Abstract
A novel biochar carried nanoscale zero-valent iron (NZVI) particles were synthesized by means of liquid phase reduction method that can overcome the aggregation of NZVI particles. The Composite material of the biochar-NZVI can effectively improve the removing rate of hexavalent chromium Cr(VI) in the application of water treatment. The experimental results showed that the remove rate of Cr(VI) was up to 96.8% by using biochar-NZVI when the mass ratio of biochar and NZVI was of 5:1, the rate was enhanced by about 35.9% than that of the same dose pure NZVI. The analyzed results of TEM and BET showed that the biochar-NZVI had higher dispersion and specific surface area than pure NZVI, which was the key reason for biochar-NZVI with high removing efficiency of Cr(VI). The apparent rate constant kobs decreases from 0.1041 to 0.0235 min−1 as the initial concentration of Cr(VI) increased from 25 to 125 mg/L in the solution, indicating that the reaction velocity decreases with the increased initial concentration of Cr(VI) in the solution. The removal efficiency reached to 92.1% when the pH value of the solution containing biocha-NZVI treating Cr(VI) increased from 4.48 to 8.36, showing that the biochar carried NZVI has high removing rate of Cr(VI) in a wide range of pH value.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
Introduction
Nanoscale zero-valent iron (NZVI) with a larger specific surface area and a high reactivity, can effectively remove heavy metal pollutants. Recently years, it reported that nanoscale zero-valent iron (NZVI) attached on montmorillonite [1], bentonite [2], activated carbon and other materials maintain its strong reduction characteristics in the field of environmental remediation, and enhance its stability and suitable for engineering operation. Compared with above carriers, biochar is a kind of a easy to gat raw material, low cost, superior adsorption performance and environmentally friendly materials [3]. At the same time, it has not only good pore structure and large specific surface area, which easily disperse NZVI particles. Obviously, NZVI carried by biological carbon may have good engineering application prospects [4]. However, the studies and related reports on biological carbon carrying NZVI and the research on removal of heavy metals are few.
Six valence chromium Cr(VI) is a common pollutants in water environment, which can easily invade into human body through the digestive tract, respiratory tract and skin contacts, harm to human health. With the development of economy and the change of consumption structure, more and more chromium wastewater discharged into the environment through tanning, electroplating, metallurgy and other industries. Therefore, The effectively removing methods of Cr(VI) in water received wide attention [5, 6]. NZVI is an efficient reducing agent have got much attention because can effectively reduce Cr(VI) to trivalent chromium Cr(III) that is of low toxicity. In the present study, we studied the properties of biological carbon carried NZVI to remove Cr(VI) in solution using liquid phase reduction method. The results will provide some theoretical basis for engineering application.
Materials and Methods
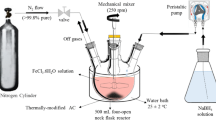
Materials Preparation. The prepared biochar was over a 100 mesh screen, immersed in 1.0 mol/L hydrochloric acid 12 h to remove impurities, and washed it with deionized water to neutral water, then dried for use. The preparing and attaching of NZVI on the cleaned biochar was operated under anaerobic conditions. 1.0 g nano zero valent iron was made by solving 5 g FeSO4·7H2O in a 100 mL ethanol and deionized water intermixture with the alcohol water ratio of 4:1 (v/v), and dropping 50 mL 20 g/L KBH4 solution one by one with stirring under nitrogen protection. Then 5.0 g biochar was soaked in FeSO4·7H2O solution 60 min with stirring velocity 200 r/min to fully mixed. The remaining steps the same as above. Finally, 6.0 g biochar carried NZVI with a carbon iron mass ratio of 5:1 was made. The chemical equation of the reaction as
After the reaction, washing the obtained material a few times with deionized water and absolute ethanol, which were purged nitrogen 1 h to remove oxygen before using, finally dried it under nitrogen protection.
