Abstract
Intercropping with hyperaccumulators/accumulators is a promising alternative to enhance phytoextraction of heavy metal(loid)s in contaminated soil. In this research, a pot experiment was conducted to evaluate the influences of intercropping As hyperaccumulator Pteris vittata L. with Cd hyperaccumulator Sedum alfredii Hance or accumulator Hylotelephium spectabile (Boreau) H. Ohba on the plant growth, As and Cd phytoextraction, and rhizosphere bacterial microbiota. The results indicated that intercropping can promote the growth of plants. The total biomass of P. vittata, S. alfredii, and H. spectabile in intercropping systems was improved by 19.9–34.1%, 16.8%, and 11.5%, respectively, in comparison with corresponding plant monoculture. The As content in rhizoid and frond of P. vittata when intercropped with S. alfredii was significantly increased by 28.3% and 19.0% (P < 0.05), respectively, as compared with P. vittata monoculture, and this treatment acquired the maximum As and Cd accumulation with 2032 μg·pot−1 and 397 μg·pot−1, respectively. Intercropping enhanced the soil bacterial community diversity. The genera of Lysobacter in P. vittata rhizosphere and Massilia and Arthrobacter in S. alfredii rhizosphere had higher abundance in the intercropping system of P. vittata and S. alfredii. There were significantly positive correlation relationships between Massilia and Arthrobacter with plant Cd content and Lysobacter with plant As content, indicating that they may play important roles in As and Cd phytoextraction. The results suggested that intercropping P. vittata with S. alfredii could be a potential strategy for phytoextraction of As and Cd from co-contaminated soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil polluted with heavy metals (metalloids) has been one of the global environmental challenges due to their persistence and toxicity to animals, inhibition to the growth of plants, and microorganisms in the soil environment (Zhou et al. 2004; Antoniadis et al. 2017). There was more than 10 million km2 contaminated soil in the world, and about 1.0 million km2 contaminated soil with approximately 80% of those caused by heavy metal(loid)s has been reported in China (He et al., 2015). Among those heavy metals, high concentrations of cadmium (Cd) and arsenic (As) in soils are frequently reported (Xiao et al. 2008; Huang et al. 2016). China nationwide surveys have been shown that Cd and As ranks the first (7.0%) and third (2.7%) in the percentage of soil samples overrunning the Ministry of Environmental Protection limit among the 8 heavy-metal elements (Ni, Hg, Cu, Pb, Zn, As, Cd, and Cr) (Zhao et al. 2015). A document retrieval database with heavy metal contents in soils obtained from 1443 industrial and agricultural sites in China has been reported that As and Cd were determined as the priority control pollutants (Yang et al. 2018a). Arsenic and Cd have been the category one carcinogens claimed by the World Health Organization (WHO 2017). Moreover, agricultural soil polluted with As and Cd damages human health through the food chain (Mu et al. 2019; Kumar et al. 2019). Thus, it is urgent to find an efficient and cost-effective technique for the remediation of As and Cd co-contaminated soil.
Phytoextraction utilizes the hyperaccumulators with the accumulation ability for high content of metal(loid)s in aboveground part to remove pollutants in soil and has been one of the most widespread and alternative phytoremediation techniques (Patra et al. 2020). There are 664 hyperaccumulator species for the 8 metals of As, Cd, Cu, Pb, Cr, Zn, Mn, and Ni, which were obtained from the global hyperaccumulator database in 2020 (hyperaccumulators.smi.uq.edu.au/collection/) (Xu et al. 2020). Some heavy metal accumulators with high shoot biomass such as Linum usitatissimum L. and Hylotelephium spectabile (Boreau) H. Ohba were also used to remediate soil (Yang et al. 2018b; Guo et al. 2020). However, there was little information on As and Cd co-hyperaccumulators, so it is a great challenge for phytoextraction of As and Cd in co-contaminated soil due to their different opposite geochemical behavior.
Intercropping, one of the most representative agronomical practices, is helpful for the improvement of the structure of planting system and the efficiency of resource utilization (Bedoussac et al. 2015). Some researchers have reported that intercropping hyperaccumulators with other plant species could improve the tolerance of plants to heavy metal(loid)s and/or enhance the comprehensive phytoextraction efficiency (Desjardins et al. 2018). For example, the intercropping of hyperaccumulator Sedum plumbizincicola and moso bamboo (Bian et al. 2017), Sedum alfredii and oilseed rape (Cao et al. 2020), and Pteris vittata L. and Morus alba L. (mulberry) (Wan et al. 2017) could simultaneously remediate soil and obtain safe crop products. The higher accumulation of different heavy metals by intercropping with diverse plant species was owing to the effect of root exudates and soil microorganisms on metal speciation and bioavailability in rhizosphere soil (Tang et al. 2017; Yu et al. 2016; Li et al. 2019). In addition, there were positive relationship between soil microorganism response and intercropping plants. For instance, the toxicity of heavy metals to soil microbial community structures could be efficiently alleviated by intercropping herbs with woody plants for phytoremediation of contaminated soil compared with monoculture (Zeng et al. 2019a). Cao et al. (2020) have founded that the variations of bacterial community in rhizosphere soil were very important for Cd phytoextraction in the intercropping system of Sedum alfredii and oilseed rape. Hence, when soils contaminated with multiple metals are remediated, intercropping with two or more different heavy metal(loid)s hyperaccumulators/accumulators might be an effective measure to solve this problem (Lin et al. 2018).
Arsenic hyperaccumulator P. vittata has been widely applied for the phytoextraction of As-contaminated soil (Wan et al. 2020). S. alfredii Hance was a Cd/Zn hyperaccumulator and was also used for phytoextraction of multiple-metal (Cd, Pb, and Zn) soils (Yang et al. 2004; Liang et al. 2019a, b). Hylotelephium spectabile (Boreau) H. Ohba, a potential Cd accumulator with high shoot biomass, could make up for a relatively low Cd content in aboveground parts (Zhou et al. 2020; Guo et al. 2020). Several studies about P. vittata or S. alfredii intercropping with other plant species to remediate only As or Cd contaminated soils have been reported (Wan et al. 2017; Cao et al. 2020). To date, there are some studies on phytoextraction of As and Cd co-contaminated soil by intercropping of As hyperaccumulator Pteris cretica var. nervosa or Pteris vittata and Cd hyperaccumulator Solanum nigrum (Xiong et al. 2013). Moreover, the application of phytohormones could significantly improve the remediation efficiency of intercropping Pteris vittata with Solanum nigrum (Cong et al. 2021). However, there is scarce information about the simultaneous phytoextraction As and Cd from co-contaminated soil by intercropping of P. vittata or S. alfredii. Moreover, the influences of intercropping hyperaccumulators on rhizosphere microbial response and their effects on plant performance and heavy metal accumulation still remain unknown (Cao et al. 2020). In this study, a greenhouse experiment was conducted to study the phytoextraction potential of As and Cd in co-contaminated soil by intercropping P. vittata with S. alfredii/H. spectabile. The aims were to (1) investigate the impact of intercropping on plant biomass and physiological response; (2) compare the As and Cd content and accumulation in plants under the different intercropping systems; and (3) determine the changes in rhizosphere microflora and explore the correlation between response of rhizosphere soil microorganisms and heavy metal removal efficiency. The outcome in this study may helpful for establishing hyperaccumulator/accumulator intercropping systems to remediate soil co-polluted by As and Cd.
