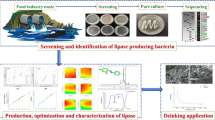
Abstract
Lipases are important biocatalysts having the third largest global demand after amylases and proteases. In the present study, we have screened 56 potential lipolytic Pseudomonas strains for their lipolytic activity. Pseudomonas plecoglossicida S7 showed highest lipase production with specific activity of 70 U/mg. Statistical optimizations using Plackett Burman design and response surface methodology evaluated fourteen different media supplements including various oilcakes, carbon sources, nitrogen sources, and metal ions which led to a 2.23-fold (156.23 U/mg) increase in lipase activity. Further, inoculum size optimization increased the overall lipase activity by 2.81-folds. The lipase was active over a range of 30–50° C with a pH range (7–10). The enzyme was tolerant to various solvents like chloroform, methanol, 1-butanol, acetonitrile, and dichloromethane and retained 60% of its activity in the presence of sodium dodecyl sulfate (0.5% w/v). The enzyme was immobilized onto Ca-alginate beads which increased thermal (20–60 °C) and pH stability (5–10). The purified enzyme could successfully remove sesame oil stains and degraded upto 25.2% of diesel contaminated soil. These properties of the lipase will help in its applicability in detergent formulations, wastewater treatments, and biodegradation of oil in the environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lipases are serine hydrolases that catalyze the conversion of triglycerides into glycerol and free fatty acids. It is due to this unique catalysis that lipases have applications in food, pharmaceutical, and biofuel production industries (Bayramoglu et al. 2015; Devi et al. 2020). Additionally, they take part in inter-esterification, esterification, aminolysis, and alcoholysis activities which makes the lipases one of the most sought after enzymes in the industries (Chandra et al. 2020). Microbial lipases are one of the most studied enzymes due to their ease of genetic manipulation, non-toxicity, no harmful residues, scale-up production in fermenters, and eco-friendly approach. In order to enhance the cost–benefit ratio and to meet the global demands, new sources of lipases are constantly being explored and reported (Ahmad et al. 2019; Coelho et al. 2020). With the aim to meet the energy demands of the ever growing population, countries have shifted their focus from the depleting non renewable to renewable energy sources (Ogino and Amoah 2019; Putri et al. 2020). Lipases have a major role in that context as they help in cost effective biofuel production and help in reduction of import duties of a country (Geetha et al. 2020). Biodiesel (fatty acid alkyl esters) is one such alternative liquid fuel which is almost similar to petro-based diesel and the role of lipases in processing glycerides and fatty acids from animal or plant fats/oil sources is indispensable and eco-friendly. The global markets of lipases were projected at $ 590.5 million by 2020 with Asia–Pacific region as the largest market. Increase in the Compound Annual Growth Rate (CAGR) of lipases at 6.5% from 2015 to 2020 has being attributed to the rising awareness for animal health and numerous feeds for livestock.
Various microbial sources of lipases have been reported such as Candida rugosa, Pseudomonas sp., Arthrobacter sp., Chromobacterium sp., Serratia sp., and Aspergillus niger (Rios et al. 2019a; Phukon et al. 2020). Pseudomonas spp. such as P. fluorescens, P. cepacia, and P. aeruginosa have been known for their versatile nature and as source of industrially important enzymes (Paggiola et al. 2020; Verma et al. 2020). They are chemoorganotrophs and can catabolize a large number of organic compounds. In response to fluctuating external nutrients, they secrete a number of extracellular enzymes including lipases (Paggiola et al. 2020; Verma et al. 2020). Pseudomonas spp. have another advantage as they easily survive on low cost substrates like agro-industrial wastes, drainage spills, and sludge (Kezrane et al. 2020; Rocha e Silva et al. 2014).
Optimization of carbon and energy sources, media components, temperature, pH, and other factors are required to check the yield of an enzyme in proportion to the cost of production (Musa et al. 2019). Various statistical designs are used for the optimization experiments (Yele and Desai 2014; Balaji et al. 2020; Sahoo et al. 2020). Low productivity and stability issues of the enzyme presents a great challenge towards the applicability and availability on a large scale. Immobilization of Pseudomonas lipases have also been reported to increase the stability, storability, and efficiency of the enzyme (Pereira et al. 2019; Rios et al. 2019b). The choice of the carrier depends on the chemical and thermal stability, capability of easy rejuvenation, biocompatibility and reusability as well as cost effectiveness (Chandra et al. 2020).
The present study aims to optimize the production of lipase from Pseudomonas plecoglossicida S7 by using various substrates and increase its downstream applicability by immobilization.
Materials
Microbial cultures
Forty isolates of Pseudomonas spp. were obtained from National Agriculturally Important Microbial Culture Collection (NAIMCC), Maunath Bhanjan, India (Table 1). Sixteen Pseudomonas cultures were generously provided by Dr. Pandiyan Kuppusamy (ICAR-NBAIM, Mau). The cultures were revived and maintained on King’s B media for further use.
Chemicals and reagents
All the media, buffer components, and p-nitrophenyl acetate were purchased from HiMedia Laboratories, India. The solvents used in the study were of analytical grade and procured from Sigma-Aldrich Co. (St. Louis, MO, USA). Supplementary Table 1 contains the details of all the chemicals used in the study.
Screening for lipolytic isolates and lipase assay
The cultures were streaked on media containing beef extract (3.0 g), peptone (5.0 g), sodium chloride (5.0 g), CaCl2. 2H2O (0.1 g), Tween 80 (1% v/v), and agar (2% w/v) per liter of distilled water (Sierra 1957). The lipolytic activity was indicated by the appearance of a visible precipitate, resulting from the deposition of crystals of the calcium salt formed by the fatty acid liberated by the enzyme (Mehta et al. 2018).
