Abstract
Qinghai Lake is the largest inland saltwater lake in China, with a drainage area of 29,661 km2. This study sought to conduct an ecological and human health risk assessment of metals and heavy metals, including copper, as well as investigate their concentration, distribution, and source distribution. In terms of seasonal variation, the increases in Fe, Cr, As, Pb, and Hg were relatively large, and the spatial distribution of metals presented a three-level stepped distribution trend, gradually increasing from east to west. By further exploring the source and migration path of pollutants, our study found that the source of metals in the sediments of Qinghai Lake is mainly controlled by five rivers entering the lake. Enrichment factor (EF) calculations indicated that the metal accumulation or enrichment capacity of the three central points in Qinghai Lake Basin was strong. Interestingly, the enrichment capacity of Cu and Zn was the strongest among all metals but occurred at low and medium concentration levels, respectively. The Igeo and \({E}_r^i\) ecological risk assessment results indicated that the individual metals posed little to no ecological risks to the Qinghai Lake Basin. However, the multi-element environmental risk comprehensive index (RI) indicated that Hg (RI = 147.97) represented a slight ecological hazard, Mn (RI = 181.13) posed moderate ecological hazards, and Zn (RI = 386.66) posed strong ecological hazards. The human health risk assessment results showed that the heavy metals in the surface sediments of Qinghai Lake currently do not pose a threat to human health. This information may facilitate the implementation of more stringent monitoring programs in the aquatic ecosystem by the relevant regulatory authorities.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As an important part of the terrestrial hydrosphere, lakes have many functions, such as regulating regional climate, improving the regional environment, maintaining river runoff, maintaining regional balance, providing water for production activities, living, and agricultural irrigation, and breeding aquatic organisms (Guo et al. 2015; Ra et al. 2011; Hansen 2012). In recent decades, with the rapid development of China’s industry, agriculture, and other socioeconomic activities, human activities have intensified (e.g., development of tourism resources, overgrazing, disorderly reclamation, road construction, and transportation) (Wang et al. 2014), and the discharge of solid waste, engineering waste, and sewage water has increased each year. This has resulted in an influx of metals and toxic substances into lakes through various channels, which in turn has led to the deterioration of aquatic environments (Nriagu and Pacyna 1988; Bergbäck et al. 2001; Förstner et al. 2004). Therefore, the Qinghai Lake Basin is currently facing huge ecological and environmental pressure. Among the contaminants that affect this basin, heavy metals have a wide range of sources, long residence times, and non-biodegradability (Dong et al. 2011; Li et al. 2014), and have thus become among the most important pollutants in aquatic environments (El-Sayed et al. 2015; Milenkovic et al. 2005; Mwamburi 2014; Hernández et al. 2020). However, few studies have assessed the level, source, and ecological risk of heavy metals in China’s largest inland lake. In recent years, most studies on Qinghai Lake have focused on the impact of climate change on the lake area, dissolved organic matter (Li et al., 2021), and the source and distribution of microplastics (Xiong et al. 2018). Moreover, a few studies have characterized heavy metals in sediments but only consider small river sections. Therefore, these small-scale studies cannot meet the monitoring and evaluation requirements of the entire Qinghai Lake basin. In this study, Qinghai Lake, the largest inland saltwater lake in China, was selected as the research object. The basin is located in the transition zone of the northwest arid area, the eastern monsoon area, and the Qinghai Tibet high cold area in China. The basin is located in a sensitive global climate change area and is a typical example of a fragile ecosystem area (Lu et al. 2015). The environmental quality of the basin is directly related to the sustainable social and economic development of the region. Moreover, the basin has an important impact on the ecology of the region and the regional development of agriculture and animal husbandry (Jiang et al. 2015). Therefore, to protect the Qinghai Lake Basin, the levels of eight heavy metal elements (Cr, Mn, Fe, Hg, Cu, Zn, As, and Pb) were analyzed in the sediments of the study area. Specifically, this study evaluated the pollution degree, pollution characteristics, and ecological risk of heavy metals in the lakes in the study area. Moreover, we evaluated the source of the heavy metals and compared the rivers and lakes entering the lake in the basin to explore the impact of different types and intensities of human activities on heavy metal levels in lake sediments. Our findings will be of great significance for the sustainable socio-economic development in the region and provide a scientific basis for the effective control of heavy metal pollution in lakes, as well as for environmental governance.
