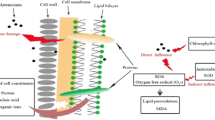
Abstract
Environment-friendly algaecides based on allelopathy have been widely used to control harmful algal blooms. In this research, micro and nano scale artemisinin sustained-release algal inhibitor was prepared, the optimal preparation conditions were explored, and the inhibitory mechanism of artemisinin algaecides was turned perfect. The results showed that when the particle size of artemisinin sustained-release microspheres (ASMs) was 2/10,000 of artemisinin sustained-release granules (ASGs), the inhibitory effect was more remarkable. The optimal concentration of ASMs was 0.2 g L−1, and the inhibitory effect reached 99% on the 10th day. The algal density and chlorophyll a both showed a downward trend, indicating that ASGs and ASMs could promote the degradation of chlorophyll a. The inhibition rate of ASGs was faster than that of ASMs on the 4th day, and the inhibitory effect of ASMs was more significant after the 5th day. The activities of superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT) increased rapidly at first and then decreased, which indicated that ASGs and ASMs caused oxidative damage to Microcystis aeruginosa and inhibited the activity of antioxidant enzymes. Furthermore, the content of the oxygen free radical (O2−) and malondialdehyde (MDA) continued to rise after the 5th day, and the protein, nucleic acid, and conductivity in the culture medium increased. These results showed that lipid peroxidation occurred in the algal cell membrane, and the permeability of the membrane increased. In summary, the ASMs had a significant sustained inhibitory effect while the ASGs had a better short-term effect. The main inhibitory mechanism of artemisinin algaecides is the irreversible damage of cell membrane.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water eutrophication is a widespread phenomenon, and cyanobacteria bloom has become a threat to our water bodies necessitating a corresponding action (Zhao et al. 2010). The ever-increasing Microcystis aeruginosa (M. aeruginosa) reproduction rate is the main reason for the outbreak of harmful blooms (Feng et al. 2013; Zhu et al. 2012). The large-scale outbreak of M. aeruginosa leads to the decrease in dissolved oxygen in water, which breaks the stability and balance of the ecosystem (Greenfield et al. 2014; Harke et al. 2016). Therefore, it is imperative to control algae reproduction (Jancula and Marsalek 2011). Allelopathy has been considered as an important method to solve the algal succession and bloom. Many studies have shown that allelopathy can effectively inhibit the growth of cyanobacteria (Wu et al. 2010; Xiao et al. 2010). Phenolic acids have been successfully extracted and purified from plants, making them the most widely investigated bioactive compounds. Allelopathic inhibition of algae has been proven by catechin (Laue et al. 2014), ginkgolic acids (Zhang et al. 2014), linoleic acid (Ni et al. 2018), and ferulic acid (Guo et al. 2013). Nonetheless, finding alternatives is necessary due to the potential negative effects of phenolic acids on non-target organisms and the complex transformation in the environment (Xie et al. 2014).
Artemisinin extracted from Artemisia annua has been proven to inhibit algae. Artemisinin can cause growth stagnation, injury, or death of the receptor organism through affecting cell proliferation, ultrastructure, protein synthesis, and biochemical reaction (Guo et al. 2019). Many researchers have begun to pay attention to the allelopathic effect of artemisinin on aquatic organisms. Ni et al. (2012) isolated and identified artemisinin from Artemisia annua and confirmed that artemisinin could cause physiological functional disorder of algal cells through algal inhibitory test. According to Wu et al. (2013), the allelopathic effect of artemisinin may hinder water productivity around the Artemisia annua production region, and the growth of Chlorella pyrenoidosa and Scenedesmus obliquus was significantly inhibited. Finaurini et al. (2012) found that the allelopathic effect of artemisinin on water hyacinth is reflected in its serious impact on the photosynthetic efficiency of water hyacinth. Therefore, the allelopathic effect of artemisinin on algae provides a new idea for algal inhibition, and its inhibitory mechanism needs to be explored. Most researchers discussed the influence of allelochemicals on photosynthetic system (Xing et al. 2018; Zhu et al. 2010), cell membrane (Shao et al. 2009), and enzyme activity (Lu et al. 2016; Zhang et al. 2010) while studying the inhibitory mechanism of allelochemicals. The inhibitory mechanism of artemisinin’s consistency with the inhibitory mechanism of other allelochemicals still needs to be further explored.
