Abstract
Index of biotic integrity (IBI) based on fish has been applied globally. However, few have considered that fish assemblages change among different aggregate ecoregions when conducted their health assessment. Indeed, some comprehensive indices, such as functional and phylogenetic diversity indices and ABC curve, can be used to identify aspects that are not captured by traditional metrics. Consequently, we try to integrate comprehensive indices and spatial patterns of fish assemblages to develop IBI systems and then verified their effectiveness and accuracy for assessing the environmental health of the Chishui River basin. The comprehensive disturbance index (CDI), based on 11 water quality parameters and 4 human land use, was set to distinguish reference sites and impaired sites. According to the spatial patterns of fish assemblages, the 40 sites were finally divided into 2 aggregate ecoregions, include wadeable streams and nonwadeable rivers. 97 candidate metrics were selected to develop our IBI systems based on the systematic screening method. The result also showed that our IBI systems performed well in discriminating anthropogenic disturbances at both aggregate ecoregions, which suggests that our systems could provide a reliable evaluation. The mean IBI score of the Chishui River basin was 72.09 ± 16.58, and was classified as good status. However, S1 (Chishuiyuan Town), Baisha River, Tongzi River, and Xishui River were disturbed by various human activities. We conclude that the spatial patterns of fish assemblages should be combined with more comprehensive indices to assess river health. On the other hand, we do believe that the process of developing and verifying our IBI systems could be regarded as a reference for biomonitoring in more mountain river systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent decades, freshwater ecosystems have become increasingly threatened by various stressors, such as pollution, land use changes, dam construction, water extraction, and overfishing (Ormerod et al. 2010; Gomes-Silva et al. 2020; Ngor et al. 2018). As a result, health assessment of freshwater ecosystem is attracting increasing attention globally (Ruaro and Gubiani 2013), and there are now multiple approaches for evaluating such effects. In recent years, increasing aquatic biota have been selected as indicators, such as fish, macroinvertebrates, algae, and microbiota. Fish, factually, have proven to be important and efficacious indicators due to their widespread distributions, relatively long lifespan, sensitivity to environmental changes, and occupy multiple trophic levels that represent a greater proportion of rivers (Karr 1981; Mendonça et al. 2005; Maceda-Veiga et al. 2014; Chen et al. 2020). The index of biotic integrity (IBI) is an important tool for evaluating and managing river ecosystem and was proposed by Karr (Karr 1981). Karr initially set out to develop a IBI system with twelve fish community parameters belong to two categories: species composition and richness, and ecological factors (Karr 1981). In addition, Karr argued that (1) criteria that only emphasize physical and chemical attributes of water are unsuccessful as surrogates for measuring biotic integrity; (2) since an ability to sustain a balanced biotic community is one of the best indicators of the potential for beneficial use, sophisticated monitoring programs should seek to assess “biotic integrity”; (3) by carefully monitoring fishes, one can rapidly assess the health (“biotic integrity”) of a local water resource; and (4) IBI system could be expanded continuously, as long as those metrics are adjusted by biogeographic and stream-size considerations (Karr 1981). Unfortunately, although the approaches of IBI based on fish (F-IBI) have been reported frequently in past decades (Voss et al. 2012; Aura et al. 2017), some deficiencies remain in actual situation. For example, they have not taken into account naturally occurring geographic variation of fish assemblages, nor considered divergence of life-history information (e.g., tolerance, reproduction, habitat) of these fish. In addition, few comprehensive indices in monitoring systems often miss many of the man-induced perturbations that may be critical to assessment of biotic impacts (Saito et al. 2015). In short, many scholars developed IBI systems without considering variations of fish assemblages or using more comprehensive indices (Voss et al. 2012; Lin et al. 2014; Aura et al. 2017). Therefore, the modification and improvement of biomonitoring tools as a critical step should be the first priority.
To protect and manage the aquatic ecosystem, the accuracy, efficiency, and universality are required for ecological assessment methods. As mentioned above, Karr had highlighted that biogeographic and stream-size should be taken into account when developed IBI system (Karr 1981). In other words, it mandated that IBI methods should be combined with spatial patterns of aquatic assemblages. In fact, much literature on variations of fish assemblages is of authoritative value for referring. Streams exhibit a gradient of natural resources and physical environment from small headstreams to large rivers (Shelford 1911), and the River Continuum Concept (RCC) has highlighted a variation in aquatic communities in response to such habitat changes (Vannote et al. 1980). Similarly, the significantly variations of fish assemblage had been observed in many rivers in the past few years (Matthews 1998; Lasne et al. 2007; Ferreira and Petrere 2009; Liu et al. 2020). For the development of effective and accurate IBI system, there is an urgent need to understand the organization mechanisms of fish assemblages. Besides, although not any comprehensive indices were used in Karr’s IBI system yet, many follow-up researchers thought that more comprehensive indices require clear relationships between biological attributes and anthropic disturbance, and integration into traditional IBI methods to mirror different aspects (Chen et al. 2020; Shi et al. 2020; Saito et al. 2015). To date, Pielou’s index, Shannon-Weiner index, and Simpson’s index (Boltzmann 1970; Shannon 1948; Simpson 1949) have been widely used in IBI systems for evaluating species diversity (Cai et al. 2012; Wang et al. 2018; Zhu et al. 2021). In recent years, functional and phylogenetic diversity metrics have attracted attention from ecologists recently because they consider aspects of ecological or evolutionary differences among species that are not captured by traditional species diversity indices (Saito et al. 2015). Functional diversity indices, such as functional richness, functional evenness, and functional divergence, measure the diversity of functional traits, i.e., characteristics of organism phenotype that influence ecosystem services, and organism survival (Mason et al. 2005). Phylogenetic diversity indices, such as “variation in taxonomic distinctness” (VarTD, Λ +) and “average taxonomic distinctness” (AvTD, ∆ +), measure the evolutionary relationship among species through the taxonomic tree connecting every pair of species in the list (Clarke and Warwick 1994). In addition, the abundance biomass comparison (ABC) method was initially proposed by Warwick (Warwick 1986) and has been used to assess levels of disturbance on fish community in the past decades (Bianchi et al. 2001; Sun et al. 2019; Vanna et al. 2020). Theoretically, the ABC curve combines dominance of species abundance and biomass may create 3 possible forms, representing undisturbed, moderately disturbed, or strongly disturbed communities, and is based on classical evolutionary theory of r- and K-selection (Warwick 1986; Coeck et al. 1993). All of them have truly withstood the test of time and are still generally regarded as the important tools of ecological field.
The Chishui River is the last free-flowing tributary of the upper Yangtze River and provides an ideal model to test river ecological principles because of its undammed mainstream (Liu et al. 2020; Wu et al. 2011). In addition, the “National Nature Reserve for Rare and Endemic Fishes of the upper Yangtze River” was established by the Chinese Government in 2005, aiming to protect fish resources that suffered from hydropower engineering, such as Three Gorges Dam, Xiluodu Dam, and Xiangjia Dam (Wu et al. 2011). Complementarily, the Chishui River is an important part of this national natural reserve and become a fish-conservation hotspot. In recent years, increasing research reports about the Chishui River have been proposed to capture the fish diversity patterns, community biology, and conservation biology (Liu et al. 2020; Wu et al. 2011), including a few studies of ecological health. For example, Lin et al. developed a simple F-IBI of the Chishui River based on three sampling sites and M-IBI (Macroinvertebrate) was constructed by Wang soon after (Lin et al. 2014; Wang et al. 2018). Although these studies have reflected ecological status in some aspects, there are still some deficiencies, such as lacking of comprehensive indices, few sampling sites were set, focusing only on mainstream, or developing IBI without considering spatial patterns of aquatic communities along the river. Moreover, as a typical mountain river system, Chishui River basin includes wadeable streams and nonwadeable rivers. Liu et al. had confirmed that fish assemblages varied significantly along the longitudinal gradient of the Chishui River mainstream (Liu et al. 2020), but not covering more sites of its important tributaries.