Materials and methods
Study area and sampling design
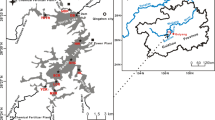
Qinghai Lake is the largest inland saltwater lake in China and a representative plateau wetland inland lake. The basin is located in the northeast of Qinghai Tibet Plateau, with a drainage area of 29,661 km2. The lake area spans from 97° 50′ E–101° 20′ E to 36° 15′ N–38° 20′ N (Zhang et al. 2021; Wang et al. 2010a, 2010b). Qinghai Lake is located at the intersection between the eastern monsoon region, the northwest arid region, and the southern Qinghai Tibet alpine region in China, and therefore exhibits unique regional climate characteristics (Chang et al. 2017). The annual average temperature in the Qinghai Lake area is −1.0–1.5 °C, the maximum monthly average temperature is 16.0–20.0 °C, and the absolute maximum temperature is 26 °C. The minimum monthly average temperature is −18.0 to −23.0 °C, and the absolute minimum temperature is −35.8 °C (Zhu et al. 2013). The average annual precipitation in the Qinghai Lake area is generally between 300 and 400 mm, but reaches up to 500 mm in some wet years. The Qinghai Lake area is a semi-arid area with frequent winds all year round. Therefore, the area exhibits a high evaporation capacity, with an average annual evaporation capacity of approximately 1300–2000 mm (Zhang et al. 2021; Chang et al. 2017; Zhu et al. 2013; Wang et al. 2010a, 2010b).
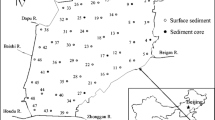
In this study, sampling was conducted in Qinghai Lake before the tourism season in July 2020 and in the frozen season in September to evaluate the impact of human factors on the region. A total of 25 sampling points (including 12 N1–N13 in the lake body, 7 R1–R8 in the river entering the lake, and 6 B1–B6 at the entrance of the lake) were set across Qinghai Lake and the five major rivers entering the lake (Fig. 1). The distribution pattern of heavy metals in the sediments of Qinghai Lake from 2020 to 2021 was analyzed, the sources of heavy metals were evaluated, and the pollution degree, pollution characteristics, and ecological risk of heavy metals in the lakes in the study area were assessed.
Sample collection, storage, and preservation
To prevent anthropogenic riverbed disturbances, the samples were collected from the middle of the river by wading (R1–R8) or from a sampling boat (N1–N13, B1–B6). Moreover, to minimize variations between samples, three parallel samples were collected at each point for mixing. The samples were collected using a mky-1 / 40 Peterson grab dredger, and the sample container was made of polyethylene. Before each use, the containers were soaked with (1+2) nitric acid for 2–3 days, cleaned with deionized water, and thoroughly dried before use. The mixed samples were packed in double polyethylene bags and placed in a portable refrigerator at −4 °C until required for downstream analyses. Before the test, the sample was ground with an agate mortar, passed through a 100-mesh nylon sieve, and transferred to a pre-numbered wide-mouth bottle until required for downstream analyses.
Sample laboratory analysis
The samples were further analyzed in the laboratory. Eight heavy metal elements (Cr, Mn, Fe, Hg, Ni, Cu, Zn, As, and Pb) in sediments were analyzed. These metals were selected because they are crustal metals (soil source) that are common in urban areas—Fe and Mn—and anthropogenic metals (from human activities): Cu, Zn, Pb, Cr, Hg, and As. For the extraction of total heavy metals in sediments, we followed the United States Environmental Protection Agency (US EPA) 3051 method (EPA., 2004) and used the acid digestion system of HNO3+H2O2 for microwave digestion (microwave digester, ECM, USA). After digestion, the sample was filtered through a 0.45-μm mixed fiber membrane as described by the EPA (EPA., 2001). An Agilent 7800 inductively coupled plasma mass spectrometer (ICP-MS, Agilent, USA) was used to determine the content of heavy metals. During the analysis, all glass and polyethylene utensils were fully soaked in 10% HNO3 for more than 24 h before use. In the process of sample pretreatment and analysis, all reagents were of superior purity to ensure the accuracy of the experimental data. In terms of quality assurance (QA) and quality control (QC), the national sediment standard GSD-7 (GBW-07366) was followed, and blank samples and parallel samples were used for quality control (QC). The total recovery rate of the standard material was 94–115%, and the detection limit of each metal element was 0.02–1.82 μg·L−1. The relative standard deviation (RSD) between the parallel samples was less than 5%, meaning that the accuracy and reproducibility of the analytical method were acceptable.
Statistical data analysis
SPSS 21.0, Python, and MATLAB were used for data analysis, using ArcGIS 10.2, Origin 2018, SigmaPlot 10.0, and other software for data visualization and graphing. Kriging interpolation and other geostatistical methods were used to analyze the spatial heterogeneity of the research area. Ecological risk assessment was conducted based on the Enrichment Factor (EF), geoaccumulation index (Igeo), and potential ecological hazard index (RI) (Müller 1979; Hakanson 1980; Brady et al. 2014; Gao et al. 2014; Knox et al. 2016). The US EPA health risk assessment model (US EPA 1996) was used to evaluate the carcinogenic risk of metal exposure in the study area.
