Abstract
The current study has been designed to observe the coloring efficacy of wild turmeric–based natural yellowish colorant for wool dyeing under microwave (MW) treatments. Extracts and fabrics have been exposed to MW treatment for up to 10 min. Surface morphology and changes in the fabric’s chemical nature before and after radiation have been studied through SEM and FTIR, respectively. The results obtained after a series of experiments show that using 45 mL of aqueous extract (pH = 5) in the presence of 1.5g/100mL of table salt as an exhausting agent at 75°C for 45 min has displayed outstanding color depth (K/S) onto microwave-treated wool fabric. On applying biomordants, it has been found that acacia extract (1.5%), pomegranate (2%), and pistachio extracts (1.5%) before dyeing, whereas acacia (1%), pomegranate (1%), and pistachio extracts (2%) after dyeing, have shown colorfast shades of high strength. Comparatively, salts of Al (1.5%) and Fe (1%), and T.A (2%) before dyeing, while salts of Al (1%) and Fe (1.5%) and T.A (1.5%) after dyeing, have given the best results. Generally, it has been originated that salt of Fe (1.5%) as a post-chemical mordant and pomegranate extract (1.5%) as a post-bio-mordant have displayed wonderful color strength. It very well may be inferred that MW treatment, being naturally protected, has just superior the varying strength of colorants on wool fabric. Adding biomordants has transformed the strategy into a more sustainable one.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sustainably using natural dyes necessitates using natural products that are both economically and environmentally viable (Baseri 2022; Adeel et al. 2022a). Natural color that is renewable, biodegradable, sustainable, and non-toxic is becoming increasingly popular worldwide (Haji and Naebe 2020; Nathan and Rani 2021; Patel and Kanade 2019). Surprisingly, their feces can be recycled and turned into fertilizer (Rather et al. 2019). The widespread consciousness of zero liberation emissions, global warming, and ecosystem health has sparked a worldwide interest in green products (Mia et al. 2022). Textile dyeing is one of the most ecologically constructive and destructive businesses. In synthetic color dyeing methods, extreme quantities of water and chemicals are used (Khattab et al. 2020; Singh and Sheikh 2021). Because of the numerous negative environmental resurgences of industrial development, such as universal heating and air and water pollution (Haque et al. 2022; Hasan et al. 2022), it is vital to consider the environmental dimension of manufacture and research in every arena (de Marco et al. 2019; Haji et al. 2020). Most synthetic dyes contain toxic and cancer-causing environments, and when released into the environment, they contaminate the environment (Mansour and Ben Ali 2021). These industries shed potentially dangerous chemicals into bodies of water and the environment, causing allergic, poisonous, carcinogenic, and severe skin reactions (Alebeid and Pei 2020; Gan et al. 2021). So, after careful observation, international environmental organizations have started to spread awareness about the acute toxic effects of effluent carcinogens and the benefits of green products to shift their focus to natural products. Natural dyes have distinct color chemistry compared to other natural materials (Thakker 2021; Haji 2020; Agrawal and Chopra 2020). Natural dyes have become more appealing because they provide special colors (Hu et al. 2019; Haji and Rahimi 2020). Natural dyes applied to wool-dyed cloth with natural mordants such as pomegranates improve dye uptake and produce the desired color tone (Kiran et al. 2020; Eser and Yavas 2021). Furthermore, these dyes have anti-inflammatory, anti-hemolytic, antipyretic, antifungal, antioxidant, and antibacterial characteristics, making them useful in medicine (Rai et al. 2020; Hosseinnezhad et al. 2021). These dyes are a safe and environmentally friendly way to color textiles and other materials (Rodiah et al. 2022). However, it has been found that these dyes have some issues, such as low production yield and poor fastness rating. Although old methods have been utilized for rapid treatment with efficient results, modern methods have been included in green products.