Materials and methods
Soil sample and plant seedlings
The tested soils were sampled from a deserted agricultural land of the surface layer (0–20 cm) near a closed As smelting plant in Changde city of Hunan Province, China. After the scrap and plant residues were removed, the soil samples were air-dried, pestled, and passed through a 5-mm nylon sieve for pot experiments. The soil physicochemical properties were pH, 8.10; soil organic matter content, 16.6 g∙kg−1; available content of nitrogen (N), phosphorous (P), and potassium (K) with 59.5, 30.0, and 109 mg∙kg−1, respectively. The total content of As (410 mg·kg−1) and Cd (3.0 mg·kg−1) was far higher than the recommended content of As (25 mg·kg−1) and Cd (0.6 mg·kg−1) listed in the Risk Control Standard for Soil Contamination of Agricultural Land (GB15618-2018) (pH > 7.5).
Seedlings of P. vittata and H. spectabile were acquired from a nursery base in Shimen County, Hunan Province and a nursery base in Hebei Province established by the Institute of Geographic Sciences and Natural Resources Research, Chinese Academy of Sciences, respectively. The S. alfredii seedlings sampled from a Pb/Zn mine site in Zhejiang Province were planted in the unpolluted farm soil for pot experiments.
Experimental design and plant incubation
Six kilogram air-dried experimental soil mixed with the basic fertilizer including 0.27 g CO(NH2)2∙kg−1, 0.05 g KH2PO4∙kg−1, and 0.1 g KNO3∙kg−1 soil was placed into a container (30 cm length × 22cm width × 12 cm height). The soil was sprayed with deionized water to maintain at equilibration with 70% water-holding capacity for 2 weeks. Then, the healthy seedlings with uniform size of P. vittata, S. alfredii, and H. spectabile were transplanted into each pot. The intercropping patterns were listed in Table S1, which included (1) monoculture treatment of P. vittata (PM), S. alfredii (SM), and H. spectabile (HM) with 4 seedlings, respectively, and (2) intercropping of 2 P. vittata with 2 S. alfredii seedlings (PS) or 2 H. spectabile seedlings (PH). The unplanning soil was used as a control treatment (CK). Each treatment has 4 replicates. The pot experiment was conducted in a greenhouse under the controlled conditions of 14 h photoperiod with photon flux of 260–350 mmol m−2∙s−1 and a day/night temperature of 30 °C/22 °C. Deionized water was added into each pot daily to maintain plant’s growth.
After 120-day cultivation, plants in each pot were harvested and divided into rhizoids and fronds for P. vittata and shoots and roots for S. alfredii and H. spectabile. The plant samples were clean washed and weighed as fresh biomass. Some fresh leaves/pinna (for P. vittata) was placed in 4 °C for the analysis of malondialdehyde (MDA) and soluble protein contents. The rest plant parts were de-enzymized for 30 min at 105 °C and dried at 65 °C until a constant weight. The samples were ground and screened by a 1-mm nylon sieve for further determination. Besides, the rhizosphere soil samples from each pot were collected as described by Wang et al. (2018). The 7 rhizosphere soil samples were recorded as PM, SM, and HM (monoculture treatment), PS-P (P. vittata in PS treatment), PS-S (S. alfredii in PS treatment), PH-P (P. vittata in PH treatment), and PH-H (H. spectabile in PH treatment). These soil samples were used for the analysis of pH, available As and Cd content, and microbial community.
Soil and plant sample analysis
Soil physiochemical properties were measured following the methods of Lu (1999). The content of soluble protein in fresh leaves/pinna was measured using coomassie brilliant blue G-250 and bovine serum albumin and then counted and presented as mg∙g−1 FW (fresh weight) (Aitken and Learmonth 2009). The MDA content was analyzed according to the thiobarbituric acid (TBA) reaction following the method of Velikova et al. (2000).
The plant and soil samples were digested in the acid mixture of HNO3:HClO4 (5:1) (Yang et al. 2012) and mixture of HNO3–H2O2 (USEPA, 1996) to determine total As and Cd content, respectively. Blank and standard reference materials of plant (GBW-07603) and soil (GBW-08303) were used to assess the quality control of sample analysis. The recovery efficiency of As and Cd was calculated based on the whole recycling process in comparison to the total content, and the recovery values were within the range of 90–105%. The available As and Cd content in soil was extracted using NaHCO3 solution (Woolson et al. 1971) and the diethylene-triaminepenta acetic acid (DTPA) solution (Lindsay and Norvell 1978), respectively. The Cd and As contents in digested and extracted solutions were measured through the inductive coupled plasma-optical emission spectrometer (ICP-OES, Thermo, USA) and hydrogen generation-atomic fluorescence spectrometer (AFS-2202E, Haiguang Instrument Company of Beijing, China), respectively.
The DNA extraction from the rhizosphere soil samples was performed by the method of Moffett et al. (2003) and detected using 1.0% agarose gel electrophoresis. The V3-V4 regions of the bacterial 16S rRNA gene were amplified with the polymerase chain reaction, which was performed using the 341F/806R primer set. All PCR products were recovered and purified with 2% agarose gel and AxyPrepDNA Gel Extraction Kit (Thermo Scientific, USA), eluted with Tris-HCl buffer solution, and detected with QuantiFluor™-ST (Promega, USA) blue fluorescence quantitative system. Purified amplicons were sequenced according to an Illumina platform (Illumina Inc., San Diego, CA, USA) at Novogene Co., Ltd, Beijing, China.