Enrichment factor method
The enrichment factor method (EF) can be used to reflect the amount of heavy metal absorption, accumulation, or enrichment capacity in different environmental media. This method is often used to determine the accumulation capacity of metal elements in surface water sediments and coastal soils such as bays, lakes, and rivers (Zhang et al. 2016), and its calculation formula is as follows (Brady et al. 2014; Gao et al. 2014):
where EF represents the enrichment coefficient, Cn refers to the measured concentration value of the nth heavy metal in the sample, Cref is the measured content value of the calibration element, Bn is the background value of each heavy metal element in the environmental medium, and Bref is the background value of the calibration element in the environmental medium. Due to a lack of evidence from manmade sources, Fe was selected as the calibration element in this study. “Metal/Fe sample” is the sample’s metal to Fe ratio and “Metal/Fe background” is the natural background value (Table 5). Fe was used as a normalizer to account for the lack of evidence of anthropogenic sources and compensate for the lack of information regarding the grain size of the sediments, as reported in several previous studies (Neto et al. 2000; Aloupi and Angelidis 2001; Mucha et al. 2003; Varol and Şen 2012; Silva et al. 2017b). Al, K, Sc, Ga, Zr, Cs, Be, Ti, Mn, and Si have also been used as normalizers in other previous studies (Middleton and Grant 1990; Xu et al. 2017b; Zhuang et al. 2018; Pavlović et al. 2019). The background values play an important role in interpreting geochemical data because they reflect the heavy metal concentration expected to occur naturally (Rubio et al. 2000; Turekian and Wedepohl 1961; Abrahim and Parker 2008; Silva et al. 2017a; Dung et al., 2013). The EF values were interpreted according to Xu et al. (2017a) as summarized in Table 2.
Ground accumulation index method
Igeo is widely used to evaluate sediment pollution. The following equation, proposed by Müller (1979) (Hanif et al. 2016), was used to quantify the degree of pollution in the sediments.
where Cn is the measured content of element n, in milligrams per kilogram; Cn is the background value of corresponding elements, in milligrams per kilogram; and the constant K is the natural fluctuation of the content of heavy metals during diagenesis, whose value is typically 1.5. A correction factor of 1.5 was employed to analyze the possible fluctuation of background values (BGV) due to the lithogenic phenomenon (Muller et al. 1981; Krishnakumar et al. 2017; Arisekar et al. 2021; Wei et al. 2019; Youssef et al. 2020; Magni et al. 2021). Based on the Muller (1981, 1969) classification, the Igeo values were classified into seven classes, as summarized in Table 2.
Potential ecological hazard index
The potential ecological hazard index method, also known as the Håkanson method, is a way to evaluate heavy metal pollution and ecological damage based on the principle of sedimentology. This approach is mainly affected by the concentration, toxicity level, and type of heavy metals in surface sediments and the sensitivity of the water body to heavy metal pollution. Moreover, this method can evaluate the synergy of multiple heavy metal elements and consider the toxic pollution of a single metal (Hakanson 1980; Lin et al. 2019; Tang et al. 2017). The potential ecological risk index (\({E}_r^i\)) of single heavy metals can be expressed as follows:
where \({E}_r^i\) is the environmental risk index of the ith heavy metal; \({C}_f^i\) is the pollution coefficient of heavy metal I relative to the reference ratio; \({C}_s^i\) is the measured concentration of heavy metal I; \({C}_n^i\) is the evaluation reference ratio of heavy metal I; and \({T}_r^i\) is the heavy metal I toxicity response coefficient, which mainly reflects the toxicity level of heavy metals and the sensitivity of the environment to heavy metal pollution. The multi-element comprehensive environmental risk index (RI) is expressed as follows (1.6):
In this study, the toxicity coefficient of heavy metals proposed by Håkanson (Table 1) was used as the reference ratio in the calculation formula of the potential ecological risk index. Due to the lack of data on the evaluation reference ratio and toxicity response coefficient of heavy metals, our study only assessed the ecological risk of six elements: Hg, Cr, Cu, Zn, As, and Pb. The calculation results of \({E}_r^i\) and RI are shown in Table 5, and the classification is shown in Table 2 (Hakanson 1980; Lin et al. 2019; Tang et al. 2017; Wang et al. 2010a, 2010b; Li et al. 2020).
Human health risk assessment
US EPA Health Risk Assessment Model (US EPA 1996): We adopted the health risk model and recommended standards proposed by the US EPA to assess the human health risk of heavy metal pollution in lake surface sediments.
ADD ing, ADDinh, and ADDderm are acceptable daily ingested, inhaled, and dermal intake doses in units of mg·(kg*day)−1. C is the content of heavy metals in soil, in mg·kg−1. The parameter in Formulas (1.7), (1.8), and (1.9) were selected from the data in Table 3, which in turn were obtained from previous literature (Wang et al. 2018).