Summing up, the objectives of our study were to determine the following: (1) Separate all the sampling sites of the Chishui River basin into reasonable aggregate ecoregions based on spatial patterns of fish assemblages, (2) try to integrate more candidate metrics such as, species, functional and phylogenetic diversity metrics, ABC curve into our IBI systems, and test different properties, and verify the accuracy and feasibility of them; (3) identify the biological condition of rivers throughout the Chishui River basin; and (4) provide a reference for biomonitoring in more mountain river systems.
Materials and methods
Study region
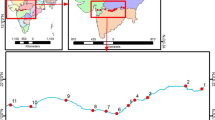
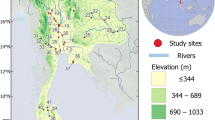
We sampled 40 sites on the Chishui River basin (primary-and second-order streams of the upper Yangtze River), including the Chishui River mainstream and its eleven tributaries (in order from upstream to downstream: Zhaxi River, Daoliu River, Tongche River, Baisha River, Erdao River, Wuma River, Tongzi River, Gulin River, Tongmin River, Datong River and Xishui RiverR) of the Chishui River basin (27°20′–28°50′N; 104°45′–106°51′E) with a drainage area of 20,440 km2 (Fig. 1). The Chishui River originates from the Wumeng Mountains in Yunnan province, flows through Yunnan, Guizhou, and Sichuan provinces for nearly 436.5 km before converging into the upper Yangtze River in Hejiang County, Sichuan Province, southwest of China. These eleven second-order tributaries have lengths ranging from of 35 to 150 km. All of these streams are located in the eastern Yungui Plateau, Sichuan Basin or the transitional area between them. With a subtropical monsoon climate, the annually average rainfall of the Chishui River was about 1000 mm. The Chishui River contains lots of laterite soil, which could lead to extensive erosion; thus, it is the origin of the name “Chishui” (i.e., “red river” in Chinese). Karst landforms are mainly distributed in the upper and midstream of the river, and the river’s downstream areas belong to the Sichuan Basin (Jiang et al. 2011; Wu et al. 2011; Liu et al. 2020).
Sampling
Field surveys were conducted biannually in September–October 2019 and May–June 2020. Depending on sampling operability, habitat types, and streams’ morphology, fish at different water layers were sampled from main stream and eleven tributaries, which makes a total of 40 sampling sites along the Chishui River (Mainstream = 18, Tributaries = 22). Owing to the high heterogeneity of the river habitat, multiple sampling techniques were adopted during surveys. On each sampling occasion, fish specimens were caught with the assistance of professional fishermen by using a backpack electrofishing gear (CWB-2000 P, China; 12 V import, 250 V export) for 1 h, 10 stationary gillnets (8 m long × 1.2 m high, 5 cm mesh size), and 5 shrimp pots for 12 h. According to authoritative monographs (Wu 1989; Ding 1994; Chen 1998), fish were identified to the species level, and then measured length (to the nearest 1 mm) and weight (to the neatest 0.1 g) before releasing from the sites, while individuals that could not be identified immediately were preserved in 10% formalin for further taxonomic determination.
Eleven (11) physicochemical water quality parameters were analyzed following the standard methods (APHA 2005). conductivity (Cond, μS/cm), dissolved oxygen (DO, mg/L), oxidation–reduction potential (ORP, mV), water temperature (WT, °C), and pH that were measured in situ by portable multi-parameter water quality meter (YSI Professional Plus, USA). For other parameters, water samples at 0.5 m below water surface were collected from each sampling site. 2-L water samples were collected with a 5-L plexiglass sampler at each site and preserved in 1-L polyethylene bottles. The samples were kept in a cooler with ice and then transported to the laboratory on the same day by a designated person for further analysis (within 48 h) to determine total nitrogen (TN, mg/L), total phosphorus (TP, mg/L), soluble reactive phosphorus (SRP, mg/L), total dissolved nitrogen (TDN, mg/L), total dissolved phosphorus (TDP, mg/L), and chlorophyll a (Chl-a, mg/L) according to the Water and waste water monitoring and analysis method (fourth edition). Laboratory analytic quality assurance and quality control including blanks, replicates, calibration blank, and standards were performed according to the standard protocol (APHA 2005)”.
Land use data were used to reflect habitat quality, including 4 major types (farmland, forested land, shrub and grassland, construction area), were extracted for each sampling site with ArcGIS software (v.10.6.1) based on 30 m-resolution satellite image databases, and the percentages of each land use type were then calculated. The whole area with 40 km2 (2000 m length * 500 m width * 40 sampling sites) were extracted for the description of the Chishui River basin, and the mean distance to the rivers was 250 m.
Construction of IBI
Reference sites and impaired sites
Fifteen parameters pertaining to water quality and land use were aggregated to calculate the comprehensive disturbance index (CDI) (Table S4). Our starting point of developing CDI is to divide reference and impaired sites, to better understand how IBI responds in varying levels of comprehensive disturbance. To evaluate the degenerated level of all sites, as many parameters as possible were set to refer to existing standards (Kocer and Sevgili 2014; Pesce and Wunderlin 2000; Wu et al. 2018) and the other parameters were finally categorized into four groups by considering the 25th, 50th, and 75th percentiles (Chen et al. 2020; Li and Zeng 2020). Therefore, the data for all these parameters were classified into four grades as follows: (4) excellent, (3) good, (2) fair, and (1) poor (Table S1). Then, the sum of these scores were used as the final CDI value to reflect anthropogenic interference and distinguish reference sites and impaired sites with 75th percentiles since it was difficult to find an ideal nondisturbed site in the extensive landscape (Stoddard et al. 2006).
Metric development and data treatment
To determine the spatial patterns of fish assemblages in the Chishui River basin, species abundance data were used in the following analysis (Wang et al. 2018): Analysis of similarity (ANOSIM) was carried out to determine the differences between different site-groups. Then, similarity of percentage analysis (SIMPER) was used to identify species that were principally responsible for similarities within site-groups and those that contributed to 60% of the total average similarity considered as typifying species (Liu et al. 2020). Moreover, cluster analysis (a group average hierarchical sorting strategy) and nonmetric multidimensional scaling (NMDS) ordination analysis were used to determine the Spatial patterns of fish assemblages in the Chishui River basin (Liu et al. 2020; Wang et al. 2018; Oksanen et al. 2019). All these steps were performed with the PRIMER 5 software package (Clarke and Warwick 1994).