Material Characterization. Biochar-NZVI mixed with 2.0 mol/L hydrochloric acid and hydroxylamine hydrochloride solution, dissolved iron and the solution heated in a water bath at 100°C, then filtered with 0. 22 μm membrane. The filtrate was measured with phenanthroline colorimetric method to determination the content of ferrous ion, and converted to the mass of NZVI. The mass ration of iron and carbon in the biochar was obtained by compared the dried filter residue with the mass of NZVI.
The size of the material particles was observed by Hitachi HT7700 transmission electron microscope (TEM) under 100 kV operating voltage. The surface functional groups of biochar were measured using NICOLET iN10 MX Fourier transform infrared spectrometer (FT-IR); the crystal structure was analyzed using thermo ARL X’TRA X-ray differaction (XRD), and the specific surface area and aperture parameters of material were measured by ASAP 2020 surface area meter.
Experiment Methods. The Cr(VI) water samples used in the experiment were prepared using K2Cr2O7 to a stock solution with a concentration of 150 mg/L. First, the removing effect to Cr(VI) in solution was investigate by comparing the results of biochar, nano zero valent iron, and biochar-NZVI with the mass ration of iron and carbon of 3:1, 5:1, 7:1, noted as 3BC-Fe, 5BC-Fe and 7BC-Fe, respectively. The initial concentration of Cr(VI) used in the experiment was 50 mg/L, the average mass of NZVI in various materials is 0.5 g/L,the reaction temperature was 25 °C, and the solution initial pH was 4.5.
The influence of the initial concentration of Cr(VI) on the removing effect of biochar-NZVI to Cr(VI) was analyzed using 5BC-Fe solution. The initial concentration of Cr(VI) was set as 25, 50, 75, 100 and 125 mg/L; the amount of biochar-NZVI was 3.0 g/L, the reaction temperature was 25°C,and the solution initial pH was 4.5.
The influence of the initial pH in the solution to the remove effect of Cr(VI) was selected 5BC-Fe solution. The initial pH was set as 4.5, 6.5, 7.0, 8.5, and 10.5; the amount of biochar-NZVI was 3.0 g/L, and the reaction temperature was 25 °C.
Third, the effect of temperature on remove Cr(VI) was selected 5BC-Fe solution. The temperature was set as 15, 25, 35, 45 and 55 °C, The initial concentration of Cr(VI) was 50 mg/L; the amount of biochar-NZVI was 3.0 g/L, and the solution initial pH was 4.5.
Each experiment was carried in a 250 mL conical flask stirred by amagnetic stirrer with a speed of 200 r/min. Samples was took in the set time point and filtered using 0.22 μm membrane. The concentration of Cr(VI) was determined using two benzoyl hydrazine spectrophotometry by means of Alpha-1106 spectrophotometry of 540 nm wavelength light.
Result and Discuss
Materials Characterization. First, we validate the mass ration of iron and carbon in biochar-NZVI. The results show that the mass ration of iron and carbon was 1: 6.84, 1:4.92 and 1: 2.88 in the 7BC-Fe, 5BC-Fe and 3BC-Fe samples, basically consistent with the mass ratio of iron carbon settled in the original experimental. Figure 1a–c are the appearance pictures observed in biochar, NZVI, and BC-Fe by TEM. We can see that biochar takes the shape of flakes, NZVI is of spherical particles with a size about 30–70 nm. In Fig. 1c shows that many NZVI particles are attached on the surface of biochar, indicating that biochar successfully carried NZVI partiles. The shape of NZVI is characteristic of a chain because of their larger specific surface area leading to a large attraction between particles [7]. In the TEM picture we observed that almost each the biochar flake is attached on NZVI particles. The measured specific surface area for biochar, NZVI Fe, 3BC-Fe, 5BC-Fe and7BC-Fe is 205.4, 20.9, 138.1, 142.8 and 126.6 m2/g, respectively. The decrease of the specific surface area of biochar may be related to the holes and channels of biochar filled up by NZVI particles.