Statistical analysis
The translocation factor (TF) of As and Cd in plant was counted as TF = As or Cd content in shoots(fronds)/As or Cd content in root (rhizoid) (Liang et al. 2019a). The As and Cd phytoextraction efficiency was described as the plant metal accumulations, which are based on the biomass of root (rhizoid) and shoot (frond) multiplied by As and Cd content in corresponding plant part. The data were analyzed by Microsoft Excel 2016 and shown as the mean values ± standard deviations. All statistical analyses were conducted using SPSS 18.0 software. One-way analysis of variance (ANOVA) with Duncan’s test at the significance level of P < 0.05 was applied for the comparison of differences in plant growth and As and Cd uptake among different intercropping treatments.
The Majorbio cloud platform was used for online data analysis of diversity gene sequencing. Venn diagram was drawn to highlight similarities between the monoculture and intercropping treatments. Community bar plot was drawn to show the relative abundance of microorganisms. Heat map analysis was performed using Heml 1.0. Redundancy analysis (RDA) was applied to analyze the correlation between the bacterial communities and selected soil environmental factors or the phytoremediation parameters, various R packages (http://www.r-project.org).
Results and discussion
Plant growth, MDA, and soluble protein content in leaf/pinna
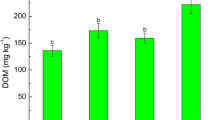
The plant growth was positively affected by intercropping treatment (Fig. 1). Compared with monoculture treatment (PM), the rhizoid and frond biomass of P. vittata was enhanced by 20.1% and 13.2% for PS treatment and 34.1% and 24.3% for PH treatment; consequently, the total biomass was significantly increased by 19.9% and 34.1%, respectively (P < 0.05). The shoot biomass of S. alfredii in PS treatment was significantly improved by 21.1%, and that of H. spectabile from PH treatment improved by 11.5% as compared with SM and HM, respectively (P < 0.05). This is consistent with previous results that intercropping could protect neighboring companion plants and enhance plant growing on heavy-metal contaminated soil (Wan et al. 2017; Zeng et al. 2019b; Cao et al. 2020). This may be contributed to the improvement of the rhizosphere environment where the soil As efficiently extracted by P. vittata (Wan et al. 2017). Hong et al. (2017) confirmed that different plant species show different temporal and spatial resource requirements, so intercropping can obtain essential growth resources more conveniently than monoculture plant.
The content of MDA in pinna of P. vittata from the intercropping system of PS and PH was significantly dropped by 15.3% and 17.3% (P < 0.05) compared with PM, respectively (Table 1). The result was in consistent with former findings that intercropping with Solanum nigrum or Solanum photeinocarpum significantly decreased the MDA content in eggplant in comparison to monoculture (Tang et al. 2017). The reason may be related to that intercropping alleviates the oxidative stress of metal(loid)s to decrease the degree of leaf lipid peroxidation and promote the P. vittata growth (Du et al. 2020). The soluble protein is beneficial to reduce water loss and maintain the main function of cellular membranes stressed by heavy metals (Pan et al. 2018). Intercropping treatments of PS and PH significantly increased the soluble protein content of P. vittata compared with PM (P < 0.05) (Table 1), which was consistent with the result of higher P. vittata biomass from the intercropping system (Fig. 1). The difference in the leaf MDA and protein content of S. alfredii and H. spectabile between intercropping and monoculture treatment was not significant, indicating that intercropping has no obvious influence on their physiological response.
Arsenic and Cd uptake by plants
Uptake and transport of As and Cd
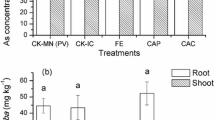
The As content in rhizoid and frond of P. vittata from intercropping treatments was significantly increased by 10.9–28.3% (P < 0.05) in contrast with PM, and the PS treatment showed the highest As content in rhizoid (245 mg∙kg−1) and frond (1339 mg∙kg−1) of P. vittata. The Cd content in root and shoot of S. alfredii in PS treatment was also significantly enhanced by 13.3% and 25.2% in comparison to monoculture, respectively (P < 0.05) (Table 2). Similar studies have been confirmed that As content in P. vittata rhizoid was significantly improved when intercropping with Morus alba and Broussonetia papyrifera L. (Wan and Lei 2018; Zeng et al. 2019b), and Cd content in S. alfredii intercropped with pakchoi was significantly improved (Ma et al. 2020). These results may be due to the interactions of root exudates which increased the heavy metal phytoavailability in rhizosphere soil (Yang et al. 2006). Unfortunately, the content of Cd in P. vittata frond and that of As in S. alfredii shoot was very low with the maximum value of 2.00 mg∙kg−1 and 19.0 mg∙kg−1, respectively. This may be related to that most hyperaccumulators strongly accumulate specific metals (Mahar et al. 2016). The difference in the TFshoot value of As for P. vittata and Cd for S. alfredii between intercropping and corresponding monoculture treatment was not significant. The content of Cd in root and shoot of H. spectabile from intercropping treatment was slightly decreased, while shoot As content was significantly increased compared with HM (Table 2). Though the content of Cd and As in H. spectabile was low, the TFshoot value of Cd was high than 1.0, suggesting that it was effective in Cd uptake. Our previous study has shown that the maximum Cd content in shoots of H. spectabile grown in 5 mg Cd∙L−1 solution reached up to 603 mg∙kg−1 with the TFshoot value of 5.62 (Zhou et al. 2020). This may be owing to low soil Cd content in this study (Yang et al. 2018a).
Arsenic and Cd accumulation in plants
The As accumulation in fronds of P. vittata was significantly enhanced by 27.5% and 23.4% (P < 0.05) after intercropping with S. alfredii and H. spectabile compared with PM, respectively (Table 3). Similarly, the Cd accumulation in S. alfredii from PS treatment was significantly increased by 14.6% in comparison to monoculture (P < 0.05). Previous studies also have been reported that intercropping can enhance As accumulation in P. vittata intercropped with wood species of Morus alba L. or Broussonetia papyrifera L. (Zeng et al. 2019b). Cao et al. (2020) have demonstrated that co-planting with oilseed rape can improve Cd phytoextraction of S. alfredii due to reducing intra-species competition for nutrients and water. The As accumulation of H. spectabile from PH treatment was significantly higher than monoculture (P < 0.05). It is possible that more root exudates (organic acids) secreted between intercropped plants, which could increase the phytoavailable As and Cd contents in rhizosphere soil and promote heavy metal extraction by corresponding plant (Kim et al. 2013; Li et al. 2019). Generally, the As accumulation from PS and PH treatments was close with 2065 μg∙pot−1 and 1988 μg∙pot−1, respectively, and the Cd accumulation for PS (397 μg∙pot−1) was far higher than that from PH (19.0 μg∙pot−1) treatment, suggesting that the Cd removal was more effective when P. vittata intercropped with S. alfredii than H. spectabile. Thus, intercropping of P. vittata and S. alfredii may be more effective in simultaneous phytoextraction As and Cd in co-contaminated soil.