The W is a standard statistic of ABC method and based on the relative position of the two K-dominance curves for biomass and abundance, respectively (Lambshead et al. 1983; Clarke et al. 2014). For mild-disturbed communities, the biomass curves above the numbers curve throughout its entire length (W > 0). At moderate disturbance, the biomass and number curves are close and may intersect. As disturbance increases, the abundance curve is above the biomass curve throughout its length (W < 0). W values were calculated in the PRIMER 5 software package, formula 1. In addition, to calculate functional diversity indices, fish database included maximum length, growth rate, life span, age at first maturity, length at first maturity, trophic level, habitat type, food consumption, and body type were collected from our surveys and supplemented through https://fishbase.mnhn.fr/search.php and authoritative monographs (Wu 1989; Ding 1994; Chen 1998). Phylogenetic diversity indices were calculated using fish species presence-absence database involving five different levels of the taxonomic hierarchy: class, order, family, genus, and species. All the three dimensions of alpha diversity indices were calculated in R (v. 4.0.3, vegan package) (Niu et al. 2015; Xia 2021).
where W is the standardized sum of the differences between each pair of species cumulative biomass Bi and cumulative abundance Ai value ranked in decreasing order.
With reference to the previous studies (Chen et al. 2020; Whittier et al. 2007; Yu et al. 2018), a dataset of candidate metrics was first composed of seven categories: species composition, habitat guilds, trophic guilds, reproductive guilds, tolerance guilds, health and size, and comprehensive indices. Four size-based indicators (mean length, mean weight, maximum length and maximum weight in the community) were used to reflect size of species in the community. Dominance was calculated using the Index of relative importance (IRI%) (Pinkas et al. 1971) (formula 2). Then, a list of 97 candidate metrics (included one rarely ABC curve, eight comprehensive indices, and eighty-eight functional metrics) were obtained. To reflect the different aspects of the metrics, four types of descriptors, including numerical variables based on the number of species, proportional variables for individual number and total biomass, and IRI% for dominance, were considered (Table S2). Then, calculate the candidate parameter values for each sampling site (Pinkas et al. 1971).
where Wi% is the biomass percentage of the ith Functional group species in the catch, Ni% is the individual number percentage of the ith Functional group species in the catch, and Fi% is the occurrence rate in the community.
Metric screening
All the candidate metrics were screened with subsequent analyses (Fig. 2). First, metrics with a narrow range (percentage metrics with the coefficient of variation CV < 10%, richness metrics with a range < 5 and metrics with > 70% of values equal to zero) were excluded from the IBI system because they would decrease the separating capacity (Whittier et al. 2007; Yu et al. 2018; Cai et al. 2020). Prior to the subsequent analysis, all metrics were corrected for normality and heteroscedasticity using log (x + 1) for count variables and the arcsine square root transformation for all proportional variables except the comprehensive indices except the comprehensive indices (Chen et al. 2020). Second, to prevent the sensitivity of each metric to distinguish anthropogenic interference, metric values were calculated using a t-test when the normality assumption was met, while the Wilcoxon rank sum test was used for nonnormally distributed data (Stojkovic et al. 2014). Metrics that significantly differed (p < 0.05) between reference sites and impaired sites were retained for subsequent analyses. Finally, Pearson correlation analyses were used to test redundancy among the remaining metrics. When a pair of metrics showed strong redundancy (R > 0.80, R < − 0.80) were deleted (Xue et al. 2019).
All R version 4.0.3 (R Core Team), Excel version 2019 and SPSS version 22 were used in these statistical analyses, and the level of significance was set at 0.05.
Metric scoring and validation
To avoid the subjectivity of discrete scoring, all retained metrics were scored on a continuous scale that ranged from 0 to 100 (Wang et al. 2018; Lin et al. 2014). For positive metrics, which increase with an increase in the CDI, calculated as formula 3 (ceiling scores were valued as the 95th quantile for all sites, and floor scores were valued as the 5th quantile for reference sites). For negative metrics, which decrease with an increase in the CDI, calculated as formula 4 (ceiling scores were valued as the 95th quantile for reference sites, and floor scores were valued as the 5th quantile for all sites) (Stoddard et al. 2008). Then, scores for each metric were summed to obtain IBI scores, which were divided into five grades (excellent, good, fair, poor, and badly) based on the 95th, 75th, 50th, and 25th percentages of the highest value.
The final IBI scores and CDI scores, represented the fish community and physical habitat respectively, were used to validate the rationality of the IBI systems. To validate the performance of the IBI systems, general linear regression models and box-plots considering the IBI scores as a predictor variable and the CDI scores as response variables.
Results
Fish composition and structure
A total of 125 fish species belonging to 7 orders and 17 families were collected across the 40 sites during the sampling period (Table S3). Among them, 32 species were endemic to the upper Yangtze River, accounting for 25.60% of the total species.
According to the spatial patterns of fish assemblages, all the 40 sites could be divided into two site-groups, corresponding to group 1: wadeable streams (S1–S5, S19–S36, S39, S40) and group 2: nonwadeable rivers (S6–S18, S37, S38), which are shown by Fig. 3 and Table S5 based on cluster and ordination analysis. ANOSIM (p < 0.01) and the stress value of NMDS was 0.13 (between 0.1 and 0.2) further confirmed the significant differences between two site-groups (Wang 2018).
Comprehensive disturbance
The CDI values showed that twenty-one of the sites were divided into reference sites because their higher CDI values and indicating minimal levels of disturbance. Nineteen were judged to be impaired, with significant anthropogenic stressors (Fig. 4; Table S4). Among all sampling sites, five sampling sites S1 (Chishuiyuan Town), S30 (Gaoqiao Town), S31 (Guancang Town), S37 (Changsha Town), and S39 (Shibao Town) were rated as the highest-disturbed conditions because of their lowest CDI values, 31, 33, 30, 32, and 33, respectively, while S14 (Hushi Town), S17 (Mixi Town), S25,(Malu Town), and S36 (Baoyuan Town) had better condition with CDI values more than 46. Among all tributaries, Tongzi River and Xishui River had the lowest average CDI values of 35 and 36, respectively.
IBI systems development
Independent IBI system was constructed for each of the group respectively since their unique fish assemblages. In group 1, according to the screening procedures shown in Fig. 2, thirty-four metrics were initially rejected from the range test due to their small ranges, following which forty-six metrics were excluded from the sensitivity test since they showed weak capability to distinguish between reference sites and impaired sites of human perturbation. In the redundancy testing step, five metrics were removed since they showed high correlation with other metrics (Table S2). Twelve metrics, including percent of biomass of Cyprinidae species, number of Cobitidae species, percent of biomass of the endemic fishes of the Yangtze River, IRI% of herbivorous species, number of adhesive egg species, percent of individuals of sensitive species, mean length in community, Shannon-Weiner index, W value of the ABC curve, functional richness, functional evenness, and taxonomic distinctness indices, were finally used to develop the IBI-G1 (Table 1). Correspondingly, metrics in group 2 were performed the same IBI development steps as described above (Fig. 2), corresponding to IBI-G2. The final result showed that fourteen metrics were used to develop IBI-G2 (Table 2), and to calculate the IBI scores.