Microwave radiation is one of the most cutting-edge approaches among modern technologies (Baig et al. 2021). These rays use a solid-liquid transfer technique to maximize biomolecule interaction with the solvent (Chen et al. 2020). Furthermore, this sustainable process of microwave heating has the largest benefit that it yields (Arain et al. 2021; Phan et al. 2020. This procedure fine-tunes the cloth surface, ready to take the dye molecule, resulting in the maximum dyeing rate (Majumder et al. 2020). These rays disintegrate clusters of dye molecules into small units, which sorb into voids of surface-modified fibers promisingly. Hence, MW rays are extremely fast and uniform, resulting in a procedure that saves energy and time. Another benefit is that the size of the equipment, waste, and utilization of solvent materials is reduced (Buyukakinci et al. 2021; Jafari et al. 2019). Mordanting is an old art to improve shade depth, but some toxic salts acting as bridging agents must be replaced with green anchors. These green anchors have mostly planted phenolics that produce new colorfast shades and transfer their good characteristics into fabric upon dyeing. By creating complexes with the natural dyes in the fiber, biomordants are traditionally used in the dyeing process to increase fiber affinity and hence improve the color fastness of dyed textiles.
Wild turmeric (Curcuma aromatica) is a fragrant therapeutic plant that belongs to the Zingiberaceae family. It has a wide range of pharmacological properties. It is well known in India for ethnobotanical uses such as stimulant, carminative, snakebite remedy, and beautifying uses (Behura et al. 2021). Its extract has been used to treat gastrointestinal issues, stiff pain, provocative disorders, injuries, skin contaminations, and insect stings, among other things (Umar et al. 2020). The aim of current research is as follows:
-
a)
To use MW rays for improving the color yield from wild turmeric.
-
b)
To observe changes at surface of wool and changes in the functional point, i.e., amido linkage of wool through SEM and FTIR.
-
c)
To check the validity of data under central composite design through statistical tool.
-
d)
To get the mordanting level for uniform shades to assent fastness of shades as per standard ISO methods.
Materials and methods
Collection of materials
Wild turmeric (Curcuma aromatica) is a source of natural yellow dyes. Interestingly, pomegranate rinds (Punica granatum), pistachio (Pistacia vera), and acacia bark residue (Acacia nilotica) as a wellspring of polyphenolic anchors have been procured from the store, handled, and utilized. Chemical mordants such as Al, Fe, and tannic acid salts have been procured and employed for shade fastness. Wild turmeric rhizomes for the detachment of natural color and biomordants have been grounded finely and through a filter of 20 lattice size to acquire a powder of constant molecule size. Wool fibers have been washed with nonpartisan cleanser at 80°C for 30 min to prepare them for shading.
Irradiation and extract process
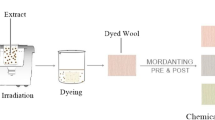
Fine plant powder divided (4g) has been boiled with 100mL of aqueous and acidic media for 45 min to isolate colorants, having a media to powder the ratio of 25:1. The extract and fabric have been microwaved for up to 10 min, utilizing a powerful level (NRWF). Treated and untreated extracts (NRE) have been utilized to color-treated (RWF) and untreated fabrics by keeping an extract to fabric proportion of 25:1 at 75°C for 45 min; the complete arrangement of work is given in Fig. 1.
Optimization of dyeing conditions
Different coloring boundaries like temperature, time of coloring, pH of coloring, and extract capacity improved statistically by utilizing the response surface methodology. To see the impact of temperature, coloring was completed at 65–85°C for 35–65 min, utilizing 35–65mL of concentrates of 3–7 pH, having 1g/100mL of salt as a debilitating agent concluded a sequence of 32 experiments under the statistical model given in Table 3.
Optimization of mordanting conditions
To further develop the color complexity and colorfastness of fabric dyed under ideal circumstances, pre and post-mordanting were completed at 80 °C for 45 min (Habib et al. 2022). For this reason, 0.5, 1, 1.5, 2, and 2.5 g/100 mL of eco-friendly salt of Al (Alum), Fe (iron sulfate), and tannic acid (T.A.) have been used under selected conditions (Fig. 2). In parallel observations, 0.5–2.5 g/100 mL of concentrate of biomordants like pomegranate rinds (Punica granatum), pistachio (Pistacia vera), and acacia bark powder (Acacia nilotica) have been utilized at 80 °C for 45 min, keeping the mordant to fabric proportion of 25:1 before and after dyeing.
