Abstract
Anthropogenic activities impacted the ecological health of rivers by altering the physical habitat and water flow as well as by pollution. Monitoring of biotic groups for gauging the river health is a prerequisite for assessing the extent of degradation and formulating management guidelines for river restoration. An assessment using fish-based index of biotic integrity (IBI) was carried out in the Central Indian river, Tapti, for probing its health status. For the multimetric index, twelve metrics were adopted under five categories: taxonomic richness, habitat composition, tolerance indicators, species resilience, and trophic composition. Among the studied sites, Betul in the upper stretch was selected as the reference site for River Tapti, which almost meets the upper expectation of the metrics explored. Continuous scoring method was applied to evaluate the biotic integrity in the selected sites of the river. The IBI score based on the pooled fish abundance data in River Tapti ranged from 33 to 60. Assessment of the ecological health revealed that three-fourth of the river stretch was moderately impaired (25–50% of impairment) and the most deteriorated site was Kamrej with 45% of impairment which might be due to its location in the urban area with high influx of domestic sewage and industrial effluents. The IBI scores were plotted and compared with an independent estimate of water quality. The CCA with environmental and IBI variables revealed higher correlation with each other and the functional groups such as carnivores, herbivores, and fishes with high population doubling time (PDT) were found in close association with nitrate-N, total alkalinity, and specific conductivity. The study urges the need for the adoption of proper management and mitigation measures to restore the health and wealth of aquatic ecosystem.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rivers have been the lifeline of human existence from the time immemorial. They play a prominent role starting from the birth of civilization to meet up the basic requirements of mankind by providing water supply for drinking, domestic, and industrial activities (Bock et al. 2018). Rivers are considered as an integral part in maintaining and proper functioning of ecosystem and ecological services (Brauman et al. 2014). Rather than a natural purifier and a cleanser, rivers are the treasure trove for the biological wealth and germplasm reserves (Usha 2014). India, being one of the agrarian economies, immensely depends on the river-based livelihood activities. Indian riverine system comprises of Ganga, Brahmaputra, Indus river basins, and also peninsular river systems (Jhingran 1991). The peninsular component consists of east coast rivers, viz., Mahanadi, Godavari, Krishna, and Cauvery and west coast rivers, viz., Narmada and Tapti (Ayyapan et al. 2011).
Rivers are among the most threatened ecosystems of the world (Sendzimir and Schmutz 2018) and there is an array of issues rivers face globally. Wastewater discharge from human activities negatively impacts aquatic ecosystems (Wen et al. 2017). Organic pollution in rivers stimulates microbial growth, which cause oxygen depletion and disrupt the entire river ecosystems (Sirota et al. 2013). The proliferation of river projects and the resultant river fragmentation alters the habitat structure, ecosystem functions, and processes, posing a great threat to biodiversity (Jumani et al. 2022). The cumulative effects of industrialization and urbanization together with climate change and anthropogenic perturbations have damaged the very sensitive riverine systems immensely (Banerjee and Gupta 2013; Jain and Singh 2020). It is a key fact that the anthropogenic pressures and drivers play a paramount role in determining the physical and chemical quality of rivers (Hughes and Noss 1992) and thereby affects the aquatic biodiversity.
In order to assess and quantify impacts of environmental degradation, monitoring programs for river ecosystems have existed for many decades which initially included water quality indicators exclusively and later on included biological indicators. The concept of fish as a bioindicator to identify and monitor the ecosystem health status is widely recognized (Karr 1991), as they reflect integrated perception about their environment over longer periods of time (Chovanec et al. 2003). The presence or absence, abundance, diversity, and distribution status of various species can provide a screen shot of the habitat quality of a particular ecosystem (Lawton and Gaston 2001). Among the different approaches of using fish as ecological indicators, index of biotic integrity (IBI) is extensively used for assessing the aquatic ecosystem health by multiple metrics representing quantifiable attributes of fish communities (Sendzimir and Schmutz 2018). Biotic integrity is defined as “the capability of supporting and maintaining a balanced, integrated, adaptive community of organisms having species composition, diversity and functional organization comparable to that of the natural habitat of the region” (Karr and Dudley 1981). The IBI based on multimetric approach, originally developed by Karr (1981), measures how far an ecosystem has moved from the pristine condition and it comprises various metrics associated with biological attributes, i.e., taxa richness and composition, trophic structure, niche, and others. Each of the multiple metrics incorporated in the IBI can predict specific impacts caused by ecological alterations and by combining them within the IBI framework can reflect and quantify the degree of anthropogenic interferences on the ecosystem (Barbour et al. 1996).
