Abstract
Sardine co-products can represent an interesting source of bioactive compounds, such as polyunsaturated fatty acids and in particular omega-3. This study aimed to investigate extraction of oil from sardine co-products by enzymatic hydrolysis using two proteases: commercial Alcalase and protease Bb from a local fungal strain (P2) of Beauveria bassiana, which overproduces proteases. Despite a higher degree of hydrolysis (41.34%) than Alcalase (24.28%), protease Bb allowed the extraction of approximately the same oil content. Resulting oil from both processes had the same fatty acid profile. Interestingly, the all-produced oil displayed an attractive w6/w3 ratio, an indicator of nutritional quality, of the order of 0.16. The safety of the generated oils was also assessed by treating two groups of Wistar rats with the fish oil administered by oral gavage at the doses (30 mg/kg and 300 mg/kg body weight) for 14 days using olive oil as a vehicle. Compared to controls used, both treated groups showed no statistically significant differences. Consequently, the acute oral toxicity evaluated by hematological, biochemical, and histological studies showed the safety of the oil generated using B. bassiana protease.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dietary fish oil supplements are convenient for the enrichment of diets with low omega-3 fat, which are a key family of long-chain polyunsaturated fatty acids (Rizliya and Mendis 2014). Fish oil or lipid is also a rich source of vitamins, including vitamins A, D, E, and K. The nutritional and health implications of marine oils have been shown to prevent cardiovascular diseases and atherosclerosis (Yates et al. 2014) and to improve the cognitive functions as well as the development of the central nervous system (Moro et al. 2016). Substantial quantities of fat are present in fish co-products (head, viscera, and skin) that are generated by the fishery and aquaculture product processing industry; however, they remain very poorly recovered (Tacias-Pascacio et al. 2021).
The processing techniques involved in the commercial production of fish oils vary according to the type of raw material and its use. There are a number of processes used to convert raw fish and fish trimmings into fish oil. These processes can be classified into three categories: physical, biological, and chemical. Physical extraction processes include homogenizing, heating, pressing, and filtering (Jayasinghe and Hawboldt 2012). Some of these methods may affect the properties of fatty acids including a thermal degradation of long-chain polyunsaturated fatty acids and an increase of the trans-fatty acid content (Fournier et al. 2006). Lipids are generally extracted by chemical methods based on their solubility in organic solvents, which allows them to be isolated from other non-lipid constituents (Haedrich et al. 2020). The main disadvantage of the latter traditional methods is that the high temperatures used can degrade the naturally occurring heat-sensitive and labile compounds, and the toxic solvents used may still be present in the final product (Mercer and Armenta 2011).
Over the past 20 years, green extraction methods have been recognized as a promising alternative to physical or chemical technologies. Enzymatic hydrolysis uses exogenous proteolytic enzymes to digest materials to release oil and is thus one of the green extraction methods (Ramakrishnan et al. 2013) that meet the principles of the circular bio-economy (Alkhawli et al. 2019). Enzymatic hydrolysis has several characteristics that justify its adoption in the treatment of fishery processing waste as it is considered a rapid and easily reproducible method (Araujo et al. 2020).
In this study, we used two proteases: a commercial one named Alcalase and another secreted by a local fungal strain (P2) of Beauveria bassiana, a spontaneous mutant hyper-producer of protease activities (Borgi and Gargouri 2014). In the case of Alcalase obtained initially from Bacillus subtilis and called “subtilisin Carlsberg,” it is considered a “serine endopeptidase” as it contains a serine in its catalytic site, known as the classical catalytic triad of amino acids (aspartic acid, histidine, and serine). Particular interest can be addressed to its use for the treatment of waste raw materials from different industries, such as the processing of vegetable oil, fish, or poultry. It has impressive and very suitable potential in the production of peptides with very different bioactivities (Tacias-Pascacio et al. 2020). The aim of this study is to extract high omega-3 fish oil from Sardinella aurita co-products by an enzymatic method due to its advantages over chemical oil extraction. Benefits include low energy consumption, no solvent usage, and low investment for a large-scale process. More specifically, for a low investment, we will compare the oil yield using a commercial protease (Alcalase) and another produced locally (local fungal strain (P2) of B. bassiana) therefore normally at a lower cost. We also planned to determine the amount of omega-3 fatty acids (primarily EPA + DHA) as they have been associated with healthy aging throughout life as well as fetal development, cardiovascular function, and Alzheimer’s disease (Swanson et al. 2012); and determine the quality of the extracted oil according to health criteria (ratio ω-6/ω-3). Finally, we assess the safety of green fish oil extraction in Wistar rats as an animal model.
Materials and methods
Materials
The co-products of the Sardinella aurita fish were collected from a local fish shop mainly composed of medium-sized fish heads, skins, viscera, and mutilated muscles.
Two proteases were used in this work: a commercial Alcalase, and a locally produced enzyme. Proteolytic Alcalase enzyme is produced from Bacillus licheniformis with optimal working conditions for temperatures between 50 and 70° C and pH values of 6.5 to 8.5. Alcalase has been used extensively in other studies on the hydrolysis process, for fish oil and protein hydrolysates (Slizyte et al. 2005a, b), and has shown one of the highest degrees of hydrolysis of several substrates (Ovissipour et al. 2009). On the other hand, the enzyme locally produced is in fact the extracellular juice of the fungal strain (P2) of Beauveria bassiana (BP), a spontaneous hyper-producing mutant of proteases. The optimal working conditions for Bb protease are a temperature of 50–60 °C and a pH of 7–8.5 (Borgi and Gargouri 2014).
Chemical analysis
According to AOAC methods (2005), the dry matter content of the Sardinella aurita co-product was determined by gravimetry after drying the samples in an oven at 105 °C until the weight became constant. Subsequently, ash content was quantified after incubation at 650 °C for 3 h (AOAC 1984).
Total lipids were extracted from the Sardinella co-products according to the Soxhlet method using chloroform as extraction solvent (AOAC 2005). Crude protein content was determined by Kjeldahl procedure using a nitrogen-to-protein conversion factor of 6.25 (AOAC 2005).