In addition, it is worth mentioning that adding artemisinin directly into water has the disadvantages of being easily lost and is unable to reach the optimum concentration for algal inhibition. The encapsulation techniques provide a regulated medication delivery system by adsorbing active compounds into polymer skeleton materials and achieving long-term drug release. Ion crosslinking is widely used in the preparation of chitosan/sodium alginate particles. Chitosan, a natural hydrophilic basic polysaccharide, is an easy-to-form polycation electrolyte under acidic conditions and interacts with sodium alginate by electrostatic force to form tightly packed particles (Lee et al. 2011; Gokce et al. 2014). Compared with other methods, the encapsulation method is stable, mild, and non-toxic (without using organic chemical reagents), which reduces the additional toxicity of sustained-release carrier to a certain extent and safe. Meanwhile, research has shown that using two different types of embedding materials with differing properties might improve the embedding rate and the materials’ sustained-release impact (Li et al. 2020). As a result, alginate-chitosan microcapsule technology has emerged as one of the most effective and safe ways to prevent algae from spreading on a big scale (Ni et al. 2018). The sustained-release granules have the ability to release drugs for an extended period of time, resulting in a long-term effect of algal inhibition, and are widely used (Ni et al. 2015).
In a previous research, artemisinin sustained-release algal inhibition granules (ASGs) were successfully prepared by emulsification method, and their algal inhibitory effect and inhibitory mechanism were explored. In this research, we aim to prepare artemisinin sustained-release algal inhibition microspheres (ASMs) by ion crosslinking method combined with micro nanotechnology, reducing the particle size, swelling rate, and increasing the specific surface area of the sustained-release particles. The main objectives of this paper are (1) to determine the optimal preparation conditions of ASMs, (2) to compare the algal inhibitory effect of two artemisinin sustained-release algal inhibitors, and (3) to make the inhibitory mechanism of artemisinin sustained-release algal inhibitors perfect.
Materials and methods
Algal cultivation
M. aeruginosa FACHB-905 was obtained from the Freshwater Algae Culture Collection at the Institute of Hydrobiology (China). It was cultured at Hohai University with sterilized BG-11 medium for 1 week and grew to logarithmic phase before the test. The algae were cultivated at 25℃ under 40–60 μmol photons m−2 s−1 (14 h light/10 h dark) (Ni et al. 2013) and had to be shaken several times a day.
Preparation of ASGs and ASMs
The drop preparation method was used to prepare ASGs, following the steps by Ni et al. (2013). The ASMs were produced with the ion crosslinking preparation method, combined with micro nanotechnology. Chitosan and sodium alginate were used as carrier materials to encapsulate artemisinin to prepare novel artemisinin sustained-release algal inhibition microspheres. The specific steps are shown in Fig. S1. In brief, calcium chloride (6 mg mL−1, w/v) solution was slowly dripped (at a mixture speed of 700–1000 rpm) into sodium alginate (1 mg mL−1, w/v) solution to obtain a pregel, which was stirred for 30 min, crushed for 10 min, then successively dripped artemisinin solution (10 mg mL−1, w/v) and aqueous acetic acid (1%, v/v) solution containing chitosan (0.6 mg mL−1, w/v) into the pregel, slowly stirring for 60 min, and chitosan colloidal microspheres were formed which were then crushed for 1 min. After centrifugating at 12,000 rad/min for 30 min, rinsing several times with distilled water, and freeze-drying, the ASMs were then collected. This experiment was carried out with orthogonal test, and the optimal preparing conditions of ASMs were tested by high-performance liquid chromatography with a photodiode array (HPLC–PDA).
The artemisinin microspheres were washed three times with double-distilled water, grind-mixed with diatomite, and then dissolved in solvent to obtain artemisinin sustained-release solution. HPLC–PDA (Waters 2695 HPLC) was used to determine the artemisinin content of the sustained-release microspheres and to calculate the encapsulation efficiency based on the initial amount of ASMs. The HPLC–PDA method was performed on a Waters C18 column. The mobile phase was 0.02 mol L−1 KH2PO4:acetonitrile (90:10 v/v) solution at a flow rate of 1 mL min−1, with a column temperature of 30℃.
To determine the optimal preparation of ASMs, the concentrations (in %, w/v) of materials mentioned above were dependent on an orthogonal test. The size of microspheres was calculated by dynamic light scattering (DLS) analysis (Mastersizer 3000, UK). The encapsulation efficiency (%) of the artemisinin was calculated using the following equation:
where W1 is the measured amount of encapsulated artemisinin in the sustained-release microspheres, and W0 is the initial amount of ASMs.