The IBI concept based on integrity of fish communities has very high plasticity and can be used on a variety of aquatic habitats in different zoo-geographical regions all over the world (Belpaire et al. 2000). Although the modified versions of IBI follow same principle, they vary according to the context of targeted biotic group, the reference conditions, the scoring criteria, and the composition of metrics (Sendzimir and Schmutz 2018). The index of biotic integrity-based approach for estimating the aquatic health has been carried out by various researchers across the continents (Ruaro and Gubiani 2013; Kim and An 2015; Atique and An 2018; HaRa et al. 2019; Kim et al. 2019; Wu et al. 2020; Zhu et al. 2021; Achieng et al. 2021) and has already been introduced in legislative acts related to aquatic ecosystem management of many developed countries. Under the Water Framework Directive, the European Fish Index (EFI) was developed based on the concept of IBI under the FAME consortium for assessing the ecological status of flowing waters of EU countries (Schmutz et al. 2007) and was later developed as EFI+, a spatial extension of EFI (EFI+ Consortium, 2009). IBI-Jucar, a modified version of IBI, was formulated for evaluating biological integrity throughout Iberian Peninsula, as an effective tool for determining environmental quality, even in species-poor Mediterranean streams (Aparicio et al. 2011). In USA, unique state-specific fish-based IBI versions were developed for assessing biological integrity of rivers under United States Environmental Protection Agency (MPCA, 2014). The pioneer work on index of biotic integrity based on fish assemblage in Indian rivers was developed by Ganasan and Hughes (1998) for the rivers Khan and Kshipra of central India. Subsequently, the tool has been effectively utilized for assessing the other rivers (Das and Samanta, 2006; Das et al. 2013; Das et al. 2014; Das et al. 2016; Sajina et al. 2022). Sajina et al. (2022) developed an IBI version comprising 13 metrics, based on major ecological stressors and ichthyofaunal characteristics identified for Indian rivers that can effectually reflect human perturbations on river structures and processes.
Tapti or Tapi, a west flowing river in semi-arid region of central India, is one of the major river basins of Indian Peninsula. More than 20 million population depends on the river for meeting the basic needs directly or indirectly (Sharma et al. 2019). The river has been subjected to pollution by the dumping of industrial wastes and domestic sewages, especially, the lower stretches in southern part of Gujarat where core industrial areas for textile mills, fertilizers, chemical plants, and diamond processing units have established (Dubey and Ujjania 2013). Based on the literature survey, we found that systematic information on the ecological integrity of the River Tapti is lacking. Moreover, previous investigations on this river and its tributaries mainly focused on the status of biodiversity, water quality, and pollution (Karamchandani and Pisolkar 1967; Dubey and Ujjania 2013; Patole 2013; Khade et al. 2017). With this background, the present study attempts to fill this knowledge gap using the IBI as a tool to understand the fish assemblages and ecological health status, by adopting the IBI version developed by Sajina et al. (2022) for River Tapti. The adoption of IBI necessitates deep understanding about environmental parameters and hence, effort was given to unveil the effects of water quality variables on the fish assemblages and community structure of River Tapti.
Materials and methods
Description of study area
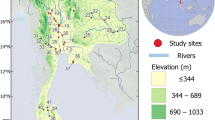
River Tapti, with a total length of 724 km covering geographical area of 65,145 km2 (Sharma et al. 2019), is the water source of three states: south of Gujarat, north of Maharashtra, and west of Madhya Pradesh. The fish assemblage data used for the study are based on the results of surveys in Tapti River, carried out during the period 2017–2020. The river has its origin in Multai in Betul district of Madhya Pradesh, which traverses through Burhanpur town of Madhya Pradesh and finds the way through dense forest areas of Maharashtra and Gujarat. The selected study area represents the entire stretch of the river starting from the origin of river, Multai (Madhya Pradesh) to Kamrej (Gujarat), after which it is emptying to the Arabian Sea. The eleven sampling sites selected were Multai (S1), Betul (S2), Dedtalai (S3), Nepanagar (S4), Burhanpur (S5), Changdev (S6), Bhusawal (S7), Savkheda (S8), Sarangkheda (S9), Singalkhanch (S10), and Kamrej (S11) (Fig. 1 and Online Resource 1). The station codes S1 to S5 represent upper stretch, S6 to S8 represent selected middle stretch, and S9 to S11 represent the lower stretch of the river. Although the lowermost sampling site Kamrej (S11) was at a distance of <60 km from the Arabian Sea mouth, a barricade constructed below the site kept the entire river stretch as freshwater.