Soil pH and available contents of As and Cd after remediation
The rhizosphere soil pH was slightly varied from 7.84 to 7.99 (Fig. 2), which was coincided with previous findings that soil pH under the intercropping of P. vittata and castor bean slightly altered (Yang et al. 2017), suggesting that acidification is not the mobilization mechanism of soil metals in present study (Liang et al. 2019b). The available As content in PS-S rhizosphere soil was significantly higher than that from unplanted soil (CK) and other planting treatments except for PS-P and PH-H rhizosphere soil. The available Cd content in SM and PS-P rhizosphere soil was higher than that in HM, PH-P, and PH-H rhizosphere soil, indicating that intercropping P. vittata and S. alfredii could enhance As and Cd mobility in soil (Fig. 2). Organic acids secreted by hyperaccumulators could facilitate the transformation of heavy metals to an exchangeable fraction, thus indirectly resulting in high phytoremediation efficiency (Zu et al. 2020). Previous researches have been confirmed that organic acids like oxalic acid as a predominant root exudate of both S. alfredii and P. vittata could trigger soil As and Cd availability and enhance uptake by plant (Tao et al. 2016; Das et al. 2017; Liang et al. 2021). Also, Xia et al. (2018) have reported that the secretion components in soil from intercropping systems with Conyza canadensis, Cardamine hirsuta, and Cerastium glomeratum are significantly more complex than those from monoculture treatments, which have effects on heavy metal accumulation. Therefore, further research is needed to investigate organic acids in the intercropping system of P. vittata and S. alfredii. Additionally, the available As content in HM rhizosphere soil and that of Cd in PH-H rhizosphere was lower than that from other treatments, which might explain the results of lower As and Cd content in H. spectabile (Table 3).
Soil microbial diversity and community structure
Soil bacterial community diversity
Generally, intercropping enhanced the soil bacterial community diversity. The bacterial α-diversity indices of ACE, Chao 1 and Shannon in intercropping treatments were significantly more than in SM and HM (Table S2), which could help to maintain the stability of soil microbial structure, indicating that these corresponding species could be intercropped. There were 590 OTUs common to CK and planting treatments (Fig. S1a). The PM, SM, and PS treatments shared higher OTUs of 646, and 632 operational taxonomic units (OTUs) common to PM, HM, and PH treatments were found (Fig. S1b, c). However, the unique OTUs in CK and planting treatments were very low, which from intercropping treatments were higher than monoculture. This agreed with former research that the microbial composition from the intercropping system of P. vittata with M. alba or B. papyrifera was more complex (Zeng et al. 2019a). Different plants have distinct rhizosphere soil condition due to its own spectrum and specificity of root exudates (Deng et al. 2018). Bian et al. (2021) have found that acetic acid, malic acid, and n-hexadecanoic acid were closely correlated with multiple bacterial species in rhizosphere for intercropping system of Moso bamboo with Sedum plumbizincicola.
Bacterial community structure
The phylum Proteobacteria was the dominant bacterial community in all treatments, accounting for 31.0–36.4% of total phyla in soil (Fig. 3a). The abundance of Proteobacteria was increased in intercropping system compared with monoculture, and that in S. alfredii rhizosphere soil of PS treatment was highest. Similar result has been reported that Proteobacteria predominated in bacterial phyla with more than 31% relative abundances from intercropping treatment with Moso bamboo and Sedum plumbizincicola (Bian et al. 2021). Proteobacteria was important in resistance to metal(loid)s toxicity and significantly correlated with soil nutrients such as carbon, nitrogen, and phosphorus contents (Zhang et al. 2016; Zhao et al. 2019). These results suggested that Proteobacteria could help plants increase the environmental quality and intensify the biological function of metal(loid) contaminated soil, in turn the presence of plants could provide a better living condition for microorganisms. Additionally, the other predominant phyla included Actinobacteria, Chloroflexi, Acidobacteria, Bacteroidetes, and Gemmatimonadetes, which was in consistent with prior research reported by Chen et al. (2018), and they are important for the microbial community reconstruction in metal(loid)-contaminated soil (Zhai et al. 2020).
The genera Massilia, Lysobacter, and Sphingomonas, belonging to the phylum Proteobacteria, could adapt to extreme soil conditions (Yang et al. 2019; Jiao et al. 2019). Massilia, Sphingomonas, Lysobacter, Arthrobacter, and norank_c__Subgroup_6 with an average abundance more than 1% were the main genera in soil after phytoremediation (Fig. 3b). Lysobacter can resist the pathogens by generating extracellular enzymes and affect the bacterial behaviors (Expósito et al. 2015; Feng et al. 2019), and Massilia could effectively enhance soil phosphate mobilization (Zheng et al. 2017). In the present study, Massilia in S. alfredii rhizosphere soil and Lysobacter in P. vittata rhizosphere soil of PS intercropping system were the richest genera. Zhang et al. (2018a) have found that intercropping of Morus alba L. and Medicago sativa L. had a positive impact on bacterial taxa with soil nutrients cycling such as Bacillus, Bradyrhizobium and Sphingomonas as compared to monoculture. The results may be related to that root exudates such as hexadecenoic, acetic acid, and malic acid of different cropping treatments may alter the bacterial community and rhizosphere conditions (Bian et al. 2021).