Figure 2 shows the spectrum of Fourier transform infrared spectroscopy. The absorption peak at 3400 cm−1 is attributed to the stretching vibration caused by –OH group; the peak at 1710 cm−1 is caused by the vibration of C=O band in carboxyl group; and the peak at 1103 cm−1 corresponding to the stretching vibration of C=O bands in phenol, alcohol and other substances [8]. In the preparation of biochar-NZVI, these groups can absorb Fe(II) of the solution, which help to the carrying of NZVI.
Figure 3 shows the XRD spectrum of the three kinds samples [9]. Obviously, there is a main peak at 44.5° from NZVI Fe, corresponding to the characteristic diffraction peak of α-Fe [10, 11], indicating that the main form of iron in the samples is α-Fe. The peak at 2θ = 22.7° is characteristics a amorphous structure of carbon from the characteristic peak of the biochar samples [12]. The two characteristic peaks of the biochar-NZVI samples in the XRD spectrum shows that NZVI Fe and biochar both exist simultaneously in the samples.
Biochar-NZVI removing Cr(VI). Figure 4 shows the remove behaviors of the NZVI and biochar-NZVI materials to Cr(VI). It is seen that the removal reaction to Cr(VI) basically achieves to a balance about 120 min. In 120 min, the removing effect of biochar with mass concentration of 2.5 g/L to Cr(VI) is not obvious. The removal rate is only about 16.1%. This because Cr(VI) in the solutions exists in the forms of \( {\text{HCr}}_{2} {{\text{O}}_{7}}^{ - } ,\,{\text{Cr}}_{2} {{\text{O}}_{7}}^{2 - } \,{\text{and}}\,{{\text{CrO}}_{4}}^{2 - } \) etc. anionics. Although biochar has porous structure and large specific surface area, the carried surface negative charges are mutually exclusive with \( {\text{HCr}}_{2} {{\text{O}}_{7}}^{ - } ,\,{\text{Cr}}_{2} {{\text{O}}_{7}}^{2 - } \,{\text{and}}\,{{\text{CrO}}_{4}}^{2 - } \) etc. anionics, leading to low removing efficiency to Cr(VI).
The removing efficiency of NZVI sample to Cr(VI) is about 60.9% in 120 min. Clearly, biochar-NZVI sample significantly increases the removal capacity of Cr(VI). The removing efficiency of Cr(VI) enhanced from 60.9 to 92.0, 96.8 and 85.0% for 3BC-Fe, 5BC-Fe and 7BC-Fe samples, respectively. This because NZVI particles attached on biochar can effectively overcome the defects of NZVI, increasing the removing efficiency of biochar-NZVI to Cr(VI). At the same time, biochar has a certain adsorption capacity to Cr(VI), which can adsorb Cr(VI) on the surface of it. The larger concentration of NZVI is the fast the remove reaction does.
The result of Fig. 4 shows that the ratio of carbon and iron in biochar-NZVI has influence the removing effect to Cr(VI). Compared the removal efficiency of the biochar-NZVI samples to Cr(VI), we found that the efficiency of 5BC-Fe sample is larger about 4.8% than that of 3BC-Fe sample. However, it reduced about 11.8% for 7BC-Fe than that of 5BC-Fe. As a carrier biochar can overcome the aggregation defect of NZVI, then increases the reaction rate. But too much biochar will lead reunion between biochars [13] that lows the specific surface area of material, finally leading to low the removal efficiency of Cr(VI). Chen et al. [14] thought that the reaction rate reduced because too much biochar will occupy the activity position of NZVI. Therefore, there is an optimum proportion of iron and crrbon in the biochar-NZVI material. The results found that the removal effect of biochar-NZVI Fe to Cr(VI) is better than that of other materials when the ration of iron and carbon is 5:1.
