Abstract
Uranium (U) is an important strategic resource as well as a heavy metal element with both chemical and radiotoxicity. At present, the rapid and efficient removal of uranium from wastewater remains a huge challenge for environmental protection and ecological security. In this paper, phosphate-modified biochar supporting nano zero-valent iron (PBC/nZVI) was triumphantly prepared and fully characterized. The introduction of polyphosphate can greatly increase the specific surface area of biochar pores, and then the zero-valent iron can be evenly distributed on the surface of material, thus leading to excellent removal performance of the PBC/nZVI for U(VI). The theoretical maximum U(VI) removal capacity of PBC/nZVI was up to 967.53 mg/g at pH 5. The results of adsorption kinetics, isotherm, and thermodynamics showed that the adsorption of uranium by PBC/nZVI was a monolayer physical adsorption and endothermic reaction. And the PBC/nZVI has favorable selectivity toward uranium against the interference of coexisting metal ions. Further mechanism studies show that the excellent uranium removal performance of PBC/nZVI is mainly attributed to the synergistic effect of physical adsorption and chemical reduction. This work proves that the PBC/nZVI has a wide application prospect in the field of uranium wastewater treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Uranium, as the most important metal element of nuclear energy, is with both chemical and radiological toxicity (Dai et al. 2021). Nowadays, with the rapid development of the nuclear industry, as clean energy does not release CO2 (Wang et al. 2021; Wu et al. 2021), more uranium contamination was produced, which would be discharged into the aquatic environment and then may lead to DNA damage, cancer, and the destruction of natural balance (Tang et al. 2021; Liao et al. 2021; Lu et al. 2020). Therefore, there is an urgent need to approach the efficient method for U(VI) removal. Currently, known methods of U(VI) removal mainly included adsorption, membrane separation, ion exchange, co-precipitation, and bioconcentration (Guo et al. 2018; Zhang et al. 2022a, b; Sun et al. 2021; Li et al. 2019a, b; Liu et al. 2021a, b; Wen et al. 2022), most of which were with the limitation of high cost or complex operation.
Adsorption is widely used for uranium removal because of its advantages of low-costing, simple operation, high efficiency, and portability (Wang et al. 2019a, b). Up to now, many adsorbents are utilized to remove U(VI) including covalent organic framework (COF), graphene oxide (GO), biochar (BC), and silica microspheres (Yang et al. 2019; Li et al. 2019a, b; Dutta and Nath 2018; Li et al. 2022a, b, c). Compared with the other adsorbents, biochar has the broad prospect in U(VI) removal owing to its advantages of low cost, high stability, favorable specific surface area, and negatively charged surface (Zhou et al. 2020). However, pure biochar is generally less capable of removing uranium, so biochar is often modified by acid, alkali, or salt to maximize the removal efficiency. For example, the adsorption capacity of FMAR biochar modified by potassium permanganate (KMnO4) was significantly higher than that of the original, and the theoretical maximum U(VI) adsorption capacity of activated biochar obtained from activated longan shells by ferric chloride activation is 331.13 mg/g (Li et al. 2019a, b; Zhang et al. 2021a, b).
Ammonium polyphosphate (APP) is an environmentally friendly flame retardant with a high ratio of nitrogen and phosphorus (Ran et al. 2019; Li et al. 2021). APP can affect the properties of biochar in many ways. First of all, APP acts as a flame retardant, limiting the decomposition of biomass. Specifically, in the pyrolysis process of APP-modified biomass, the pore structure will not be destroyed, and the nitrogen-containing groups released from APP will promote the specific surface area and pores. Next, some groups bound to biochar in APP are favorable U(VI) adsorption sites including nitrogen-containing and phosphorus-containing functional groups (Fan et al. 2020; Yang et al. 2020). The nitrogen-containing and phosphorus-containing groups introduced by polyphosphate-modified biochar (PBC) can remove U(VI) from wastewater by physical adsorption, electrostatic attraction, ion exchange, and especially the complexation (Ahmed et al. 2021a, b; Zhang et al. 2021a, b; Hu et al. 2020; Ahmed et al. 2021a, b).
Nano zero-valent iron (nZVI) is widely used to deal with a variety of contaminants, such as heavy metals, antibiotics, and nitrates (Zhou et al. 2022). From previous research, it was known that the use of highly agglomerative and oxidized nZVI alone would limit the stability of nZVI and then reduce their reaction efficiency (Li et al. 2022a, b, c; Zhang et al. 2022a, b; Li et al. 2022a, b, c). Many studies have been performed to reduce the nZVI aggregation and oxidation by utilizing modifiers, such as thiols, silica, biochar, polyvinylpyrrolidone (PVP), and carboxymethyl cellulose (Hua et al. 2021; Li et al. 2020; Wang et al. 2019a, b; Zhao et al. 2016; Ye et al. 2021). Dispersing nZVI with biochar can give full play to the uranium removal advantages of both biochar and nZVI.