Relationship between rhizosphere microecological characteristics and phytoextraction efficiency
The redundancy analysis (RDA) showed that the relative abundance of Massilia was positively correlated with shoot/frond biomass of plant. This was in agreement with former study that intercropping effectively improves the functional diversity of soil microbial communities and increases the yields of plants (Zhang et al. 2018b). The relative abundance of Massilia, Arthrobacter, and Lysobacter were positively correlated with NaHCO3-As and DTPA-Cd content in rhizosphere soil (Fig. 4a), and positive correlations between the relative abundance of Massilia and Arthrobacter and Cd content and the abundance of Lysobacter and As content in plant tissues were found (Fig. 4b). Massilia and Arthrobacter contain some strains with tolerance to heavy metal, which could enhance the phytoremediation efficiency through alleviating metal toxicity to plant and increasing the phytoavailability to heavy metals (Ma et al. 2016; Rojjanateeranaj et al. 2017). In Sedum alfredii-oilseed rape intercropping system, rhizosphere bacterial community could influence plant Cd uptake, and it appears to be important for phytoextraction (Cao et al. 2020). Bian et al. (2021) have reported heavy metal uptake in intercropping plants of Moso bamboo, and Sedum plumbizincicola was correlated with the abundances of Acidobacteria, Gemmatimonadetes, and Proteobacteria, which were the core functional phyla in the contaminated sites (Chen et al. 2016, 2018). Additionally, Lin et al (2021) found that Lysobacter had the highest abundance in Cr contaminated soil and contributed to Cr accumulation by Trifolium repens L. In present study, intercropping P. vittata with S. alfredii increased the relative abundance of Massilia and Arthrobacter in S. alfredii rhizosphere and Lysobacter in P. vittata rhizosphere and improved the availability of As and Cd in rhizosphere, As uptake by P. vittata, and Cd uptake by S. alfredii. This agrees with previous findings that co-cropping Vicia sativa with a Ni hyperaccumulator (Alyssum murale) had the potential to improve both soil bacterial diversity and soil functions, and metal accumulation could be enhanced (Saad et al. 2018a, b). Therefore, rhizosphere-associated microorganisms could play a crucial in regulating phytoextraction, and further studies on application of the critical genus of microorganisms in phytoremediation with intercropping of P. vittata and S. alfredii/H. spectabile are warranted.
a Redundancy analysis (RDA) of the soil pH, available As and Cd content, and bacterial communities at genus level, b RDA of the phytoremediation parameters and bacterial communities at genus level. NaHCO3-As, soli available As content; DTPA-Cd, soli available Cd content; SB, shoot/frond biomass; RB, root/rhizoid biomass; SAs, As content in shoot/frond; SCd, Cd content in shoot/frond; RAs, As content in root/rhizoid; RCd, Cd content in root/rhizoid
Conclusions
Intercropping of P. vittata with S. alfredii /H. spectabile can effectively alleviate the oxidative stress of As and Cd on plant growth in contaminated soil. The total biomass of P. vittata, S. alfredii and H. spectabile in intercropping systems was significantly improved compared with monoculture by decreasing MDA content and increasing soluble protein content in leaves/pinna. Intercropping of P. vittata and S. alfredii could simultaneously obtain greater As and Cd accumulation than monoculture and intercropping P. vittata with H. spectabile. The Venn diagram, heatmap, and RDA analysis showed that the relative abundance of Massilia and Lysobacter in plant rhizosphere was the richest from intercropping system of P. vittata and S. alfredii and significantly correlated with plant Cd and As content. Thus, intercropping of P. vittata and S. alfredii was a promising strategy to simultaneously phytoextract As and Cd from co-contaminated soil.
Data availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
Aitken A, Learmonth MP (2009) Protein determination by UV absorption. In: The Protein Protocols Handbook. Humana Press, Totowa. https://doi.org/10.1385/1-59259-169-8:3
Antoniadis V, Shaheen SM, Boersch J, Frohne T, Laing GD, Rinklebe J (2017) Bioavailability and risk assessment of potentially toxic elements in garden edible vegetables and soils around a highly contaminated former mining area in Germany. J Environ Manag 186:192–200. https://doi.org/10.1016/j.jenvman.2016.04.036
Bedoussac L, Journet EP, Hauggaard-Nielsen H, Naudin C, Corre-Hellou G, Jensen ES, Prieur L, Justes E (2015) Ecological principles underlying the increase of productivity achieved by cereal-grain legume intercrops in organic farming. A review. Agron Sustain Dev 35:911–935. https://doi.org/10.1007/s13593-014-0277-7
Bian F, Zhong Z, Zhang X, Yang C (2017) Phytoremediation potential of moso bamboo (Phyllostachys pubescens) intercropped with Sedum plumbizincicola in metal-contaminated soil. Environ Sci Pollut Res 24:27244–27253. https://doi.org/10.1007/s11356-017-0326-2
Bian F, Zhong Z, Li C, Zhang X, Gu L, Huang Z, Gai X, Huang Z (2021) Intercropping improves heavy metal phytoremediation efficiency through changing properties of rhizosphere soil in bamboo plantation. J Hazard Mater 416(62-68):125898. https://doi.org/10.1016/j.jhazmat.2021.125898
Cao X, Luo J, Wang X, Chen Z, Liu G, Khan MB, Kang Y, Feng Y, He Z, Yang X (2020) Responses of soil bacterial community and Cd phytoextraction to a Sedum alfredii-oilseed rape (Brassica napus L. and Brassica juncea L.) intercropping system. Sci Total Environ 723:138152. https://doi.org/10.1016/j.scitotenv.2020.138152
Chen Q, An X, Li H, Su J, Ma Y, Zhu Y (2016) Long-term field application of sewage sludge increases the abundance of antibiotic resistance genes in soil. Environ Int 92:1–10. https://doi.org/10.1016/j.envint.2016.03.026