Fatty acid analysis
The fatty acid analysis reported here is ISO/IEC 17,025–2017 accredited. Fatty acid methyl esters (FAMEs) are prepared using the derivatization method with sulfuric acid hydrolysis of lipids and methylation of fatty acids with methanol. FAMEs can also be analyzed via multiple detection modes. Margaric acid (C17:0, Sigma, France) was added as an internal standard to eliminate losses that occurred during derivatization and injection. A volume of 100 µL (40 µg) of sour margarine (C17:0, 400 µL/mL) was added to the sample for methylation (400 µg total lipid). The solvent was evaporated under nitrogen, then 5 mL of 2% concentrated sulfuric acid in anhydrous methanol was added, and the tube was capped. After cooling overnight at 50 °C, we added 1 mL of distilled water followed by 2 mL of hexane. After stirring the mixture and settling, the hexane phase (upper phase) was taken out and analyzed by gas chromatography (Sigma-Aldrich, Steinheim, Germany). The chromatography system is equipped with a flame ionization detector (FID), a spitless injector, and a polar INNOWAX fused silica capillary column (30 m × 0.25 mm id × 0.25 μm film thickness). The injector and detector temperatures were 220 °C and 275 °C, respectively. Helium was used as carrier gas with a flow rate of 1.5 mL.min−1. Peaks were identified by comparing their retention times with those of known mixtures of standard fatty acids. We used 37 fatty acid esters in standard mixture: the mixed standard (Sigma ref CRM47885, Supelco 37 component FAME Mix Sigma-Aldrich, Laramie, WY, USA) is certified in accordance with ISO 17034 and ISO/IEC. The results are expressed as percentages of the methyl esters of total fatty acids.
Enzymatic hydrolysis
The minced fish co-products of the S. aurita sample (400 g) were homogenized with water (ratio 1/1, w/v) in a 2-L bioreactor (cylindrical glass reaction vessels, with the ability to control and modify agitation, temperature, and pH online).
Before hydrolysis, the mixture was preheated to the reaction temperature (50 °C) and adjusted to the desired pH level (8) with 1 M NaOH. Enzymatic hydrolysis was started by adding Alcalase or Bb protease. During the process, the temperature was controlled using a thermo-static bath and pH was maintained constant by the manual addition of 1 N NaOH. The hydrolysis was carried out for 120 min with stirring (300 rpm, Stuart SS20). The Alcalase and Bb proteases hydrolyzed proteins at the optimum temperature (50 °C) and pH8 and cleave specific peptide bonds, resulting in the liberation of amino acids and peptides of different sizes (Wubshet et al. 2019). The hydrolysis time was fixed at 120 min because longer times do not cause a significant increase in the degree of hydrolysis. After the enzymatic hydrolysis, samples were centrifuged at 5000 g for 30 min. Four phases were generated: oil on the top layer, emulsion, hydrolyzed fish proteins, and sludge on the bottom (see Fig. 1).
The degree of hydrolysis (DH) is determined according to the formula (Adler-Nissen 1986):
where V is the volume (mL) of sodium hydroxide consumed during hydrolysis; M is the molarity of sodium hydroxide; α is the average degree of dissociation factor of α-NH2 groups released during the hydrolysis = 0.88 at 50 °C (Adler-Nissen 1986); Mp is the initial mass (g) of protein in the raw material introduced into the system; and htot is the total number of peptide bonds in protein = 8.6 eq g kg−1 of protein in fish samples (Novozymes 2001).
The degree of hydrolysis (DH) is defined as the ratio between the total number of peptide bonds cleaved and the total number of peptide bonds in the protein substrate, expressed as a percentage (Ravallec-Ple et al. 2000). The supernatant oily phase is then recovered, blown in with an inert gas such as nitrogen, and stored at − 20 °C until analysis.
Percentage of oil extracted
The oil was obtained as a top layer during the extraction process and was collected with a pipette and weighed using a digital balance. The weight was used to calculate the percentage of oil recovered, as follows:
The oil yield was defined as the ratio of the recovered oil to the estimated fat content (determined by the Soxhlet method) in the raw material multiplied by 100 and was calculated as follows:
Acute toxicity assays in rats
Male Wistar rats (weighing approximately 200–220 g) were purchased from the Pasteur Institute in Tunis (Tunis, Tunisia) and transported to Toxicology-Environmental Microbiology and Health Laboratory (Faculty of Sciences, Sfax, Tunisia). Animals were quarantined for 14 days before starting the experiment. Rats were divided into three groups (eight rats per group) as follows: two treated groups were fed daily by fish oil by oral gavage (G1: 300 mg/kg body weight and G2: 30 mg/kg body weight) and the other considered controls (G3: “positive control” given only olive oil) throughout the 14-day experimental period, rats were inspected daily to check their general health and the development of any physical abnormalities. Food and drinking water consumption was estimated by subtracting the amount of food and water left in the cages from the total amount supplied each day. After 24 h from the last day of treatment, all rats were sacrificed by cervical decapitation, and blood and organs (liver and kidney) were immediately collected for further analysis. Animal handling and experimental procedures followed the guidelines of the European Federation for Laboratory Animal Sciences for the handling of laboratory animals (Nicklas et al. 2002).
Hematological parameters
Shortly after the rats were sacrificed, blood samples, collected with an EDTA anticoagulant, were analyzed for hematologic parameters (red blood cell count (RBC), hemoglobin (Hb), hematocrit (Ht), white blood cell count (WBC), mean corpuscular hemoglobin (MCH), mean corpuscular volume (MCV), mean corpuscular hemoglobin concentration (CMHC), and platelets (PLT)) using a Sysmex KX21N automated hematology analyzer (Sysmex Corporation, Japan) in the Hematology Laboratory, CHU Habib Bourguiba (Sfax, Tunisia).
Biochemical parameters
Blood samples were centrifuged at 3500 rpm for 20 min and the serum was separated. Total protein, alkaline phosphatase (ALP), alanine amino-transferase (ALT), aspartate amino-transferase (AST), and lactate dehydrogenase (LDH) were evaluated using a commercial enzymatic kit and analyzed by spectrophotometry at the polyclinic laboratory of the CNRPS-Sfax.