The formula for calculating the carcinogenic and non-carcinogenic effects of heavy metals on human health (Wang et al. 2018) is as follows:
HI is the non-carcinogenic risk index of multiple substances or multiple exposure modes of a substance, which has no dimension. HQi is a non-carcinogenic health risk index of non-carcinogenic heavy metal i, which has no dimension. ADDij and RfDij are the daily exposure and reference doses of non-carcinogenic heavy metal i in the j exposure pathway, respectively, in milligrams per kilogram. The RfD and SF reference values of each heavy metal are shown in Table 4 (He et al. 2020). When HI ≤1, there is no risk of non-carcinogenic influence. When HI >1, there is a risk of non-carcinogenic influence, and the likelihood of non-carcinogenic outcomes increases with the increase in the HI value.
Results and discussion
Analysis of spatial and temporal changes and pollution characteristics of heavy metals
In the context of environmental pollution, the origin of pollutants and their transport pathways are important for effective pollution mitigation. Understanding these factors allows decision-makers to determine whether to adopt a source-control or transport-control approach for the design of effective pollution mitigation strategies. Therefore, our study also sought to characterize the variations in crustal and anthropogenic metal load patterns and how these types of metals behave along the river.
The content of heavy metals in the surface sediments of Qinghai Lake presents a three-step distribution pattern in space, gradually increasing from east to west. The spatial distribution characteristics of the contents of As and Cr are similar, but different from the other 10 heavy metals. This indicates that the spatial fluctuation of As and Cr in Qinghai Lake is relatively large, and there may be pollution from point source discharge in these areas (Zhu et al. 2013). The first step (high value) takes N1, N6, N12, and N8 as the dividing interface to the west, and the second step (average content) in the third stage (low value) takes N1, N6, N12, and N8 as the dividing interface to the east, and gradually decreases with the B4 and N13 extension lines as the dividing line. Based on the results of a field investigation, the roads around Qinghai Lake are widely distributed and far-reaching (e.g., Qinghai Tibet railway, national highway 316, national highway 109, provincial roads, county and township roads), and the urbanization, construction, and tourism industry have also increased in recent 10 years. Therefore, traffic and transportation emissions have become one of the main sources of pollutants. The hukou of Buha River and the wharf of Jiangxi ditch may be directly related to human activities such as dense population, vehicle traffic, and scenic spot tourism around the sampling point. The level of pollution at the entrance of the Hema River and Bird Island may be related to pesticides, fertilizers, and farms. This is likely due to the rapid urban development and frequent vehicle traffic in the west of Qinghai Lake. The impact of agricultural non-point sources and human activities may result in high levels of Cu, Zn, Pb, Cr, and other metals nearby. Previous studies have shown that road sections with large traffic volume and frequent brake use such as intersections and turns exhibit high levels of Cu, Zn, Pb, and Cr accumulation in nearby sediments, which is consistent with the results of the present study (Zhu et al. 2013). Additionally, the contents of Cu, Zn, Pb, Cr, and As at points N2 (Buha River estuary), N7 (three rocks), and N5 (Quanji River Estuary and Qinghai Lake farm) were significantly higher than those at other points, which is thought to be related to natural factors and human impacts such as sediment accumulation at the entrance (Wang et al. 2010a, 2010b), increased tourism activities, and agricultural production (Zhu et al. 2013) (Figs. 2 and 3).
Relatively speaking, the other nine metal elements at B1 (Sand Island), B4 (Jiangxi ditch wharf), R7 (HEMA River), and B6 (Haiyan Bay) show low values except for the high values of the crustal metal Fe. The main external reasons are that the eastern part of the lake develops slowly, the population is small, the land use in the eastern part of the lake is mainly composed of natural reserves and biodiversity reserves, and human beings are less involved. The internal mechanism is that the sediments of the island and its adjacent waters are mainly sandy, and the sand particles are large and not easy to be enriched with metals (Chang et al. 2017). The source data is summarized in Supplementary Table S1.