In this study, we developed a novel PBC supporting nZVI composite to remove U(VI). The nZVI was dispersed by biochar to reduce the degree of aggregation and maximize the use of nZVI. The synthesis process was carried out by liquid phase reduction. Mechanism studies showed that U(VI) was mainly removed by adsorption and reduction. The basic requirements of this experiment were (1) to successfully synthesize the PBC/nZVI; (2) to carry out adsorption experiments, which obtained the most suitable adsorption reaction conditions; and (3) to explore the possible adsorption mechanism. The ultimate goal of achieving these requirements was to remove uranium in actual acidic wastewater more efficiently and conveniently.
Material and methods
Chemicals
Cornstalk was obtained from Changsha, Hunan province. The ammonium polyphosphate (APP), hydrochloric acid (HCl), anhydrous ethanol (CH3CH2OH), ferrous sulfate heptahydrate (FeSO4·7H2O), and sodium borohydride (NaBH4) were analytical grade. Five hundred milligrams per liter of U(VI) stock solution was prepared by dissolving uranyl nitrate (UO2(NO3)2·6H2O) in ultrapure water solution. All metal ions (K+, Ca2+, Mg2+, Mn2+, Zn2+, Cu2+, Cd2+) were purchased from the National Non-ferrous Metal and Electronic Materials Analysis and Testing Center. All solutions were prepared with ultrapure water.
Preparation of materials
The crushed corn straw was pyrolyzed in a tubular furnace under N2 atmosphere held at a temperature of 500/600/700/800 ℃ for 2 h with the heating rate of 5 ℃/min. After cooling, the product was washed with 0.5 M HCl and subsequently with deionized (DI) water to neutralize and then dried at 60 ℃. Finally, BC was obtained. As shown in Fig. S1, it was found that pyrolysis temperature had negligible influence on the removal of uranium. Combined with the principle of energy saving and referenced to the relevant literature (Guilhen et al. 2019), 500 ℃ was selected for pyrolysis.
PBC was synthesized through the following steps: firstly, 3 g corn stover (80 mesh) and 9 g APP were added to 40 mL deionized water, stirred at room temperature for 12 h, and centrifuged; the resulting product was dried at 70 ℃ for 8 h. Next, the dried material was ground into a tubular furnace and pyrolyzed to 500 ℃ for 2 h with a heating rate of 5 ℃/min under the protection of N2. Then, the product was stirred in hydrochloric acid (0.5 M) for 4 h to remove excess ash and then washed with deionized water to neutralize. Finally, the PBC was obtained by drying at 70 ℃ for later use.
The nZVI was synthesized by liquid phase reduction (Chen et al. 2021).
The PBC/nZVI was prepared by liquid phase reduction referencing the reported method in the literature (Cheng et al. 2021). The specific process is shown in Fig. 1. The whole reaction was conducted under N2 conditions. Firstly, 1.69 g FeSO4·7H2O was added to 170 mL of deoxidized DI water and stirred for 30 min; then, 0.1 g PBC was added and stirred for 3 h continuously; then, 0.9 g of NaBH4 was dissolved into 30 mL of deoxygenated ice water, and NaBH4 solution was evenly dropped into the mixed solution using a disposable infusion bottle hanger, which was completed for 15 min; finally, after reaction for 1 h, the synthesized material was centrifuged and washed with deoxidized water and anhydrous ethanol for three times. The material was dried at 60 ℃ in a vacuum and then stored under anaerobic conditions.
Batch experiments
Batch experiments were used to evaluate the adsorption properties of PBC/nZVI and to optimize the reaction conditions. Typically, at T = 30 ℃, pH = 5, 5 mg adsorbent was added to 10 mg/L (30 mL) of UO22+. The interfering ions were K+, Ca2+, Mg2+, Cu2+, Cd2+, Mn2+, and Zn2+, and the proportion to UO22+ was 10:1. Under the condition of UO22+ of 10 mg/L and PBC/nZVI of 0.075 g/L, the adsorption kinetic experiments of the adsorption capacity (Qe) with time were carried out to explore the possible adsorption types of PBC/nZVI, and the thermodynamic experiments were carried out with the reaction temperatures ranging from 15 to 40 ℃. Under the conditions of a UO22+ concentration of 5–120 mg/L and PBC/nZVI concentration of 0.075 g/L, an isothermal adsorption model experiment was conducted to explore the removal mechanism.
Characterizations
The microstructure of PBC/nZVI was obtained by transmission electron microscopy (TEM, JEM2100PLUS, Japan), and the elements in the material were obtained by energy spectrum (EDS, JEM2100PLUS, Japan). X-ray diffraction (XRD, Bruker D8 Advance, Germany) was used to compare the crystal morphology of the materials. The concentrations of U(VI) and interfering ions were determined by UV-spectrophotometer (T6 new century, China) and flame atomic absorption spectrometer. In addition, Fourier transform infrared spectroscopy (FT-IR, Nicoletis 10, USA) and X-ray photoelectron spectroscopy (XPS, Thermo Scientific K-Alpha+, USA) were used to understand the elements and groups of materials. The specific surface area and pore diameter were analyzed by BET analyzer (BET, Tristar II 3020, USA). Zeta potential (Zetasizer Nano ZS 90, UK) could be used to explore the charge change on the surface of the material.