The lipase activity was determined using p-nitrophenyl acetate as substrate with slight modifications (Choo et al. 1998). 200 mM of p-nitrophenyl acetate solution was prepared using DMSO (Extrapure, analytical grade) as solvent and stored at 4 °C for further use. The assay mixture consisted of 0.25 mL of bacterial broth culture, 0.2 mL of toluene (Extrapure, analytical grade), 2 mL of MUB buffer (pH-7.0, 100 mM), and 1 mL of p-Nitrophenyl acetate (200 mM). The mixture was mixed and incubated at 37 °C for 1 h at 150 rpm. The mixture was then centrifuged at 10,000 rpm (4 °C) for 15 min and 0.1 mL of the supernatant (diluted with 1 mL of 0.5 M NaOH and volume made upto 10 mL using distilled water) was used for final assay. Absorbance was taken at 430 nm. A standard curve using p-nitrophenol (100–1000 µg/mL) was prepared after taking absorbance at 430 nm. One unit of lipase activity was defined as amount of enzyme required to release one micromole of p-nitrophenol from the substrate per minute under standard assay conditions. Total protein was estimated using Bradford’s method (Bradford 1976).
Medium optimization for higher lipase production
Initial screening of multiple media components
A total of fourteen different media components were taken for the screening of best lipolytic activity (Table 2). Seven lipid rich sources including four oilcakes (groundnut, sesame, Brassica nigra, and Sinapis alba), three surfactants (Tween 20, Tween 40, Triton X-100 (2X) and Triton X-100), three carbon sources (glucose, sucrose, and maltose), and one metal ion source (MgSO4. 7H2O) was used initially. The basal lipase medium reported by Sierra (1957) was amended for the experiments. The concentration for each media component used has been mentioned in Table 2. The culture (1% v/v) was inoculated in 20 mL to amend the lipase media at 37 °C for 24 h at 150 rpm and the lipase activity was recorded as described in the “Chemicals and reagents” section. The results were analyzed by Duncan’s Multiple Range Test (DMRT) using the SPSS software package 16.0 using Microsoft excel 2007 (SPSS Inc. 2007).
Screening of factors using Plackett Burman experimental design
Four best substrate screened using initial screening were subjected to Plackett Burman experimental design as depicted in Table 3. Three nitrogen sources, viz., ammonium di-hydrogen orthophosphate (ADP), urea, and potassium nitrate (PN), were also added for evaluation as substrates for the experiment. The higher limit was denoted by + 1 and lower limit by − 1 indicating the amount of substrate used per run. Eight runs were performed in duplicates using a total of seven substrates. The results were analyzed using Design-Expert software package version 9.0.6.2 (serial number: 1619–5898-8149–2390). Factors were screened using normal probability plot (Fig. 2) from where it can be seen that four factors, namely ADP, Maltose, PN, and TX-100, were having positive effects on lipase activity. Therefore, out of these four factors, for the present investigation, three important factors, namely ADP, Maltose, and TX-100, were opted for further investigation.
Central composite design for optimization of parameters
For quadratic model
Central composite design (CCD) is a second-order rotatable design (SORD) which demands fitting of a second order or quadratic polynomial for getting more precision in analyzing the results. For the present investigation, a 20 run CCD was used with these three factors (Table 5). For three factors, the second-order polynomial model generally used is as follows:
So, first as expected, a second-order quadratic model was fitted with the data. Based on second-order model fitting, it was observed that for the given dataset, the overall model was not significant even at 10% level of significance (p-value: 0.1774) although the quadratic effect of TX-100 (p-value: 0.0465) and the interaction effect of ADP and TX-100 (p-value: 0.0277) were significant at 5% level of significance. The non-significance of lack of fit error although desirable but the low p-value of 0.1938 against the lack of fit error indicated there was still scope for model improvement. The R2 = 0.6237 for the model indicates the model was able to explain only 62.37% variability which was also in lower side. The adjusted R2 = 0.2851 indicated the significant portions of variations explained by the model was only 28.51% which was low. It is to be noted here that the adjusted R2 will increase if only significant variables included in the model. The adequate precision value of 5.161 although desirable as it is more than 4 but it was still on the lower side. Thus, all these criteria suggest that the model is not appropriately representing the given data and there is a need to improve the model by adding significant terms.
Modified model
From the results obtained based on fitting of quadratic model as discussed above, it can be seen that there is still enough scope for improving the model. In case of the quadratic model although lack of fit test remains non-significant (which should remain non-significant for a goodness of fit test), the p value of 0.1938 is on the lower side. Thus, cubic model may be a good choice.
It should be note that the data under consideration were obtained based on a 20 run central composite designs with three factors which is although enough for estimating all the 10 parameters (including intercept) of a quadratic model, but the same resources were not enough to estimate all the 20 parameters (including intercept) of a cubic model. However, the existing resources can be used to estimate some additional parameters apart from all the 10 parameters of quadratic model. Therefore, in order to improve the performance of the model and keeping the resource constraint, based on hit and trial method, the following modified model with AB2 was fitted again:
All the above analysis and the optimization were carried out using Design-Expert Software package version 9.0.6.2 (serial number: 1619–5898-8149–2390). The final optimized media compositions were tested for lipase production in triplicates.
Optimization of inoculum size and effect of temperature, pH, organic solvents, and SDS on the activity and stability of lipase
Inoculum concentration for the assay was tested with a range from 1.0 to 5.0% (v/v) with an interval of 1%. The activity and stability were determined under a range of physiological and chemical factors like temperature, pH, organic solvents, and SDS. Temperature optimization was checked by incubating the substrate and enzyme at 20–60 °C (with an interval of 10° C) for 1 h and the enzyme activity was recorded. A range of pH from 5.0 to 10.0 (with an interval of 1) was taken by adjusting the pH of the MUB buffer (0.1 M) by citrate buffer (5.0–6.0, 0.1 M) and 1 M NaOH (8.0–10.0). The final concentration of all the buffers were 57.14 mM in all the experimental setups. Similarly, organic solvents like chloroform, methanol, 1-butanol, acetonitrile, and dichloromethane were tested with a concentration of 1 and 2% (v/v). Sodium dodecyl sulfate, being an important component of detergents was also used to test the enzyme activity (0.1–0.5% w/v). The above optimization experiments were checked for lipase activity after 24 and 48 h of the incubation at 150 rpm and repeated three times.