Ecological risk assessment
EF can reflect the amount of heavy metal absorption, accumulation, or enrichment capacity in different environmental media. As summarized in Table 5, the metal accumulation or enrichment capacity of points N7, N10, N12, and N13 in Qinghai Lake Basin was strong. Interestingly, the enrichment capacity of Cu and Zn was the strongest among all metals, but they occurred at low and medium concentration enrichment, respectively. Therefore, our findings indicated that Cu and Zn had little impact on the ecological environment in the basin. Among the study points, N7 mainly enriches the metals Ni (EF = 2.10), Cu (EF = 2.72), and Zn (EF = 2.19); N10 enriches Cu (EF = 2.02); N12 enriches Cu (EF = 2.23); and N13 enriches Mn (EF = 4.01), Ni (EF = 3.69), Cu (EF = 4.65), Zn (EF = 3.56), As (EF = 2.58), and Pb (EF = 2.78). The Igeo calculation results (Table 5) show that the Igeo values of metals Cr, Mn, Ni, Cu, Zn, As, and Pb were less than 0, and therefore these metals do not pose an ecological risk to the Qinghai Lake Basin. The \({E}_r^i\) calculation results indicated that the \({E}_r^i\) values of Hg, Cr, Cu, Zn, As, and Pb were ≤ 40, and the average values were 5.92, 7.25, 0.91, 15.47, 2.14, and 1.45 respectively. The order from high to low is Zn > CR > Hg > As > Pb > Cu. Combined with the calculation results of Igeo, our findings indicated that the calculation results of Igeo and \({E}_r^i\) were the same, and any given single metal posed little to no ecological risk to Qinghai Lake Basin. By further considering the toxicity level and types of heavy metals and the sensitivity of water bodies to heavy metal pollution, we calculated the multi-element environmental risk comprehensive index and found that Hg (RI = 147.97) posed a slight ecological risk, Mn (CE: Run-in header = 181.13) posed a moderate ecological risk, and Zn (CE: Run-in header = 386.66) posed a strong ecological risk. Therefore, Hg, Mn, Cu, and Zn should be listed as local priority pollution elements. Moreover, the interior of the study area and a small part of the northwest area are risk-prone areas and should thus be closely monitored.
Human health risk assessment
According to the health risk assessment model and model parameters, combined with the measured parameters of heavy metals, the average personal risk of adults and children that may be caused by the heavy metals As, Cr, Hg, and Pb in sediments can be calculated. The risk calculation is only based on the amount of food intake, without considering the carcinogenic risk of water consumption (i.e., the model assumes that the drinking water is clean). See Table 6 for the calculation results. Based on Table 6, the following conclusions were made: (1) at present, heavy metals in the surface sediments of Qinghai Lake do not pose a threat to the human health of the surrounding residents, because the HI values of metals As, Cr, Hg, and Pb are less than 1. When HI > 1, the metal pollution level poses a threat to human health. (2) The average personal risk of adults and children upon metal exposure exhibits the following order: Pb > CR > As > Hg. (3) A comprehensive comparison of HI between adults and children indicated that children are more vulnerable to the content of heavy metals in sediments than adults. Furthermore, the average risk coefficients of the four metals were greater in children than in adults. Specifically, the total health hazard risk for children is 2–3 times higher than that of adults.
Conclusion
The present watershed-scale study provides information about the widespread distribution, enrichment, ecological risk, and human health risk of heavy metals in the surface sediments of Qinghai Lake. The average concentration of heavy metals in surface sediments of Qinghai Lake does not exceed its geochemical background level. From the perspective of spatial distribution, the study site exhibited a three-level distribution pattern, gradually increasing from east to west, which may be caused by human activities (such as agriculture) around Qinghai Lake. Among the evaluated metals, the spatial distribution of As and Cr fluctuated greatly, suggesting that these heavy metals originate from point source emissions. The regional heavy metal enrichment was calculated using the EF model. Our findings indicated that the metal enrichment or enrichment capacity of points N7, N10, N12, and N13 in Qinghai Lake Basin was strong. Among the evaluated metals, Cu and Zn had the strongest enrichment capacity, but occurred at low and medium concentration enrichment. Therefore, these metals had little impact on the ecological environment of the basin. The comprehensive evaluation results of ecological risk level indicated that any single metal posed little to no ecological risk to the Qinghai Lake Basin. In other words, the metals in the surface sediments of Qinghai Lake are at a low risk level. However, the multi-element environmental risk comprehensive index (RI) shows that metal Hg (RI = 147.97) poses slight ecological risks, Mn (RI = 181.13) poses moderate ecological risks, and Zn (RI = 386.66) poses strong ecological risks. Relatively speaking, the metals Hg, Mn, Cu, and Zn in the study area should be listed as local priority pollution elements. Moreover, the interior of the study area and a small part of the northwest area are risk-prone areas and should thus be closely monitored. The human health risk assessment results indicated that the heavy metals in the surface sediments of Qinghai Lake do not currently pose a significant threat to the human health of the surrounding residents. It is also worth noting that after comprehensively comparing the HI results of adults and children, our findings indicated that children are more vulnerable to the content of heavy metals in sediments than adults, and the average risk coefficients of the four metals were greater in children than in adults. The total risk of health hazards for children was 2–3 times higher than that of adults. This study provides crucial insights into the pollution level of heavy metals in Qinghai Lake and provides a basis for the development and establishment of reasonable ecological protection measures. Nevertheless, there is still a lack of information on some pollutants and their toxicity. Therefore, future studies must fill these knowledge gaps to reveal the pollution level of metals in aquatic environments and improve the accuracy of ecological risk and human health risk calculation. Future studies should also consider metal conversion and response mechanisms from the perspective of pollutant synergy, and incorporate a wide range of metal toxicity data into ecological risk and health risk assessment methods to obtain more accurate estimations.