Scores, validation, and ecological health
For sites in group 1, the IBI scores ranged from 0 to 83.57, with a mean of 68.08 and a standard deviation of 19.65. IBI scores of sites in group 2 ranged from 60.29 to 89.28, with a mean of 78.79 (Table S5). Validation procedure: Box-plot and T-test showed that the IBI scores could efficiently distinguish the disturbance levels (reference sites and impaired sites) for both the group 1 and group 2 (p < 0.05) (Fig. 5), with the reference sites having the higher IBI scores and the impaired sites having the lower scores. Moreover, regression analysis revealed that the IBI scores was significantly positively correlated with the CDI scores for both group 1(R2 = 0.465, p = 0.003) and group 2 (R2 = 0.688, p < 0.001), although group 1 was affected by an outlier (IBI score of S1 is 0) (Fig. 6). The consistent results from these two analyses validated the expected performance of the IBI-G1 and IBI-G2 in assessing the ecological health of the Chishui River basin.
Relationship between the IBI (index of biotic integrity)) scores and CDI (comprehensive disturbance index) scores. Points in red color are the group 1 (wadeable streams) data, and points in green color are the group 2 (nonwadeable rivers) data. Lines in the corresponding colors are the linear fitting lines for them
The final IBI scores of 40 sites ranged from 0 to 89.28, with the mean ± SD of 72.09 ± 16.85, and were divided into five categories, excellent: 84.82 to 100, good: 71.42 to 84.81, fair: 53.57 to 71.41, poor: 22.32 to 53.56, and badly: 0 to 22.31. The mean score more than 71.41, which revealed that the ecological health of the Chishui River basin was classified as good: 3 sites were excellent, 27 sites were good, 5 sites were fair, 4 sites were poor, and 1 site was badly (Fig. 7). Among them, the IBI score of S1 was 0 as no fish were caught during sampling, while S8, S14, and S17 were classified as having excellent status with high IBI scores. Besides, the health conditions (mean IBI scores) for the Chishui River mainstream and its eleven tributaries were Xishui River: 71.25 (fair), Baisha River and Tongzi River: 49.62 and 49.04, respectively (poor) and the other rivers: 73.15–83.17(good) (Table S5).
Health status of the 40 sites in the Chishui River basin. Sites with black border are the group 2 (nonwadeable rivers); the other sites are the group 1 (wadeable streams). The corresponding data can be found in table S5
Discussion
Performance of our IBI system
A more refined biotic assessment system is required for effective protection of freshwater fish resources (Karr 1981). Principal characteristics of our IBI system are that it (1) divides reference sites and impaired sites by using water quality parameters and land use; (2) classifies this basin into two aggregate ecoregions based on their distinct fish assemblages, and independent IBI system is developed for each; (3) synthesizes the cumulative metrics of a wide variety of environmental disturbances that match aggregate ecoregion; (4) is verified by CDI; and (5) measures end-response values of biological health.
Scientific division of aggregate ecoregions is the critical first step for a reasonable IBI system. To date, there are no unified rules and definitions for “headstreams” around the world since different definitions of “headstreams” are set for different research levels (Flotemersch et al. 2014; Chen et al. 2020). Conventionally, some scholars hold that the second-order tributaries and the headwater of primary-order tributary are considered “headstreams” (Meyer et al. 2007; Moore and Richardson 2003). Parallelly, headwater (S1–S5) and the most sites in 11 tributaries (S19–S36, S39, S40) were considered “wadeable streams,” while the other sites were classified as “nonwadeable rivers” (corresponding to the groups 1 and 2, respectively). Compared with nonwadeable rivers, the hydrologic changes more intense, the habitat is simple, the nutrition is poor, and the spatial variation of ecosystem is more obvious in wadeable streams, which determine that they are two significantly distinct aggregate ecoregions (Flotemersch et al. 2014). Different fish assemblages have unique classification status, function, and life habits (Lasne et al. 2007). The structure of fish assemblages changed obviously between wadeable streams and nonwadeable rivers in the Chishui River basin. Indeed, such changes in fish assemblages are largely determined by the variations of geomorphic structure along the Chishui River basin (Wang et al. 2007). For the group 1 “wadeable streams,” most sites lie in the Yungui Plateau characterized by high mountains, rocky bottoms, deep valleys, and scarce vegetative cover. Fish assemblages that fit rapids and cold water are the dominant species in this group, such as Onychostoma sima, Schizothorax graham, Acrossocheilus yunnanensis and Sinogastromyzon sichangensis. However, most sites of the Chishui River mainstream, except for headwater, were contained in group 2 “nonwadeable rivers.” This aggregate ecoregion is situated on both the Sichuan Basin and transitional area between the Yungui Plateau and the Sichuan Basin, with a relatively gentle slope, reduced water flow and lush vegetative cover. Fish assemblages, in this group, show the characteristics of complexity, with not only some typical rapids-loving fishes, such as Onychostoma sima and Spinibarbus sinensis, but also a large number of nonrheophilic fishes are found, such as Pelteobagrus nitidus, Pelteobagrus vachelli, and Leiocassis crassilabris. As expected, our results also show that the metrics of final IBI systems were closely related to different aggregate ecoregions, such as metrics of rheophilic and sensitive species were used in IBI-G1 while metrics of nonrheophilic and omnivorous species were used in IBI-G2. We found that IBI systems performed well in discriminating among the anthropogenic pressure classes when they make adjustments to the corresponding fish assemblages. In short, each aggregate ecoregion has unique fish assemblages and physical environment. Therefore, we strongly recommend that fish assemblages should be considered fully to divide different aggregate ecoregions before developing a IBI system, and then developing appropriate IBI systems for them. And we have reasons to think that it is unreasonable to develop only one IBI system for Chishui River basin.
Without any doubt, division of reference and impaired sites is another key step for a reasonable IBI system. Complementarily, previous research has demonstrated that it is not appropriate to treat the single sampling sites in the headstreams as the reference sites for a river for the reason that the changes of aquatic communities along rivers are not entirely caused by disturbance (Morley and Karr 2002; Wang et al. 2018). In a survey of the recent research reports, dividing reference and impaired sites based on the levels of comprehensive disturbance, including water quality and human activities, has become a popular research method (Chen et al. 2020; Li and Zeng 2020; Xue et al. 2019). In our research, correspondingly, high-resolution land-cover classification were used to test human activities (Hill et al. 2000) and typical water quality parameters were used to reflect the degrees of pollution (Qu et al. 2020). Given increasing urbanization, ecological habitats have been altered and compressed in numerous ways and eventually cause nondisturbed sites to become less and less (Olguin et al. 2000). Therefore, we classify least-disturbed sites as reference sites, while impaired sites mean high-disturbed conditions. In short, the Chishui River basin have an ideal state since reference sites are distributed in both the two aggregate ecoregions and most of the reference sites were primarily in forested watersheds with low human activities.