Algal inhibitory test
M. aeruginosa was introduced into the culture medium of 250-mL flasks, and 2 ~ 3 × 106 cells mL−1 was set as the initial cell density to simulate the stationary phase of the algal growth. The concentration range of the ASMs was determined by pre-experiment, and then the algal inhibitory experiment was carried out. The blank group (without algal inhibitor) and the ASM groups with different concentrations (the dosages of ASMs were 0.1 g L−1, 0.2 g L−1, 0.4 g L−1, 0.8 g L−1, and 1 g L−1, respectively) were set. The culture condition was the same as in the section “Algal cultivation.” During the 30-day culture phase, algal density was assessed on a regular basis using a conversion of optical density to reflect the growth of M. aeruginosa. By determining the algal inhibition rate of different concentrations of ASMs, the optimal effective dosage of sustained-release microspheres was determined.
Inhibitory mechanism studies
The dosage of ASG algal inhibitor was based on earlier research (Ni et al. 2013). Three experimental groups were set up in this research: blank control group, ASG algal inhibitor experimental group, and ASM algal inhibitor experimental group. The three experimental groups were in triplicates. In this research, the initial algal density was 2 × 106 cells mL−1, the optimal dosage of ASG algal inhibitor was 0.54 g L−1, and the optimal dosage of ASMs was determined by the results of algal inhibitory test. The content of chlorophyll a and the activities of antioxidant enzymes (superoxide dismutase (SOD), catalase (CAT), and peroxidase (POD)) were measured every 5 days during the 30-day culture period. The degree of damage on cell membrane was characterized by the content of protein, nucleic acid, MDA (degree of lipid peroxidation), oxygen free radical (O2−), and the changes in conductivity.
Algal density and chlorophyll a content assays
The cell density of M. aeruginosa was determined by optical density method. The microscope counting method and the optical density at 680 nm were used to get a regression equation relating to algal density, that is
where X is the absorbance value and Y × 105 cells mL−1 represents the density of algae.
The relative inhibition rate of algal inhibitor release on M. aeruginosa is as follows:
where N is the algal density of treatment group, and N0 is the algal density of blank group.
The content of chlorophyll a was determined by ethanol extraction (Li et al. 2019). The absorbance of the extract and blank reference was measured at 665 nm and 750 nm, respectively. The calculation formula is as follows:
Cell membrane assays
The content of MDA was determined by thiobarbituric acid (TBA) spectrophotometry (Zhang et al. 2016a). The culture medium was treated with AP-TEMED system, and then the concentration of O2− was determined by measuring its absorbance at 530 nm. The contents of protein and nucleic acid in the culture medium were mainly obtained by measuring the absorbance values of the supernatant at 280 nm and 260 nm, respectively (Zhang et al. 2016b). Conductivity is mainly measured by a conductivity meter.
Antioxidant enzyme activity assays
The total SOD activity was determined by NBT photoreduction method as described by Charles and Irwin (1971). The activity of CAT was determined by its ability to decompose H2O2, and CAT activity was determined according to Aebi et al. (2010). POD can catalyze the reaction of peroxyphenol, and the activity detection method of POD referred to that of Liu et al. (2010).
Statistical analysis
All statistical analyses were performed using SPSS 22.0, and graphs were prepared in Origin 9.0. p values were derived from the one-way ANOVA test to determine differences between groups with normally distributed data. A p value < 0.05 was considered statistically significant.
Results and discussion
Optimal manufacture of ASGs and ASMs
The optimal preparation method of ASGs was based on our previous research (Ni et al. 2013), where the average particle size of ASGs was 5 mm, with a maximum encapsulation efficiency of 52%. In this research, the optimal preparation concentrations (in %, w/v) of ASMs are as follows: chitosan (0.06), sodium alginate (0.1), calcium chloride (0.6), and artemisinin (1). Under the optimal preparation conditions, the microsphere size of ASMs is shown in Fig. 1. It is obvious that the microsphere size of ASMs is in the micro/nano level, which is far smaller than that of ASGs. According to the experimental data, the average particle size of ASMs was 1189.5 nm, and the encapsulation efficiency was 52.4%. It is well known that greatly reduced particle size is beneficial to reduce the swelling rate of the sustained-release algal inhibitor. Therefore, the novel preparation method used in this research is conducive to further improve the algal inhibitory effect.