Evaluation of fabric and extract
The surface morphology of optimal irradiated and un-irradiated fabrics was investigated using scanning electron microscopy (SEM model Tescan; 5 kV, image size= x 1000). The variations in the distinctive peaks of the functional element of wool were observed using FTIR analysis. Finally, all the colored fabrics have been investigated in the Lab and LCh system using data color SF 600 (USA). For fastness rating, ISO standard methods of light (ISO 105-B02), washing (ISO 105-C03), and rubbing (ISO 105-X12) have been used and results have been observed as per the greyscale.
Results and discussion
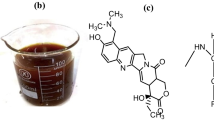
Microwaves in natural products are being used based on their fast leveling, uniform, and energy-cost-effective nature (Gala et al. 2022; Wang et al. 2021). These rays evolve the functional molecule at a particular level by rupturing its boundary without altering the chemical nature of a particular bio-potent molecule (Ticha et al. 2021; Hayat et al. 2022). These rays work via the principle of the powder-solvent mechanism, which takes less solvent for the interaction of colorant to enhance mass transfer kinetics and gives a promising yield (Adeel et al. 2021). This good yield cannot be possible to get if a low level (<6 minutes) of rays is given to the isolation system because the rays cannot break the cell wall (Xie et al. 2019). Similarly, over the level (> 6 minutes) of rays also disturbs the actual function of colorant because, along with potentially isolated curcumin, other bio-molecules are also evolved, which during application affects the actual yield (Gong et al. 2022). The same situation has been observed in our studies. The results reveal that before MW rays, the water-born extract (aqueous) has given good color strength (NRE/NRWF=24.941); upon irradiation up to 6 min, the application of treated extract (RE) has given an excellent yield (K/S=26.393) onto the treated wool (Fig. 3a). Similarly, up to 10 min, the treatment of both extract and fabric has given a low yield (K/S=21.209), which might be due to the involvement of other molecules isolated along with curcumin. During dyeing, their appearance has affected shade strength (colorant) strength. The color coordinates given in Table 1 show that before the treatment of extract and fabric (NRE/NRWF), the shade is brighter (L*=71.47) in appearance and reddish yellowish (a*=8.06; b*=87.54). After 6 min of exposure, the shades become darker (L*=68.68), having a reddish-yellow tone (a*=8.68; b*=84.68). Using an acidic medium before treatment of extract and fabric, the shade strength was low (K/S=15.483), having a darker tone (L*=68.31) but with a less reddish (a*=5.40) yellow hue (b*=71.73) given in Table 1 although shade strength was proved to be little (K/S=15.500), having a dark tone (L*=68.20) with more red but little yellow hue (a*=6.02; b*=68.01) shown in (Fig. 3b). Hence, the acidic medium did not support the isolation of curcumin from wild turmeric followed by wool dyeing.
The other aspect is the irradiation of fabric, which imparts its role promisingly to get a high yield after tuning. Work shows that MW rays have scratched the fiber surface, which helps improve its uptake ability. The wool fabric observed under the scanning electron microscope (SEM) shows that in Fig. 4a and b, after irradiation, the wool fabric was peeled, which has given promising results after dyeing. Hence, MW rays have modified the surface physical spectral images before and after irradiation for up to 6 min. The ATR-FTIR spectral images of wool fabric results given in Fig. 5a and b show the stretching peak of –NH (3000–3500 cm−1), the stretching peak C=C (900–1100 cm−1), and the C=O peak (1500–1700 cm−1). After irradiation, their position cannot be altered after microwave treatment for up to 6 min (Reference). The ATR-FTIR spectral images of curcumin extract results given in Fig. 6a and b show that before irradiation, the functional spectral peak of –OH was observed near about 3500 cm−1 (O–H stretching of the phenol group), C=C was observed at 1599 cm−1 (aromatic C=C stretching), and 1501 cm−1 (C=O stretching). After irradiation, the spectral peak was not position changed. The functional peaks of –OH, C=C, and C=O were observed simultaneously. The physiochemical analysis shows that MW rays add value to the coloration of wool when dyed with curcumin in the aqueous extract of wild turmeric without altering the chemistry of the fabric. Conclusively, it is recommended that for isolation, aqueous extract, and dyeing, the wool fabric should be MW treated for up to 6 min to get excellent results. Hence, the result in Fig. 3a shows that for excellent color characteristics, isolation should be done in an aqueous medium followed by treatment for up to 6 min for wool dyeing.