Histological studies
For histological examination, liver and kidney tissues were dissected and fixed in 10% neutral formalin for 24 h. Then the samples were treated with a series of graduated ethanol and embedded in paraffin. Paraffin sections were cut into slices of 5–6 µm thick and stained with hematoxylin and eosin for examination under a light microscope. Sections were viewed and photographed using an Olympus optical microscope (Olympus BX51, Tokyo, Japan) with a camera attached (Olympus E-330, Olympus Optical Co. Ltd., Japan). Ten slides were prepared from each liver tissue. All sections were assessed for the degree of mononuclear cell infiltration, sinusoidal dilation, and necrosis.
Statistics
All data obtained were expressed as means ± standard deviation (mean ± SD). Differences between treatments were analyzed using one-way ANOVA, followed by Tukey’s post hoc test for multiple comparisons. Statistical significance was defined at P < 0.05. These analyses were performed using version 20 of Statistical Product and Service Solutions (SPSS).
Results and discussion
Chemical composition of the raw material
The chemical composition of sardine co-products found in this study expressed as g/100 g of fresh weight was represented by 72.79 ± 0.04% moisture, 15.00 ± 0.06% proteins, 8.29 ± 0.01% lipids, and 3.75 ± 0.04% ash. These values were more or less comparable to the chemical composition of sardine (Sardina pilchardus) co-products (heads, viscera) reported by Batista et al. (2009) (with moisture, proteins, lipids, and ash contents of 67.3 ± 0.7, 12.7 ± 0.8, 14.5 ± 2.1, and 4.9 ± 0.9% respectively by fresh weight). The determination of the chemical composition of fish and fishery products is very important to develop the adequate processing technology (both commercially and industrially). However, it should also be noted that the proximate chemical composition undergoes large fluctuations depending on the marine organisms studied and the sampling period (Kacem et al. 2011).
Proteolytic hydrolysis by supplemented enzymes
Enzymatic hydrolysis is an ideal way to recover oil and protein from fish and fishery processing wastes. The enzymatic extraction of oil was used in this study because of its advantages over chemical oil extraction, including low energy requirement, no use of solvent, and low investment for a large-scale process.
For this study, minced fish co-products were subjected to enzymatic hydrolysis during 2 h using the two alkaline proteases: Alcalase and the extracellular protease from Beauveria bassiana called protease Bb. Generally, hydrolysis is conducted in a short duration (mainly between 1 and 3 h) (Liaset et al. 2003; Sathivel et al. 2003). During hydrolysis, the sardine co-products were converted from a viscous chopping into a free-flowing liquid. The hydrolytic curves shown in Fig. 2 show a first rapid reaction corresponding to the rapid consumption of NaOH, followed by a slowing phase. The shapes of these curves are similar to those reported for the enzymatic hydrolysis of other fish co-product substrates (Aspmo et al. 2005; Liaset et al. 2003). Notice that some studies on hydrolysis kinetics show that the plateau is not reached (Kristinsson and Rasco 2000c; Sathivel et al. 2003). After 2 h, the degree of hydrolysis (DH) of the sardine co-product was 41.34% using protease Bb, significantly higher than the DH of 24.28% using Alcalase, knowing that DH is the percentage ratio of the number of peptide bonds cleaved over the total number of peptide bonds in the substrate.
These values are higher and faster than most of previously published marine co-products. Nguyen et al. (2011) reported values of 32.2% and 22.2% of DH (for head and tail of tuna respectively) after 12 h of hydrolysis. The work of Batista et al. (2009), using Alcalase enzyme, reported DH values of 20% for raw co-products of sardine after 2 h of hydrolysis. Interestingly, they found only 15% of DH by Alcalase when using cooked co-products over the same period of hydrolysis, suggesting the participation of endogenous enzymes in the proteolysis. Using another protease, the value dropped to only 10% (Batista et al., 2009). In fact, DH is an important criterion in controlling the proteolytic reaction (Addler-Nissen 1986). As reported by Ravallec-Ple et al. (2000), the level of DH depends on the number of peptide bonds present in the preparation. Moreover, the hydrolysis time is the second most influential factor, but it leads to different effects depending on the response. Longer hydrolysis leads to high DH. However, it is not useful to carry out a long hydrolysis to obtain the greatest quantity of released lipids. Previous studies rather obtained contrary results with long hydrolysis (Diniz and Martin 1996; Ravallec-Plé 2000).
Oil yield
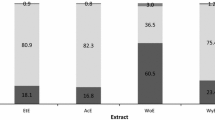
According to Fig. 3, the quantity of lipids recovered using the Alcalase enzyme is 15.72 g per 400 g of fresh co-products, i.e., the recovered oil representing 3.93% of the biomass (according to Eq. 2) giving an oil yield of 47.41% (according to Eq. 3), whereas for the protease Bb, the oil produced was 15.4 g, representing 3.85% of recovered oil (Eq. 2) and an oil yield of 46.44% (Eq. 3; Fig. 3). Thus, despite a fairly different DH for each enzyme, about ≈ 50% of the oil was extracted by both proteases. We could try to explain this by evoking Parmentier et al. (2004) who reported a certain lipid loss in the aqueous layer following the centrifugation step. Therefore, in order to achieve a better oil separation, a centrifugal force greater than 9000 g is recommended to remove the oil from the slurry. In this study, water and NaOH were added to maintain the desired pH. The addition of buffer during the hydrolysis process increased the recovery of soluble proteins in the system. Thereby, it increased the formation of emulsion and reduced the amount of the lipid released from fish (Ramakrishnan et al. 2013). Previous studies by Dauksas et al. (2005) and Slizyte et al. (2005a, b) also suggest the same phenomenon upon water supplementation. Thus, the weaker emulsion and the larger amount of oil separated prove that it would have been more effective if no water or buffer was added to the system (Ramakrishnan et al., 2013).