Candidate metrics that obtained directly from sampling (some traditional metrics) and that requested subsequent mathematical calculations, such as three dimensions of alpha diversity indices and ABC curve were included in our IBI systems. It is worth mentioning that metrics based on size and dominance had distinct responsiveness to human disturbance, but few appeared in previous studies. Differing from the traditional candidate metrics systems, numerical variables, IRI% for each functional group, proportional variables, and total biomass of each metric were included to reflect the different functional guilds of them in our IBI systems. And four metrics related to size, such as MEL (mean length in community), MEW (mean weight in community), MAL (maximum length in community), and MAW (maximum weight in community) were included, which was considered to be more comprehensive and reasonable (Chen et al. 2020). According to historical records (Wu 1989; Ding 1994; Chen 1998), PERCIFORMES and SILURIFORMES had so scant families in the Chishui River that they were directly used as metrics, while Cyprinidae, Cobitidae, and Homalopteridae of CYPRINIFORMES were used as metrics respectively because of their rich species (Liu et al. 2020; Wu et al. 2011; Yu et al. 2018). However, three metrics related to PERCIFORMES (NPES, PPEI and PPEB) did not appear in the final IBI systems since only seven PERCIFORMES species in the Chishui River basin and were excluded in the range test. And the results obtained by using these metrics are difficult to translate into values meaningful to the general public. Fortunately, some comprehensive indices performed well and were used in the final IBI systems. Shannon-Weiner index, functional richness, functional evenness, and taxonomic distinctness exhibited low inter-correlation that were used in our IBI systems and can be explained by their complementarity (Gascon et al. 2009). Among biodiversity metrics, variation in taxonomic distinctness was least correlated with all others, suggesting this metric provides different information on ecosystem conditions, which was consistent with previous studies (Gallardo et al. 2011). As an uncommonly used metric, W value of ABC curve was considered potential indicators and decreased basically with human disturbances in our study: the mean W value of reference sites and impaired sites were 0.084 and 0.011, respectively. Moreover, 80.96% of the reference sites were subject to low human disturbances (sites with W > 0) and only 19.04% of them suffered from high human disturbances (sites with W < 0), while 42.11% and 57.89% of the impaired sites were subject to low and high human disturbances respectively. However, some W values did not show a significant positively associated with the CDI values, such as S15 (Fuxing Town), S17 (Mixi Town), and S18 (Hejiang County) had high CDI values (> 40) and low W values (< 0). Fabian et al. thought that the size of dominant species in a community has an important influence on the ABC curve (Fabian et al. 2004). As dominant species of S15, S17, and S18, Pelteobagrus nitidus, Leiocassis crassilabris, and Hemibarbus labeo were small size fish and would cut down W values here, which also supports this explanation.
Ecological status of the Chishui River basin
Unlike most rivers in China, as a recognized ecological river basin, the Chishui River basin was generally classified as good ecological status with the mean IBI score of 72.09 based on our results. Previous researches had shown that sites with relatively low human population density and lush forest were healthy generally, while sites with a poor status were densely populated with a high degree of cluster, and certain human activities around which had serious impact on them (Li and Zeng 2020; Shen et al. 2006). In our study, similarly, we found that sites with “excellent” and “good” status were all distributed in sparsely populated areas with a good vegetation coverage, and few industrial and agricultural distribution, such as S4 (Shuitian Town), S8 (Qingchi Town), S14 (Hushi Town), and S25 (Malu Town). It might be attributed to little anthropogeni c impact on these sites (Li and Zeng 2020). On the contrary, sites with a “poor” and “badly” status differed primarily in extent of urbanization, industrial and agricultural distribution, such as S24(Jianzhu Town), S30(Gaoqiao Town), S31(Guancang Town) and S37(Changsha Town).
Mainstream had an average IBI score of 76.28, which means that it is healthy. Unfortunately, some problems still existed in several sites (S1, S9, and S12) and were reflected by their lower IBI score (0, 75.44, and 73.79, respectively). S1 (Chishiuiyuan Town) had small runoff and was seriously polluted by local domestic sewage. Based on our investigation, S1 has high values of total nitrogen (TN, 3.08 mg/L), total phosphorus (TP, 0.13 mg/L), conductivity (Cond, 468 μS/cm), and the lowest value of dissolved oxygen (DO, 6.54 mg/L). The smelly and polluted water body of S1 had leaded to not any fish here. We found that excessive domestic sewage of Chishiuiyuan Town had injected into the river channel and destroyed the ecological balance. Now, the local government has taken measures to remedy, for example, prohibiting the discharge of domestic sewage into river channel and the construction of sewage treatment plants proximity to the stream channel. Moreover, although S9 (Maotai Town) and S12 (Taiping Town) were classified as good ecological status with marginal IBI scores of 75.44 and 73.79, respectively (IBI score of good status: 71.42 to 84.81), suffered from frequent industries. Maybe it will become “fair” status in the near future without any management measures. As we all know, Maotai Town was famous for its white spirit all over the world (Wu et al. 2011). In recent years, growing wineries pollution and excessive construction activities had enveloped Maotai Town and further damaged the river health, which is in accordance with a recent assessment of this area by Wu (Wu et al. 2011). While commercial shipping and industrial and mining enterprises of Taiping Town also had an impact on river health. The above are the hidden problems that currently exist in the main stream of the Chishui River basin.
Of eleven tributaries of the Chishui River basin, streams with a “good” condition accounted for 72.72%, while streams with a “fair” and “poor” status accounted for only 9.09% and 18.18% respectively, and no streams were rated “badly.” Among them, the Datong River, Erdao River and Daoliu River are healthier than the others with the highest IBI scores, mainly due to large areas of forest, undeveloped industry and agriculture and less anthropogenic impact (Li and Zeng 2020). Regrettably, Tongzi River, BaiSha River, and Xishui River had poor ecological status, with the average IBI scores of 49.04, 49.62, and 71.25 respectively. As the largest tributary of the Chishui River, several upstream sites of Tongzi River, such as S30 (Huoshigang Town) and S31 (Guancang Town) were classified as poor status, with IBI scores of 43.00 and 42.4, respectively. Tongzi River was characterized by high dams and large reservoirs, such as Yangjiayuan Dam and Yuanmanguan Dam. The dominant species of S30 and S31 were Zacco platypus, Hemiculter leucisculus and Misgurnus anguillicaudatus, which are considered as nonrheophilic and pollution-tolerant species (Chen et al. 2020). Moreover, S30 and S31 suffered by both industrial sewage (coal industries) and domestic sewage. S24 (Jianzhu Town) of Baisha River suffer severely from two invasive fishes, Rhynchocypris oxycephalus and Barbatula toni (Tang et al. 2021). These two invasive fishes account for 92.67% of total quantity and 69.14% of total biomass of S24, which prove their absolute dominance. Similarly, Hall argued that increased dominance of small, fast-growing species, and general reductions in species diversity are symbols of unhealthy ecosystem (Hall 1999). Besides, S37 (Changsha Town) and S39 (Shibao Town) were affected by constructions of the dams and there are at least 15 hydropower stations that have been built on the Xishui River (Liu et al. 2020). It has proved that these cascade dams not only blocked the migration routes of fishes and reduced the heterogeneity of the habitat but also can cause frequent and irregular fluctuations in water level, habitat size, and food resources (Liu et al. 2019). More seriously, the reach of Xishui River below the Gaodong Dam was often dehydrated. It is worth mentioning that Tongmin River and Gulin River are suffering from different kinds of human disturbance, in spite of their good condition currently. To the best of our knowledge, growing construction activities are emerging in Tongmin River, while domestic sewages are gradually polluting the river channel of Gulin River. Therefore, to deal with these problems, effective measures need to be implemented immediately.