Results and discussion
Adsorbents characterization
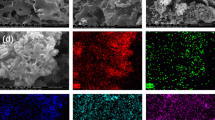
The TEM images of PBC/nZVI and PBC/nZVI-U as shown in Figs. 2 and 3. Figure 2a clearly show that most of nZVI was evenly dispersed on PBC except for a small portion of agglomeration. The corresponding EDS mapping and energy spectrum of the TEM image in Fig. 2b-h show that the elements C, H, O, N, P of PBC, and Fe of nZVI were contained in the PBC/nZVI. The existence of N and P elements indicated that PBC was successfully synthesized, and the Fe element was also introduced into PBC/nZVI. From the TEM image of PBC/nZVI-U (Fig. 3a), it could be seen that nZVI was disintegrated and dispersed and formed a thin film on the surface. This film formed mainly of U(VI) bound to the surface of PBC/nZVI, which suggested that nZVI had participated in the removal of U(VI). The corresponding EDS mapping and energy spectrum of the TEM image in Fig. 3b-i showed that the reacted PBC/nZVI had contained U, indicating that PBC/nZVI had bound with U.
The XRD diffraction patterns of nZVI, PBC/nZVI, and PBC/nZVI-U are shown in Fig. 4. nZVI appeared at its weak characteristic peak at 44.7° (Tang et al. 2021). For PBC/nZVI, the characteristic peak of nZVI still existed, which was located at 44.6°, and there was a slight position shift, indicating that the PBC/nZVI has been synthesized successfully. As for PBC/nZVI-U, the characteristic peak belonging to nZVI disappeared, and many characteristic peaks belonging to Fe(OH)3 appeared, with the strongest diffraction peaks at 14.18°, 26.95°, 36.15°, and 46.73°, respectively. The disappearance of nZVI and the appearance of Fe(OH)3 indicated that nZVI could be oxidized to Fe(OH)3 and might also be reduced to U(VI). By comparing the XRD data obtained with the database, it could be seen that the nZVI has been successfully synthesized in the solution with PBC and could participate in the reaction and be oxidized.
Figure 5 shows the FT-IR spectra of BC, PBC, PBC/nZVI, and PBC/nZVI-U, respectively. From the FT-IR spectrum of BC, it could be seen that the -OH and -C = O bonds were located in 3347.96 cm−1 and 1608.41 cm−1, respectively (Zhou et al. 2020). The existence of the carboxyl group and hydroxyl group were beneficial to the adsorption of U(VI), so the pure biochar had a certain effect on the removal of U(VI). In the infrared spectrum of PBC, in addition to the groups contained in BC, new bonds of -P-O-C, P-N–C, and -P = O at 997.98, 1081.92, and 1182.20 cm−1 were introduced, respectively. These groups indicated that the successful introduction of APP into BC could further promote the adsorption of U(VI) (Devi et al. 2020). For PBC/nZVI, the peak intensity of the phosphorus-containing groups originally located between 990 and 1200 cm−1 was reduced. In fact, phosphate can disperse nZVI into PBC through synergistic interaction with nZVI, so some phosphorous groups could be utilized by nZVI (Ruan et al. 2022). But there were still most groups that could be used to remove U(VI) in PBC. When the PBC/nZVI reacted with U(VI), there were U–O bonds at 896.77 cm−1, phosphorus-containing groups decreased, and -OH and -C = O bonds were moved to high waves, which proved that these groups were involved in the adsorption of U(VI). In short, the FT-IR results showed that PBC/nZVI had contained a variety of groups for U(VI) removal. At the same time, the adsorption of PBC for U(VI) was also explained.
XPS analysis was further used to characterize the elemental composition of PBC/nZVI. Figure 6 shows the XPS patterns of Fe 2p, O 1 s, C 1 s, P 2p, and N 1 s of PBC/nZVI. The XPS spectrum of Fe 2p (Fig. 6b) showed that the peaks of Fe(III) were located at 726.87 eV and 712.71 eV, the peaks of Fe(II) were located at 723.44 eV and 710.26 eV, and the peaks of Fe0 were located at 719.25 eV and 706.17 eV. It showed that nZVI was successfully synthesized, and that iron oxide still existed on the synthesized nZVI. Therefore, the U(VI) could be reduced by Fe(II) and Fe0. Figure 6c shows the C–C, C–OH, and -COOH in C 1 s were located in 284.01 eV, 285.23 eV, and 288.67 eV, respectively. O 1 s spectrum could be simulated by the = O and metal–oxygen groups at 530.86 eV and 529.33 eV (Fig. 6d). Combining the above C 1 s and O 1 s spectrum, it can be seen that PBC/nZVI also had groups contained on BC. In N 1 s and P 2p spectra (Fig. 6e, f), -NH2 and -N = were at 399.42 eV and 397.54 eV, and phosphates were at 132.78 eV. These results indicated that the APP was bound to BC and that the nZVI was successfully synthesized and dispersed by PBC. XPS results also provided a basis for further analysis of the mechanism of U(VI) removal by PBC/nZVI.