Inhibitory effect of ASMs on M. aeruginosa
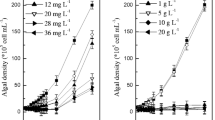
The inhibitory effect on algae differs due to the dosages of algaecides (Ni et al. 2018). To investigate the effects of ASMs, algal density was determined and the results (Fig. 2) showed that the inhibitory effect of ASMs with higher concentration on M. aeruginosa was more remarkable and the inhibition time was longer than that with low concentration. As shown in Fig. 2, the blank control group showed an S-shaped growth curve (Li et al. 2010). At the dosage of 0.1 g L−1, ASMs showed a transient inhibitory effect on M. aeruginosa. The inhibition rates were below 30% after the 2nd day, and the inhibitory efficiency was only 10.20% at the end of the experiment (30 days). The growth of cyanobacteria was clearly suppressed with doses above 0.1 g L−1. The dosages of 0.2 g L−1 and 0.4 g L−1 showed a very similar pattern. The growth of algae was in a constant inhibition state from the 4th to the 10th day and maintained high inhibitory efficiency (> 99%) after the 10th day. When the dosage was higher than 0.8 g L−1, the inhibition rate was up to 99% on the 5th day. Considering the perspective of algal inhibitory test and economy, 0.2 g L−1 of ASMs was selected as the optimal algal inhibitory concentration under laboratory conditions.
Effects of ASGs and ASMs on chlorophyll a
As an important index reflecting the photosynthetic capacity of algae, the content of chlorophyll a was affected by the growth of algal cells and photosynthesis (Maqbool et al. 2018). In this research, the effects of ASGs and ASMs on the density and chlorophyll a content of M. aeruginosa were investigated under the optimal algal concentration. The results (Fig. 3) were consistent with the results of many researchers (Espinoza-Avalos 2006), and the content of chlorophyll a in the blank control group was approximately positively correlated with the density of algae. At day 10 after being exposed to the two algal inhibitors, there were significant changes between the treatment groups and the blank control group (p < 0.05). In addition, chlorophyll a remained at a low level during the whole experiment. At the beginning of the experiment (the first 4 days), ASGs had more apparent inhibitory effect and faster inhibition rate. After day 5, the intensities of the inhibitory effects of ASGs and ASMs were reversed. Both showed persistent and strong algal inhibitory efficiency after 10 days, with ASMs having a higher inhibitory intensity. At the end of the experiment (30 days), M. aeruginosa had no trace of recovery. The ASMs had a stronger long-term inhibitory effect, which could be due to the smaller particle size of ASMs compared to ASGs, combined with the multi-space structure on their surface, which considerably increased the effective contact area with M. aeruginosa. In addition, ASGs easily swell and sink in water, which reduces the contact time with M. aeruginosa. It is worth mentioning that in the previous research, the apparent sudden release phenomenon of ASGs in the initial stage may be the real reason for the faster inhibition rate of ASGs. In general, both algal inhibitors were highly effective in inhibiting algae, although ASMs had a longer-lasting effect on algal inhibition and were more damaging to chlorophyll a.
Effects of ASGs and ASMs on cell membrane permeability
Cell membrane is an important structure of cells, and its important components are phospholipid bilayer and protein. It can keep the cell microenvironment stable and has selective permeability function (Jonas et al. 2016). Generally, allelochemicals damage the integrity of cell membrane, reduce its selective permeability function, and make algal cells exude a lot of endolysates (Li et al. 2007). Therefore, we often use the change of cell membrane permeability to characterize the degree of membrane damage. Figure 4 shows the effects of the two algal inhibitors on the protein and nucleic acid content in the culture medium. The experiment was accompanied by natural apoptosis in algae, which was higher in the early stage than in the late stage, albeit being in a relatively low range (Zhou et al. 2009). The protein and nucleic acid concentration in the blank control group decreased at first, then increased, and then remained constant at a low level throughout. The two algal inhibitors induced a substantial rise in protein and nucleic acid content in M. aeruginosa (p < 0.05), and the protein and nucleic acid content in the ASG group was higher in the beginning of the experiment. It was reversed in the middle and later periods. Both of them appeared to be able to continuously release artemisinin in order to replenish the anti-algal active components in the algal inhibition system, but the damage effect of ASGs on cell membrane was faster, and the sustained inhibitory effect of ASMs was more impressive, which was consistent with their inhibition pattern. Protein deficiency in algae can damage or disrupt normal physiological metabolic processes, even resulting in algal death (Ni et al. 2013). As a result of the persistent stress of the two algal inhibitors, both algal cell membranes were severely damaged, and the decrease in membrane selective permeability led to the gradual leakage of protein and nucleic acid outside the cells. The degree of damage to algal cells increased over time as artemisinin was released, and the damage caused by ASMs on the cell membrane was more effective and long-lasting.