Conclusions
The modification and improvement of biomonitoring tools has been an ongoing issue; comprehensive and effective methods are needed. In this study, we try to integrate comprehensive indices and spatial patterns of fish assemblages to develop IBI systems and then verified their effectiveness and accuracy for assessing the environmental health of the Chishui River basin. The comprehensive disturbance index (CDI), based on 11 water quality parameters and 4 human land use, was set to distinguish reference sites and impaired sites. According to the spatial patterns of fish assemblages, the 40 sites were finally divided into 2 aggregate ecoregions, including wadeable streams and nonwadeable rivers. 97 candidate metrics were selected to develop our IBI systems based on the systematic screening method. The result also showed that our IBI systems performed well in discriminating anthropogenic disturbances at both aggregate ecoregions, which suggests that our systems could provide a reliable evaluation. The basin was given a mean IBI score of 72.09 ± 16.58 and classified under the status “good.” However, S1 (Chishuiyuan Town), Baisha River, Tongzi River, and Xishui River were disturbed by various human activities. We conclude that the spatial patterns of fish assemblages should be combined with more comprehensive indices to assess river health. On the other hand, we do believe that the process of developing and verifying our IBI systems presented herein will be helpful for ecosystem assessment not only in this region but also in other mountain river systems with similar characteristics related to human disturbance.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
APHA (2005) Standard methods for the examination of water and wastewater 21st edition American Public Health Association, American Water Works Association, Water Environment Federation, Washington, DC, USA,https://doi.org/10.2105/AJPH.56.4.684-a
Aura CM, Kimani E, Musa S, Kundu R, Njiru JM (2017) Spatio-temporal macroinvertebrate multi-index of biotic integrity (MMiBI) for a coastal river basin: a case study of River Tana, Kenya. Ecohydrol Hydrobiol 17:13–124. https://doi.org/10.1016/j.ecohyd.2016.10.001
Bianchi G, Hamukuaya H, Alvheim O (2001) On the dynamics of demersal fish assemblages off Namibia in the 1990s. S Afr J Mar Sci 23:419–428. https://doi.org/10.2989/025776101784528737
Boltzmann L (1970) Weitere studien uber das warmegleichgewicht unter gasmolekalen. Vieweg+Teubner Verlag, 66: 275–370. https://doi.org/10.1007/978-3-322-84986-1_3
Cai K, Qin CY, Li JY, Zhang Y, Niu ZC, Li XW (2012) Preliminary study on phytoplanktonic index of biotic integrity (P-IBI) assessment for lake ecosystem health: a case of Taihu Lake in Winter. Acta Ecol Sin 36(5):1431–1441. https://doi.org/10.5846/stxb201407211483
Cai W, Xia J, Yang M et al (2020) Cross-basin analysis of freshwater ecosystem health based on a zooplankton-based index of biotic integrity: models and application - ScienceDirect. Ecol Indic 114:106333. https://doi.org/10.1016/j.ecolind.2020.106333
Chen YY (1998) Fauna sinica, rganization, cypriniformes II. Science Press, Beijing, China
Chen K, Jia Y, Xiong X et al (2020) Integration of taxonomic distinctness indices into the assessment of headwater streams with a high altitude gradient and low species richness along the upper Han River, China. Ecol Indic 112:106106. https://doi.org/10.1016/j.ecolind.2020.106106
Clarke KR, Gorley R, Sommerfield PJ (2014) Warwick, R.M. Change in marine communities: an approach to statistical analysis and interpretation, 3rd edn. Plymouth, UK, PRIMERE
Clarke KR, Warwick RM (1994) Change in marine communities: an approach to statistical analysis and interpretation. Plymouth Marine Laboratory, Plymouth, UK, 144.
Coeck J, Vandelannoote A, Yseboodt R, Verheyen RF (1993) Use of the abundance/biomass method for comparison of fish communities in regulated and unregulated lowland rivers in Belgium. Regul Rivers: Res Manage 8:73–82. https://doi.org/10.1002/rrr.3450080111
Ding RH (1994) The fishes of Sichuan. Sichuan Publishing House of Science and Technology, Chengdu, China
Fabian B, Leloc’ h F, Hily C et al (2004) Fishing effects on diversity, size and community structure of the benthic invertebrate and fish megafauna on the Bay of Biscay coast of France. Mar Ecol Prog 280:245–251. https://doi.org/10.1023/A:1004105820586
Ferreira FC, Petrere MJ (2009) The fish zonation of the Itanhaém river basin in the Atlantic Forest of southeast Brazil. Hydrobiologia 636:11–34. https://doi.org/10.1007/s10750-009-9932-4
Flotemersch JE, North S, Blocksom KA (2014) Evaluation of an alternate method for sampling benthic macroinvertebrates in low-gradient streams sampled as part of the National Rivers and Streams Assessment. Environ Monit Assess 186(2):949–959. https://doi.org/10.1007/s10661-013-3426-6
Gallardo B, Gascon S, Quintana X et al (2011) How to choose a biodiversity indicator — redundancy and complementarity of biodiversity metrics in a freshwater ecosystem. Ecol Ind 11(5):1177–1184. https://doi.org/10.1016/j.ecolind.2010.12.019
Gascon S, Boix D, Sala J (2009) Are different biodiversity metrics related to the same factors? A case study from Mediterranean wetlands. Biol Cons 142:2602–2612. https://doi.org/10.1016/j.biocon.2009.06.008
Gomes-Silva G, Cyubahiro E, Wronski T, Riesch R, Apio A, Plath M (2020) Water pollution affects fish community structure and alters evolutionary trajectories of invasive guppies (Poecilia reticulata). Sci Total Environ 730:138912. https://doi.org/10.1016/j.scitotenv.2020.138912
Hall SJ (1999) The effects of fishing on marine ecosystems and communities. Blackwell Science, Oxford.