Oil yield (according to Eq. 3) from Alcalase and Bb protease hydrolysis treatments. Performed with an E/S ratio of 1.0% (w/w) at 50 °C. pH8
Emulsion is not a desired fraction after hydrolysis. Therefore, reducing or avoiding the formation of an emulsion layer is one of the aims in the modelling of the hydrolysis. According to Slizyte et al. (2005a, b), the most important factor influencing the yield of the different fractions was added water rather than the type of enzyme used. The formation of emulsion can be avoided or reduced by using enzymes, which are more efficient in breaking lipid/protein emulsion. Moreover, the reduction of emulsion can be reached by the reduction and/or elimination of water supplementation into hydrolysis mixture. In addition, Slizyte et al. (2005a, b) used Alcalase and Lecitase Ultra to extract lipids from cod by-products, and found that heating, to inactivate the endogenous enzyme, affected the oil yield. The type of treatment of raw material and addition of water played a significant role in determining the amount of oil and emulsion. The results indicate that the highest amount of oil was obtained from hydrolysis using Alcalase (after initial heating and without addition of water), which lowered the emulsifying properties of fish protein. The reports also suggested that Alcalase was the best enzyme for oil extraction (Batista et al. 2009).
Fatty acid profile determination
The fish oil extracted by protease Bb and Alcalase showed no significant difference in fatty acid composition. The fatty acid profiles were as follows: 46.158–44.850% saturated fatty acids (SFA), 24.041–24.665% monounsaturated fatty acids (MUFA), and 26.365–27.059% of polyunsaturated fatty acids PUFAs, for Alcalase and Bb protease respectively as shown in Table 1.
The most abundant individual fatty acids were myristic acid (C14: 0), palmitic acid (C16: 0), palmitoleic acid (C16: 1 n-7), oleic acid (C18: 1 n-9), eicosapentaenoic acid EPA (C20: 5 n-3), and docosahexaenoic acid (DHA) (C22: 6 n-3) in the lipids of Sardinella aurita by-product. We recall that the group of omega-3 acids (ω3) includes alpha-linolenic acid (ALA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA), while the group of omega-6 (ω6) acids includes linoleic acid (LA), gamma-linolenic acid (GLA), and arachidonic acid (AA).
Palmitic acid (C16: 0) was predominant among saturated fatty acids (SFAs) and is the major fatty acid among all other fatty acids in sardine oil (28.56%).
The EPA and DHA content of fish oil is an important nutritional quality parameter of this product. In this study, the amounts of EPA and DHA were respectively 9.34–8.81% and 8.75–8.79% in the oils extracted by Bb and Alcalase. EPA + DHA (17.61% and 18.1%) were predominant in total ɷ-3PUFAs (26.36% and 27.06%) in sardine fish oil. Ozogul et al. (2007, 2009) and Li et al. (2011) also reported that these fatty acids are prevalent in marine species.
Fish oil contains a high level of unsaturated fatty acids (PUFA + MUFA > SFA). Unsaturated fatty acids are more heat labile, and as the degree of unsaturation increases, they generally become less stable (Sioen et al. 2006). PUFA ω-3 and ω-6 are fundamental for the formation of important structural lipids of cell membranes. In addition, these PUFAs are precursors of eicosanoids, which influence the inflammatory process and immune reactions (Calder 2010). These findings were similar to those found by Alnahdi et al. (2019) who reported that the biological properties attributed to ω-3 will function as potential therapeutic tools for diseases such as neuro-inflammation and cerebrovascular disorders. They have opposite physiological functions and their balance is important for normal growth and development. Omega-3 and omega-6 fatty acids are called essential fatty acids since they are not synthesized by the human body (Leaf and Weber 1988) and thus must be supplied in appropriate amounts.
ω6/ω3 ratio of oils obtained
The ɷ-6/ɷ-3 ratio has been suggested as a useful indicator for comparing the relative nutritional value of fish oils (Bag and Chattopadhyay 2018). This study shows that fish oil is richer in ɷ-3 than in ɷ-6 (21.023 versus 3.519% for Alcalase and 21.653 versus 3.52% for Bb respectively). When calculating the ω-6/ω-3 ratio, the values were of the order of 0.16 for both enzyme treatments. The ratio of total lipids in 13 different seafood species caught on the Northeastern Mediterranean coast ranged from 0.02 in European squid to 0.48 in striped piggy (Durmuş 2019) and similar results were found in 34 fish species from the Mediterranean Sea (Ozogulet al.2009). Similarly, AyasD and YazganH (2013) reported that the ω6/ω3 ratio of shrimp varied between 0.2 and 0.7.
Nutrition experts have recommended a ω-6/ω-3 ratio of less than 4 (Valencia et al. 2006). According to current WHO recommendations, the daily ω-6/ω-3 ratio in the total human diet should not be greater than 5 (Vujkovic et al. 1999) as this is the key factor for balanced synthesis of eicosanoids in the body (Steffens et al. 1999). Based on studies related to the evolutionary aspects of diet, modern hunter-gatherers and traditional diets, according to which the genetic makeup of human beings is adapted to a diet with a ω6/ω3 ratio of about 1 (Simopoulos and Robinson 1999; Simopoulos 2006; 2008). Such ratio may vary from 15 to 17 in Western diets, and is accompanied by several chronic diseases such as diabetes, cancer, obesity, autoimmune diseases, rheumatoid arthritis, asthma, and depression (Simopoulos 2008; Patterson et al. 2012). Accordingly, increasing ω-3 (EPA and DHA) in the diet to have a balanced ω6/ω3 ratio around 1 by including healthy oils (such as fish oil extracted in this study) can be considered an interesting strategy to promote optimal health conditions (Durmuş 2019). However, not only should the chemical quality of fish oils in syrup form be improved and their reliability in terms of fatty acid content be increased, but it also seems important to track heavy metals more strictly in fish oil products (Ozyurt et al. 2022).
Physiognomy of rats fed with the fish oil produced
Regular observation of rats receiving both oil doses (30 mg/kg bw/day and 300 mg/kg bw/day) showed no notable change in general behavior and no adverse clinical signs. In addition, there was no change in the nature of the stools, urine, or eye color of the animals. No mortality was observed in the different groups of rats given fish oil via oral route.