Figure 5 shows the change in electrical conductivity under the action of the two artemisinin sustained-release algal inhibitors. In general, the electrical conductivity of the blank control group did not fluctuate significantly, but the ASM group increased significantly after the 7th day (p < 0.05). On the 2nd day, the conductivity of the treatment group culture medium was higher than that of the blank control group under the stress of the two sustained-release algal inhibitors. After the 7th day, the conductivity of the culture medium under the stress of ASMs was gradually higher than that of ASGs. The results showed that under the sustained stress of the two artemisinin sustained-release algal inhibitors, the degree of damage on cell membrane was gradually aggravated, and its permeability was also gradually increased, which led to the leakage of electrolytes including K+, PO42−, and other ions in the algal cell to the external environment. These phenomena indicated that artemisinin active substance could cause irreversible damage to cell membrane (Peng et al. 2016).
Oxidative damage
Under the condition of environmental stress, the production and elimination of O2− in the algal cell system will be out of balance. A large number of O2− will destroy the biological macromolecules in the algal cells and cause membrane lipid peroxidation (Huang et al. 2013a; Saed-Moucheshi et al. 2014). MDA is the product of membrane lipid peroxidation. Membrane lipid peroxidation can destroy the structure of cell membrane and make it abnormal (Huang et al. 2013b). The higher the content of MDA, the higher the degree of lipid peroxidation and the more serious the damage of cell membrane (Ali et al. 2006). As shown in Fig. 6, the content of O2− and MDA in the blank control group was nearly invariable, which indicated that M. aeruginosa did not suffer membrane lipid peroxidation and grew in a normal state. In addition, the change in trend for O2− and MDA content in the two artemisinin algal inhibitor groups was basically the same, showing different degrees of upward trend, which indicated that the algae experienced different degrees of lipid peroxidation after adding the two sustained-release algal inhibitors, and the algal cells were damaged in different degrees. Specifically, the increase rate of O2− and MDA content in the ASG group was higher than that in the ASM group 4 days prior to the experiment, but it was reversed after the fifth day. On the 10th day of the experiment, the content of MDA and O2− in the ASM group increased significantly, while the significant increase appeared in the ASG group on the 15th day (p < 0.05). This phenomenon was consistent with the algal inhibitory effect and the abovementioned pattern of intracellular solute extravasation. These phenomena indicated that the algal inhibitory effect of ASGs was better than that of ASMs in a short time while ASMs had better sustained algal inhibitory effect. To some extent, the results indicated that the smaller the particle size of the algal inhibitor, the more favorable the contact between the algal inhibitor and the algal cell, and the algal inhibitory effect was more remarkable.
Inhibition of antioxidant enzyme activities
Antioxidant system is the protective system of organism against harsh environment. The key antioxidant enzymes are SOD, POD, and CAT (Gulzar and Siddiqui 2015). Antioxidant enzymes usually eliminate excessive reactive oxygen species (ROS) to ensure the homeostasis of internal oxidation metabolism. SOD is the key protective enzyme of the body, which can reflect the degree of damage and the ability to resist the adverse environment (Blackhall et al. 2004). CAT and POD play an important role in the antioxidant system of algal cells, which can promote the decomposition of H2O2 and stabilize the ROS in the organism at a normal level, in order to avoid damage to cells (Tang et al. 2010).
The activities of SOD, CAT, and POD were measured to evaluate the effect of the two artemisinin sustained-release algal inhibitor on antioxidant enzymes (Fig. 7). The activities of SOD, POD, and CAT in the two artemisinin sustained-release algal inhibitor groups showed a trend of rapid increase and then decrease, showing a dynamic change process from induction to inhibition. The SOD activity of treatment groups decreased gradually after reaching the maximum, and the ASG group and ASM group reached maximum on the 5th day and 10th day, respectively, and then declined sharply (p < 0.01) on the 25th day. The SOD activity of treatment groups was lower than that of the blank control group on the 15th day. Furthermore, the decrease in SOD activity in the ASM group was much greater than that in the ASG group. The activities of POD and CAT reached the maximum on the 5th day, then decreased sharply after 15 days (p < 0.01) until beyond the detection range. Excess ROS was created as a result of the continual release of artemisinin, preventing algal cells from developing normally and even destroying the antioxidant system. The activity of antioxidant enzymes decreased and eventually inactivated as intracellular components (nucleic acid, proteins, ions, etc.) were lost (Hejl and Koster 2004).
SOD can convert O2− into H2O2 and O2 specifically to avoid cell injury (Ni et al. 2018). CAT and POD can remove excessive hydrogen peroxide in algal cells, so the change in trend for the three antioxidant enzymes was consistent, until the antioxidant enzymes were inactivated. To sum up, under the action of algal inhibitory substances, ROS increased, and the contents of SOD, CAT, and POD increased. With the constant release of artemisinin, antioxidant enzymes were gradually inactivated, resulting in the damage of algal cells, thus inhibiting the growth of algae (Li et al. 2013).