The N2 adsorption and desorption isotherms of BC, PBC, and PBC/nZVI were further understood by BET analysis. The characterization results are shown in Fig. 7 and Table 1. The PBC, which was produced by APP-modified BC (3.1247 m2/g), had a larger specific surface area, reaching 392.436 m2/g. According to the above characterization results, APP released not only phosphorus-containing groups, but also nitrogen-containing groups into the biochar. Due to the presence of nitrogen, some gases (NH3, etc.) can be released from nitrogen-containing groups in the pyrolysis process of modified biomass, which could inhibit the large-scale destruction of pore structure at a high temperature and promote the development of microporous pores (Lu et al. 2021). As could be seen from Table 1, the pore size decreased from 12.75 nm of BC to 2.06 nm of PBC. It also showed that the microporous structure of PBC was developed. However, after the introduction of nZVI, the specific surface area of PBC/nZVI decreased greatly. Due to the encapsulation of nZVI by PBC, part of the pore structure of PBC was occupied by nZVI, resulting in the reduction of specific surface area (Zhang et al. 2021a, b). It could be seen that the specific surface area and pore of APP-modified biochar were promoted, which was more conducive to the adsorption and reduction of U(VI).
Combined with the characterization results of TEM, EDS, XRD, FT-IR, XPS, and BET, it could be proved that the PBC/nZVI was synthesized successfully. PBC had a certain ability to encapsulate nZVI, which was used to slow down the oxidation rate of nZVI and could be adsorped U(VI). And the nZVI could reduce U(VI) to U(IV) at the same time. Moreover, PBC/nZVI was magnetic, which facilitated recovery from wastewater. In a word, of the functional groups contained in PBC/nZVI that could be classified and explained by XPS analysis, the characterization results of XRD and FT-IR showed that both nZVI and APP modification could be used to promote the removal of U(VI).
Effects of reaction conditions on U(VI) removal
The removal effect of BC, PBC, nZVI, and PBC/nZVI for U(VI) in wastewater was compared. Therefore, the U(VI) removal experiment about time was carried out to select the best uranium removal material. The time curve is shown in Fig. 8a. Compared with BC, PBC, and nZVI, PBC/nZVI had a higher removal efficiency for U(VI) with a U(VI) concentration of 10 mg/L. The excellent U(VI) removal performance of PBC/nZVI lies not only in the larger specific surface area and more adsorption sites brought by APP-modified BC, but also in the strong reducibility brought by nZVI. It could also be seen from the figure that the removal of U(VI) by PBC/nZVI reached equilibrium within 30 min. The reaction time to reach equilibrium was very short, better than most adsorption materials, such as PAOS-BC, MB, and Fe3O4/MB (Ding et al. 2018; Wang et al. 2020). Thus, 30 min was selected as the reaction time in subsequent experiments.
Figure 8b showed the effects of pH values from 3 to 9 on U(VI) removal by PBC/nZVI. The removal rate of U(VI) by PBC/nZVI increased with the increase of pH value from 3 to 5. When the pH was 5 to 7, the removal rate was high and stable, all of which were above 92%. But the removal rate decreased from 7 to 9. Specifically, the reasons for these results could be analyzed in combination with zeta potential (Fig. S2): (1) when pH < 5, U(VI) mainly existed in the form of positive charge, such as UO22+, UO2(OH)+, and (UO2)2(OH)22+ (Zhao et al. 2022), and the surface of PBC/nZVI was positively charged, thus generating electrostatic repulsion with U(VI) complex, leading to the decline of U(VI) removal rate in this pH range; (2) when pH > 7, U(VI) mainly existed in the form of negative charge, for instance, UO2(OH)2, UO2(OH)3−, and (UO2)3(OH)7− (Liu et al. 2021a, b), and the surface of PBC/nZVI was mainly negatively charged, which produced electrostatic repulsion with U(VI). However, when pH was between 5 and 7, the positive charge gradually decreased or even became negative due to the deprotonation of the PBC/nZVI surface, while U(VI) existed in the form of positive or neutral charge, so the electrostatic repulsion was weakened in favor of uranium removal (Zhao et al. 2022; Liu et al. 2021a, b). Based on acidic wastewater, pH = 5 was selected for follow-up experiments.
The influence of U(VI) concentration and temperature on the experiment was also considered, and the results are shown in Fig. 9. As could be seen from the curve of Fig. 9a that with the increase of concentration, the removal rate of U(VI) by PBC/nZVI firstly increased and then decreased. When the concentration of U(VI) was 10 mg/L, the highest removal rate exceeded 93%. Accordingly, 10 mg/L U(VI) solution was selected as the initial concentration of the subsequent reaction. The effects of temperature on PBC/nZVI removal U(VI) are shown in Fig. 9b. With the increase of temperature, U(VI) removal increased sharply at first and then slowly. It could be seen that the increase of temperature could promote the removal of U(VI), and 30 ℃ was selected as the reaction temperature in combination with the temperature in the actual environment.