The Influence Factors of Biochar-NZVI Fe to Cr(VI). Figure 5 shows the relationship of removal effect with the initial concentration of Cr(VI). In 120 min reaction time, the removal rate of Cr(VI) in solution decreases with the initial concentration increasing of Cr(VI). The rate is 100, 96.8, 92.8, 83.7 and 78.8% when the initial concentration is 25, 50, 75, 100 and 125 mg/L, respectively. The removing process of biochar-NZVI to Cr(VI) can be divided into two stages: in the first 60 min the removing speed is very fast. In this case Cr(VI) ions in the solution were quickly absorbed on the adsorption sites of the material surface. With the reaction proceeding, Cr(VI) began to diffuse into the pores of biochar because the surface adsorption site decreases gradually. Since the iron oxide formed in previous reaction might block the pores, leading to the removing speed to Cr(VI) decreases in the second stage. Zhu [15] found the similar result in the supported nano iron removing As(V) in drinking water.
a The influence of the initial concentration of Cr6+ on the removing efficiency of Cr6+ by BC-Fe; b The pseudo-first-order kinetics fitting for different initial concentration of the Cr6+ reduced by BC-Fe; c The influence of the initial pH on the removing of Cr6+ by BC-Fe; d The pseudo-first-order kinetics fitting of the reduction Cr6+ by BC-Fe in different initial pH; e The influence of temperature on the removing of Cr6+ by BC-Fe; f The pseudo-first-order kinetics fitting of the reduction of Cr6+ by BC-Fe at different temperature
Generally, the process of removing Cr(VI) using NZVI or bimetallic materials took place on the surface of materials is a heterogeneous reactions. The Kinetic process can be well fitted using the pseudo first order reaction kinetics model. The pseudo first order reaction as
where, c is the concentration (mg/L) of Cr(VI); c0 is the initial concentration of Cr(VI) (mg/L); kobs is the apparent rate constant, it can be obtained by linear fitting to ln (c/c0) and t.
Figure 5b is the fitting results using pseudo first order kinetic each reaction for different initial concentration of Cr(VI). kobs is 0.1041, 0.0584, 0.0449, 0.0306 and 0.0235 min−1 when initial concentration of Cr(VI) is 25, 50, 75, 100 and 125 mg/L, respectively. Clearly, kobs decreases with the increasing of the initial concentration of Cr(VI). This because NZVI deduction Cr(VI) is a surface controlled reaction process. The reaction speed has related to the number of surface active sites of NZVI as it is not affected by the mass transfer. At a low concentration of Cr(VI) in the solution, the surface active sites of NZVI are not saturated, the reaction speed is larger. With the increasing concentration of Cr(VI), the surface active sites of NZVI tends saturate, the reaction near zero order kinetics and the reaction rate declines [16]. It can be seen that kobs has a good linear relationship with Cr(VI) concentration when the concentration is between 50 and 125 mg/L.
kobs increase sharply when the initial concentration of Cr(VI) is 25 mg/L that is smaller about 10 times than that of the concentration of NZVI. This because Cr(VI) is reduced to Cr(III) when Cr(VI) reacts with NZVI carried by biochar. At the same time, NZVI is reduced to Fe(III). Cr(III)-Fe(III) co-deposition were created, which hinder the reaction continually when they attached on the surface of NZVI [16]. Melitas et al. [17] found that the increasing concentration of Cr(VI) will promote the formation of passivation layer, thereby reduced the reaction rate.
Figure 5c shows that the relationship between the removal efficiency and the initial pH value in the solution. The removal rate of Cr(VI) by biochar-NZVI is 96.8, 93,692,992.1 and 79.7% when the pH value is 4.5, 6.5, 7.0, 8.5 and 10.5 in 120 min. Obviously, the removal rate decreases gradually with the increasing PH. Comparing the removal rate of the four groups, we found that removal rate biochar-NZVI have good removal effect when PH is from acidic to weakly alkaline range.
Figure 5d shows that the pseudo first order kinetic fitting relationship between the removal effect and the initial pH of the solution. The five groups show a better linear correlation (the correlation coefficient r2 over 97%). It is found that the initial PH has some influence to the apparent rate constant. kobs researches the maximum when pH = 4.5, and it has the best removal effect. The removal rate of Cr(VI) drops gradually when kobs decreases from 0.0307 to 0.0143 min−1, and the initial pH increases from 4.5 to 10.5.