Chen Y, Jiang Y, Huang H, Mou L, Ru J, Zhao J, Xiao S (2018) Long-term and high-concentration heavy-metal contamination strongly influences the microbiome and functional genes in Yellow River sediments. Sci Total Environ 637-638:1400–1412. https://doi.org/10.1016/j.scitotenv.2018.05.109
Cong C, Yang N, Wang H, Wang H (2021) Enhancing arsenic and cadmium accumulation in Pteris vittata and Solanum nigrum by combined application of indoleacetic acid and kinetin: a field experiment. Ecol Environ Sci 30(6):1299–1309
Das S, Chou ML, Jean JS, Yang HJ, Kim PJ (2017) Arsenic-enrichment enhanced root exudates and altered rhizosphere microbial communities and activities in hyperaccumulator Pteris vittata. J Hazard Mater 325:279–287. https://doi.org/10.1016/j.jhazmat.2016.12.006
Deng S, Ke T, Li L, Cai S, Zhou Y, Liu Y, Guo L, Chen L, Zhang D (2018) Impacts of environmental factors on the whole microbial communities in the rhizosphere of a metal-tolerant plant: Elsholtzia haichowensis Sun. Environ Pollut 237:1088–1097. https://doi.org/10.1016/j.envpol.2017.11.037
Desjardins D, Brereton N, Marchand L, Brisson J, Pitre FE, Labrecque M (2018) Complementarity of three distinctive phytoremediation crops for multiple-trace element contaminated soil. Sci Total Environ 610-611:1428. https://doi.org/10.1016/j.scitotenv.2017.08.196
Du J, Guo Z, Li R, Ali A, Guo D, Lahori AH, Wang P, Liu X, Wang X, Zhang Z (2020) Screening of Chinese mustard (Brassica juncea L.) cultivars for the phytoremediation of Cd and Zn based on the plant physiological mechanisms. Environ Pollut 261:114–213. https://doi.org/10.1016/j.envpol.2020.114213
Expósito R, Postma J, Raaijmakers JM, Bruijn ID (2015) Diversity and activity of Lysobacter species from disease suppressive soils. Front Microbiol 6(145):1243. https://doi.org/10.3389/fmicb.2015.01243
Feng T, Han Y, Li B, Li Z, Yu Y, Sun Q, Li X, Du L, Zhang X, Wang Y (2019) Interspecies and intraspecies signals synergistically regulate Lysobacter enzymogenes twitching motility. Genet Mol Biol 85(23):e01742–e01719. https://doi.org/10.1128/AEM.01742-19
Guo Y, Qiu C, Long S, Wang H, Wang Y (2020) Cadmium accumulation, translocation, and assessment of eighteen Linum usitatissimum L. cultivars growing in heavy metal contaminated soil. Int J Phytoremed 22:490–496. https://doi.org/10.1080/15226514.2019.1683714
Hong Y, Heerink N, Jin S, Berentsen P, Zhang L, Werf W (2017) Intercropping and agroforestry in China – current state and trends. Agric Ecosyst Environ 244:52–61. https://doi.org/10.1016/j.agee.2017.04.019
Huang M, Zhu Y, Li Z, Huang B, Luo N, Liu C, Zeng G (2016) Compost as a soil amendment to remediate heavy metal-contaminated agricultural soil: mechanisms, efficacy, problems, and strategies. Water Air Soil Pollut 227(10):359. https://doi.org/10.1007/s11270-016-3068-8
Jiao S, Xu Y, Zhang J, Hao X, Lu Y (2019) Core microbiota in agricultural soils and their potential associations with nutrient cycling. Msystems. 4(2):e00313–e00318. https://doi.org/10.1128/mSystems.00313-18
Kim J, Lee Y, Chung J (2013) The role of organic acids in the mobilization of heavy metals from soil. KSCE J Civ Eng 17(7):1596–1602. https://doi.org/10.1007/s12205-013-0323-z
Kumar S, Prasad S, Yadav KK, Shrivastava M, Malav L (2019) Hazardous heavy metals contamination of vegetables and food chain: role of sustainable remediation approaches - a review. Environ Res 179:108792. https://doi.org/10.1016/j.envres.2019.108792
Li Z, Wang J, An L, Tan J, Zhan F, Wu J, Zu Y (2019) Effect of root exudates of intercropping Vicia faba and Arabis alpina on accumulation and sub-cellular distribution of lead and cadmium. Int J Phytoremed 21:4–13. https://doi.org/10.1080/15226514.2018.1523867
Liang Y, Wang X, Guo Z, Xiao X, Peng C, Yang J, Zhou C, Zeng P (2019a) Chelator-assisted phytoextraction of arsenic, cadmium and lead by Pteris vittata L. and soil microbial community structure response. Int J Phytoremed 21:1032–1040. https://doi.org/10.1080/15226514.2019.1594685
Liang Y, Zhou C, Guo Z, Huang Z, Peng C, Zeng P, Xiao X (2019b) Removal of cadmium, lead, and zinc from multi-metal-contaminated soil using chelate-assisted Sedum alfredii Hance. Environ Sci Pollut Res 26:28319–28327. https://doi.org/10.1007/s11356-019-06041-w
Liang Y, Xiao X, Guo Z, Peng C, Zeng P, Wang X (2021) Co-application of indole-3-acetic acid/gibberellin and oxalic acid for phytoextraction of cadmium and lead with sedum alfredii hance from contaminated soil. Chemosphere 285:131420. https://doi.org/10.1016/j.chemosphere.2021.131420
Lin L, Chen F, Wang J, Liao M, Lv X, Wang Z, Li H, Deng Q, Xia H, Liang D, Tang Y, Wang X, Lai Y, Ren W (2018) Effects of living hyperaccumulator plants and their straws on the growth and cadmium accumulation of Cyphomandra betacea seedlings. Ecotoxicol Environ Saf 155:109–116. https://doi.org/10.1016/j.ecoenv.2018.02.072
Lin H, Liu C, Li B, Dong Y (2021) Trifolium repens L. regulated phytoremediation of heavy metal contaminated soil by promoting soil enzyme activities and beneficial rhizosphere associated microorganisms. J Hazard Mater 402:125898. https://doi.org/10.1016/j.jhazmat.2020.123829
Lindsay WL, Norvell WA (1978) Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci Soc Am J 42:421–428. https://doi.org/10.2136/sssaj1978.03615995004200030009x
Lu R K (1999) Soil and agro-chemistry analytical methods. China Agriculture Science and Technology Press, Beijing
Ma Y, Rajkumar M, Zhang C, Freitas H (2016) Beneficial role of bacterial endophytes in heavy metal phytoremediation. J Environ Manag 174:14–25. https://doi.org/10.1016/j.jenvman.2016.02.047
Ma L, Wu Y, Wang Q, Feng Y (2020) The endophytic bacterium relieved healthy risk of pakchoi intercropped with hyperaccumulator in the cadmium polluted greenhouse vegetable field. Environ Pollut 264:114796. https://doi.org/10.1016/j.envpol.2020.114796