The common wastewater was a solution in which many metal ions coexist. In order to explore the selectivity of U(VI) removal by PBC/nZVI and the maximum degree of removal U(VI), the interference of 10 mg/L competitive ions on the removal of U(VI) by PBC/nZVI was studied, and the ratio of concentration to U(VI) was 10:1. The selected coexisting ions were K(I), Ca(II), Mg(II), Mn(II), Cu(II), Cd(II), and Zn(II). The reaction was carried out at pH = 5 and T = 30 ℃. The result of the reaction is shown in Fig. 9c, which showed that PBC/nZVI could be removed more than 98% of U(VI) and had almost no effect on K(I), Ca(II), Mg(II), Mn(II), Cd(II), and Zn(II), but could still be removed about 79% of Cu(II). Because of the small radius of copper ions and strong coordination competition on its surface, the adsorption and reduction ability to Cu(II) was strong in PBC/nZVI. The above results showed that PBC/nZVI had good selectivity for uranium in the presence of many kinds of ions. It also showed that it was beneficial to remove U(VI) in a practical environment.
Mechanism study
Adsorption kinetic
In order to explore the adsorption kinetics of PBC/nZVI and to analyze the possible interaction mechanism, U(VI) removal experiments were carried out. The results are shown in Fig. 10a. Pseudo-first-order and pseudo-second-order models were used to analyze the U(VI) adsorption kinetics (Tian et al. 2021). Table 2 displays the simulatively obtained parameters. The removal of U(VI) by PBC/nZVI could be better fitted by the pseudo-first-order model (R2 = 0.970) than the pseudo-second-order model (R2 = 0.927), indicating that the rate-controlled mechanism for U(VI) removal was dominated by physisorption.
Isotherms and thermodynamics studies
Figure 10b shows the removal behavior of U(VI) by PBC/nZVI at different initial concentrations. In order to understand the removal mechanism of U(VI) by PBC/nZVI, Langmuir and Freundlich’s isothermal models were used to fit the adsorption effects. Langmuir’s isothermal adsorption model was expressed as the monolayer adsorption homogenized on the material surface, while Freundlich’s isothermal adsorption model was expressed as multilayer adsorption (Fu et al. 2022). Table 3 presents the relevant parameters calculated by the two models. R2 indicated the degree of fitting. It could be seen that the removal of U(VI) by PBC/nZVI was more suitable for the Langmuir model, and the theoretical maximum adsorption capacity could reach 967.53 mg/g. These phenomena also indicated that the binding energy of PBC/nZVI was uniform over the entire surface. Therefore, the removal of U(VI) by PBC/nZVI was monolayer adsorption.
The effects of reaction temperature (288.15–313.15 K) on U(VI) removal by PBC/nZVI were studied by thermodynamic analysis. Thermodynamic parameters included Gibbs free energy change (ΔG), enthalpy change (ΔH), and entropy change (ΔS) were calculated as formula ΔG = − RTlnKc, ΔG = ΔH–TΔS, lnKc = ΔS/R–ΔH/RT. ΔG was used to determine the direction and limit of the reaction; the reaction was in the forward direction when ΔG < 0. ΔH was related to the internal energy and determined whether the reaction was endothermic or exothermic; the reaction was an endothermic reaction when ΔH > 0. ΔS was used to indicate the spontaneity of the reaction; the reaction was carried out spontaneously when ΔS > 0. Where the related parameters T was the absolute temperature (K), R was gas constant (8.314 J/mol/K), and Kc (Qe/Ce) depends on the adsorption capacity and concentration at equilibrium, at the same concentration and different temperature (Sun et al. 2022). The results of U(VI) removal by PBC/nZVI in the range of 288.15–313.15 K are shown in the Fig. 10c. Combined with the relevant parameters of Table 4, when ΔH and ΔS were both > 0, ΔG became smaller and smaller with the increase of reaction temperature. This suggested that the removal of U(VI) by PBC/nZVI was positive for a spontaneous endothermic reaction. The results showed that the removal of U(VI) from wastewater by PBC/nZVI could be promoted efficiently with the increase of temperature.
XPS study
XPS analysis was used to explain the mechanism of U(VI) removal by PBC/nZVI. According to the results in Fig. 11, the U 4f peaks presented broad U 4f7/2 and U 4f5/2 lines separately situated at 381.18 and 392.08 eV. The potential difference between the two was 10.9 eV. U(VI) peaks could be found in 392.15 and 381.28 eV. The peaks of U(IV) were fitted into 391.04 and 379.86 eV. Synchronously, satellites of U(IV) at 383.81 eV had been discovered. XPS analysis showed the presence of U(IV), which proved that U(VI) was successfully reduced.
Combined with XPS analysis of PBC/nZVI before, the reaction showed that Fe(0), Fe(II), oxygen-containing groups, and iron (hydr) oxides on PBC/nZVI could be used for the reduction and adsorption of U(VI). It was worth noting that the reduced U(IV) by PBC/nZVI could be easily reoxidized to U(VI), so the uranium on the surface of PBC/nZVI existed in two valence forms.
In conclusion, the removal of U(VI) by PBC/nZVI was a monolayer uniform physical adsorption, which was a spontaneous endothermic reaction. Combined with the characterization results of FT-IR, XRD, and XPS, it could be seen that the efficient removal of U(VI) by PBC/nZVI was the coexistence result of many ways, including (1) Fe0 and Fe(II) reduced U(VI); (2) various oxygen-containing groups on PBC, such as phosphates and -COOH, complexed adsorption for U(VI); (3) metal oxides contained in PBC/nZVI adsorbed U(VI); and (4) using the solution pH and the charge on the surface of the PBC/nZVI attracted U(VI) for electrostatic attraction.