Purification of lipase and SDS-PAGE
After optimization, the selected isolate was grown in the optimized media at pH 7 for 2 days. The cells were harvested by centrifugation at 10,000 g for 20 min. The cell-free supernatant was subjected to precipitation by 50–70% ammonium sulfate at 37 °C with continuous stirring (150 rpm) followed by centrifugation at 10000 g for 30 min. Ammonium sulfate precipitate was dialyzed against 100 mM phosphate buffer in a dialysis membrane (HiMedia) with a molecular weight cutoff of 12–14 kDa (Priyanka et al. 2019). The dialyzed fraction was further purified by ion exchange chromatography using a DEAE-cellulose column (HiMedia, India) equilibrated with 100 mM phosphate buffer (with 0.002 M sodium azide) at pH 7. The enzyme was eluted at a flow rate of 0.5 ml/min with linear gradient of 0.1–1.0 M NaCl in the same buffer. Lipase activity and protein concentration of the eluted fraction were measured spectrophotometrically, and the active fractions were pooled together and stored for further analysis. The molecular weight of the active fraction was determined by SDS-PAGE, as mentioned by Laemmli (1970).
Entrapment in Ca-alginate beads using ionotropic gelation
The biopolymer solution was prepared using 3% (w/v) sodium alginate in 10 mL of the cell-free crude as well as purified enzyme extracts separately. Using an intravenous syringe (20 mL), the biopolymer solution was added drop wise to an aqueous solution of calcium chloride (0.14 M) while keeping the system on a magnetic stirrer at 500 rpm for 5 min (Pereira et al. 2019). After the encapsulation process, the beads were washed with 0.1 M phosphate buffer and dried at 4 °C. The dried microcapsules were used to check the enzyme activity retention after encapsulation along with its reusability. The beads were used three times subsequently to analyze the retention of their enzyme activity and efficiency. Beads were also prepared without containing crude/purified enzyme to check the effect of alginate and calcium chloride on the substrate. Also, the microcapsules were characterized at different temperature, pH, organic solvents, and SDS as performed for the free enzyme. A schematic representation of the immobilization process has been depicted in Fig. 1.
The effect of commercial detergent on lipase stability, laundry additive, and oil stain removal
Stability of lipase in the presence of detergents was investigated using five commercial detergent powders: Tide® and Ariel® (Procter & Gamble home products Ltd., India), Vidhisa® (Vidhisa Group, Kanpur, India), Surf excel® (Hindustan Unilever Limited-Mumbai, India), and Active Wheel® (Hindustan Unilever Limited-Mumbai, India). Solution of 1% (w/v) commercial detergents was prepared by dissolving each detergent in distilled water and boiling for 15 min to inactivate the enzyme present in the detergent formulation (Sahay and Chouhan 2018). A positive control without detergent was run under similar conditions to calculate the residual lipase activity of each test sample. Lipase activity was analyzed as method described earlier and residual enzyme activity was calculated with respect to control (without detergent) as 100%.
The purified lipase was tested for its ability to remove oil stains from fabrics. Polycotton fabric was cut into pieces (3 cm × 3 cm) and each piece was stained with two drops of commercially available sesame oil (Organic India) used for deep frying. The fabric pieces were allowed to dry and subjected to various oil-destaining treatments such as distilled water, commercially available detergent 1% (w/v) and water + lipase (Das et al. 2016). The oil-stained fabric pieces were separately soaked in each treatment solution taken in a petri-plate and gently agitated. Post-incubation for 30 min, the fabric pieces were removed, dried, and observed for residual oil stains. The efficiency of lipolytic activity was evaluated by visualization of the oil stain.
Determination of diesel degradation through gravimetric analysis
Soil samples were collected from the fields of Indian Institute of Seed Science, Mau. It was sterilized thrice through tyndallization before proceeding with the experiment. Diesel was procured from a nearby fuel station and filter sterilized prior to use. P. plecoglossicida S7 was raised in broth media and the following treatments were set up: (1) T1 consisted of 25 g soil + 1 mL diesel, (2) T2: 25 g soil + 1 mL diesel + 100 mL P. plecoglossicida S7 bacterial suspension, and (3) MS medium (control) in sterile 250-mL flasks. The treatments were incubated at 37 °C for 10 days at 120 rpm. Residual diesel was extracted using n-hexane as described earlier by Zakaria et al. (2021). The degradation rate was determined using the following equation:
Results
Screening of isolates
Out of fifty six isolates used in the study, thirty nine formed a clear halo zone on the Tween 80 supplemented media (Fig. 2a). Upon quantitative screening of the 39 positive cultures, 27 showed lipase activity in a range between 1.083 and 28.89 µmole p-NP/min (Table 4). Pseudomonas plecoglossicida S7 (NAIMCC-B: 00,397) showed a maximum activity of 28.9 µmole p-NP min−1 and a specific activity of 70 U/mg protein. Hence, it was selected for further optimization and analysis.
a The isolate Pseudomonas plecoglossicida S7 formed a halo zone on Tween 80 supplemented media. b The effect of various substrates on the production of lipase. Magnesium sulfate and sucrose was excluded as it had a negative impact on lipase production. The letters placed above the bars are DMRT ranks. Different letters present significantly different mean values. GOC, groundnut oilcake; SOC, sesame oilcake; BNOC, Brassica nigra oilcake; SAOC, Sinapis alba oilcake
Substrate optimization
Screening with multiple substrates
DMRT ranking showed four substrates, viz., groundnut oilcake, sesame oilcake, Triton-X 100, and maltose, which enhanced the production of lipase significantly as compared to control substrate Tween 80 (Fig. 2b). Hence, these substrates were selected for the Plackett Burman design along with three nitrogen sources, viz., ADP, potassium nitrate, and urea.