Dyeing parameters in wool dyeing always give a promising tint because low or high levels always hinder good results. Dyeing of fabric using a particular volume (45mL) of 5 pH containing 1.5g/100mL of salt has given excellent results when an employee dyed irradiated wool at 75 °C for 45 min after MW treatment for 6 min (Table 2). The low value of extract provides low molecules onto fabric, whereas the high value of extract causes the gathering of aggregates onto fabric. These aggregates do not enter the voids of wool fibers, which mainly remain on the fabric after washing; these unfixed molecules are washed away and give a low tint. Hence, 45mL of aqueous extract should be used after irradiation to get good results. Proteinous fabric dyeing also influences protein extract nature (pH). The highly acidic nature of the extract does not give excellent results. In contrast, moving towards the alkalinity (pH>5), the amido linkage of wool hinders its interaction with the OH of curcumin from wild turmeric. Hence, 45mL of aqueous extract at 5 pH should be used to get excellent results. Salt always acts as a leveling agent, particularly in wool dyeing. The results in Table 2 show that 1.5g/100mL of table salt has exhausted colorant from the extract towards the fabric. This excellent exhaustion has provided an interactive atmosphere between fabric, colorant, and solvent within a short range of attractive forces to give excellent shade strength. A low amount cannot exhaust dye well. In contrast, the utilization of salt above 1.5g /100mL has given over-exhaustion, which has led to the gathering of colorant molecules in the form of aggregates or clusters onto fabric. Poorly fixed colorant molecules are washed away, and a low yield is observed. Hence, it is recommended that 45mL of aqueous extract of 5 pH containing 1.5 g/100mL salt should be used to get effective results. Similarly, dyeing wool with plant pigment also requires particular contact levels. It can be seen that dyeing of irradiated fabric at 75°C for 45 min, using 45mL of aqueous extract of 5 pH containing 1.5g /100mL of salt as an exhaustion agent, has given better results. At low contact levels, the dyeing rate is less, whereas at optimal levels (45 min at 75°C), the equilibrium of the dye bath is disturbed, and low shade strength is found. Statistical analysis given in Table 3 shows that model used for significance of results is fit and linear. For single interaction, the role of extract volume (mL) is pretty significant (P= 0.018), whereas the role of contact of extract with fabric, i.e., dyeing time is also highly significant (P=.002). For two-way interaction (Table 3), heating level in combination with salt amount for exhaustion has been found significant also (P= 0.046) . Overall, MW rays have reduced extract volume, time, and salt amount, which show its effective treatment nature. Thus, a high-yielding 45mL extract of 5 pH should be used to dye wool at 75°C for 45 min.