The removal effect of pH to Cr(VI) is related to the reaction process. The reaction equation of Cr(VI) reduced to low toxicity Cr(III) by Fe as following
where, Fe2+ still has reducibility, it can continually reduced Cr(VI) to Cr(III), that is,
Combined the two reaction equations as
From the above analysis, we can get the main reason of pH effect to the remove reaction of Cr(VI) is that Fe(III)–Cr(III) hydroxide co-precipitation were formed on surface of NZVI, which blocked the internal electron of NZVI transfer to outward [4] when PH is too high. In addition, Cr(VI) exists in a Cr2O 27 negatively charge form in the solution, high PH will lead to the increase of the negative charge on the surface of NZVI. It will generate a rejection to Cr2O72 that is unfavorable to the reaction. At the same time, too high PH will prevent zero valent iron from turning to Fe(II) (etching process), thus inhibiting the reduction of Cr(VI).
The removal reaction of biochar-NZVI to Cr(VI) is well accordance with the pseudo first order kinetics. According to the theory of chemical reaction dynamics, temperature can affect the rate of first order reaction kinetics, as shown in Fig. 5e. The removal rate of Cr(VI) reaches 91.9, 96.8, 99.2, 100 and 100% when the reaction temperature is 15, 25, 35, 45 and 55 °C, respectively. In Fig. 5f the apparent rate constant increases from 0.0222 to 0.0715 min−1 when the reaction temperature increases from 15 to 55 °C. It shows that the thermal motion of molecules increases that makes the number of activated molecules increase also, thus the reaction is speed up [18].
Summary
The removal effect of biochar-NZVI to Cr(VI) by biochar carried NZVI using liquid phase reduction preparation was investigated. The results showed that biochar-NZVI has better dispersion and higher specific surface area than that of pure NZVI, and overcomes the agglomeration defects of NZVI Fe. The removal rate of 5BC-Fe sample to Cr(VI) reached 96.8% at the initial concentration of Cr(VI) 50 mg/L and in the same initial concentration of NZVI. It was about 35.9% higher than that of the pure NZVI dosage, indicating that biochar-NZVI has better removal effect to Cr(VI) that favors to engineering application
The removal of biochar-NZVI to Cr(VI) accords with the pseudo first order kinetics. The study of the theory of chemical reaction dynamics shows that the removal rate of biochar-NZVI to Cr(VI) in solution decrease with the increase of initial concentration and initial pH value. In addition, the increase of reaction temperature can improve the removal rate of biochar-NZVI to Cr(VI) in the solution.
The removal rate of biochar-NZVI samples to Cr(VI) in the solution is all over 92.1% when the initial pH of the reaction solution is between 4.48 and 8.36, indicating that the synthesized materials have good removal effect on Cr(VI) in acidic to weakly alkaline pH conditions.