Conclusions
In this study, the PBC/nZVI was obtained by binding the agglomerated and oxidized nZVI with phosphate-modified biochar easily. The obtained PBC/nZVI had both the reduction performance of nZVI and the adsorption performance of PBC, so it could be used to effectively remove U(VI) in acidic wastewater. The maximum removal capacity of U(VI) for PBC/nZVI was 967.53 mg/g, and the reaction could reach equilibrium within 30 min, which showed rapid and efficient uranium removal performance. The adsorption data were fitted with Langmuir’s isotherm and the pseudo-first-order kinetic model well. In a word, the removal of U(VI) by PBC/nZVI was efficient and stable and showed great potential in extracting uranium from practical acid wastewater.
Data availability
All the data and materials applied in the study could be available from the corresponding author only on academic or other non-business requests.
References
Ahmed W, Mehmood S, Nunez-Delgado A, Ali S, Qaswar M, Khan ZH, Ying H, Chen DY (2021) Utilization of citrullus lanatus l. Seeds to synthesize a novel MnFe2O4-biochar adsorbent for the removal of U(VI) from wastewater: insights and comparison between modified and raw biochar. Sci Total Environ 771:144955
Ahmed W, Mehmood S, Qaswar M, Ali S, Khan ZH, Ying H, Chen DY, Nunez-Delgado A (2021) A. Nunez-Delgado, Oxidized biochar obtained from rice straw as adsorbent to remove uranium (VI) from aqueous solutions. J Environ Chem Eng 9(2):105104
Chen C, Zhang XW, Jiang TJ, Li M, Peng Y, Liu XD, Ye J, Hua YL (2021) Removal of uranium(VI) from aqueous solution by Mg(OH)2-coated nanoscale zero-valent iron: Reactivity and mechanism. J Environ Chem Eng 9(1):104706
Cheng YJ, Dong HR, Hao TW (2021) CaCO3 coated nanoscale zero-valent iron (nZVI) for the removal of chromium(VI)in aqueous solution. Sep Purif Technol 257:117967
Dai ZR, Zhen Y, Sun YS, Li L, Ding DX (2021) ZnFe2O4/g-C3N4 S-scheme photocatalyst with enhanced adsorption and photocatalytic activity for uranium(VI) removal. Chem Eng J 415:129002
Devi P, Kothari P, Dalai AK (2020) Stabilization and solidification of arsenic and iron contaminated canola meal biochar using chemically modified phosphate binders. J Hazard Mater 385:121559
Ding L, Tan WF, Xie SB, Mumford K, Lv JW, Wang HQ, Fang Q, Zhang XW, Wu XY, Li M (2018) Uranium adsorption and subsequent re-oxidation under aerobic conditions by leifsonia sp - coated biochar as green trapping agent. Environ Pollut 242:778–787
Dutta DP, Nath S (2018) Low cost synthesis of SiO2/C nanocomposite from corn cobs and its adsorption of uranium (VI), chromium (VI) and cationic dyes from wastewater. J Mol Liq 269:140–151
Fan YH, Wang H, Deng LY, Wang Y, Kang D, Li CZ, Chen H (2020) Enhanced adsorption of pb(II) by nitrogen and phosphorus co-doped biochar derived from camellia oleifera shells. Environ Res 191:110030
Fu MT, Ao JX, Ma L, Kong DX, Qi SM, Zhang P, Xu G, Wu MH, Ma HJ (2022) Uranium removal from waste water of the tailings with functional recycled plastic membrane. Sep Purif Technol 287:120572
Guilhen SN, Mašek O, Ortiz N, Izidoro JC, Fungaro DA (2019) Pyrolytic temperature evaluation of macauba biochar for uranium adsorption from aqueous solutions. Biomass Bioenergy 122:381–390
Guo XJ, Chen RR, Liu Q, Liu JY, Zhang HS, Yu J, Li RM, Zhang ML, Wang J (2018) Superhydrophilic phosphate and amide functionalized magnetic adsorbent: a new combination of anti-biofouling and uranium extraction from seawater. Environ Sci Nano 5(10):2346–2356
Hu R, Xiao J, Wang TH, Chen GC, Chen L, Tian XY (2020) Engineering of phosphate-functionalized biochars with highly developed surface area and porosity for efficient and selective extraction of uranium. Chem Eng J 379:122388
Hua Y, Wang W, Hu N, Gu TH, Ling L, Zhang WX (2021) Enrichment of uranium from wastewater with nanoscale zero-valent iron (nZVI). Environ Sci: Nano 8(3):666–674
Li F, Yu XY, Fang HS, Zong RW (2021) Influence of polymerization degree on the dynamic interfacial properties and foaming ability of ammonium polyphosphate (APP)-surfactant mixtures. J Mol Liq 335:116175
Li HL, Li Y, Zhou YZ, Li BL, Liu DB, Liao HY (2019a) Efficient removal of uranium using a melamineitrimesic acid-modified hydrothermal carbon-based supramolecular organic framework. J Colloid Interface Sci 544:14–24