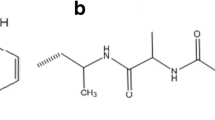
Mordanting in natural dyeing is a key element because this art helps in developing colorfast shades; usually, salts of Al+3, Fe+2, Cr+3, Co+2, Ni+2, etc. are used, but owing to carcinogenic aspects and environmental regulation, only salts of Al and Fe as well as tannic acid and cream of tartar (COT) are preferred (Adeel et al. 2022b). In our studies, the salts of Al, Fe, and tannic acid (T.A.) have been used. The results given in Fig. 7a show that the application of 1.5g/100mL of Al salt, 1g/100mL of Fe salt, and 2g /100mL of tannic acid before dyeing irradiated wool (RW=6 min) with 45 mL of aqueous extract (5pH) at 75°C for 45 min has given excellent results. Similarly, after dyeing, 1g/100mL of Al salt, 1.5g/100mL of Fe salt, and 1.5g/100mL of tannic acid (T.A.) have developed excellent colorfast shades of promising color depth Fig. 8a. The color coordinates presented in Table 4. show that mostly dyed fabrics are darker in shades and more reddish yellow in hue when obtained upon dyeing after mordanting. The shade after application of 1.5g/100mL of Al salt is darker (L* = 69.78), reddish (a* = 4.92), and yellow (b*= 54.03) in tone. On using 1g/100mL of Fe salt, shades have become darker (L* = 65.85) and more reddish yellow in tone (a* = 2.41) and (b* = 34.10). Similarly, using 2g/100mL of tannic acid, the shade becomes brighter (L* = 70.50) but greenish-yellow in hue (a* =−0.98) and (b*= 53.11). The color coordinates given in Table 4. for post-mordanted dyed fabrics are brighter in the shade and reddish yellow in the hue. The post-mordanted dyed fabric using 1g/100mL of Al salt is much brighter (L* = 80.01) but less reddish and yellow in hue (a* =−2.82; b* 57.56). Using 1.5/100mL of Fe salt, the dyed fabric is darker (L*= 63.99) in the shade, having a reddish-yellow hue (a*=6.20; b*= 46.60). Similarly, using 1.5g/100mL of tannic acid, the shade is brighter (L* = 70.97), having less reddish (a*= −3.49) but more yellow hue (b* = 45.78). Overall, 2g/100mL of tannic acid as pre-mordant and 1.5g/100mL of Fe salt as post-mordant have given excellent results. In chemical mordants, Al salt only increases the brightness in the shade and causes a reddish-yellow hue to rise. Iron utilizes its d-orbital electron to interact with e of O.H. from curcumin and amido linkage of wool, due to which darker shades are obtained through a coordinate covalent bond. Using tannic acid, there is H bonding among –O.H. of colorant, (–NHCO–) of wool and tannic acid, which results in variable shades. The proposed interaction of mordant with colorant and the wool fabric is given in Table 3. The proposed interaction of the metal dye with fabric has been shown in Fig. 9a.
Using plant phenolics, the functional molecules also have anti-viral, antioxidant, and antibacterial characteristics, which can be transferred into fabric upon dyeing. In this study, the extract of acacia, pomegranate, and pistachio hull has been utilized as an alternative to toxic chemical anchors. The results Fig. 7b show that extracts from 1.5g/100mL of acacia, 2g/100mL of pomegranate, and 1.5g/100mL of pistachio hulls before dyeing have given excellent results. Similarly, extract from 1g/100mL of acacia, 1g/100mL of pomegranate, and 2g/100mL of pistachio hulls after dyeing has given excellent color characteristics shown in Fig. 8b. Overall, extract from 2g/100mL of pomegranate as pre and 1g/100mL of pomegranate as post-bio-mordant has developed colorfast shades. The color coordinates given in Table 4. show that using acacia shades is a dark yellow, whereas using pomegranate and pistachio hulls is a bright yellow. Using 1.5g/100mL of acacia extract, the shade is brighter (L* = 70.57), having less reddish (a*=−2.97) but a more yellowish hue (b*= 61.06). Using 2g/100mL of pomegranate extract before dyeing, the shade is much brighter (L*= 75.54), having a less reddish but more yellow hue (a*=-4.18; b*= 68.91). Similarly, at 1.5g/100mL of pistachio hull, the shade is more bright (L*=77.12), having less reddish (a*=−3.51) but a yellower hue (b*=62.75). Similarly, the application after dyeing, 1g/100mL of acacia has developed a darker shade (L*=66.46) with a reddish-yellow tone (a*=0.40; b*=47.74). They use 1g/100mL of pomegranate extract after dyeing has developed a much darker shade (L*= 72.21) with a less reddish but more yellow hue (a*=-2.24; b*= 67.34). On using 2g/100mL of pistachio extract after dyeing, the developed shade is brighter (L*=72.80), less red (a*=−1.97), and yellower in tone (b*=57.74). The proposed structure of the bio-mordanted fabric and dye extract is shown in Fig. 9b.