Hill KE, Botsford E, Booth DB (2000) A rapid land cover classification method for use in urban watershed analysis. Washington Water Resour 11:7–9
Jiang XM, Xiong J, Xie ZC, Chen YF (2011) Longitudinal patterns of macroinvertebrate functional feeding groups in a Chinese river system: a test for river continuum concept (RCC). Quatern Int 244:289–295. https://doi.org/10.1016/j.quaint.2010.08.015
Karr JR (1981) Assessment of biotic integrity using fish communities. Fisheries 6:21–27. https://doi.org/10.1577/1548-8446(1981)006%3c0021:AOBIUF%3e2.0.CO;2
Kocer MAT, Sevgili H (2014) Parameters selection for water quality index in the assessment of the environmental impacts of land-based trout farms. Ecol Ind 36:672–681. https://doi.org/10.1016/j.ecolind.2013.09.034
Lambshead PJD, Platt HM, Shaw KM (1983) The detection of differences among assemblages of marine benthic species based on an assessment of dominance and diversity. J Nat Hist 17:859–874. https://doi.org/10.1080/00222938300770671
Lasne E, Bergerot B, Lek S, Laffaille P (2007) Fish zonation and indicator species for the evaluation of the ecological status of rivers: Example of the Loire basin (France). River Res Appl 23:1–14. https://doi.org/10.1002/rra.1030
Li Z, Zeng B (2020) Health assessment of important tributaries of Three Georges Reservoir based on the benthic index of biotic integrity. Sci Rep 10(1):18743. https://doi.org/10.1038/s41598-020-75746-7
Li Y, Gao L, Niu L et al (2021) Developing a statistical-weighted index of biotic integrity for large-river ecological evaluations. J Environ Manage 277:111382. https://doi.org/10.1016/j.jenvman.2020.111382
Lin PC, Liu F, Gao X et al (2014) Development of a fish index of biotic integrity (F-IBI) and its application to the Chishui River. Freshw Fish 44(6):7. https://doi.org/10.3969/j.issn.1000-6907.2014.06.014
Liu F, Wang J, Zhang FB et al (2020) Spatial organization of fish assemblages in the Chishui River, the last free-flowing tributary of the upper Yangtze River. China Ecol Freshw Fish 30(1):1–13. https://doi.org/10.1111/eff.12562
Liu F, Yang GH, Liu DM, Li L, Wang X, Zhang Z, Liu HZ (2019) Current situation and conservation strategies of fish resources in the Xishui River. Freshw Fish 49, 36–43. CNKI:SUN:DSYY.0.2019–05–006
Maceda-Veiga A, Green AJ, De Sostoa A (2014) Scaled body-mass index shows how habitat quality influences the condition of four fish taxa in north-eastern Spain and provides a novel indicator of ecosystem health. Freshw Biol 59:1145–1160. https://doi.org/10.1111/fwb.12336
Mason N, Mouillot D, Lee WG et al (2005) Functional richness, functional evenness and functional divergence: the primary components of functional diversity. Oikos 111(1):112–118. https://doi.org/10.1111/j.0030-1299.2005.13886.x
Matthews WJ (1998) Patterns in freshwater fish ecology. Chapman and Hall, London, UK. https://doi.org/10.1023/A:1008891703274
Mendonça FP, Magnusson WE, Zuanon J (2005) Relationships between habitat characteristics and fish assemblages in small streams of Central Amazonia. Copeia 4:751–764. https://doi.org/10.1643/00458511(2005)005[0751:RBHCAF]2.0.CO;2
Meyer JL, Strayer DL, Wallace JB et al (2007) The contribution of headwater streams to biodiversity in river networks. J Am Water Resour Assoc 43(1):86–103. https://doi.org/10.1111/j.1752-1688.2007.00008.x
Moore RD, Richardson JS (2003) Progress towards understanding the structure, function, and ecological significance of small stream channels and their riparian zones. Can J Forest Res-Revue Canadienne De Recherche Forestiere 33(8):1349–1351. https://doi.org/10.1139/x03-146
Morley SA, Karr JR (2002) Assessing and Restoring the Health of Urban Steams in the Puget Sound Basin. Conserv Biol 16(1):498–1509. https://doi.org/10.1046/j.1523-1739.2002.01067.x
Ngor PB, McCann KS, Grenouillet G, So N, McMeans BC, Fraser E, Lek S (2018) Evidence of indiscriminate fishing effects in one of the world’s largest inland fisheries. Sci Rep 8:1–12. https://doi.org/10.1038/s41598-018-27340-1
Niu L, Li Y, Wang P, Zhang W, Wang C, Wang Q (2015) Understanding the linkage between elevation and the activated-sludge bacterial community along a 3600 meters elevation gradient in China. Appl Environ Microbiol 19:6567–6576. https://doi.org/10.1128/AEM.01842-15
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Solymos P (2019) Vegan: community ecology package. R package version 2.5–6
Olguin HG, Salibian A, Puig A (2000) Comparative sensitivity of Scenedesmus acutus and Chlorella pyrenoidosa as sentinel organisms for aquatic ecotoxicity assessment: studies on a highly polluted urban river. Environ Toxicol 15:14–22. https://doi.org/10.1002/(SICI)1522-7278(2000)15:1%3c14::AID-TOX3%3e3.0.CO;2-6
Ormerod S, Dobson M, Hildrew A, Townsend C (2010) Multiple stressors in freshwater ecosystems. Freshw Biol 55:1–4. https://doi.org/10.1111/j.1365-2427.2009.02395.x
Pesce SF, Wunderlin DA (2000) Use of water quality indices to verify the impact of Cordoba City (Argentina) on Suquia River. Water Res 34(11):2915–2926. https://doi.org/10.1016/S0043-1354(00)00036-1
Pinkas L, Oliphant MS, Iverson ILK (1971) Food habits of albacore, bluefin tuna, and bonito in California waters. Fish Bull 152:1–105
Qu X, Chen YS, Han L et al (2020) A holistic assessment of water quality condition and spatiotemporal patterns in impounded lakes along the eastern route of China’s South-to-North water diversion project. Water Res 2020(185):116275. https://doi.org/10.1016/j.watres.2020.116275
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Higher Education Press, China
Ruaro R, Gubiani ÉA (2013) A scientometric assessment of 30 years of the Index of Biotic Integrity in aquaticecosystems: aapplications and main flaws. Ecol Ind 29:105–110. https://doi.org/10.1016/j.ecolind.2012.12.016
Saito VS, Siqueira T, Alaíde Ap, Fonseca-Gessner (2015) Should phylogenetic and functional diversity metrics compose macroinvertebrate multimetric indices for stream biomonitoring? Hydrobiologia 745(1): 167-179.https://doi.org/10.1007/s10750-014-2102-3
Shannon CE (1948) A mathematical theory of communication. Bell Syst Tech J 27(379–423):623–656. https://doi.org/10.1002/j.1538-7305.1948.tb01338.x
Shelford VE (1911) Ecological succession I Stream fishes and the method of physiographic analysis. Biol Bull 21:9–35. https://doi.org/10.2307/1535983
Shen Z, Zhang Q, Yue C, Zhao J, Hu Z, Lv N, Tang Y (2006) The spatial pattern of land use/land cover in the water supplying area of the Middle Route of the South to North Water Diversion Project. Acta Geogr Sin 61:633–644. https://doi.org/10.1109/MILCOM.2006.302259
Shi L, Zhu H, Ye SW et al (2020) Fish assemblage and biotic integrity assessment in Shihoudian Lake. Chin J Ecol 39(8): 2646–2656. 1535–1459(2006)22:8<923:FAABIO>2.0.TX;2–5
Simpson EH (1949) Measurement of diversity. Nature 1949(163):688. https://doi.org/10.1136/thx.27.2.261