Figure 3 showed the substrates which contributed to the enhancement in lipase production. Results of Plackett Burman analyses revealed Triton X-100, ADP, maltose, and potassium nitrate to be the most dominant components. ADP was selected over potassium nitrate as it was more effective. Finally, Triton X-100, maltose, and ADP were selected for further optimization.
Response surface optimization based on CCD using modified model
Figures 4, 5, and 6 showed that keeping ADP % at intermediate level, maxima with high desirability lied towards the extremes which was towards the lower concentration of maltose and higher concentration of Triton X-100. The results of projected composition of the selected substrates are presented in Table 5. The actual factor concentrations predicted by the RSM cubic model were: Maltose: 0.75%, Triton X-100: 0.81%, and ADP: 1.25% with a prediction maxima of 77.22 µmole p-NP min−1 of lipase and a desirability of 0.939. The final lipase activity obtained was 70.32 µmole p-NP min−1 with a specific activity of 156.23 U/mg protein. An increase by 38% in the lipase production was achieved after the optimization of substrate concentrations.
The effect of inoculum size, temperature, pH, organic solvents, and SDS of the optimal lipase
Maximum lipolytic activity (197 U/mg protein) was obtained with 4% (v/v) inoculum increasing the enzyme production by 1.26-folds (Fig. 7). There was no activity at acidic pH of 5 and 6 whereas it retained ~ 43% activity at pH 10 when compared to neutral pH (Fig. 8a). Likewise, the enzyme retained its activity in the temperature 30–50 °C with optimal activity at 37 °C (Fig. 8b). The lipase showed good organo-tolerance to a variety of organic solvents and could tolerate 0.5% of SDS retaining 60% of its activity (Fig. 8c, d).
a–d A comparative profile of the free and bound (Ca-alginate beads) of crude and purified lipase in the presence of organic solvents and SDS along with the varying lipase production over a range of pH and temperature. CFE, crude-free enzyme; CBE, crude bound, enzyme; PFE, purified free enzyme; PBE, purified bound enzyme
Purification of lipase enzyme and determination of molecular weight through SDS-PAGE
An overall 9.2-fold purification was obtained after purification using ion exchange chromatography; however, the final yield was ~ 11% (Table 6). The purified enzyme was eluted at 0.9 M NaCl. The purified protein was ~ 67 KDa as determined by SDS-PAGE (Fig. 9a).
Determination of activity of lipase (crude/purified) in free and bound form under various growth parameters
The immobilized enzyme beads showed greater stability as compared to the unbound enzyme in both crude and purified form. Under immobilized condition, the enzyme retained 85% of its activity at pH of 5 and 6 whereas the unbound crude enzyme lost its activity in acidic pH (Fig. 8a). Notably, the purified free enzyme showed enhanced activity even at acidic pH as compared to the free crude enzyme. Similarly, the purified immobilized enzyme showed excellent activity at 60 °C and showed sustained activity over the range 20–60 °C (Fig. 8b). The activity of both bound and unbound crude enzymes was almost the same in the case of varying concentrations of SDS (Fig. 8c) showing enhanced tolerance in purified bound form. It was noted that the activity of the bound crude enzyme lowered (27–34%) in the presence of various organic solvents but increased significantly in its purified bound form (Fig. 8d). After third re-use, the immobilized beads could retain 66.2% of its initial specific activity as compared to the initial activity. Overall, the immobilized purified enzyme beads showed maximum retention of lipase activity when subjected to various external parameters.
The effect of commercial detergents, SDS-PAGE, and oil stain removal capacity of the purified lipase
Among the five detergents tested, the purified lipase showed best compatibility retaining 99% of its activity in the presence of Vidhisa detergent powder (Fig. 10). Ariel showed the least compatibility as the enzyme could retain only 29% of its activity in its immobilized form. The rest of the three detergents were moderately compatible with the purified enzyme. As compared to the detergent + water formulation, the purified lipase + water solution proved to be more effective against the sesame oil stain on the cloth fabric as shown in Fig. 9b.
Diesel degradation using P. plecoglossicida S7
After gravimetric analysis of the different samples, it was observed that the bacterial isolate was able to degrade 25.2% of the diesel added to the soil as compared to the abiotic control.
Discussion
Pseudomonas spp. are known as potential source of diverse lipases. This is due to the versatile metabolic machinery present in Pseudomonas spp. Studies have reported application of Pseudomonas lipases in detergent, paper pulp, biodiesel production industries, etc. (Phukon et al. 2020; Nathan and Rani 2020). Optimization of the production of lipase from Pseudomonas plecoglossicida S7 was performed using sequential statistical designs with multiple substrates. Numerous studies have been undertaken to enhance the production of lipase using cost effective substrates (Jain et al. 2020; Ilesanmi et al. 2020). Also, the use of statistical models comes in quite handy while designing such experiments. Devi et al. (2020) used castor oil as lipase inducer for the optimization of lipase production (220 U/mL) from P. guariconesis. Phukon et al. (2020) applied response surface methodology using tryptone, yeast extract, and NaCl and obtained a 2.69-fold increase in lipase activity (179.3 U/mg) from P. helmanticensis HS6. Azevedo et al (2020) used cacay butter and wheat bran as lipase inducers producing 308.14 U/g of lipase from Aspergillus terrus NRRL-255. Similarly, Nema et al. (2019) used a tri-substrate mixture of rice husk, cottonseed cake, and red gram husk in the ratio of 2:1:1 to maximize the lipase production from Aspergillus niger MTCC 872. In this study, optimization of the medium resulted in production of 197 U of lipase per mg protein. The focus on agro-industrial wastes and cost effective oils helps in reducing the cost of production of the enzyme significantly. Moreover, the recycling of agricultural and industrial waste has positive impact on the environment contributing to their valorization (Federici et al. 2009). This study included various oilcakes of groundnut, sesame, Brassica nigra, and Synapis alba although they were eliminated as the Plakket Burman design experiment showed that the oilcakes had a negative impact on the lipase production during their comparative analysis with other substrates. However, amendments of media components like maltose, Triton X 100 and ammonium di-hydrogen phosphate (ADP) increased the lipase activity by 2.23-folds. Al-Dhabi et al. (2020) used six carbon sources in which maltose was reported to enhance the production of lipase by 10%. Generally Pseudomonas spp. have been reported to use glucose more effectively (Salwoom et al. 2019; Sooch and Kauldhar 2013). Triton X-100, a nonionic surfactant, showed the highest effect on lipase activity in the screening experiment and is a well-known inducer of lipase production (Mesa et al. 2018; Perna et al. 2017). The surfactant has also been reported to contribute to the stability and catalytic property of the enzyme (Mesa et al. 2018). In the present study, further optimization of inoculum amount (4% v/v) resulted in increase in activity by 1.26-folds. Priyanka et al. (2020) reported 2.5% inoculum to be optimum for lipase activity in P. brenneri. 3% inoculum was found to be most favorable for lipase production in P. yamanorum LP2 (Komesli et al. 2020).