References
Abrahim GMS, Parker RJ (2008) Assessment of heavy metal enrichment factors and the degree of contamination in marine sediments from Tamaki Estuary, Auckland, New Zealand. Environ Monit Assess 136(1–3):227–238
Aloupi M, Angelidis MO (2001) Geochemistry of natural and anthropogenic metals in the coastal sediments of the island of Lesvos, Aegean Sea. Environ Pollut 113(2):211–219
Arisekar U, Shakila RJ, Shalini R, Sivaraman B, Jeyasekaran G, Malini NAH (2021) Heavy metal concentration in reef-associated surface sediments, Hare Island, Gulf of Mannar Marine Biosphere Reserve (southeast coast of India): the first report on pollution load and biological hazard assessment using geochemical normalization factors and hazard indices. Mar Pollut Bull 162:111838
Bergbäck B, Johansson K, Mohlander U (2001) Urban metal flow —a case study of Stockholm. Review and conclusions. Water, Air, and Soil Pollution: Focus 1(3–4):3–24. https://doi.org/10.1023/A:1017531532576
Brady JP, Ayoko GA, Martens WN et al (2014) Enrichment, distribution and sources of heavy metals in the sediments of Deception Bay, Queensland, Australia[J]. Mar Pollut Bull 81(1):248–255
Chang H et al (2017) Risk assessment of heavy metal pollution in sediments in the lower reaches of Shaliu River in Qinghai Lake Basin. Geoscience 37(02):259–265. https://doi.org/10.13249/j.cnki.sgs.2017.02.012 (in chinese)
Dong J, Yu M, Bian Z et al (2011) Geostatistical analyses of heavy metal distribution in reclaimed mine land in Xuzhou, China. Environ Earth Sci 62(1):127–137. https://doi.org/10.1007/s12665-010-0507-5
Hernández E, Obrist-Farner J, Brenner M, Kenney WF, Curtis JH, Duarte E (2020) Natural and anthropogenic sources of lead, zinc, and nickel in sediments of Lake Izabal, Guatemala. J Environ Sci 96:117–126. https://doi.org/10.1016/j.jes.2020.04.020
El-Sayed SA, Moussa EMM, El-Sabagh MEI (2015) Evaluation of heavy m et al content in Qaroun Lake, El-Fayoum, Egypt. Part I: bottom sediments [J]. J Radiat Res Appl Sci 8(3):276–285
Förstner U, Heise S, Schwartz R et al (2004) Historical contaminated sediments and soils at the river basin scale: examples from the Elbe River Catchment Area. J Soils Sediments 4(4):247–260. https://doi.org/10.1007/BF02991121
Gao C, Lin Q, Bao K et al (2014) Historical variation and recent ecological risk of heavy metals in wetland sediments along Wusuli River, Northeast China[J]. Environ Earth Sci 72(11):4345–4355
Guo W, Huo S, Xi B et al (2015) Heavy m et al contamination in sediments from typical lakes in the five geographic regions of China: Distribution, bioavailability, and risk [J]. Ecol Eng 81:243–255
Lin H, Zhu X, Feng Q, Guo J, Sun X, Liang Y (2019) Pollution, sources, and bonding mechanism of mercury in street dust of a subtropical city, southern China. Hum Ecol Risk Assess 25:393–409. https://doi.org/10.1080/10807039.2018.1526631
Hansen AM (2012) Lake sediment cores as indicators of historical m et al(loid)accumulation –a case study in Mexico [J]. Appl Geochem 27(9):1745–1752
He B, Zhao H, Wang TY et al (2020) Spatial distribution and risk assessment of heavy metals in soils from a typical urbanized area. J Environ Sci 40(6):2869–2876
Jiang B, Zhang L, Zhiyun O (2015) Evaluation of Qinghai Lake wetland ecosystem service value [J]. J Appl Ecol 26(10):3137–3144 in chinese
Krishnakumar S, Ramasamy S, Chandrasekar N, Peter TS, Godson PS, Gopal V, Magesh NS (2017) Spatial risk assessment and trace element concentration in reef associated sediments of Van Island, southern part of the Gulf of Mannar, India. MarPollut Bull 115(1–2):444–450
Knox AS, Paller MH, Milliken CE, Redder TM, Wolfe JR, Seaman J (2016) Environmental impact of ongoing sources of metal contamination on remediated sediments. Sci Total Environ 563:108–117
Hakanson L (1980) An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res 14:975–1001. https://doi.org/10.1016/0043-1354(80)90143-8
Li ZY, Ma ZW, Van der Kuijp TJ et al (2014) A review of soil heavy metal pollution from mines in China: pollution and health risk assessment. Sci Total Environ 468−469:843–853. https://doi.org/10.1016/j.scitotenv.2013.08.090
Li Z, Liu J, Chen H et al (2020) Water environment in the Tibetan Plateau: heavy metal distribution analysis of surface sediments in the Yarlung Tsangpo River Basin. Environ Geochem Health 42:2451–2469. https://doi.org/10.1007/s10653-019-00409-0
Lu R, Jia F, Gao S et al (2015) Holocene aeolian activity and climatic change in Qinghai Lake Basin, Northeastern Qinghai-Tibetan Plateau[J]. Palaeogeogr Palaeoclimatol Palaeoecol 430:1–10
Milenkovic N, Damjanovic M, Ristic M (2005) Study of heavy m et al pollution in sediments from the Iron Gate (Danube River), Serbia and Montenegro [J]. Pol J Environ Stud 6(14):781–787
Magni, L.F., Castro, L.N., Rendina, A.E., 2021. Evaluation of heavy metal contamination levels in river sediments and their risk to human health in urban areas: a case study in the Matanza-Riachuelo Basin, Argentina, vol. 197. Environmental Research, p. 110979.