Mahar A, Wang P, Ali A, Awasthi MK, Lahori AH, Wang Q, Li R, Zhang Z (2016) Challenges and opportunities in the phytoremediation of heavy metals contaminated soils: a review. Ecotoxicol Environ Saf 126:111–121. https://doi.org/10.1016/j.ecoenv.2015.12.023
Moffett JR, Price RA, Anderson SM, Sipos ML, Moran AV, Tortella FC, Dave JR (2003) DNA fragmentation in leukocytes following subacute low-dose nerve agent exposure. Cell Mol Life Sci 60:2266–2271. https://doi.org/10.1007/s00018-003-3238-x
Mu T, Wu T, Zhou T, Li Z, Ouyang Y, Jiang J, Zhu D, Hou J, Wang Z, Luo Y, Christie P, Wu L (2019) Geographical variation in arsenic, cadmium, and lead of soils and rice in the major rice producing regions of China. Sci Total Environ 677:373–381. https://doi.org/10.1016/j.scitotenv.2019.04.337
Pan G, Liu W, Zhang H, Liu P (2018) Morphophysiological responses and tolerance mechanisms of Xanthium strumarium to manganese stress. Ecotoxicol Environ Saf 165:654–661. https://doi.org/10.1016/j.ecoenv.2018.08.107
Patra DK, Pradhan C, Patra HK (2020) Toxic metal decontamination by phytoremediation approach: concept, challenges, opportunities and future perspectives. Environ Technol Innov 18:100672. https://doi.org/10.1016/j.eti.2020.100672
Rojjanateeranaj P, Chirawee S, Prapagdee B (2017) Enhanced cadmium phytoremediation of Glycine max L. through bioaugmentation of cadmium-resistant bacteria assisted by biostimulation. Chemosphere. 185:764–771. https://doi.org/10.1016/j.chemosphere.2017.07.074
Saad RF, Kobaissi A, Amiaud B, Ruelle J, Benizri E (2018a) Changes in physicochemical characteristics of a serpentine soil and in root architecture of a hyperaccumulating plant cropped with a legume. J Soils Sediments 18:1994–2007. https://doi.org/10.1007/s11368-017-1903-1
Saad RF, Kobaissi A, Echevarria G, Kidd P, Calusinska M, Goux X, Benizria E (2018b) Influence of new agromining cropping systems on soil bacterial diversity and the physico-chemical characteristics of an ultramafic soil. Sci Total Environ 645:380–392. https://doi.org/10.1016/j.scitotenv.2018.07.106
Tang Y, He J, Yu X, Xie Y, Lin L, Sun G, Li H, Liao M, Liang D, Xia H, Wang X, Zhang J, Liu Z, Tu L, Liu L (2017) Intercropping with Solanum nigrum and Solanum photeinocarpum from two ecoclimatic regions promotes growth and reduces cadmium uptake of eggplant seedlings. Pedosphere 3:638–644. https://doi.org/10.1016/S1002-0160(17)60358-8
Tao Q, Hou D, Yang X, Li T (2016) Oxalate secretion from the root apex of Sedum alfredii contributes to hyperaccumulation of Cd. Plant Soil 398:139–152. https://doi.org/10.1007/s11104-015-2651-x
USEPA(1996) Method 3050B: Acid digestion of sediments, sludges, and soils, revision 2. Washington, DC. http://www.epa.gov/sw-846/pdfs/3050b.pdf
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci 151:59–66. https://doi.org/10.1016/S0168-9452(99)00197-1
Wan X, Lei M (2018) Intercropping efficiency of four arsenic hyperaccumulator Pteris vittata populations as intercrops with Morus alba. Environ Sci Pollut Res 25:12600–12611. https://doi.org/10.1007/s11356-018-1366-y
Wan X, Lei M, Chen T, Yang J (2017) Intercropped Pteris vittata L. and Morus alba L. presents a safe utilization mode for arsenic-contaminated soil. Sci Total Environ 579:1467–1475. https://doi.org/10.1016/j.scitotenv.2016.11.148
Wan X, Lei M, Yang J, Chen T (2020) Three-year field experiment on the risk reduction, environmental merit, and cost assessment of four in situ remediation technologies for metal(loid)-contaminated agricultural soil. Environ Pollut 266:115193. https://doi.org/10.1016/j.envpol.2020.115193
Wang R, Xiao Y, Lv F, Hu L, Wei L, Yuan Z, Lin H (2018) Bacterial community structure and functional potential of rhizosphere soils as influenced by nitrogen addition and bacterial wilt disease under continuous sesame cropping. Appl Soil Ecol 125:117–127. https://doi.org/10.1016/j.apsoil.2017.12.014
WHO (2017) The World Health Organization (WHO) International Agency for research on cancer carcinogens list. https://www.nmpa.gov.cn/xxgk/mtbd/20171030163101383
Woolson EA, Axley JH, Kearney PC (1971) Correlation between available soil arsenic, estimated by six methods, and response of corn (Zea mays L.). Soil Sci Soc Am J 35:101–105. https://doi.org/10.2136/sssaj1971.03615995003500010030x
Xia H, Liang D, Chen F, Liao M, Lin L, Tang Y, Lv X, Li H, Wang Z, Wang X, Wang J, Liu L, Ren W (2018) Effects of mutual intercropping on cadmium accumulation by the accumulator plants Conyza canadensis, Cardamine hirsuta, and Cerastium glomeratum. Int J Phytoremed 20:855–861. https://doi.org/10.1080/15226514.2018.1438356
Xiao X, Chen T, An Z, Lei M, Huang Z, Liao X, Liu Y (2008) Potential of Pteris vittata L. for phytoremediation of sites co-contaminated with cadmium and arsenic: the tolerance and accumulation. J Environ Sci 20:62–67. https://doi.org/10.1016/S1001-0742(08)60009-1
Xiong G, He Y, Luan J, Pan Y, Wang H (2013) Cd-, As- and Pb- polluted farmland remediation potentials of Solanum nigrum, Pteris cretica var. nervosa and Tephrosia candida. J Ecol Rural Environ 29(4):512–518
Xu W, Xiang P, Liu X, Ma LQ (2020) Closely-related species of hyperaccumulating plants and their ability in accumulation of As, Cd, Cu, Mn, Ni, Pb and Zn. Chemosphere 251:126334. https://doi.org/10.1016/j.chemosphere.2020.126334
Yang X, Long X, Ye H, He Z, Calvert DV, Stoffella PJ (2004) Cadmium tolerance and hyperaccumulation in a new Zn-hyperaccumulating plant species (Sedum alfredii Hance). Plant Soil 259:181–189. https://doi.org/10.1023/B:PLSO.0000020956.24027.f2
Yang X, Li T, Yang J, He Z, Lu L, Meng F (2006) Zinc compartmentation in root, transport into xylem, and absorption into leaf cells in the hyperaccumulating species of Sedum alfredii Hance. Planta. 224:85–195. https://doi.org/10.1007/s00425-005-0194-8
Yang M, Xiao X, Miao X, Guo Z, Wang F (2012) Effect of amendments on growth and metal uptake of giant reed (Arundo donax L.) grown on soil contaminated by arsenic, cadmium and lead. Trans Nonferrous Metals Soc China 22:1462–1469. https://doi.org/10.1016/S1003-6326(11)61342-3