Lipases have a broad range of action acting on various substrates demanding different environmental conditions like temperature, pH, tolerance to organic solvents, and detergents. A wide number of thermophilic enzymes have been characterized such as amylases, xylanases, proteases, esterases, and lipases (Chakdar et al. 2016; Curci et al. 2019; Gazali and Suwastika 2018). Similarly, pH stability becomes an important factor during the mass production of an enzyme (Dumorné et al. 2017). Variations in pH change the conformation of the catalytic site, as well as extreme changes, may completely alter the enzyme structure. Lipase produced by P. plecoglossicida S7 showed an optimum activity at 37 °C at neutral pH. Molecular weight of the purified lipase was determined to be ~ 67 KDa. Pseudomonas lipases with high molecular weight have been reported previously (Ramani et al. 2010). The enzyme retained its activity over a range of 30-–50° C (Fig. 8) whereas it retained 43% of its activity at pH 10. Similar results have been reported earlier showing alkaline nature of Pseudomonas lipases and optimal activity at temperature ranging from 30 to 45 °C C (Devi et al. 2020; Azevedo et al. 2020). The catalytic activity of enzymes is altered or lost by the presence of ionic detergents like SDS and CTAB. P. plecoglossicida S7 lipase retained 60% of the activity at 0.5% concentration of SDS. Hu et al. (2018) reported that the lipase activity of P. aeruginosa HFE733 was lost at 10 mM/L concentration of SDS. Also, P. plecoglossicida S7 lipase activity increased by 4% in the presence of chloroform (2% v/v) as compared to control without organic solvents. In the presence of other organic solvents like dichloromethane, 1-butanol, methanol, and acetonitrile, the lipase activity was lost by 28.6, 21.3, 13, and 17% respectively (Fig. 8c). The bound enzyme showed relative stability (27–34% less activity) in the presence of organic solvents (1% & 2% v/v) (Bayramoglu et al. 2022; Acıkgoz-Erkaya et al. 2021). Klibanov (2001) discussed the applicability and commercialization of enzyme-catalyzed reactions in organic solvents as compared to natural aqueous reaction media. Sonkar and Singh (2020) reported that the organic solvents showed inhibitory effect on enzyme activity of P. punonensis except in the case of acetone and 2-propanol. Bisht et al. (2014) attributed this loss of activity to the hydrophilic nature of some organic solvents. Purification of lipase led to 9.2-fold purification in specific activity of the protein. Previous studies have used column chromatographic techniques to enhance the purification of lipase enzyme (Priyanka et al. 2020; Hu et al. 2018). Hu et al. (2018) achieved 9.9-folds of alkaline lipase from Pseudomonas aeruginosa HFE733. Mehta et al. (2018) reported a 6.9-fold purification of lipase from Aspergillus fumigatus with a yield of 11.03% using Octyl Sepharose chromatography.
In order to have industrial applications of an enzyme, stability over broad range of temperature and pH is important for downstream processing. Since all natural enzymes are not suitable for large-scale production, they are immobilized/bound to inert substances to enhance their downstream processing including selectivity, stability, and kinetics (Velasco-Lozano 2020). Reusability of the bound enzyme also contributes to enhancing cost–benefit ratio. In this study, we carried out the ionotropic gelation of lipase onto Ca-alginate beads. As compared to the free enzyme, the stability of the bound enzyme improved in terms of both pH and temperature. The free crude enzyme lost its activity at acidic pH whereas the bound enzyme retained 84% of its activity at pH 5 as compared to the activity of free enzyme at neutral pH. The enzyme bound to Ca-alginate beads showed considerable thermal tolerance at 60 °C retaining 72% of its activity. Importantly, the purified bound form showed a more improved and sustained lipase stability when subjected to varying temperature ranges. Immobilization of lipases has been reported to improve the range of thermo stability as well as stability due to changes in pH. Pereira et al. (2019) showed that the bound enzyme in alginate-chitosan beads shifted lipase optimum pH (to 8.5 from 7) and temperature (to 40 °C from 35 °C). Notably, both the free and bound enzymes were stable in the presence of SDS which makes it a suitable candidate for usage in detergent industries. The bound enzyme could retain 66.2% of its activity which highlighted the fact that effective immobilization could save repeated production of the lipase for industrial applications. Marín-Suárez et al. (2019) analyzed the reusability of immobilized Novozyme 435 on environment-friendly biodiesel production. Further, the present study evaluated the compatibility of the purified lipase with commercially popular detergents and found that it worked extremely well in the presence of them. Earlier studies also point to the fact that enzymes compatible with commercial products prove more useful on an industrial scale (Das et al. 2016; Prazeres et al. 2006). Additionally, the action of lipase on stubborn oil stains proved the versatility of the purified enzyme (Fig. 10a). Also, the applicability of P. plecoglossicida S7 lipase to degrade diesel in soil can have major impact on environmental friendly removal of diesel spills. Sharma et al. (2014) reported that 66% of the diesel contaminated soil could be degraded by augmenting P. aeruginosa. Looking at the use of diesel by various industries and research stations, biodegradation of diesel spills becomes more relevant (Brooks et al. 2018; Zakaria et al. 2021). The use of enzymes is restricted to their natural, aqueous reaction media, and the scope of industrial bioconversions, especially for the production of speciality chemicals and polymers gets limited (Klibanov 2001). Clearly, the purified immobilized lipase in this study showed great promise to be utilized for industrial applications.