Conclusions
In summary, the inhibitory effects of the two artemisinin sustained-release inhibitors were both significant. The results showed that the optimal concentration of ASMs was 0.2 g L−1, and the inhibition rate was up to 99% on the 10th day. Under the optimal conditions, the algal inhibitory effect of ASMs with small particle size was more effective than that of ASGs on the whole, and the ASMs had significant sustained inhibitory effect while ASGs had better short-term effect.
The inhibitory mechanism of ASGs was consistent with that of ASMs. They inhibited the growth of M. aeruginosa by destroying the photosynthetic system, causing oxidative damage, inhibiting antioxidant enzyme activity, and destroying cell membrane. The decrease in chlorophyll a content indicated that both algal inhibitors could inhibit the photosynthesis of algal cells. Under the stress of algal inhibitor, the algal cells were stimulated to generate a large amount of O2−, which resulted in lipid peroxidation of cell membrane and irreversible damage to the permeability of algal cell membrane. At the same time, the production of a large amount of O2− can induce the increase of SOD, POD, and CAT activities. With the prolongation of inhibition time, the generated excess ROS prevented the algal cells from growing normally and ultimately led to the inactivation of antioxidant enzymes. The accumulation of MDA showed that the cell membrane was damaged to a certain extent, which led to the leakage of substances in the cell, resulting in the increase of protein, nucleic acid, and electrical conductivity in the culture medium. ASMs had a remarkable anti-algal effect, and the preparation method was environmentally friendly, and economical to produce, which provided an unlimited possibility for the application of ASMs in controlling red tide in natural lakes in the future.
Data availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
References
Aebi H, Wyss SR, Scherz B, Skvaril F (2010) Heterogeneity of erythrocyte catalase II. Isolation and characterization of normal and variant erythrocyte catalase and their subunits. FEBS J 48:137–145
Ali MB, Hahn EJ, Paek KY (2006) Antioxidative responses of in bioreactor liquid cultures. Enzyme Microb Technol 39:982–990
Blackhall ML, Coombes JS, Fassett R (2004) The relationship between antioxidant supplements and oxidative stress in renal transplant recipients: a review. ASAIO J 50(5):451–457
Charles B, Irwin F (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Espinoza-Avalos QY (2006) Reduction of zooxanthellae density, chlorophyll a concentration, and tissue thickness of the coral Montastraea faveolata (Scleractinia) when competing with mixed turf algae. Limnol Oceanogr 51:1159–1166
Feng Y, Chang X, Zhao L, Li X, Li W, Jiang Y (2013) Nanaomycin A methyl ester, an actinomycete metabolite: algicidal activity and the physiological response of Microcystis aeruginosa. Ecol Eng 53:306–312
Finaurini S, Basilico N, Corbett Y, D’Alessandro S, Parapini S, Olliaro P, Haynes RK, Taramelli D (2012) Dihydroartemisinin inhibits the human erythroid cell differentiation by altering the cell cycle. Toxicology 300:57–66
Gokce Y, Cengiz B, Yildiz N, Calimli A, Aktas Z (2014) Ultrasonication of chitosan nanoparticle suspension: influence on particle size. Coll Surf A Physicochem Eng Aspects 462:75–81
Greenfield DI, Duquette G, Keppler CJ, Williams SH, Brock LM (2014) The effects of three chemical algaecides on cell numbers and toxin content of the cyanobacteria Microcystis aeruginosa and Anabaenopsis sp. Environ Manage 54:1110–1120
Gulzar A, Siddiqui M (2015) Allelopathic effect of Calotropis procera (Ait.) R. Br. on growth and antioxidant activity of Brassica oleracea var. botrytis. J Saudi Soc Agric Sci 16(4):375–382
Guo YL, Fu HY, Huang GH, Gao PF, Chai T, Yan B, Liao H (2013) Allelopathy effects of ferulic acid and coumarin on Microcystis aeruginosa. Environ Sci 34:1492–1497
Guo MW, Liu FM, Lv JZ (2019) Advances in research on the allelopathic effects of artemisinin. Crop Res 33(01):82–85
Harke MJ, Steffen MM, Gobler CJ, Otten (2016) A review of the global ecology, genomics, and biogeography of the toxic cyanobacterium, Microcystis spp. Harmful Algae 54:4–20