Yang J, Yang J, Huang J (2017) Role of co-planting and chitosan in phytoextraction of As and heavy metals by Pteris vittata and castor bean - a field case. Ecol Eng 109:35–40. https://doi.org/10.1016/j.ecoleng.2017.09.001
Yang J, Guo J, Yang J (2018a) Cadmium accumulation and subcellular distribution in populations of Hylotelephium spectabile (Boreau) H. Ohba. Environ Sci Pollut Res 25:30917–30927. https://doi.org/10.1007/s11356-018-3065-0
Yang Q, Li Z, Lu X, Duan Q, Huang L, Bi J (2018b) A review of soil heavy metal pollution from industrial and agricultural regions in China: pollution and risk assessment. Sci Total Environ 642:690–700. https://doi.org/10.1016/j.scitotenv.2018.06.068
Yang E, Cui D, Wang W (2019) Research progress on the genus Massilia. Microbiol China 46(6):1537–1548. https://doi.org/10.13344/j.microbiol.china.180573
Yu HY, Ding X, Li F, Wang X, Zhang S, Yi J, Liu C, Xu X, Wang Q (2016) The availabilities of arsenic and cadmium in rice paddy fields from a mining area: the role of soil extractable and plant silicon. Environ Pollut 215:258–265. https://doi.org/10.1016/j.envpol.2016.04.008
Zeng P, Guo Z, Xiao X, Peng C (2019a) Effects of tree-herb co-planting on the bacterial community composition and the relationship between specific microorganisms and enzymatic activities in metal(loid)-contaminated soil. Chemosphere. 220:237–248. https://doi.org/10.1016/j.chemosphere.2018.12.073
Zeng P, Guo Z, Xiao X, Peng C, Feng W, Xin L, Xu Z (2019b) Phytoextraction potential of Pteris vittata L. co-planted with woody species for As, Cd, Pb and Zn in contaminated soil. Sci Total Environ 650:594–603. https://doi.org/10.1016/j.scitotenv.2018.09.055
Zhai W, Dai Y, Zhao W, Yuan H, Qiu D, Chen J, Gustave W, Maguffin SC, Chen Z, Liu X, Tang X, Xu J (2020) Simultaneous immobilization of the cadmium, lead and arsenic in paddy soils amended with titanium gypsum. Environ Pollut 258:113790. https://doi.org/10.1016/j.envpol.2019.113790
Zhang C, Liu G, Xue S, Wang G (2016) Soil bacterial community dynamics reflect changes in plant community and soil properties during the secondary succession of abandoned farmland in the Loess Plateau. Soil Biol Biochem 97:40–49. https://doi.org/10.1016/j.soilbio.2016.02.013
Zhang X, Ning T, Han H, Sun T, Geng L, Li ZJ, Lai R (2018a) Effects of waxy maize relay intercropping and residue retention on rhizosphere microbial communities and vegetable yield in a continuous cropping system. Pedosphere. 28:84–93. https://doi.org/10.1016/S1002-0160(17)60332-1
Zhang M, Wang N, Hu Y, Sun G (2018b) Changes in soil physicochemical properties and soil bacterial community in mulberry (Morus alba L.)/alfalfa (Medicago sativa L.) intercropping system. MicrobiologyOpen. 7(2):e555. https://doi.org/10.1002/mbo3.555
Zhao F, Ma Y, Zhu Y, Tang Z, McGrath SP (2015) Soil contamination in China: current status and mitigation strategies. Environ Sci Technol 49:750–759. https://doi.org/10.1021/es5047099
Zhao X, Huang J, Lu J, Sun Y (2019) Study on the influence of soil microbial community on the long-term heavy metal pollution of different land use types and depth layers in mine. Ecotoxicol Environ Saf 170:218–226. https://doi.org/10.1016/j.ecoenv.2018.11.136
Zheng B, Bi Q, Hao X, Zhou G, Yang X (2017) Massilia phosphatilytica sp. nov., a phosphate solubilizing bacteria isolated from a long-term fertilized soil. Int J Syst Evol Microbiol 67(8):2514–2519. https://doi.org/10.1099/ijsem.0.001916
Zhou Q, Cheng Y, Zhang Q, Liang J (2004) Quantitative analyses of relationships between ecotoxicological effects and combined pollution. Sci China Ser C Life Sci 47(4):332–339. https://doi.org/10.1360/03yc0042
Zhou C, Xiao X, Guo Z, Peng C, Zeng P, Bridget FA (2020) Physiological responses, tolerance efficiency, and phytoextraction potential of Hylotelephium spectabile (Boreau) H. Ohba under Cd stress in hydroponic condition. Int J Phytoremed 23:1–9. https://doi.org/10.1080/15226514.2020.1797628
Zu Y, Qin L, Zhan F, Wu J, Li Y, Chen J, Wang J, Hu W (2020) Intercropping of Sonchus asper and Vicia faba affects plant cadmium accumulation and root responses. Pedosphere. 30:457–465. https://doi.org/10.1016/S1002-0160(17)60484-3
Funding
This work was sponsored by the Environment Production Scientific Research Project of Hunan Province ([2019] 0011) and National Natural Science Foundation of China (Grant No. 41771532), the Fundamental Research Funds for the Central University of Central South University (No. 2019zzts509).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Xiaohui Wang and Cong Zhou: conceiving and designing the experiments. Xiaohui Wang, Cong Zhou, and Xiaoyan Wang: performing the experiments. Xiaohui Wang and Cong Zhou: analyzing the data and writing original draft. Xiyuan Xiao, Zhaohui Guo, and Chi Peng: reviewing and editing, funding acquisition, project administration. All authors commented on previous versions of the manuscript, and all authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Elena Maestri
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
1. As and Cd phytoextraction by intercropping hyperaccumulators/accumulator was investigated.
2. Intercropping P. vittata with S. alfredii acquired the maximum As and Cd accumulation.
3. Soil microbial community diversity was improved by intercropping and important for heavy metal uptake.
Supplementary information
ESM 1
(DOCX 393 kb)
Rights and permissions
About this article
Cite this article
Wang, X., Zhou, C., Xiao, X. et al. Phytoextraction potential of arsenic and cadmium and response of rhizosphere microbial community by intercropping with two types of hyperaccumulators. Environ Sci Pollut Res 29, 91356–91367 (2022). https://doi.org/10.1007/s11356-022-21994-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-21994-1