Food intake, body and organ weights in rats fed fish oils
The acute toxicity of fish oils extracted with protease Bb was assessed by its administration to Wistar rats via oral gavage using two different oil doses daily (300 mg/kg bw and 30 mg/kg bw for G1 and G2 groups, respectively). In fact, there were variable changes in the body weights of G3 control group. Interestingly, final body weights of G1 group showed a significant decrease compared to all other rats (G2 and G3) (P < 0.05) (Table 2). Pellizzon et al. (2002) noted that diets rich in n-3 PUFAs, such as those contained in fish oils, tend to reduce weight gain. Moreover, El-Gerbed (2013) showed that rats fed fish oil (menhaden oil) exhibited an insignificant decrease in body weight compared to control animals.
It was also reported by Pellizon et al. (2002) that mice fed a diet based on fish oil gained less weight than those fed soybean oil (mainly n-6 PUFA) or oil palm (mainly AGS).
While relative liver and kidney weights showed no significant difference between control and treated groups, absolute ones of G1 group showed a significant decrease compared to all rats (P < 0.05) (Table 2). In contrast with the work reported by Seonhye and Yongsoon (2009), rats fed fish oil had significantly lower abdominal fat weights, but significantly higher liver and kidney absolute ones, as compared to those fed soybean oil.
Food intake data showed that food consumption increased gradually for all animals (Fig. 4), but it was more pronounced in the G3 control group (besides, it was remarkably noted that food consumption of G1 and G2 treated rats was significantly different from that of the control group (G3)) (P < 0.05). Similarly, Seonhye and Yongsoon (2009) reported that rats fed fish oil had significantly lower abdominal fat weight. Benefits of dietary supplementation with marine n-3 PUFA are well documented and may therefore offer a logical approach for preventing or treating obesity and obesity-induced complications (Wang and Huang 2015).
Regarding the consumption of drinking water throughout the experiment, there was no significant difference for all groups of rats.
Hematological study
Hematological parameters, shown in Table 3, revealed no significant differences between fish oil–treated groups (G1, G2) and control (G3). In addition, we noticed normal red blood cell (RBC) morphology in groups G1 and G2 compared to control rats (G3) as illustrated in Fig. 5. These results were in agreement with those of Mas Rizky et al. (2020). These authors mentioned that the total WBC number, hemoglobin, hematocrit, and platelet count of the control group did not significantly differ from the fish oil and virgin coconut oil groups.
Peripheral blood film of treated male Wistar rats with fish oil extracted and administered by oral gavage using oil olive vehicle in dose (G1: 300 mg kg−1 BW and G2: 30 mg kg−1 BW) compared to positive control G3 (olive oil added in diet), showing normal red blood cell (RBC) morphology in all groups
Biochemical parameters
All biochemical parameters were not significantly changed after the treatment with fish oil (Table 4). Liver enzymes and other biomarkers referred to as a liver panel including AST, ALT, ALP, LDH, GGT, bilirubin, and total bilirubin showed no statistically significant difference between all groups. We recall that the above-cited parameters are recommended to monitor the structural integrity of the liver and aid in the clinical diagnosis of anhepatic toxicity state (Simon-Giavarotti et al. 2002). The group G1 of mice receiving 300 mg/kg showed a significantly higher level of serum ALP (alkaline phosphatase) compared to the G3 control group. Nevertheless, no significant histopathological changes were observed in the liver and kidneys of treated mice compared to control mice (see below). Similar results were obtained in other studies (Tamsir et al. 2019). Interestingly, the levels of AST and ALT were reduced in the G1 group, to a lesser extent in the G2 group, compared to the control G3 group, but not significantly enough. In the literature, fish oil supplementation of rats showed significant difference as compared to the standard diet-fed normal control in ALT and AST enzymes activities (Lashin et al. 2020). In this context, it should be also noticed that both activities are also significantly reduced in cisplatin-induced hepato-renal toxicity in rats when supplemented with lycopene and N-acetylcysteine (Elsayed et al. 2021).
No significant differences (P > 0.05) were observed in all measured proteins, urea, and creatinine between fish oil–treated groups (G1, G2) and control (G3) (Table 4). Urea and creatinine are considered to be good indicators of normal kidney functioning. These findings were similar to those found by Lashin et al. (2020) for proteins in the fish oil group compared to the control group.
Histology of liver and kidney sections
In order to evaluate the toxicity of fish oil in more detail, liver and kidney samples from different groups were examined under light microscopy. In all rat organ sections, there was normal histological architecture (Figs. 6, 7).
In fact, in liver sections, there was preservation of the lobular architecture, normal hepatocytes with appropriate nuclear to cytoplasmic ratio, normal central vein, capsule with no evidence of adhesion or inflammation, and portal tracts with appropriate number of bile ducts and blood vessels (Fig. 6).
Similar to our results, El Kalawy et al. (2017) reported that histological examination of liver sections from omega-3 group revealed normal liver parenchyma with preserved architecture of hepatic lobules.
Likewise, according to Fig. 7, kidney sections from all groups displayed normal renal tissue structure, complete renal tubular epithelial cells, and no obvious pathological changes in glomerular or renal interstitium (Fig. 7).
Our results are in agreement with Moghadamnia et al. (2016), who had mentioned that the omega-3 fish oil–supplemented group displayed similar kidney appearance compared to those of control rats. Moreover, Fassett et al. (2010) proved that omega-3 fatty acids were beneficial in kidney disease treatment.