Hejl AM, Koster KL (2004) Juglone disrupts root plasma membrane H+-ATPase activity and impairs water uptake, root respiration, and growth in soybean (Glycine max) and corn (Zea mays). J Chem Ecol 30:453–471
Huang X, Liang YS, Yang LT, Chen RF, Li YR (2013a) Effects of abscisic acid and its biosynthesis inhibitor on the activities of antioxidant system of sugarcanes treated by cold stress. J South China Agric Univ 34(03):356–361
Huang Y, Yu B, Yan W, Kong H (2013b) Allelopathic effects of the extracts from an invasive species Solidago canadensis L. on Microcystis aeruginosa. Lett Appl Microbiol 57(5):451–458
Jancula D, Marsalek B (2011) Critical review of actually available chemical compounds for prevention and management of cyanobacterial blooms. Chemosphere 85:1415–1422
Laue P, Bahrs H, Chakrabarti S, Steinberg CEW (2014) Natural xenobiotics to prevent cyanobacterial and algal growth in freshwater: contrasting efficacy of tannic acid, gallic acid, and gramine. Chemosphere 104:212–220
Lee YH, Chang JJ, Lai WF, Yang MC, Chien CT (2011) Layered hydrogel of poly(γ-glutamic acid), sodium alginate, and chitosan: fluorescence observation of structure and cytocompatibility. Colloids Surf, B 86:409–413
Li FM, Hu HY, Chong YX, Guo MT, Men YJ (2007) Effects of allelochemical isolated from Phragmites communis on algal membrane permeability. Environ Sci 28:2453–2456
Li FT, Qi JM, Zhang GY, Lin LH, Fang PP, Tao AF, Xu JT (2013) Effect of cadmium stress on the growth, antioxidative enzymes and lipid peroxidation in two kenaf (Hibiscus cannabinus L.) plant seedlings. J Integr Agric 12:610–620
Li BH, Yin YJ, Kang LF, Feng L, Liu YZ, Du ZW, Tian YJ, Zhang LQ (2020) A review: Application of allelochemicals in water ecological restoration-algal inhibition. Chemosphere 267:128869
Li XY, Wang JQ, Wei HY, Bu DP, Hu H, Zhou LY (2010) Bovine mammary epithelial cells culture methods in vitro and its application. Acta Agriculturae Boreali-Sinica 25(S2):31–35
Li PF, Sun X, Yang L, He FF, Wang X (2019) Optimization of extraction protocol of chlorophyll a from algae. CIESC J 70(9):3421–3429
Liu J, Zhang X, Sun Y, Lin W (2010) Antioxidative capacity and enzyme activity in Haematococcus pluvialis cells exposed to superoxide free radicals. Chin J Oceanol Limnol 28(1):1–9
Lu Z, Zhang Y, Gao Y, Liu B, Sun X, He F, Zhou Q, Wu Z (2016) Effects of pyrogallic acid on Microcystis aeruginosa: oxidative stress related toxicity. Ecotoxicol Environ Saf 132:413–419
Maqbool A, Ali S, Rizwan M, Ishaque W, Rasool N, Mzu R, Bashir A, Abid M, Wu L (2018) Management of tannery wastewater for improving growth attributes and reducing chromium uptake in spinach through citric acid application. Environ Sci Pollut Res Int 25:10848
Ni L, Acharya K, Ren G, Li S, Li Y, Yong L (2013) Preparation and characterization of anti-algal sustained-release granules and their inhibitory effects on algae. Chemosphere 91:608–615
Ni L, Jie X, Wang P, Li S, Acharya K (2015) Characterization of unsaturated fatty acid sustained-release microspheres for long-term algal inhibition. Chemosphere 120:383–390
Ni L, Rong S, Gu G, Hu L, Wang P, Li D, Yue F, Wang N, Wu H, Li S (2018) Inhibitory effect and mechanism of linoleic acid sustained-release microspheres on Microcystis aeruginosa at different growth phases. Chemosphere 212:654–661
Ni L, Acharya K, Hao X, Li S (2012) Isolation and identification of an anti-algal compound from Artemisia annua and mechanisms of inhibitory effect on algae. Chemosphere 88(9):1051–1057
Peng GY, Chen YL, Han YZ, Zhang TT (2016) The inhibitory effect of lactic acid on Microcystis aeruginosa and its mechanisms. China Environ Sci 36:1167–1172
Saed-Moucheshi A, Shekoofa A, Pessarakli M (2014) Reactive oxygen species (ROS) generation and detoxifying in plants. J Plant Nutr 37:1573–1585
Shao J, Wu Z, Yu G, Peng X, Li R (2009) Allelopathic mechanism of pyrogallol to Microcystis aeruginosa PCC7806 (cyanobacteria): from views of gene expression and antioxidant system. Chemosphere 75:924–928