Li J, Fana MJ, Li M, Liu X (2020) Cr(VI) removal from groundwater using double surfactant-modified nanoscale zero-valent iron (nZVI): effects of materials in different status. Sci Total Environ 717:137112
Li N, Yin ML, Tsang DCW, Yang ST, Liu J, Li X, Song G, Wang J (2019b) Mechanisms of U(VI) removal by biochar derived from ficus microcarpa aerial root: a comparison between raw and modified biochar. Sci Total Environ 697:134115
Li S, Tang JC, Yu C, Liu QL, Wang L (2022a) Efficient degradation of anthracene in soil by carbon-coated nzvi activated persulfate. J Hazard Mater 431:128581
Li TL, Gao CL, Wang W, Teng YX, Li X, Wang HT (2022b) Strong influence of degree of substitution on carboxymethyl cellulose stabilized sulfidated nanoscale zero-valent iron. J Hazard Mater 425:128057
Li ZY, Zhu RM, Zhang PL, Yang M, Zhao RQ, Wang YL, Dai X, Liu W (2022c) Functionalized polyarylether-based cofs for rapid and selective extraction of uranium from aqueous solution. Chem Eng J 434:134623
Liao H, Zhu WK, Duan T, Zhang YD, He GQ, Wei YX, Zhou J (2021) Beaded segments like bi-metallic nano-zero-valent iron-titanium for the fast and efficient adsorption and reduction of U(VI) in aqueous solutions. Colloids Surf A Physicochem Eng Aspects 613:126080
Liu SC, Wu MB, Ye H, Liu L, Ma LL, Yao JM (2021a) Amidoximated cellulose microspheres synthesized via homogenous reactions for high-performance extraction of uranium from seawater. Chem Eng J 426:131378
Liu T, Zhang XB, Wang H, Chen MW, Yuan YH, Zhang RQ, Xie ZJ, Liu YJ, Zhang HQ, Wang N (2021b) Photothermal enhancement of uranium capture from seawater by monolithic mof-bonded carbon sponge. Chem Eng J 412(9):128700
Lu W, Dai ZR, Li L, Liu JQ, Wang SP, Yang HX, Cao C, Liu L, Chen T, Zhu BY, Sun L, Chen L, Li HQ, Zhang P (2020) Preparation of composite hydrogel (PCG) and its adsorption performance for uranium (VI). J Mol Liq 303:112604
Lu Z, Zhang H, Shahab A, Zhang K, Zeng HT, Bacha AUR, Nabi I, Ullah H (2021) Comparative study on characterization and adsorption properties of phosphoric acid activated biochar and nitrogen-containing modified biochar employing eucalyptus as a precursor. J Clean Prod 303:127046
Ran GW, Liu XD, Guo J, Sun J, Li HF, Gu XY, Zhang S (2019) Improving the flame retardancy and water resistance of polylactic acid by introducing polyborosiloxane microencapsulated ammonium polyphosphate. Compos Part B Eng 173:106772
Ruan Y, Zhang HM, Yu ZJ, Diao ZH, Song G, Su MH, Hou LA, Chen DY, Wang S, Kong LJ (2022) Phosphate enhanced uranium stable immobilization on biochar supported nano zero valent iron. J Hazard Mater 424:127119
Sun YB, Zhang HY, Yuan N, Ge YL, Dai Y, Yang Z, Lu L (2021) Phosphorylated biomass-derived porous carbon material for efficient removal of U(VI) in wastewater. J Hazard Mater 413:125282
Sun YW, Zeng BY, Dai YT, Liang XJ, Zhang LJ, Ahmad R, Su XT (2022) Modification of sludge-based biochar using air roasting-oxidation and its performance in adsorption of uranium(VI) from aqueous solutions. J Colloid Interface Sci 614:547–555
Tang HP, Cheng WC, Yi YP, Ding CC, Nie XQ (2021) Nano zero valent iron encapsulated in graphene oxide for reducing uranium. Chemosphere 278:130229
Tian Y, Liu LJ, Ma FQ, Zhu XY, Dong HX, Zhang CH, Zhao FB (2021) Synthesis of phosphorylated hyper-cross-linked polymers and their efficient uranium adsorption in water. J Hazard Mater 419:126538
Wang BL, Li YY, Zheng JL, Hu YW, Wang XJ, Hu BW (2020) Efficient removal of U(VI) from aqueous solutions using the magnetic biochar derived from the biomass of a bloom-forming cyanobacterium (Microcystis aeruginosa). Chemosphere 254:126898
Wang D, Xu YB, Xiao DF, Qiao QG, Yin P, Yang ZL, Li JX, Winchester W, Wang Z, Hayat T (2019a) Ultra-thin iron phosphate nanosheets for high efficient U(VI) adsorption. J Hazard Mater 371:83–93
Wang FH, Xu X, Xia YP, Dong BB, Ke NW, Hao LY, Bi L, Xu X, Liu W (2021) A novel CO2-tolerant Ba0.5Sr0.5Co0.8Fe0.1Ta0.1O3-δ cathode with high performance for proton-conducting solid oxide fuel cells. Int J Hydrogen Energ 46(67):33561–33571
Wang SS, Zhao MY, Zhou M, Li YC, Wang J, Gao B, Sato S, Feng K, Yin WQ, Igalavithana AD, Oleszczuk P, Wang XZ, Ok YS (2019b) Biochar-supported nZVI (nZVI/BC) for contaminant removal from soil and water: a critical review. J Hazard Mater 373:820–834