Estimation of water quality index
For comparison of the estimated IBI scores with an independent estimate of the ecological status and to evaluate the efficacy of assessment process, water quality index (WQI) was estimated. It was estimated using parameters including water temperature, dissolved oxygen, pH, free CO2, alkalinity, hardness, conductivity, PO4-P, and NO3-N as per Pesce and Wunderlin (2000) and Wu et al. (2018).
where K is a subjective constant, Pi is the relative weight assigned to each parameter, and Ci is the value assigned to each parameter after normalization.
Fish sampling
Seasonal sampling for 3 years (2017–2020) during pre-monsoon (March–May), monsoon (June–September), and post-monsoon (October–January) was performed. In order to obtain a cross section of the standing population through sampling and representative numbers of each fish species, electro fishing is the most widely used sampling method for IBI studies. As the present study was carried out in the main river channel and some of the sites were not wadable for electro fishing, we used a wide array of netting and capture methods targeting all possible habitat types. Similar sampling strategy was followed in most of the IBI works from Asia, South America, and Africa (Hocutt et al. 1994; Bhat 2003; Pinto et al. 2006; Qadir and Malik, 2009) .
Fishes were sampled along all the stations by employing uniform fishing effort and duration using combination of various gears such as seine net (5 mm stretched mesh size), gill net (monofilament nylon nets of less than 60 mm during pre-monsoon and 60–150 mm during monsoon), cast net (10–30 mm; mostly during pre-monsoon), drag net with mesh size of 2–10 mm, scoop net (zero mm; pre-monsoon and post monsoon), hook and line (no. 25 to no. 10), and various traps were used to obtain representative samples of fish fauna. All fishes were counted and identified using the available literature on taxonomy (Talwar and Jhingran 1991; Jayaram 1991, 1999, 2010). The representative specimens of species in ambiguity were preserved in 10% formalin before transporting to the laboratory. The water quality parameters were estimated using standard methods (APHA 2017).
Metrics composition of IBI
It is pertinent to modify, delete, or add metrics for developing IBI for a particular geographic region and ecosystem to reflect regional difference in fish distribution and assemblage structure (Oberdorff et al., 2002). A recent study by Sajina et al. (2022) modified IBI for River Mahanadi, an Eastern Indian river considering the ecological stressors impacting upon the rivers and fish faunal characteristics. River Tapti and River Mahanadi traverse Indian continent in the same latitudinal range, sharing comparable biodiversity features and confront many of the ecological stressors alike. We considered all the 13 metrics used by Sajina et al. (2022) in our study and in order to remove those are redundant, we carried out a correlation analysis among the 13 metrics. As the metric indicating the percentage of omnivores showed a very high correlation with other metrics (Online Resource 2), we excluded it and hence we have used only 12 metrices in the present study. The IBI metrics used in the study come under the five broader categories: taxonomic richness, habitat composition, tolerance indicators, species resilience, and trophic composition as listed in Table 1.
The distribution of total number of native fish species (metric 1) proposed by Karr (1981) and native families (metric 2) were used based on the assumption that species and family level diversities seem to be preserved in undisturbed environments. As the environmental degradation increases, the native fish species may disappear (Karr et al. 1986). Metric 3 is the % of individual as non-native species which can determine the degree of invasion of alien species thereby provide the biological pollution aspects (Joy and Death 2004) and indicate the degraded conditions.
Number of benthic species (metric 4), number of column species (metric 5) assigned by Araujo (1998) and % of individual rheophiles (metric 6) (Harris 1995), and % of individual migratory (metric 7) can also explain the health of the habitat it occupies. Measure of benthic and column species indicates the disturbance of habitat as they are sensitive to siltation, turbidity reduced oxygen content, and toxic chemicals (Ganasan and Hughes 1998). Rheophiles can monitor the degradations of lotic areas (Oberdoff 1996). Most of the riverine fishes are potamodromous and they need sufficient volume of water as well as the habitat continuity where they can spawn, feed, or take shelter at extreme temperature. The low water level and poor water quality, which affects the fish migration, also indirectly measure the ecological health.
Number of intolerant species (metric 8) and % of individual tolerant (metric 9) are used to assess the sensitivity of fishes to habitat changes when occupied in a particular guild. Species once abundant and become occasional later due to environmental degradations is considered as intolerant (Lyons et al. 2000) which cause the dominance of tolerant species in terms of number and biomass (Ganasan and Hughes 1998).