Conclusions
The present study has used sequential statistical optimization to increase lipase production in Pseudomonas plecoglossicida S7. Inoculation of 4% (v/v) P. plecoglossicida S7 culture in basal media supplemented with Maltose-0.75% (w/v), ADP-1.25% (w/v), and Triton X-100–0.81% (v/v) followed by growth at 37 °C with pH 7 resulting in 2.81-fold increase in lipase production. The free enzyme was considerably organo and detergent tolerant and active at alkaline pH. The purified enzyme when bound to Ca-alginate beads showed significant increase in the stability over a wide range of temperature (20–60 °C) and pH (5–10) with distinct efficiency and reusability. The results of this study will be very helpful to utilize P. plecoglossicida for mass production of lipase for industrial applications such as in dairy, pharmaceuticals, animal feed, detergent formulations, and environmental engineering like wastewater treatments, biodegradation of oil, and diesel spills in the environment.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Acıkgoz-Erkaya I, Bayramoglu G, Akbulut A, Arica MY (2021) Immobilization of Candida rugosa lipase on magnetic biosilica particles: hydrolysis and transesterification studies. Biotechnol Bioprocess Eng 26:827–840
Ahmad FB, Zhang Z, Doherty WOS et al (2019) The prospect of microbial oil production and applications from oil palm biomass. Biochem Eng J 143:9–23. https://doi.org/10.1016/j.bej.2018.12.003
Al-Dhabi NA, Esmail GA, Ghilan AKM et al (2020) Isolation and screening of Streptomyces sp. Al-Dhabi-49 from the environment of Saudi Arabia with concomitant production of lipase and protease in submerged fermentation. Saudi J Biol Sci 27(1):474–479. https://doi.org/10.1016/j.sjbs.2019.11.011
Balaji L, Chittoor JT, Jayaraman G (2020) Optimization of extracellular lipase production by halotolerant Bacillus sp. VITL8 using factorial design and applicability of enzyme in pretreatment of food industry effluents. Prep Biochem Biotechnol 50(7):708–716. https://doi.org/10.1080/10826068.2020.1734936
Bayramoglu G, Akbulut A, Ozalp VC, Arica MY (2015) Immobilized lipase on micro-porous biosilica for enzymatic transesterification of algal oil. Chem Eng Res Des 95:12–21
Bayramoglu G, Celikbicak O, Kilic M, Arica MY (2022) Immobilization of Candida rugosa lipase on magnetic chitosan beads and application in flavor esters synthesis. Food Chem 366:130699
Bisht D, Yadav SK and Darmwal NS (2014) An oxidant and organic solvent tolerant alkaline lipase by P. aeruginosa mutant: downstream processing and biochemical characterization. Braz J Microbiol 44:1305–1314. https://doi.org/10.1590/S1517-83822013000400040
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brooks ST, Jabour J, Sharman AJ, Bergstrom DM (2018) An analysis of environmental incidents for a national Antarctic program. J Environ Manage 212:340–348
Chakdar H, Kumar M, Pandiyan K et al (2016) Bacterial xylanases: biology to biotechnology. 3 Biotech 6(2):150. https://doi.org/10.1007/s13205-016-0457-z
Chandra P, Enespa SR, Arora PK (2020) Microbial lipases and their industrial applications: A comprehensive review. Microb Cell Fact 19:169. https://doi.org/10.1186/s12934-020-01428-8
Choo DW, Kurihara T, Suzuki T et al (1998) A cold-adapted lipase of an Alaskan psychrotroph, Pseudomonas sp. strain B11–1: gene cloning and enzyme purification and characterization. Appl Environ Microbiol 64(2):486–497. https://doi.org/10.1128/aem.64.2.486-491.1998
Coelho ALS, Orlandelli RC (2020) Immobilized microbial lipases in the food industry: a systematic literature review. Crit Rev Food Sci Nutr 1–15. https://doi.org/10.1080/10408398.2020.1764489
Curci N, Strazzulli A, De Lise F et al (2019) Identification of a novel esterase from the thermophilic bacterium Geobacillus thermodenitrificans NG80-2. Extremophiles 23:407–419. https://doi.org/10.1007/s00792-019-01093-9
Das A, Shivakumar S, Bhattacharya S et al (2016) Purification and characterization of a surfactant-compatible lipase from Aspergillus tamarii JGIF06 exhibiting energy-efficient removal of oil stains from polycotton fabric. 3 Biotech 6:131. https://doi.org/10.1007/s13205-016-0449-z
de Azevedo WM, de Oliveira LFR, Alcântara MA et al (2020) Turning cacay butter and wheat bran into substrate for lipase production by Aspergillus terreus NRRL-255. Prep Biochem Biotechnol 50(7):689–696. https://doi.org/10.1080/10826068.2020.1728698
Devi R, Madhavan Nampoothiri K, Sukumaran RK et al (2020) Lipase of Pseudomonas guariconesis as an additive in laundry detergents and transesterification biocatalysts. J Basic Microbiol 60(2):112–125. https://doi.org/10.1002/jobm.201900326
Dumorné K, Córdova DC, Astorga-Eló M et al (2017) Extremozymes: a potential source for industrial applications. J Microbiol Biotechnol. https://doi.org/10.4014/jmb.1611.11006
Federici F, Fava F, Kalogerakis N et al (2009) Valorisation of agro-industrial by-products, effluents and waste: concept, opportunities and the case of olive mill waste waters. J Chem Technol Biotechnol 84(6):895–900. https://doi.org/10.1002/jctb.2165