References
G. Quan, J. Zhang, J. Guo, Y. Lan, Removal of Cr (VI) from aqueous solution by nanoscale zero-valent iron grafted on acid-activated attapulgite. Water Air Soil Pollut 225, 1979–1989 (2014)
C. Yan, W. Chen, L. Pan, Q. Wang, Removal of lead in water by nano zero valent iron loaded on bentonite. J. Water Resour Water Eng (in Chinese), 24, 20–24 (2013)
Q. Zhao, Q. Zhao, H. Wang, H. Yu, Z. Hui, Review of biochar soil improvement mechanism and the application prospect in our country tropical area. Chin. J. Trop. Agric. (in Chinese), 34, 53–57 (2014)
Y. Zhou, B. Gao, A.R. Zimmerman, H. Chen, M. Zhang, X. Cao, Biochar-supported zero valent iron for removal of various contaminants from aqueous solutions. Bioresour Technol 152, 538–542 (2014)
M. Avila, T. Burks, F. Akhtar, M. Göthelid, P.C. Lansåker, M.S. Toprak, M. Muhammed, A. Uheida, Surface functionalized nanofibers for the removal of chromium (VI) from aqueous solutions. Chem. Eng. J. 245, 201–209 (2014)
Y. Wang, Z. Fang, B. Liang, E.P. Tsang, Remediation of hexavalent chromium contaminated soil by stabilized nanoscale zero-valent iron prepared from steel pickling waste liquor. Chem. Eng. J. 247, 283–290 (2014)
H. Jabeen, V. Chandra, S. Jung, J.W. Lee K.S. Kim, S.B. Kim, Enhanced Cr(VI) removal using iron nanoparticle decorated graphene. Nanoscale 3, 3583–3585 (2011)
Z. Liu, F. Zhang, J. Wu, Characterization and application of chars produced from pinewood pyrolysis and hydrothermal treatment. Fuel 89, 510–514 (2010)
J. Yan, L. Han, W. Gao, S. Xue, M. Chen, Biochar supported nanoscale zerovalent iron composite used as persulfate activator for removing trichloroethylene, Biores Technol 175, 269–274 (2015)
Q. Wang, S. Snyder, J. Kim, Aqueous Ethanol modified nanoscale zerovalent iron in bromate reduction: synthesis, characterization, and reactivity. Environ. Sci. Technol. 43, 3292–3299 (2009)
X. Wang, C. Chen, H. Liu, J. Ma, Characterization and evaluation of catalytic dechlorination activity of Pd/Fe bimetallic nanoparticles. Indus. Eng. Chem. Res. 47, 8645–8651 (2008)
Z. Dai, J. Meng,. N. Muhammad, X. Liu, H. Wang, Y. He, P.C. Brookes, J. Xu, The potential feasibility for soil improvement, based on the properties of biochars pyrolyzed from different feedstocks. J. Soils Sed. 13, 989–1000 (2013)
J. Chen, J. Zhu, Z. Da, H. Xu, J. Yan, H. Ji, H. Shu, H. Li, Improving the photocatalytic activity and stability of graphene-like BN/AgBr composites. Appl. Surf. Sci. 313, 1–9 (2014)
Z. Chen, Y. Cheng, Z. Chen, M. Mallavarapu, N. Ravendra, Kaolin-supported nanoscale zero-valent iron for removing cationic dye-crystal violet in aqueous solution. J. Nanoparticle Res. 14, 899 (2012)
H. Zhu, Y. Jia, X. Wu, H. Wang, Removal of arsenic from water by supported nano zero-valent iron on activated carbon. J. Hazard. Mat. 172, 1591–1596 (2009)
R. Venkatapathy, D.G. Bessingpas, S. Canonica, J.A. Perlinger, Kinetics models for trichloroethylene transformation by zero-valent iron. Appl. Catal B: Environ. 37, 139–159 (2002)
N. Melitas, J.P. Wang, C.M. Peggy, F. James, Understanding soluble arsenate removal kinetics by zerovalent iron media. Environ. Sci. Technol. 36, 2074–2081 (2002)
X. Li, J. Cao, W. Zhang, Stoichiometry of Cr (VI) immobilization using nanoscale zerovalent iron (nZVI): a study with high-resolution X-ray photoelectron Spectroscopy (HR-XPS). Indus. Eng. Chem. Res. 47, 2131–2139 (2008)
Acknowledgements
This work is supported in parts by the National Natural Science Foundation of China (No. 31570515) and the Scientific Project Program of Suzhou City (No. SYN201511).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Shi, W., Song, X. (2018). Removal of Hexavalent Chromium from Aqueous Using Biochar Supported Nanoscale Zero-Velent Iron. In: Han, Y. (eds) Advances in Energy and Environmental Materials. CMC 2017. Springer Proceedings in Energy. Springer, Singapore. https://doi.org/10.1007/978-981-13-0158-2_89
Download citation
DOI: https://doi.org/10.1007/978-981-13-0158-2_89
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-0157-5
Online ISBN: 978-981-13-0158-2
eBook Packages: EnergyEnergy (R0)