Population doubling time (PDT) indicates the time taken for a population to double its size or number, which can be considered as a measure of species resilience. Thus, % of individual with high PDT (metric 10) is incorporated based on the concept that population size is robustly related to the local ecological conditions (Brook et al. 2006) and population size of species with long life span, delayed maturation, and long generation time may be affected by the poor ecological health and subjected to greater vulnerability. Species resilience was estimated using four levels of productivity (Musick 1999): very low PDT (less than 13 months), low PDT (1.4–4.4 years), medium PDT (4.5–14 years), and high PDT (>14 years). The data source necessary for evaluating species resilience was taken from FishBase (Froese and Pauly 2022).
The percentage of individuals as carnivores (metric 11) and percentage of individuals as herbivores (metric 12) included by Araujo et al. (2009) were incorporated in the IBI metrics as these trophic metrics are used to assess the ecological attributes; carnivores indicate the capacity of food web to maintain top predators (Bozzetti and Schulz 2004) and herbivores are sensitive to physico-chemical alterations that diminish the plant assemblages or vegetation composition (Ganasan and Hughes 1998).
Reference condition
The appropriate reference site selection is a critical step in developing multimetric index. The reference site chosen for the study should be a non-impaired condition with minimal anthropogenic disturbance (Jun et al. 2012), which can make possible judgment between reference and impaired sites (Resh et al. 1995). Various authors have followed the concept of using minimally disturbed sites as the reference site for evaluation of IBI (Ganasan and Hughes 1998; Das and Samanta 2006; Das et al. 2013). As there is a paucity of complete data set on the historical information about fish assemblage pattern of River Tapti, we followed reference-based approach to estimate the biotic integrity. Reference sites may not be pristine or completely undisturbed, but the variations in physical and chemical parameters were not enough to affect the fish fauna significantly (Pont et al. 2007). As there is a paucity of historic data of fish diversity on River Tapti, we assumed that one of the sampling sites can be referred as the reference site that might contain the best standard conditions, which could meet the upper expectations of the metrics. The site S2 (Betul) was chosen as the reference site as minimally disturbed condition (MDC, Stoddard et al. 2006) because of absence of significant anthropogenic disturbances and minimally disturbed, and the downstream sites from S3 to S11 were subjected to various human interferences including influx of domestic sewage and industrial pollutants. MDC is the most appropriate reference-site approach which can give a best estimate of biotic integrity as it is impractical to find a truly undisturbed condition without human interferences (Stoddard et al. 2006).
Scoring criteria of IBI for River Tapti
The basic biological information required to modify the scoring criteria for IBI for a new freshwater eco-region, Narmada-Tapti, was determined from the database of FishBase (Froese and Pauly, 2022) and Talwar and Jhingran (1991). The scientific names of fishes are presented according to Fricke et al. (2022). The IBI scores were calculated for each sampling site following the methods outlined by Fausch et al. (1984), Karr et al. (1986), and Ganasan and Hughes (1998). Continuous scoring was followed and the scoring criteria were developed as per the methods of Hughes et al. (1993). The qualitative evaluation of the IBI scores was done following Hughes et al. (1998), Karr et al. (1986), and Ganasan and Hughes (1998), based on a continuous scale scoring criteria (5-4-3-2-1) with best score given as 5 and worst as 1. The metrics and scoring criteria are summarized in Table 1. Four levels of integrity classes were classified based on IBI scores, where the percent of impairment less than 10% was considered “acceptable”; 10–25% as “slightly impaired”; 25–50% as “moderately impaired”; and more than 50% as “severely impaired.” In order to test the effectiveness of various metrics scores for categorizing sites in to different integrity classes, post hoc test (Duncan) was performed to comprehend the significant variation between the sites.
Relationship of IBI variables with environmental parameters
Canonical correspondence analysis (CCA) was performed between the selected metrics of the IBI (hereafter referred as IBI variables) and the water quality parameters to comprehend the interactions of different functional groups with environment. A total of nine environmental variables (temperature, dissolved oxygen, pH, free CO2, total alkalinity, total hardness, specific conductivity, phosphate-P, nitrate-N) and thirteen IBI variables were considered for the analysis. The CCA was performed employing PAST ver. 4.08.