Gazali FM, Suwastika IN (2018) Thermostable α-Amylase activity from thermophilic bacteria isolated from Bora Hot Spring, Central Sulawesi, In: Journal of Physics: Conference Series. The 2nd International Conference on Science (ICOS), Makassar, Indonesia. 979: 012001 https://doi.org/10.1088/1742-6596/979/1/012001
Geetha SJ, Al-Bahry S, Al-Wahaibi Y et al (2020) Recent update on biodiesel production using various substrates and practical execution. In: Srivastava N, Srivastava M, Mishra P, Gupta V (eds) Substrate Analysis for Effective Biofuels Production. Clean Energy Production Technologies. Springer, Singapore. https://doi.org/10.1007/978-981-32-9607-7_5
Hu J, Cai W, Wang C et al (2018) Purification and characterization of alkaline lipase production by Pseudomonas aeruginosa HFE733 and application for biodegradation in food wastewater treatment. Biotechnol Biotechnol Equip. https://doi.org/10.1080/13102818.2018.1446764
Ilesanmi OI, Adekunle AE, Omolaiye JA, et al (2020) Isolation, optimization and molecular characterization of lipase producing bacteria from contaminated soil.Scientific African 8:p.e00279. https://doi.org/10.1016/j.sciaf.2020.e00279
Jain R, Pandey N, Pandey A (2020) Aggregation properties of cold-active lipase produced by a psychrotolerant strain of Pseudomonas palleroniana (GBPI_508). Biocatal Biotransformation 38(4):263–273. https://doi.org/10.1080/10242422.2019.1666829
Kezrane I, Harouna BM, Hamadache M et al (2020) Use of hydrocarbons sludge as a substrate for the production of biosurfactants by Pseudomonas aeruginosa ATCC 27853. Environ Monit Assess 192:1–16. https://doi.org/10.1007/s10661-020-08269-3
Komesli S, Akbulut S, Arslan NP et al (2020) Waste frying oil hydrolysis and lipase production by cold-adapted Pseudomonas yamanorum LP2 under non-sterile culture conditions. Environ Technol 1-9https://doi.org/10.1080/09593330.2020.1745297
Klibanov AM (2001) Improving enzymes by using them in organic solvents. Nature 409:241–246. https://doi.org/10.1038/35051719
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227(5259):680–685. https://doi.org/10.1038/227680a0
Marín-Suárez M, Méndez-Mateos D, Guadix A, Guadix EM (2019) Reuse of immobilized lipases in the transesterification of waste fish oil for the production of biodiesel. Renew Energy 140:1–8
Mehta A, Grover C, Gupta R (2018) Purification of lipase from Aspergillus fumigatus using Octyl Sepharose column chromatography and its characterization. J Basic Microbiol 58(10):857–866. https://doi.org/10.1002/jobm.201800129
Mesa M, Pereañez JA, Preciado LM, Bernal C (2018) How the Triton X-100 modulates the activity/stability of the Thermomyces lanuginose lipase: insights from experimental and molecular docking approaches. Int J Biol Macromol 120(Pt B):2410–2417. https://doi.org/10.1016/j.ijbiomac.2018.09.009
Musa H, Hafiz Kasim F, Nagoor Gunny AA et al (2019) Enhanced halophilic lipase secretion by Marinobacter litoralis SW-45 and its potential fatty acid esters release. J Basic Microbiol 59(1):87–100. https://doi.org/10.1002/jobm.201800382
Nema A, Patnala SH, Mandari V et al (2019) Production and optimization of lipase using Aspergillus niger MTCC 872 by solid-state fermentation. Bull Natl Res Cent 43:82. https://doi.org/10.1186/s42269-019-0125-7
Nathan VK, Rani ME (2020) A cleaner process of deinking waste paper pulp using Pseudomonas mendocina ED9 lipase supplemented enzyme cocktail. Environ Sci Pollut Res 27:36498–36509. https://doi.org/10.1007/s11356-020-09641-z
Ogino C, Amoah J (2019) Energy production: biodiesel. In: Ueda M (eds) Yeast Cell Surface Engineering. Springer, Singapore. pp. 43–61. https://doi.org/10.1007/978-981-13-5868-5_4
Paggiola G, Derrien N, Moseley JD et al (2020) Application of bio-based solvents for biocatalysed synthesis of amides with Pseudomonas stutzeri lipase (PSL). Pure Appl Chem 92(4):579–586
da Pereira AS, Diniz MM, De Jong G et al (2019) Chitosan-alginate beads as encapsulating agents for Yarrowia lipolytica lipase: morphological, physico-chemical and kinetic characteristics. Int J Biol Macromol 139:61–630. https://doi.org/10.1016/j.ijbiomac.2019.08.009
Perna RF, Tiosso PC, Sgobi LM et al (2017) Effects of Triton X-100 and PEG on the catalytic properties and thermal stability of lipase from free and immobilized on glyoxyl-agarose. Open Biochem J 11:66–76. https://doi.org/10.2174/1874091x01711010066
Phukon LC, Chourasia R, Kumari M et al (2020) Production and characterisation of lipase for application in detergent industry from a novel Pseudomonas helmanticensis HS6. Bioresour Technol 309:123352. https://doi.org/10.1016/j.biortech.2020.123352
dos Prazeres JN, Cruz JAB, Pastore GM (2006) Characterization of alkaline lipase from Fusarium oxysporum and the effect of different surfactants and detergents on the enzyme activity. Braz J Microbiol 37:505–509. https://doi.org/10.1590/S1517-83822006000400019
Priyanka P, Tan Y, Kinsella GK et al (2019) Solvent stable microbial lipases: current understanding and biotechnological applications. Biotechnol Lett 41:203–220. https://doi.org/10.1007/s10529-018-02633-7