The colorfastness of optimally dyed and mordanted wool fabrics was investigated. The ratings of fastness presented in Table 5 show that the shades developed were faster after chemical and biomordanting followed by dyeing at selected conditions. This is because MW radiation increased the dye ability of the colorant and caused it to travel closer to the fabric, apparent by forming a strong link between the fabric and the coloring portion. When rubbing, light, and washing standard methods were used, biomordants have also improved the coloring strength and fastness (Rani et al. 2020). Hence, the color performance of wild turmeric as source of yellow natural dye for wool fabric dyeing was improved by microwave treatment in terms of color rating and fastness. In nut shell, the biomordanting has been identified as a new state-of-the-art technology for shade production and for making fabric dyeing more eco-friendly and sustainable.
Conclusion
Microwave irradiation has a lot of promise for isolating natural colorants under mild conditions and then dyeing wool fabric without affecting the physiological characteristics of the colorant. In this work, MW-assisted wool dyeing with a 3 pH wild turmeric extract made from 4g of wild turmeric powder at 80°C for 45 min resulted in high color strength, indicating that dyeing variables were decreased. The curcumin from wild turmeric was extracted in an aqueous solution and microwaved for 6 min, giving best results than the natural yellow color on irradiated wool fabric, according to the instructions. The use of biomordants in this study is a sustainable approach for plant-based coloring of natural fabrics, which has elevated the value of this cultural art.
References
Adeel S, Habib N, Batool F, Rahman A, Ahmad T, Amin N (2022b) Eco-friendly approach towards isolation of colorant from Esfand for bio-mordanted silk dyeing. Environ Sci Pollut Res 29:13523–13533
Adeel S, Hasan MU, Batool F, Ozomay M, Hosseinnezhad M, Amin N, Hussaan M (2022a) Eco-friendly bio-dyeing of bio-treated nylon fabric using Esfand (P. harmala) based yellow natural colorant. J Eng Fibers Fabrics 17. https://doi.org/10.1177/15589250221091265
Adeel S, Rehman FU, Hussaan M, Amin N, Majeed A, Pervaiz M, Rehman HU (2021) Microwave assisted green isolation of laccaic acid from Lac Insect (Kerria laca) for wool dyeing. Prog Color Color Coat 14(4):293–299
Agrawal A, Chopra S (2020) Sustainable dyeing of selected natural and synthetic fabrics using waste teak leaves (Tectona Grandis L.). Res J Text Appa 24(4):357–374
Alebeid OK, Pei L, Zhou W, Wang J (2020) Sustainable wool fibers dyeing using henna extract in non-aqueous medium. Environ Chem Lett 18:489–494
Arain RA, Ahmad F, Khatri Z, Peerzada MH (2021) Microwave assisted henna organic dyeing of polyester fabric: a green, economical and energy proficient substitute. Nat Prod Res 35:327–330
Baig U, Khatri A, Ali S, Sanbhal N, Ishaque F, Junejo N (2021) Ultrasound-assisted dyeing of cotton fabric with natural dye extracted from marigold flower. J Text Inst 112:801–808
Baseri S (2022) Ecological dyeing of cotton fabric with Matricaria recutita L. in the presence of human hair keratins as an alternative copartner to metallic mordants. Sustain. Mater Technol 32:e00405
Behura S, Sahoo A, Singh S, Jena S, Kar B, Nayak S (2021) Variation in essential oil yield and volatile constituents of curcuma aromatica rhizome from different regions of eastern and southern india. J Essent Oil-Bear Plants 24:1248–1255
Buyukakinci YB, Guzel ET, Karadag R (2021) Organic cotton fabric dyed with dyer's oak and barberry dye by microwave irradiation and conventional methods. Ind Textila 72:30–38
Chen TL, Kim H, Pan SY, Tseng PC, Lin YP, Chiang PC (2020) Implementation of green chemistry principles in circular economy system towards sustainable development goals: Challenges and perspectives. Sci Total Environ 716:136998