Middleton R, Grant A (1990) Heavy metals in the Humber estuary: Scrobicularia clay as a pre-industrial datum. Proc Yorks Geol Soc 48(1):75–80
Mucha AP, Vasconcelos MTS, Bordalo AA (2003) Macrobenthic community in the Douro estuary: relations with trace metals and natural sediment characteristics. Environ Pollut 121(2):169–180
Müller G (1979) Heavy metals in sediment of the Rhine-changes since 1971. Umschau in Wissenschaft Und Technik 79:778–783
Muller G (1981) The heavy metal pollution of the sediments of Neckars and its tributary: a stocktaking. Chemiker-Zeitung 105:157–164
Mwamburi J (2014) Variations in trace elements in bottom sediments of major Rivers in Lake Victoria's basin, Kenya [J]. Veritas Revista De Filosofía Y Teología 31(10):205–219
Neto BNJ, Smith BJ, McAllister JJ (2000) Heavy metal concentrations in surface sediments in a nearshore environment, Jurujuba Sound, Southeast Brazil. Environ Pollut 109(1):1–9
Hanif N, Eqani SAMAS, Ali SM, Cincinelli A, Ali N, Katsoyiannis IA, Tanveer ZI, Bokhari H (2016) Geo-accumulation and enrichment of trace metals in sediments and their associated risks in the Chenab River, Pakistan. J Geochem Explor 165:62–70
Nriagu JO, Pacyna JM (1988) Quantitative assessment of worldwide contamination of air, water, and soils by trace metals. Nature 33(6169):134–139
Pavlović P, Marković M, Kostić O, Sakan S, Đorđević D, Perović V, Pavlović D, Pavlović M, Cakmak D, Jarić S, Paunović M (2019) Evaluation of potentially toxic element contamination in the riparian zone of the River Sava. Catena 174:399–412
Ra K, Bang J, Lee J et al (2011) The extent and historical trend of m et al pollution recorded in core sediments from the artificial Lake Shihwa, Korea [J]. Mar Pollut Bull 62(8):1814–1821
Rubio B, Nombela MA, Vilas F (2000) Geochemistry of major and trace elements in sediments of the Ria de Vigo (NW Spain): an assessment of metal pollution. MarPollut Bull 40(11):968–980
Silva YJAB, Cantalice JRB, do Nascimento CWA, Singh VP, da Silva YJAB, Silva CMCAC, de Oliveira Silva M, Guerra SM (2017a) Bedload as an indicator of heavy metal contamination in a Brazilian anthropized watershed. Catena 153:106–113
Silva YJAB, Cantalice JRB, do Nascimento CWA, Singh VP, da Silva YJAB, Silva CMCAC, de Oliveira Silva M, Guerra SM (2017b) Bedload as an indicator of heavy metal contamination in a Brazilian anthropized watershed. Catena 153:106–113
Youssef M, El-Sorogy A, Osman M, Ghandour I, Manaa A (2020) Distribution and metal contamination in core sediments from the North Al-Wajh area, Red Sea, Saudi Arabia. Mar Pollut Bull 152:110924
Tang Z, Chai M, Cheng J, Jin J, Yang Y, Nie Z et al (2017) Contamination and health risks of heavy metals in street dust from a coal-mining city in eastern China. Ecotoxicol Environ Saf 138:83–91. https://doi.org/10.1016/j.ecoenv.2016.11.003
Turekian KK, Wedepohl KH (1961) Distribution of the elements in some major units of the earth’s crust. Geol Soc Am Bull 72(2):175–192
US Environmental Protection Agency (1996) Soil screening guidance: technical background document. R. Us environmental protection agency. (EPA/540/R-95/128)
Varol M, Şen B (2012) Assessment of nutrient and heavy metal contamination in surface water and sediments of the upper Tigris River, Turkey. Catena 92:1–10
Wang HZ, Bu XB, Zhang KX et al (2018) Determination of heavy metal content in soils on both sides of Chengdu city expressway and evaluation on the healthy risk. J Sichuan Environ 37(1):111–119
Wang H, Long H, Li X et al (2014) Evaluation of changes in ecological security in China’s Qinghai Lake Basin from 2000 to 2013 and the relationship to land use and climate Change[J]. Environ Earth Sci 72(2):341–354
Wei J, Duan M, Li Y, Nwankwegu AS, Ji Y, Zhang J (2019) Concentration and pollution assessment of heavy metals within surface sediments of the Raohe Basin, China. Sci Rep 9(1):1–7
Wang P, Junji C, Feng W (2010a) Background value of surface soil environment and its influencing factors in Qinghai Lake Basin. J Earth Environ 1(03):189–200 (in chinese)