Conclusion
In order to greenly recover the fish oil of Sardinella aurita co-products, they were treated with a commercial Alcalase and a local protease Bb (from Beauveria bassiana, strain P2). Interestingly, protease Bb led to a much higher degree of DH hydrolysis (41.34%) than commercial protease (24.28%). Despite such quite different DHs, about 50% of the oil was extracted by the two proteases. In addition, these latter proteases provided an attractive ω6/ω3 ratio in the oil extracted, indicative of good nutritional quality. It was also found that fish oil administered orally using an olive oil–based vehicle in two doses (300 mg/kg BW and 30 mg/kg BW) preserved hepatic and renal function in rats and no adverse clinical signs were observed. Moreover, the final body weight of rats treated with the highest dose of fish oil showed a significant decrease compared to other groups, which further confirms the anti-obesity effect attributed to pharmaceutical drugs of marine origin. Finally, the use of fish oil extracted by the protease of the local fungal strain (P2) of Beauveria bassiana as a dietary supplement should be viewed positively because it has no adverse effects on the survival of Wistar rat.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Addler-Nissen J (1986) Enzymic hydrolysis of food proteins. Elsevier, Appl Sc Publ New York, pp 110–69
AlKhawli F, Pateiro M, Domínguez R, Lorenzo JM, Gullón P, Kousoulaki K, Ferrer E, Berrada H, Barba FJ (2019) Innovative green technologies of intensification for valorization of seafood and their by-products. Mar Drugs 6(17(12)):689. https://doi.org/10.3390/md17120689
Alnahdi HS, Hanan S, Sharaf IA (2019) Possible prophylactic effect of omega-3 fatty acids on cadmium-induced neurotoxicity in rats’ brains. Environ Sci Pollut Res Int 26–30:31254–31262. https://doi.org/10.1007/s11356-019-06259-8
AOAC (1984) Official methods of analysis. 14th. Ed. Association of official analytical chemists. Arlington, Virginia 22209 USA
AOAC (2005) Official methods of analysis. 18th edn. Association of official analytical chemists; Arlington, VA, USA
Araujo J, Sica P, Costa C, Márquez MC (2020) Enzymatic hydrolysis of fish waste as an alternative to produce high value-added products. Waste Biom Valor 12:847–855. https://doi.org/10.1007/s12649-020-01029-x
Aspmo SI, Horn SJ, Eijsin VGH (2005) Enzymatic hydrolysis of Atlantic cod (Gadus morhua L) viscera. Process Biochem 40(5):1957–1966
Ayas D, Özogul Y, Yazgan H (2013) The effects of season on fat and fatty acids contents of shrimp and prawn species. Eur J Lip Sci Techn 115(3):356–362. https://doi.org/10.1002/ejlt.201200081
Bag A, Chattopadhyay R (2018) Evaluation of antioxidant potential of essential oils of some commonly used Indian spices in in vitro models and in food supplements enriched with omega-6 and omega-3 fatty acids. Environ Sci Pollut Res Int 25(1):388–398. https://doi.org/10.1007/s11356-017-0420-5
Batista I, Ramos C, Mendonça R, Nunes ML (2009) Enzymatic hydrolysis of sardine (Sardina pilchardus) by-products and lipid recovery. J Aqu Food Prod Techn 18(1):120–134. https://doi.org/10.1080/10498850802581823
Borgi I, Gargouri A (2014) Investigations on a hyper-proteolytic mutant of Beauveria bassiana: broad substrate specificity and high biotechnological potential of a serine protease. Microbiol Lett 351(1):23–31. https://doi.org/10.1111/1574-6968.12339
Calder PC (2010) Omega-3 fatty acids and inflammatory processes. Nutrients 2(3):355–374
Dauksas E, Falch E, Slizyte R, Rustad T (2005) Composition of fatty acids and lipid classes in bulk products generated during enzymatic hydrolysis of cod (Gadus morhua) by-products. Process Biochem 41:2659–2670
Diniz FM, Martin AM (1996) Use of response surface methodology to describe the combined effects of pH, temperature and E/S ratio on the hydrolysis of dog fish (Squalusa canthias) muscle. Int J Food Sci Technol 31:419–426
Durmuş M (2019) Fish oil for human health: omega-3 fatty acid profiles of marine seafood species. Food Sci Technol 39 (2)
El Kalawy S, Mostafa M, Abdelfattah L, Kamar S (2017) Histological and immunohistochemical study of the effect of omega-3 fatty acids on ifosfamide-induced liver injury in adult male albino rat. J Med Hist 2:134–145. https://doi.org/10.21608/JMH.2017.7921
El-Gerbed MSA (2013) Ameliorative effect of fish oil on the cisplatin induced hepatotoxicity and nephrotoxicity in rats. Res J Pharm Biol Chem Sci 4(4):479–491
Elsayed A, Elkomy A, Elkammar R, Gehan Y, Abdelhiee EY, Abdo W, Fadl SE, Soliman A, Aboubakr B (2021) Synergistic protective efects of lycopene and N‑acetylcysteine against cisplatin‑induced hepatorenal toxicity in rats. Sci Rep 11(1):13979. https://doi.org/10.1038/s41598-021-93196-7
Fassett RG, Gobe GC, Peake JM, Coombes JS (2010) Omega-3 poly-unsaturated fatty acids in the treatment of kidney disease. American J Kidn Disea 56(4):728–742. https://doi.org/10.1053/j.ajkd.2010.03.009
Fournier V, Destaillats F, DionisiF LP, Sébédio JL, Berdeaux O (2006) Thermal degradation of long-chain polyunsaturated fatty acids during deodorization of fish oil. Eur J Lipid Sci Technol 108:33–42
Haedrich J, Stumpf C, Denison MS (2020) Rapid extraction of total lipids and lipophilic POPs from all EU-regulated foods of animal origin: Smedes’ method revisited and enhanced. Environ Sci Eur 32:118. https://doi.org/10.1186/s12302-020-00396-5
Jayasinghe P, Hawboldt K (2012) A review of bio-oils from waste biomass: focus on fish processing waste. Renew Sustain Energy Rev 16(1):798–821. https://doi.org/10.1016/j.rser.2011.09.005