Tang B, Shang-Zhong XU, Zou XL, Zheng YL, Qiu FZ (2010) Changes of antioxidative enzymes and lipid peroxidation in leaves and roots of waterlogging-tolerant and waterlogging-sensitive maize genotypes at seedling stage. Agric Sci China 9:651–661
Van der Paal J, Neyts E, Christof (2016) Effect of lipid peroxidation on membrane permeability of cancer and normal cells subjected to oxidative stress. Chem Sci 7(1):489–498
Wu Y, Zhang S, Zhao H, Yang L (2010) Environmentally benign periphyton bioreactors for controlling cyanobacterial growth. Biores Technol 101:9681–9687
Wu YK, Ling Y, Huang JG, Li LY (2013) Allelopathic effect of artemisinin on green algae. China J Chin Materia Med 38:1349
Xiao X, Ying-Xu C, Xin-Qiang L, Li-Ping L, Xian-Jin T (2010) Effects of Tibetan hulless barley on bloom-forming cyanobacterium (Microcystis aeruginosa) measured by different physiological and morphologic parameters. Chemosphere 81:1118–1123
Xie XG, Chen Y, Bu YQ, Dai CC (2014) A review of allelopathic researches on phenolic acids. Acta Ecol Sin 34:6417–6428
Xing CY, Wu YG, Qiao JC, Zhang Y (2018) Studies on allelopathic inhibition of aquatic plant communities to algal bloom. Environ Sci Technol 41(03):35–41
Zhang TT, Zheng CY, Wei H, Xu WW, Wang HF (2010) The allelopathy and allelopathic mechanism of phenolic acids on toxic Microcystis aeruginosa. J Appl Phycol 22:71–77
Zhang C, Ling F, Yi YL, Zhang HY, Wang GX (2014) Algicidal activity and potential mechanisms of ginkgolic acids isolated from Ginkgo biloba exocarp on Microcystis aeruginosa. J Appl Phycol 26:323–332
Zhang J, Xie Z, Wang Z, Zhang J, Xie Z, Wang Z (2016a) Oxidative stress responses and toxin accumulation in the freshwater snail Radix swinhoei (Gastropoda, Pulmonata) exposed to microcystin-LR. Environ Sci Pollut Res 23(2):1353–1361
Zhang SJ, Xia WT, Yang XH, Zhang TT (2016b) Inhibition effect of aquaculture water of Salvinia natans (L.) All. on Microcystis aeruginosa PCC7806. J Hyg Res 45(1):81–86
Zhao YH, Deng XZ, Zhan JY, Xi BD, Lu Q (2010) Progress on preventing and controlling strategies of lake eutrophication in China. Environ Sci Technol 33:92–98
Zhou J, Zeng C, Wang L (2009) Study on characteristic of algae growth in tai lake based on nonlinear dynamic analysis. Acta Hydrobiol Sin 33:931–936
Zhu J, Liu B, Jing W, Gao Y, Wu Z (2010) Study on the mechanism of allelopathic influence on cyanobacteria and chlorophytes by submerged macrophyte (Myriophyllum spicatum) and its secretion. Aquat Toxicol 98:196–203
Zhu KX, Bi YH, Hu JL, Ai Y, Hu ZY (2012) Characteristics of Microcystis aeruginosa bloom in summer 2008 in Shennong River of Three Gorges Reservoir. J Lake Sci 24(02):220–226
Funding
This work was supported jointly by the Key Program of the National Natural Science Foundation of China (Grant Nos. 51779079 and 51979137), the Natural Science Foundation of Jiangsu Province (Grant No. BK20181313), the Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), and the Top-notch Academic Programs Project of Jiangsu Higher Education Institutions (TAPP).
Author information
Authors and Affiliations
Contributions
Zhiyun Jiang: writing of the original draft, formal analysis, and data curation. Lixiao Ni: funding acquisition, conceptualization, and project administration. Xianglan Li: software and methodology. Chu Xu: investigation. Xuqing Chen: conceptualization and resources. Shiyin Li: writing, with review and editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Vitor Vasconcelos
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
1. Algicide with nanometer size was successfully prepared for algal inhibition.
2. Nano scale artemisinin algal inhibitor exhibited excellent inhibitory effect.
3. Destruction of algal cells was due to the irreversible damage of cell membrane.
4. Artemisinin microspheres may be used as a potential candidate for algal control.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jiang, Z., Ni, L., Li, X. et al. Mechanistic insight into the inhibitory effect of artemisinin sustained-release inhibitors with different particle sizes on Microcystis aeruginosa. Environ Sci Pollut Res 29, 87545–87554 (2022). https://doi.org/10.1007/s11356-022-21534-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-21534-x