Wang P, Cao Junji W, Feng. (2010b) Environmental background value of topsoil in Qinghai Lake Basin and its influencing factors [J]. J Earth Environ 1(3):189–200 chinese)
Xu F, Liu Z, Cao Y, Qiu L, Feng J, Xu F, Tian X (2017a) Assessment of heavy metal contamination in urban river sediments in the Jiaozhou Bay catchment, Qingdao, China. Catena 150:9–16
Xu F, Liu Z, Cao Y, Qiu L, Feng J, Xu F, Tian X (2017b) Assessment of heavy metal contamination in urban river sediments in the Jiaozhou Bay catchment, Qingdao, China. Catena 150:9–16
Xiong X et al (2018) Sources and distribution of microplastics in China’s largest inland lake – Qinghai Lake. Environ Pollut 235. https://doi.org/10.1016/j.envpol.2017.12.081
Zhang Y, et al. (2021) Distribution and potential ecological risk analysis of heavy metals in sediments of Qinghai Lake. Environmental Science https://doi.org/10.13227/j.hjkx.202108201. (in chinese)
Zhang ZY, Li JY, Maimaiti ZLPY et al (2016) Sources identification and pollution evaluation of heavy metals in the surface sediments of Bortala River, Northwest China[J]. Ecotoxicol Environ Saf 126:94–101
Zhu Y, Xuemin C, Xiaoyong F (2013) Enrichment characteristics and sources of heavy metals in surface sediments of Qinghai Lake. J Ecol 32(07):1862–1869. https://doi.org/10.13292/j.1000-4890.2013.0380 in chinese
Zhuang Q, Li G, Liu Z (2018) Distribution, source and pollution level of heavy metals in river sediments from South China. Catena 170:386–396
Acknowledgements
The authors would like to thank all the reviewers who participated in the review and MJEditor (www.mjeditor.com) for its linguistic assistance during the preparation of this manuscript.
Data availabilty
All the data are reliable. The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Funding
The research was supported by the Chinese Academy of Environmental Sciences, Ministry of Ecology and Environment, China.
Author information
Authors and Affiliations
Contributions
Zheng Li: data collection, literature review, manuscript writing, language services. Zhenghui Fu: data collection, manuscript writing, literature review, language services. Shuhang Wang: data collection, manuscript writing, literature review, language services. Yang Zhang: data collection, literature review, language services. Jinbo Zhang: data collection, literature review, language services. Yanxiao Liu: data collection, literature review, language services. Huaicheng Guo: Provide manuscript content and ideas as well as research funding support. Pingjian Yang: literature review, language services.
Corresponding author
Ethics declarations
Ethics approval
We the undersigned declare that this manuscript entitled “Spatial dynamics, ecological risk and human health assessment of heavy metals in lake surface sediments.” However, the research content mainly includes the source analysis of heavy metal pollution, pollution path, and the methodological research of human exposure in natural basins of China, and does not involve the ethical and human experimental research.
Consent to participate
We confirm that the manuscript has been read and approved by all named authors.
Consent for publication
We confirm that the manuscript is approved by all authors for publication.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• A comprehensive pollution and health risk assessment was conducted.
• The metal types with high enrichment capacity and priority control were determined.
• Children’s risk of health hazards from heavy metals is 2–3 times that of adults.
• The content of heavy metals in lake sediments is affected by the rivers entering the lake.
• This data may help regulators adopt stringent aquatic ecosystem monitoring programs.
Supplementary information
ESM 1
(DOCX 18 kb)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Z., Fu, Z., Wang, S. et al. Spatial distribution, ecological risk, and human health assessment of heavy metals in lake surface sections — a case study of Qinghai Lake, China. Environ Sci Pollut Res 30, 5137–5149 (2023). https://doi.org/10.1007/s11356-022-22293-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-22293-5