Kacem M, Sellami M, Kammoun W, Frikha F, Miled N, Ben Rebah F (2011) Seasonal variations in proximate and fatty acid composition of viscera of Sardinella aurita Sarpa salpa and Sepia officinalis from Tunisia. J Aqu Food P Techn 20(2):233-246 https://doi.org/10.1080/10498850.2011.560365
Kristinsson HG, Rasco BA (2000) Kinetics of the hydrolysis of Atlantic salmon (Salmo salar) muscle by alkaline proteases and a visceral serine protease mixture. Process Biochem 36:131–139
Lashin FM, Rizk HA, Ahmed-Farid OA, Shehata AM (2020) A screening study of corn oil versus fish oil and coconut oil on biochemical cardiovascular risk factors in rats. Cur Sci Inter 9(3):418–430. https://doi.org/10.36632/csi/2020.9.3.36
Leaf A, Weber PC (1988) Cardiovascular effects of n-3 fatty acids. The New England J Med 318(9):549–557
Li G, Sinclair AJ, Li D (2011) Comparison of lipid content and fatty acid composition in the edible meat of wild and cultured freshwater and marine fish and shrimps from China. J Agr Food Chem 59(5):1871–1881. https://doi.org/10.1021/jf104154q
Liaset B, Julshamn K, Espe M (2003) Chemical composition and theoretical nutritional evaluation of the produced fractions from enzymatic hydrolysis of salmon frames with Protamex. Process Biochem 38(12):1747–1759. https://doi.org/10.1016/S0032-9592(02)00251-0
Mas Rizky AAS, Dinar FA, Neni A, Nia K (2020) Effect of fish oil, virgin coconut oil, and used-cooking oil consumption on hematological profile in mice. Indo J of Clinical Pharm 9(2):137–146 . https://doi.org/10.15416/ijcp.2020.9.2.137
Mercer P, Armenta RE (2011) Developments in oil extraction from microalgae. Eur J of Lipid Sci and Tech 113. https://doi.org/10.1002/ejlt.201000455
Moghadamnia D, Mokhtari M, Khatamsaz S (2016) The protective effect of omega-3 against thioacetamide induced lipid and renal dysfunction in male rats. Zahedan J Res Med Sci 18(11):4781. https://doi.org/10.17795/zjrms-4781
Moro K, Nagahashi M, Ramanathan R, Takabe K, Wakai T (2016) Resolvins and omega three polyunsaturated fatty acids: clinical implications in inflammatory diseases and cancer. World J Clin Cases 4(7):155–164. https://doi.org/10.12998/wjcc.v4.i7.155
Nguyen TM, Khalifa SSB, Randriamahatody Z, Moreno CD, Moreau J, Tran LT, Bergé JP (2011) Proteolysis of tuna by-products. Food Technol Biotechnol 49(1):48–55
Nicklas W, Baneux P, Boot R, Decelle T, Deeny AA, Fumanelli M, Illgen-Wilcke B (2002) FELASA (Federation of European Laboratory Animal Science Associations Working Group on health monitoring of rodent and rabbit colonies Recommendations for the health monitoring of rodent and rabbit colonies) in breeding and experimental units. Lab Anim 36(1):20–42. https://doi.org/10.1258/0023677021911740
Novozymes (2001) Determination of the Degree of Hydrolysis (DH) Based on OPA Reaction. Bagsvaerd, Denmark: Novozymes
Ovissipour M, Abedian A, Motamedzadegan A, Rasco B, Safari R, Shahiri H (2009) The effect of enzymatic hydrolysis time and temperature on the properties of protein hydrolysates from Persian sturgeon (Acipenser persicus) viscera. Food Chem 115:238–242. https://doi.org/10.1016/j.foodchem.2008.12.013
Ozogul Y, Ozogul F, Alagoz S (2007) Fatty acid profiles and fat contents of commercially important seawater and freshwater fish species of Turkey: a comparative study. Food Chem 103(1):217–223. https://doi.org/10.1016/j.foodchem.2006.08.009
Ozogul Y, Ozogul FH, Çiçek E, Polat A, Kuley E (2009) Fat content and fatty acid compositions of 34 marine water fish species from the Mediterranean Sea. Int J Food Sci Nut 60(6):464–547. https://doi.org/10.1080/09637480701838175
Ozyurt G, Ekmen D, Durmuş M, Ucar Y (2022) Assessment of the safety of dietary fish oil supplements in terms of content and quality. Environ Sci Pollut Res Int 29:25006–25019
Parmentier M, Guillemin S, Barba R, Linder M, Fanni J (2004) DE nouveaux procédés d’extraction des huiles pour des produits finis de haute qualité. Congrès Eurofed Lipid à Edinbourg OCL 11(6):377. https://doi.org/10.1051/ocl.2004.0377
Patterson E, Wall R,Fitzgerald GF, Ross RP, Stanton C (2012) Health Implications of High Dietary Omega-6 Polyunsaturated Fatty Acids. J Nutr Metab 2012: 539426
Pellizzon M, Buison A, Ordiz F, Ana LS, Jen K-LC (2002) Effects of dietary fatty acids and exercise on body-weight regulation metabolism in rats. Obes Res 10(9):947–55
Ramakrishnan VV, Ghaly AE, Brooks MS, Budge SM (2013) Extraction of oil from mackerel fish processing waste using alcalase enzyme. Enz Eng 2:115. https://doi.org/10.4172/2329-6674.1000115
Ravallec-Ple R, Gilmartin L, Van Wormhoudt A, Le Gal Y (2000) Influence of the hydrolysis process on the biological activities of protein hydrolysates from cod (Gadus morhua) muscle. J Sci Food Agric 80(15):2176–2180. https://doi.org/10.1002/1097-0010(200012)80:15%3c2176::AID-JSFA763%3e3.0.CO;2-G
Ravallec-Plé R (2000) Valorisation d'hydrolysats d'origine marine: optimisation de laconcentration en peptides apparentés aux facteurs de croissance et aux agentssécrétagogues. Thèse de doctorat de l'Université de Bretagne Occidentale, 171 pp
Rizliya V, Mendis E (2014) Biological, physical, and chemical properties of fish oil andindustrial applications. In: Kim, S.-K. (Ed.), Seafood Processing By-Products: Trendsand Applications. Springer Science+Business Media New York https://doi.org/10.1007/978-1-4614-9590-1-14
Sathivel S, Babbit J, Smiley S, Crapo C, Reppond KD, Prinyawiwatkul W (2003) Biochemical and functional properties of Herring (Clupea harengus) by-product hydrolysates. J Food Sci 68:2196–2200. https://doi.org/10.1111/j.1365-2621.2003.tb05746.x
Seonhye P, Yongsoon P (2009) Effects of dietary fish oil and trans fat on rat aorta histopathology and cardiovascular risk markers. Nut Res Prac 3(2):102–107. https://doi.org/10.4162/nrp.2009.3.2.102