de Marco BA, Rechelo BS, Tótoli EG, Kogawa AC, Salgado HRN (2019) Evolution of green chemistry and its multidimensional impacts: a review. Saudi Pharm J 27:1–8
Eser CO, Yavas A (2021) Effect of silk sericin pre-treatment on dyeability of woollen fabric. Ind Textila 72(2):203–209
Gala S, Sumarno S, Mahfud M (2022) Optimization of microwave-assisted extraction of natural dyes from jackfruit wood (Artocarpus heterophyllus Lamk) by response surface methodology. J Eng Appl Sci 49:29–35
Gan L, Xiao Z, Zhang J, Van Amber R, Hurren C, Xu W, Wang Y, Wang X (2021) Colored powder from colored textile waste for fabric printing application. Cellulose 28:1179–1189
Gong K, Rather LJ, Zhou Q, Wang W, Li Q (2022) Natural dyeing of merino wool fibers with Cinnamomum camphora leaves extract with mordants of biological origin: a greener approach of textile coloration. J Textile Inst 111:1038–1046
Habib N, Akram W, Adeel S, Amin N, Hosseinnezhad M (2022) Environmental-friendly extraction of Peepal (Ficus Religiosa) bark-based reddish-brown tannin natural dye for silk coloration. Environ Sci Pollut Res 1-13
Haji A (2020) Application of D-optimal design in the analysis and modelling of dyeing of plasma-treated wool with three natural dyes. Color Technol 136(2):137–146
Haji A, Ashraf S, Nasiriboroumand M, Lievens C (2020) Environmentally friendly surface treatment of wool fiber with plasma and chitosan for improved coloration with cochineal and safflower natural dyes. Fibers Polym 21(4):743–750
Haji A, Naebe M (2020) Cleaner dyeing of textiles using plasma treatment and natural dyes: a review. J Clean Prod 265:121866
Haji A, Rahimi M (2020) RSM optimization of wool dyeing with Berberis thunbergii DC leaves as a new source of natural dye. J Nat Fibers 1-14
Haque MA, Mia R, Mahmud ST, Bakar MA, Ahmed T, Farsee MS, Hossain MI (2022) Sustainable dyeing and functionalization of wool fabrics with black rice extract. Res Environ Sustain 100045
Hasan MU, Adeel S, Batool F, Ahmad T, Tang RC, Amin N, Khan SR (2022) Sustainable application of Cassia Obovata based chrysophanic acid as potential source of yellow natural colorant for textile dyeing. Environ Sci Pollut Res 29(7):10740–10753
Hayat T, Adeel S, Batool F, Amin N, Ahmad T, Ozomay M (2022) Waste black tea leaves (Camelia sinensis) as a sustainable source of tannin natural colorant for bio-treated silk dyeing. Environ Sci Pollut Res 29(16):24035–24048
Hosseinnezhad M, Gharanjig K, Jafari R, Imani H (2021) Green dyeing of woolen yarns with weld and madder natural dyes in the presences of bio-mordant. Prog Color Color Coat 14:35–45
Hu WB, Yang ZW, Wang WJ (2019) Enzymolysis-ultrasonic assisted extraction of flavanoid from Cyclocarya paliurus (Batal) Iljinskaja: HPLC profile, antimicrobial and antioxidant activity. Ind Crops Prod 130:615–626
Jafari SM, Mahdavee Khazaei K, Assadpour E (2019) Production of a natural color through microwave-assisted extraction of saffron tepal's anthocyanins. Food Sci Nutr 7:1438–1445
Khattab TA, Abdelrahman MS, Rehan M (2020) Textile dyeing industry: environmental impacts and remediation. Environ Sci Pollut Res 27:3803–3818
Kiran S, Hassan A, Adeel SHAHID, Qayyum MA, Yousaf MS, Abdullah MUHAMMAD, Habib NOMAN (2020) Green dyeing of microwave treated silk using coconut coir based tannin natural dye. Ind Textila 71(3):227–234
Majumder J, Perinban S, Singh B (2020) Effects of pre-treatment and microwave assisted extraction on natural dye from marigold (Tagetes erecta L.) and nasturtium (Tropiolum majus L.) for fabric coloration. Int J Chem Stud 8:514–521
Mansour R, Ben Ali H (2021) Investigating the use of chitosan: toward improving the dyeability of cotton fabrics dyed with Roselle (Hibiscus sabdariffa L.). J Nat Fibers 18:1007–1016