Stoddard JL, Larsen DP, Hawkins CP, Johnson RK, Norris RH (2006) Setting expectations for the ecological condition of streams: the concept of reference condition. Ecol Appl 16:1267–1276. https://doi.org/10.1890/1051-0761(2006)016[1267:SEFTEC]2.0.CO;2
Stoddard JL, Herlihy AT, Peck DV, Hughes RM, Whittier TR, Tarquinio E (2008) A process for creating multimetric indices for large-scale aquatic surveys. Freshw Sci 27:878–891. https://doi.org/10.1899/08-053.1
Stojkovic M, Milosevic D, Simic S, Simic V (2014) Using a fish-based model to assess the ecological status of lotic systems in Serbia. Water Resour Manag 28:4615–4629. https://doi.org/10.1007/s11269-014-0762-4
Sun BB, Yu CG, Liu H et al (2019) Spring and autumn shrimp and crab biodiversity in the east Nanji Islands. Biodivers Sci 27(7):787–795. https://doi.org/10.17520/biods.2019098
Tang R, Dai YX, Liu HZ et al (2021) Analysis of introduction source and ecological adaptability of exotic fishes Rhynchocypris oxycephalus and Barbatula toni in Chishui River. Chin J Zool 56(2):214–228. https://doi.org/10.13859/j.cjz.202102007
Vanna N, Sovan L, Ngor PB et al (2020) Fish community responses to human-induced stresses in the Lower Mekong Basin. Water 12(12):3522. https://doi.org/10.3390/w12123522
Vannote RL, Minshall GW, Cummins KW, Seddel JR, Cushing CE (1980) The river continuum concept. Can J Fish Aquat Sci 37:130–137. https://doi.org/10.1080/00288330.1982.9515966
Voss KA, Pohlman A, Viswanathan S, Gibson D, Purohit J (2012) A study of the effect of physical and chemical stressors on biological integrity within the San Diego hydrologic region. Environ Monit Assess 184:1603–1616. https://doi.org/10.1007/s10661-011-2064-0
Wang ZS, Jiang LG, Huang MJ, Zhang C, Yu XB (2007) Biodiversity status and its conservation strategy in the Chishui River basin. Recourse Environ Yangtze Basin 16:175–180. https://doi.org/10.1016/S1872-5791(07)60033-5
Wang Z (2018) Diversity and temporal-spatial patterns of ichthyoplankton in the mainstem of the upper Yangtze River. Dissertation Chin Acad Sci. http://dpaper.las.ac.cn/Dpaper/detail/detailNew?paperID=20148529&title=%E9%95%BF%E6%B1%9F%E4%B8%8A%E6%B8%B8%E5%B9%B2%E6%B5%81%E9%B1%BC%E7%B1%BB%E6%97%A9%E6%9C%9F%E8%B5%84%E6%BA%90%E5%A4%9A%E6%A0%B7%E6%80%A7%E4%B8%8E%E6%97%B6%E7%A9%BA%E6%A0%BC%E5%B1%80&author=%E7%8E%8B%E9%9C%87&highsearch=author_name%253A345345345%25E7%258E%258B%25E9%259C%2587345345345%2520%2520234234234234234234%2520(%2520specification_training_institution_str%253A%25E4%25B8%25AD%25E5%259B%25BD%25E7%25A7%2591%25E5%25AD%25A6%25E6%258A%2580%25E6%259C%25AF%25E5%25A4%25A7%25E5%25AD%25A6%2520123123123123123123%2520specification_training_institution_str%253A%25E4%25B8%25AD%25E5%259B%25BD%25E7%25A7%2591%25E5%25AD%25A6%25E9%2599%25A2%25E6%25B0%25B4%25E7%2594%259F%25E7%2594%259F%25E7%2589%25A9%25E7%25A0%2594%25E7%25A9%25B6%25E6%2589%2580%2520)&sortField=score%2520desc%252Cid&start=0&actionType=Browse&searchText=%E7%8E%8B%E9%9C%87
Wang J, Huang ZL, Li HY, et al (2018) Construction of macroinvertebrate integrity-based health assessment framework for the Chishui River. Environ Monit China 34(06): 69–79. CNKI:SUN:IAOB.0.2018–06–010
Warwick RM (1986) A new method for detecting pollution effects on marine macrobenthic communities. Mar Biol 92:557–562. https://doi.org/10.1007/BF00392515
Whittier TR, Hughes RM, Stoddard JL, Lomnicky GA, Peck DV, Herlihy AT (2007) A structured approach for developing indices of biotic integrity: three examples from streams and rivers in the western USA. Trans Am Fish Soc 136:718–735. https://doi.org/10.1577/T06-128.1
Wu L (1989) The fishes of Guizhou, China. Guiyang. China: Guizhou People’s Publishing House, Guangzhou.
Wu J, Wang J, He Y et al (2011) Fish assemblage structure in the Chishui River, a protected tributary of the Yangtze Rive. Knowl Manag Aquat Ecosyst 400:170–181. https://doi.org/10.1051/kmae/2011023
Wu ZS, Wang XL, Chen YW, Cai YJ, Deng JC (2018) Assessing river water quality using water quality index in Lake Taihu Basin, China. Sci Total Environ 612:914–922. https://doi.org/10.1016/j.scitotenv.2017.08.293
Xia ZJ (2021) Biodiversity pattern and metacommunity study of fish in the Chishui River. Dissertation, Chin Acad Sci. http://dpaper.las.ac.cn/Dpaper/detail/detailNew?paperID=20198635&title=%E8%B5%A4%E6%B0%B4%E6%B2%B3%E6%B5%81%E5%9F%9F%E9%B1%BC%E7%B1%BB%E5%A4%9A%E6%A0%B7%E6%80%A7%E6%A0%BC%E5%B1%80%E5%8F%8A%E9%9B%86%E5%90%88%E7%BE%A4%E8%90%BD%E7%A0%94%E7%A9%B6&author=%E5%A4%8F%E6%B2%BB%E4%BF%8A&highsearch=author_name%253A345345345%25E5%25A4%258F%25E6%25B2%25BB%25E4%25BF%258A345345345&sortField=score%2520desc%252Cid&start=0&actionType=Browse&searchText=%E5%A4%8F%E6%B2%BB%E4%BF%8A
Xue H, Zheng BH, Meng FS et al (2019) Assessment of aquatic ecosystem health of the Wutong River based on benthic diatoms. Water 11:727. https://doi.org/10.3390/w11040727
Yu FD, Gu DE, Hu YC (2018) The ecological health assessment of Nandujiang River, Hainan Island, based on species diversity and biological integrity of fish. Chin J Ecol 37(9):2717–2726. https://doi.org/10.13292/j.1000-4890.201809.016
Zhu H, Hu XD, Wu PP et al (2021) Development and testing of the phytoplankton biological integrity index (P-IBI) in dry and wet seasons for Lake Gehu. Ecol Ind 129(5):107882. https://doi.org/10.1016/j.ecolind.2021.107882
Acknowledgements
The authors thank constructive advice from Kang Chen, Xiao Qu, Zhengfei Li, and Jianghui Bao for improving the manuscript.
Funding
This research was supported by grants from the Biodiversity Survey and Assessment Project of the Ministry of Ecology and Environment, China (2019HJ2096001006), the Ministry of Agriculture and Rural Affairs of China (CJDC-2017), the China Three Gorges Corporation (0799574) and Sino BON-Inland Water Fish Diversity Observation Network.
Author information
Authors and Affiliations
Contributions
Fandong Yu, Fei Liu, and Jianwei Wang conceived and designed the investigation. Fandong Yu, Fei Liu, Zhijun Xia, Chunsen Xu, Rui Tang, Zujun Ai, and Youzhao Zhang performed field work. Fandong Yu, Zhijun Xia, and Jianwei Wang analyzed the data. Miaomiao Hou and Xinhua Zhou contributed materials and analysis tools. Fandong Yu and Jianwei Wang wrote the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All the procedures described in this study were approved by the ethics committee of the Institute of Hydrobiology, Chinese Academy of Sciences, Hubei Province, China (permit number Y216011101). After each survey, the fish were released from the sampling sites.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible editor: Boqiang Qin
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yu, F., Liu, F., Xia, Z. et al. Integration of ABC curve, three dimensions of alpha diversity indices, and spatial patterns of fish assemblages into the health assessment of the Chishui River basin, China. Environ Sci Pollut Res 29, 75057–75071 (2022). https://doi.org/10.1007/s11356-022-20648-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-20648-6