Results
Fish assemblage structure of River Tapti
A total of 80 species of fin fishes belonging to 25 families and 50 genera were recorded from different habitats and trophic guilds of River Tapti during 2017 to 2020. The dominant family was Cyprinidae with a contribution of 32.50% of the total fish fauna which was followed by Danionidae (11.25%), Bagridae (6.25%), Ambassidae (5.00%), and Channidae (5.00%). The trophic guild composition consists of 41.25% carnivores and omnivores followed by 16.25% herbivores. The niche occupied by the fishes in different habitats comprises 61.25% of column fishes, 30% of benthic fishes, and 7.5% pelagic fishes. The species resilience varies with 52.5% of medium resilient species, 32.5% high resilient, 11.25% low resilient species, and 2.5% very low resilient species. Rheophiles consists of 32.5% and 66.25% are non-rheophilic in nature.
Among the fish species reported, five were exotic species. Of the two species of tilapia, Oreochromis mossambicus was mostly distributed in the upper stretch only whereas O. niloticus was dominant exotic species in the middle and lower stretches of the river (S7 to S10). Cyprinus carpio was found to be available in the entire upper and middle stretches of the study area. Hypophthalmichthys molitrix was the dominant species in S5 where the polluted water enters into the riverine system. Pangasianodon hypophthalmus was reported from the lower stretches (S9 to S11). The invasive coefficient index (Ixi) (Singh et al. 2013) calculated for the 5 species showed that the highest Ixi (0.17) was observed for O. mossambicus at S1. Conservation status of the reported eighty species as per the International Union for Conservation of Nature (IUCN) categorization are 2 in Endangered (EN), 2 in Vulnerable (VU) and 6 in Near Threatened (NT), 66 in Least Concern (LC), 1 in Data Deficient (DD), and 1 in Not Evaluated (NE) categories. The list of fishes recorded in the study with their particulars is provided in Online Resource 3.
IBI scores of sampling sites
The IBI score based on the pooled fish abundance data in River Tapti ranged from 33 to 60. As Betul was taken as reference site, it scored the maximum score, i.e., 60. Apart from the reference site (Betul), Dedtalai and Nepanagar stretches recorded the higher IBI scores and Kamrej (S11) scored the lowest. The site-wise scores of each metrics and overall IBI scores are given in Table 2.
The present study revealed that out of the eleven stations studied, eight stations are moderately impaired, two are slightly impaired, and one in acceptable class. The percentage of impairment based on IBI scores using 12 metrics in selected stretches of River Tapti is represented in Fig. 2.
The output of post hoc test of metric scores showed that Betul, the reference site, showed significant difference from all other sites (Table 3). Kamrej, the most impaired site, varied significantly from reference site and other two slightly impaired sites. The sites under same integrity class showed similarity in the test indicating effectiveness of various metrics used in the IBI in classifying sites based on the degree of impairment.
Metrics performance
In the present study, the number of native species distributed in the eleven selected sites was found to be more or less uniform, ranging from 35 to 48. The maximum species richness was observed in upper and middle stretches (S1, S2, and S7) and minimum of 35 at Savkheda in middle stretch (S8). The native families were reported more from S2 and S7 with a highest value of 17 families. The percent of individuals as non-native was found maximum in S1 with the highest catch of tilapia, O. mossambicus. Metrics reflecting habitat composition represented by the presence of benthic and column species, % of individual rheophiles, and migratory fishes were also studied. Maximum benthic species richness was observed in S2 (20) and minimum was recorded in S8 (11). The fast-moving rheophiles were present higher in the upper stretches with more abundance in S2 (44.2%); its dominance was substantially reduced downwards with least contribution from the last station, S11 (0.51). However, migratory fishes contributed maximum from the lower stretches with maximum occurrences reported from S10 (83.48%) and least from the reference site, S2 (52.06%). The tolerance level of fishes was measured by two metrics, no. of intolerant species and % of individual tolerant. The intolerant species were reported maximum from S9 (12) and minimum from S1 (6). The abundance of tolerant species constitutes 34.7% of the total population in least disturbed sites whereas the population increased to 70.38% in most disturbed site. Metrics indicating species richness was evaluated by % of individual with high PDT and the results revealed that maximum individuals were obtained from S4 (4.40%) and minimum from the most disturbed site, S11 (0.09%). Metrics reflecting the trophic composition showed abundance of herbivores (26.34%) in reference site followed by 18.89% in S3 whereas the minimum was reported from S8; abundance of carnivores in S4 (44.11%) and least abundant in S10 (9.11%); abundance of column species in S2 (36) followed by S3 (31) and abundance of intolerant species was reported from S9 (9).