Mia R, Islam MM, Ahmed T, Waqar MA, Khanam NJ, Sultana S, Bhuiyan MSK, Uddin MN (2022) Natural dye extracted from Triadica sebifera in aqueous medium for sustainable dyeing and functionalizing of viscose fabric. Clean Eng Technol 8:100471
Nathan VK, Rani ME (2021) Natural dye from Caesalpinia sappan L. heartwood for eco-friendly coloring of recycled paper based packing material and it’s in silico toxicity analysis. Environ Sci Pollut Res 28:28713–28719
Patel B, Kanade P (2019) Sustainable dyeing and printing with natural colors vis-à-vis preparation of hygienic viscose rayon fabric. Sustain Mater Technol 22:e00116
Phan K, Van Den Broeck E, Van Speybroeck V, De Clerck K, Raes K, De Meester S (2020) The potential of anthocyanins from blueberries as a natural dye for cotton: a combined experimental and theoretical study. Dyes Pigm 176:108180
Rai S, Dutta P, Mehrotra G (2020) Natural antioxidant and antimicrobial agents from agrowastes: an emergent need to food packaging. Waste Bio Valor 11:1905–1916
Rani N, Jajpura L, Butola B (2020) Ecological dyeing of protein fabrics with Carica papaya L. leaf natural extract in the presence of bio-mordants as an alternative copartner to metal mordants. J Inst Eng (India): Series E 101:19–31
Rather LJ, Shabbir M, Li Q, Mohammad F (2019) Coloration, UV protective, and antioxidant finishing of wool fabric via natural dye extracts: cleaner production of bioactive textiles. Environ Prog Sustain Energy 38:13187
Rodiah M, Hafizah SN, Asiah HN, Nurhafizah I, Norakma M, Norazlina I (2022) Extraction of natural dye from the mesocarp and exocarp of Cocos nucifera, textile dyeing, and color fastness properties. Mater Today Proc 48:790–795
Singh A, Sheikh J (2021) Development of mosquito repellent, antibacterial, antioxidant and uv protective cotton using a novel method of azoic dyeing with Terminalia Chebula. J Nat Fibers:1–14
Thakker AM (2021) Sustainable processing of cotton fabrics with plant-based biomaterials Sapindus mukorossi and Acacia concinna for health-care applications. J Text Inst 112:718–726
Ticha MB, Slama N, Dhouibi N, Boudokhane C, Dhaouadi H (2021) Bark residues recovery of Juglans regia. for the dyeing of wool fabrics: development of microwave-assisted extraction and dyeing processes. J Nat Fibers 1-15
Umar NM, Parumasivam T, Aminu N, Toh SM (2020) Phytochemical and pharmacological properties of Curcuma aromatica Salisb (wild turmeric). J Appl Pharm Sci 10:180–194
Wang X, Yi F, Zhang W, Guo X (2021) Optimization of the application of walnut green peel pigment in wool fiber dyeing and fixing process under microwave-assisted condition. J Nat Fibers 1-14
Xie Y, Liu H, Lin L, Zhao M, Zhang L, Zhang Y, Wu Y (2019) Application of natural deep eutectic solvents to extract ferulic acid from Ligusticum chuanxiong Hort with microwave assistance. RSC Adv 9:22677–22684
Data availability
The work is from M.Phil studies.
Author information
Authors and Affiliations
Contributions
The whole experiments have been conducted by M.Phil student, Aamir Ali. Dr. Noman Habib has supervised the whole work, where Dr. Shahid Adeel and Dr. Fazal-ur-Rehman have guided scientifically for smooth running of the work. Dr. Muhammad Aftab and Ms. Asma Inayat have analyzed the data.
Corresponding author
Ethics declarations
Ethical approval
We approve that this manuscript is part of M.Phil studies.
Consent to participate and publish
We give consent to publish our work of M.Phil studies and is jointly contributed by all authors.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Habib, N., Ali, A., Adeel, S. et al. Assessment of wild turmeric–based eco-friendly yellow natural bio-colorant for dyeing of wool fabric. Environ Sci Pollut Res 30, 4570–4581 (2023). https://doi.org/10.1007/s11356-022-22450-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-22450-w