first treatment was applied on 13 March. Samples 1 and 2 were collected on 30 March and 8 May, respectively. A second treatment was applied on 11 May and 14 days later sample 3 was collected. Finally, a third treatment was applied on 1 June and sample 4 was collected 8 days later. From this basic study, several aspects can be deduced. First of all, it is important to point out that, in all cases, the concentration of chlorpyrifos in the dry land is lower than that in the irrigated land, probably caused by two factors: one of them is that when the land is irrigated, the pesticide goes deep into the soil and becomes more protected against some of the direct causes of degradation (UV light, heat and environmental factors in general); the other is the clean-up effect that takes place on the pesticide residues that remain on the plants’ leaves when the land is irrigated or even when it rains (but rain would affect equally dry land and irrigated land). Besides, the concentration in sample 2 is smaller than that in sample 1, owing to an effect of degradation. Once the second treatment had been applied, the concentration of chlorpyrifos increased in sample 3, which is similar to what happened with sample 4 collected after the third treatment. This fact shows a clear accumulation of the pesticide in the soil.
GC-NPD chromatogram corresponding to the analysis of one sample of the soil devoted to potato cultivation showing the peak corresponding to chlorpyrifos. Peak identification: 8 chlorpyrifos and IS triphenylphosphate. For experimental conditions see the legend to Fig. 1
Conclusions
A modified QuEChERS method has been developed for the determination of 11 pesticides in forestal, ornamental and agricultural soils. The extraction method consumes a low amount of organic solvent (only 20 mL of acetonitrile and 1 mL of cyclohexane). Successful linearity, repeatability, precision, accuracy, selectivity and good recovery values (with RSDs below 12% in all cases) were obtained except for malathion and malaoxon in the forestal and ornamental soils, from which they could not be extracted. The method LODs obtained are similar to the LODs of current methods or even lower. The study of matrix effects revealed that matrix-matched calibration should be used in the three soils for quantification. The method was also applied to analyse the concentration of chlorpyrifos in a treated soil used for cultivation of potatoes and a clear accumulation of the pesticide over time was found.
References
Kanazawa J (1989) Environ Toxicol Chem 8:477–484
Belfroid AC, van Drunen M, Beek MA, Schrap SM, van Gestel CAM, van Hattum B (1998) Sci Total Environ 222:167–183
Andreu V, Picó Y (2004) Trends Anal Chem 23:772–789
Anastassiades M, Lehotay SJ, Štajnbaher D, Schenck FJ (2003) J AOAC Int 86:412–431
Payá P, Anastassiades M, Mack D, Sigalova I, Tasdelen B, Oliva J, Barba A (2007) Anal Bioanal Chem 389:1697–1714
Anastassiades M, Maštovská K, Lehotay SJ (2003) J Chromatogr A 1015:163–184
Zywitz D, Anastassiades M, Scherbaum E (2004) Dtsch Lebensm Rundsch 100:140–150
Hernández-Borges J, Cabrera Cabrera J, Rodríguez-Delgado MA, Hernández-Suárez EM, Galán Saúco V (2009) Food Chem 113:313–319
Lesueur C, Knittl P, Gartner M, Mentler A, Fuerhacker M (2008) Food Control 19:906–914
Húšková R, Matisová E, Hrouzková S, Švorc Ľ (2009) J Chromatogr A 1216:6326–6334
Chen L, Li XS, Wang ZQ, Pan CP, Jin RC (2010) Ecotoxicol Environ Saf 73:73–77
Nguyen TD, Yu JE, Lee DM, Lee GH (2008) Food Chem 110:207–213
Lehotay SJ, Mastovska K, Yun SJ (2005) J AOAC Int 88:630–638
Nguyen TD, Lee BS, Lee BR, Lee DM, Lee GH (2007) Rapid Commun Mass Spectrom 21:3115–3122
Przybylski C, Segard C (2009) J Sep Sci 32:1858–1867
Cunha SC, Lehotay SJ, Mastovska K, Fernandes JO, Oliveira MBPP (2007) J Sep Sci 30:620–632
Keegan J, Whelan M, Danaher M, Crooks S, Sayers R, Anastasio A, Elliott C, Brandon D, Furey A, O’Kennedy R (2009) Anal Chim Acta 654:111–119
Nguyen TD, Lee KJ, Lee MH, Lee GH (2009) Microchem J. doi:10.1016/j.microc.2009.09.012
Walorczyk S, Gnusowski B (2009) J Chromatogr A 1216:6522–6531
Drożdżyński D, Kowalska J (2009) Anal Bioanal Chem 394:2241–2247
Dong F, Liu X, Cheng L, Chen W, Li L, Qin D, Zheng Y (2009) J Sep Sci 32:3692–3697
Shi C, Gui W, Chen J, Zhu G (2009) Bull Environ Contam Toxicol. doi:10.1007/s00128-009-9881-7
Lesueur C, Gartner M, Mentler A, Fuerhacker M (2008) Talanta 75:284–293
Page AL, Miller RH, Heeney DR (1987) Methods of soil analysis. American Society of Agronomy and Soil Science Society of America, Madison
Asensio-Ramos M, Hernández-Borges J, Borges-Miquel TM, Rodríguez-Delgado MA (2009) Anal Chim Acta 647:167–176
Poole CF (2007) J Chromatogr A 1158:241–250
Dąbrowski L, Giergielewickz-Możajska H, Górski Ł, Biziuk M, Namieśnik J, Janicki B (2002) J Sep Sci 25:290–296
Schmeck T, Wenclawiak BW (2005) Chromatographia 62:159–165
Ravelo-Pérez LM, Hernández-Borges J, Rodríguez-Delgado MA (2008) J Chromatogr A 1211:33–42
Fuentes E, Báez ME, Labra R (2007) J Chromatogr A 1169:40–46
Díaz-Cruz MS, Barceló D (2006) J Chromatogr A 1132:21–27
Sinha SN, Pal R, Dewan A, Mansuri MM, Saiyed HN (2006) Int J Mass Spectrom 253:48–57
National Institute of Standards and Technology (2009) NIST virtual library. Available via http://www.nist.gov
Acknowledgements
M.A.-R. and L.M.R.-P. wish to thank the Spanish Ministry of Education for a de FPU grant. J.H.-B. thanks the Spanish Ministry of Science and Innovation for the Ramón y Cajal contract at the University of La Laguna. The authors would also like to thank Carmen D. Arbelo Rodríguez from the Department of Edaphology and Geology of the University of La Laguna for her help and for providing the characterized forestal soil sample and also A. Padilla Cubas from the Instituto Canario de Investigaciones Agrarias (ICIA) for providing the samples of soil devoted to the cultivation of potatoes. Finally, the authors acknowledge the assistance of the Servicio General de Apoyo a la Investigación (SEGAI) of the University of La Laguna for the GC-MS measurement facilities. This work was supported by the Spanish Ministry of Science and Innovation (project AGL2008-00990/ALI).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary materials
Below is the link to the electronic supplementary material.
ESM
(PDF 472 kb)
Rights and permissions
About this article
Cite this article
Asensio-Ramos, M., Hernández-Borges, J., Ravelo-Pérez, L.M. et al. Evaluation of a modified QuEChERS method for the extraction of pesticides from agricultural, ornamental and forestal soils. Anal Bioanal Chem 396, 2307–2319 (2010). https://doi.org/10.1007/s00216-009-3440-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-009-3440-2