Simon-Giavarotti KA, Giavarotti L, Gomes LLF, Veridiano AM, Garcia EA, Mora OA, Fernandez V, Videla LA, Junqueira VB (2002) Enhancement of lindane-induced liver oxidative stress and hepatotoxicity by thyroid hormone is reduced by gadolinium chloride. Free Radic Res 36(10):1033–1039. https://doi.org/10.1080/1071576021000028280
Simopoulos AP (2006) Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: nutritional implications for chronic diseases. Biom Pharm 60(9):502–507. https://doi.org/10.1016/j.biopha.2006.07.080
Simopoulos AP (2008) The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med (maywood) 233(6):674–688. https://doi.org/10.3181/0711-MR-311
Simopoulos AP, Robinson J (1999) The omega diet: the lifesaving nutritional program based on the diet of the island of crete. Harper Collins, New York, p 400
Sioen IA, Pynaert I, Matthys C, De Backer G, Van Camp J, De Henauw S (2006) Dietary intakes and food sources of fatty acids for Belgian women, focused on n-6 and n-3 polyunsaturated fatty acids. Lipids 41(5):415–422. https://doi.org/10.1007/s11745-006-5115-5
Slizyte R, Rustad T, Storro I (2005) Enzymatic hydrolysis of cod (Gadus morhua) by-products optimization of yield and properties of lipid and protein fractions. Process Biochem 40:3680–3692. https://doi.org/10.1016/j.procbio.2005.04.007
Slizyte R, Rustad T, Storro I (2005) Enzymatic hydrolysis of cod (Gadus morhua) by-products optimization of yield and properties of lipid and protein fractions. Process Biochem 40(12):3680–3692. https://doi.org/10.1016/j.procbio.2005.04.007
Steffens W, Rennert B, Wirth M, Kruger R (1999) Effet of two lipid levels on growth feed utilisation body composition and some biochemical parameters of rainbow trout Oncorhynchus mykiss (Walbaum 1792). J Appl Ichthyol 15:159–164. https://doi.org/10.1016/j.aquaculture.2013.08.022
Swanson D, Block R, Mousa SA (2012) Omega-3 fatty acids EPA and DHA: health benefits throughout life. Adv Nutr 3(1):1–7. https://doi.org/10.3945/an.111.000893
Tacias-Pascacio VG, Morellon-Sterling R, Siar E-H, Tavano O, Berenguer-Murcia Á, Fernandez-Lafuente R (2020) Use of Alcalase in the production of bioactive peptides: A review. Int J of Biol Macr. https://doi.org/10.1016/j.ijbiomac.2020.10.060
Tacias-Pascacio VG, Castañeda-Valbuena D, Morellon-Sterling R, Tavano OL, Berenguer-Murcia Á, Vela-Gutiérrez G, Rather IA, Fernández Lafuente R (2021) Bioactive peptides from fisheries residues: a review of use of papain in proteolysis reactions. Int J of Biol Macr 184:415–428. https://doi.org/10.1016/j.ijbiomac.2021.06.076
Tamsir NM, Esa NM, Shafie NH, Hussein MZ, Hamzah H, Abdullah MA (2019) The acute effects of oral administration of phytic acid-chitosan-magnetic iron oxide nanoparticles in mice. Int J Mol Sci 20(17):4114. https://doi.org/10.3390/ijms20174114
Valencia I, Ansorena D, Astiasarán I (2006) Nutritional and sensory properties of dry fermented sausages enriched with n-3 PUFAs. Meat Sci 72(4):727–733. https://doi.org/10.1016/j.meatsci.2005.09.022
Vujkovic G, Karlovic D, Vujkovic I, Vorosbaranyi I, Jovanovic B (1999) Composition of muscle tissue lipids of silver carp and bighead carp. J Am Oil Chem Soc 76:475–480. https://doi.org/10.1007/s11746-999-0027-1
Wang Y, Huang F (2015) N-3 polyunsaturated fatty acids and inflammation in obesity: local effect and systemic benefit. Bio Res Int 2015:581469. https://doi.org/10.1155/2015/581469
Wubshet SG, Lindberg D, Veiseth-Kent E, Kristoffersen KA, Böcker U, Washburn KE, Afseth NK (2019) Bioanalytical aspects in enzymatic protein hydrolysis of by-products. Proteins: Sustain Source Process Appl 225 258 https://doi.org/10.1016/B978-0-12-816695-6.00008-8
Yates CM, Calder PC, Rainger GEd (2014) Pharmacology and therapeutics of omega-3 polyunsaturated fatty acids in chronic inflammatory disease. Pharmac Therap 141(3):272–282. https://doi.org/10.1016/j.pharmthera.2013.10.010
Acknowledgements
We deeply thank Dr. Bassem Jaouadi for offering Alcalase protease. This work is part of the agreement between the LBME laboratory of the CBS and the B3-Aqua Laboratory of the INSTM.
Funding
This work was supported financially by “Ministère de l’Enseignement Supérieur de la Recherche Scientifique et de la Technologie, Tunisia.”
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Aref Neifar, Aida Koubaa, Wassim Kammoun, Ines Borgi, and Ali Gargouri. The animal experimentations, toxicity tests, and histological analyses were performed by Meryam Chelly, Sabrine Chelly, and Hanen Bouaziz; hematological and biochemical analyses were performed by Choumous Kallel and Mohamed Boudawara. Statistical investigations were performed by Wassim Kammoun. The first draft of the manuscript was written by Aref Neifar, Aida Koubaa, and Ali Gargouri and all authors commented on previous versions of the manuscript. Project administration and supervision of the work were directed by Ali Gargouri and Hanen Bouaziz. The final writing—proofreading and editing—was achieved by Saloua Sadok and Ali Gargouri. The corresponding author is Ali Gargouri. All authors read and approved the final revised manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Ta Yeong Wu
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Aida Koubaa and Aref Neifar are considered co-first authors.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Neifar, A., Koubaa, A., Chelly, M. et al. Safety assessment of fish oil green extraction and in vivo acute toxicity evaluation. Environ Sci Pollut Res 30, 10377–10389 (2023). https://doi.org/10.1007/s11356-022-22460-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-22460-8