Wen JX, Fu WY, Ding SH, Zhang Y, Wang W (2022) Pyrogallic acid modified nanoscale zero-valent iron efficiently removed Cr(VI) by improving adsorption and electron selectivity. Chem Eng J 443:136510
Wu S, Liu YH, Wang C, Dai HL, Wang XF, Bi L (2021) Cobalt-free LaNi0.4Zn0.1Fe0.5O3-δ as a cathode for solid oxide fuel cells using proton-conducting electrolyt. Int J Hydrogen Energ 46(77):38482–38489
Yang JW, Ji GZ, Gao Y, Fu W, Irfan M, Mu L, Zhang YL, Li AM (2020) High-yield and high-performance porous biochar produced from pyrolysis of peanut shell with low-dose ammonium polyphosphate for chloramphenicol adsorption. J Clean Prod 2264:121516
Yang PP, Zhang HS, Liu Q, Liu JY, Chen RR, Yu J, Hou JD, Bai XF, Wang J (2019) Nano-sized architectural design of multi-activity graphene oxide (GO) by chemical post-decoration for efficient uranium(VI) extraction. J Hazard Mater 375:320–329
Ye Z, Xu N, Li D, Qian JC, Du CS, Chen M (2021) Vitamin C mediates the activation of green tea extract to modify nanozero-valent iron composites: enhanced transport in heterogeneous porous media and the removal of hexavalent chromium. J Hazard Mater 411:125042
Zhang DW, Zhang KJ, Hu XL, He QQ, Yan JP, Xue YW (2021a) Cadmium removal by MgCl2 modified biochar derived from crayfish shell waste: batch adsorption, response surface analysis and fixed bed filtration. J Hazard Mater 408:124860
Zhang J, Chen Y, Song X, Liu YD, Zhao JH, Wang FY (2022a) Synergistic adsorption and degradation of diclofenac by zero-valent iron modified spent bleaching earth carbon: Mechanism and toxicity assessment. J Hazard Mater 432:128753
Zhang WH, Han X, You J, Zhang XF, Pei DF, Willfor S, Li MJ, Xu CL, Li CX (2022b) Rapid and manual-shaking exfoliation of amidoximated cellulose nanofibrils for a large-capacity filtration capture of uranium. J Mater Chem a 10(14):7920–7927
Zhang Q, Wang YY, Wang Z, Zhang ZJ, Wang XD, Yang ZL (2021b) Active biochar support nano zero-valent iron for efficient removal of U(VI) from sewage water. J Alloys Compd 852:156993
Zhao L, Wang SY, Zhuang HH, Lu B, Sun LN, Wang G, Qiu JS (2022) Facile synthesis of low-cost mnpo4 with hollow grape-like clusters for rapid removal uranium from wastewater. J Hazard Mater 434:128894
Zhao X, Liu W, Cai ZQ, Han B, Qian TW, Zhao DY (2016) An overview of preparation and applications of stabilized zero-valent iron nanoparticles for soil and groundwater remediation. Water Res 100:245–266
Zhou L, Li Z, Yi YQ, Tsang EP, Fang Z (2022) Increasing the electron selectivity of nanoscale zero-valent iron in environmental remediation: a review. J Hazard Mater 421:126709
Zhou YB, Xiao J, Hu R, Wang TH, Shao XL, Chen GC, Chen L, Tian XY (2020) Engineered phosphorous-functionalized biochar with enhanced porosity using phytic acid-assisted ball milling for efficient and selective uptake of aquatic uranium. J Mol Liq 303:112659
Funding
This work was supported by the National Natural Science Foundation of China (12175103), the Key R&D Program of Hunan Province (2018SK2029), the Hunan Provincial Natural Science Foundation for Excellent Young Scholars (2020JJ3028), the Scientifc Research Fund of Hunan Provincial Education Department (21A0259), and the Fund of Hengyang Key Laboratory (202150083891).
Author information
Authors and Affiliations
Contributions
Ziwei Tang: conceptualization, methodology, validation, formal analysis, investigation, data curation, and writing—review and editing. Zhongran Dai: investigation, writing—review and editing, and supervision. Mi Gong: investigation. Hong Chen: data curation. Xiayu Zhou: investigation. Yating Wang: investigation. Cong Jiang: investigation. Wanying Yu: data curation. Le Li: validation, formal analysis, data curation, writing—review and editing, supervision, and funding acquisition.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All authors consent when it is submitted.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Tito Roberto Cadaval Jr
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tang, Z., Dai, Z., Gong, M. et al. Efficient removal of uranium(VI) from aqueous solution by a novel phosphate-modified biochar supporting zero-valent iron composite. Environ Sci Pollut Res 30, 40478–40489 (2023). https://doi.org/10.1007/s11356-022-25124-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-25124-9