Priyanka P, Kinsella GK, Henehan GT et al (2020) Isolation and characterization of a novel thermo-solvent-stable lipase from Pseudomonas brenneri and its application in biodiesel synthesis. Biocatal Agric Biotechnol 29:101806. https://doi.org/10.1016/j.bcab.2020.101806
Putri DN, Khootama A, Perdani MS et al (2020) Optimization of Aspergillus niger lipase production by solid state fermentation of agro-industrial waste. Energy Rep 6(Supplement 1):331–335. https://doi.org/10.1016/j.egyr.2019.08.064
Ramani K, Chockalingam E, Sekaran G (2010) Production of a novel extracellular acidic lipase from Pseudomonas gessardii using slaughterhouse waste as a substrate. J Ind Microbiol Biotechnol 37(5):531–535. https://doi.org/10.1007/s10295-010-0700-2
Rios NS, Arana-Peña S, Mendez-Sanchez C et al (2019a) Reuse of lipase from Pseudomonas fluorescens via its step-by-step coimmobilization on glyoxyl-octyl agarose beads with least stable lipases. Catalysts 9(5):487. https://doi.org/10.3390/catal9050487
Rios NS, Mendez-Sanchez C, Arana-Peña S et al (2019b) (2019b) Immobilization of lipase from Pseudomonas fluorescens on glyoxyl-octyl-agarose beads: improved stability And reusability. Biochim Biophys Acta - Proteins Proteomics 1867(9):741–747. https://doi.org/10.1016/j.bbapap.2019.06.005
Rocha e Silva NMP, Rufino RD, Luna JM et al (2014) Screening of Pseudomonas species for biosurfactant production using low-cost substrates. Biocatal Agric Biotechnol 3(2):132–139. https://doi.org/10.1016/j.bcab.2013.09.005
Sahoo RK, Das A, Gaur M et al (2020) Parameter optimization for thermostable lipase production and performance evaluation as prospective detergent additive. Prep Biochem Biotechnol 50(6):578–584. https://doi.org/10.1080/10826068.2020.1719513
Sahay S, Chouhan D (2018) Study on the potential of cold-active lipases from psychrotrophic fungi for detergent formulation. J Genet Eng Biotechnol 16(2):319–325. https://doi.org/10.1016/j.jgeb.2018.04.006
Salwoom L, Rahman RNZRA, Salleh AB et al (2019) Isolation, characterisation, and lipase production of a cold-adapted bacterial strain Pseudomonas sp. LSK25 isolated from Signy Island. Antarctica. Molecules. 24(4):715. https://doi.org/10.3390/molecules24040715
Sierra G (1957) A simple method for the detection of lipolytic activity of micro-organisms and some observations on the influence of the contact between cells and fatty substrates. Antonie Van Leeuwenhoek 23:15–22. https://doi.org/10.1007/BF02545855
Sooch BS, Kauldhar BS (2013) Influence of multiple bioprocess parameters on production of lipase from Pseudomonas sp. BWS-5. Brazilian Arch Biol Technol 56(5):711–721. https://doi.org/10.1590/S1516-89132013000500002
Sonkar K, Singh DP (2020) Biochemical characterization and thermodynamic study of lipase from psychrotolerant Pseudomonas punonensis. Biocatal Agric Biotechnol 28:101686. https://doi.org/10.1016/j.bcab.2020.101686
SPSS (2007) SPSS for windows version 16.0 SPSS Inc. Chicago
Sharma A, Kumar P, Rehman MB (2014) Biodegradation of diesel hydrocarbon in soil by bioaugmentation of Pseudomonas aeruginosa: a laboratory scale study. Int J Env Bioremediat Biodegrad 2:202–212
Velasco-Lozano S (2020) Immobilization of enzymes as cross-linked enzyme aggregates: general strategy to obtain robust biocatalysts. Methods in Mol Bio 2100:345–361. https://doi.org/10.1007/978-1-0716-0215-7_23
Verma N, Dollinger P, Kovacic F et al (2020) The membrane integrated steric chaperone Lif facilitates active site opening of Pseudomonas aeruginosa lipase A. J Comput Chem 41:500–512. https://doi.org/10.1002/jcc.26085
Yele VU, Desai K (2014) A New thermostable and organic solvent-tolerant lipase from Staphylococcus warneri; optimization of media and production conditions using statistical methods. Appl Biochem Biotechnol 175(2):855–869. https://doi.org/10.1007/s12010-014-1331-2
Zakaria NN, Gomez-Fuentes C, Abdul Khalil K et al (2021) Statistical optimisation of diesel biodegradation at low temperatures by an Antarctic marine bacterial consortium isolated from non-contaminated seawater. Microorganisms 9:1213
Funding
The authors received from to ICAR-NBAIM, Mau, financial assistance and infrastructural facilities.
Author information
Authors and Affiliations
Contributions
HC conceptualized the study, edited, and revised the manuscript. PC executed all experimental part and prepared the first draft of the manuscript. SV helped in screening of lipolytic Pseudomonads. PC and SV compiled all experimental results. AB designed the statistical set up for the experiments and analyzed the statistical data. HC, SS, and AKS finalized the manuscript in its final form.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare competing interests.
Disclaimer
This is a part of the Ph.D. work of the first author under supervision of the corresponding author as co-supervisor.
Additional information
Responsible Editor: Gerald Thouand
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Choudhary, P., Bhowmik, A., Verma, S. et al. Multi-substrate sequential optimization, characterization and immobilization of lipase produced by Pseudomonas plecoglossicida S7. Environ Sci Pollut Res 30, 4555–4569 (2023). https://doi.org/10.1007/s11356-022-22098-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-22098-6