Habitat quality, WQI, and influence of environmental parameters on IBI
The physical habitat characteristics and hydromorphological conditions of River Tapti were surveyed and tabulated (Table 4), indicating prominent site-wise variation of these particulars. The water quality parameters during the 3 years of study period showed that dissolved oxygen level was more than 6 mg L−1 (5.96–12.6 mg L−1) in all the sampling stations over the seasons, which is considered as congenial for fishes. There existed a point source of pollution in Burhanpur (S5) due to sewage influx. Samples from that place showed higher specific conductivity (2625 μS cm−1) which might be due to insufficient water flow for diluting the sewage during pre-monsoon; its value drastically reduced to 468 μS cm−1 during post monsoon. Similarly, higher values of alkalinity (675 mg L−1) and hardness (579 mg L−1) were also recorded from Burhanpur during pre-monsoon. At Burhanpur, the phosphate concentration in post-monsoon was 0.399 mg L−1 which is extremely high and indicated eutrophic condition. The highest nitrate level was also observed at the same place (0.432 mg L−1) in post-monsoon; however, the dissolved oxygen (6.5–8.11 mg L−1) was quite normal (Online Resource 4).
The estimated WQI scores ranged from 64.25 to 100.00 (Fig. 3). Highest score was obtained at Betul (S2), Dedtalai (S3), and Singalkhanch (S10) and the lowest score was obtained at Burhanpur (S5) and Bhusawal (S7). Out of eleven sampling sites, six were grouped under excellent, three were good, and two were moderate. The graph plotting IBI scores (adjusted to the scale of 100) with WQI scores of different sampling sites indicated that the two indices are of similar trend except at sites S1 and S8–S10 stretches (Fig. 3).
Canonical correspondence analysis of IBI and environmental variables
In the CCA, the first and second axes together explained 84.21% of the variability of the IBI and environmental variables (Fig. 4). The maximum correlation of environmental variables and location was in the order pH > NO3-N > total hardness > dissolved oxygen > temperature > total alkalinity. In axis 1, five IBI variables (NN, RH, PDT, CA, HE) were scored positively and had a gradient towards total alkalinity, total hardness, sp. cond., and NO3-N. Likewise, IBI variables (B, C, IT, and MI) were rated negatively on axis 1 and were strongly correlated with pH, temperature, and DO. Functional groups such as carnivores, herbivores, and fishes with high PDT showed their close association with nitrate-N, total alkalinity, and specific conductivity. However, non-natives showed their positive association with total hardness. The IBI variables, including migratory and intolerant fishes, showed negative gradient towards pH and DO and achieved positive values in temperature. It was reflected that the number of native species and column species was not much strongly influenced/impacted by the water variables. Overall, pH, NO3-N, total hardness, dissolved oxygen, and temperature were the influencing variables for the distribution of the IBI variables (Fig. 4).
CCA analysis of environment parameters with IBI functional metrices. Sites: MUL, Multai; BET, Betul; DED, Dedtalai; NEP, Nepanagar; BUR, Burhanpur; CHA, Changdev; BHU, Bhusawal; SAV, Savkheda, SAR, Sarangkheda; SIN, Singalkhanch; KAM, Kamrej. IBI metrices: NS, no. of native species; NN, % of non-native individuals; B, benthic species; C, no. of column species; RH, % of individual rheophiles; MI, % of individual migratory; IT, no. of intolerant species; PDT, % of individual with high PDT; CA, % of individual carnivore; HE, % of individual herbivore
Discussion
The objective of this study was to assess the ecological health status of River Tapti by adopting fish-based IBI and classifying the river stretches to different integrity classes according to the level of impairment. The study showed that around three-fourth of the river stretches was moderately impaired (25–50% of impairment) when compared to the reference site. The remaining one-fourth lying in upper stretch was in slightly impaired condition (10–25% impairment). The significant difference existing among the metric scores of sites under different integrity classes indicated IBI as a powerful diagnostic tool for river health assessment. The observed trend of decreasing IBI scores towards downstream in the present study was in agreement with study of Pinto and Araújo (2007) in the tropical river, Paraiba do Sul of Brazil. The major stressors found to have impacted the ecological health were the insufficient water flow during the lean period, the effect of industrial pollutants, dumping of domestic sewages, sand mining activities, etc. When more than one stress acts on the ecosystem, there was synergistic effect, as observed in some sites with lean flow suffering severely due to inefficient dilution of industrial effluents and large-scale eutrophication as noticed in Burhanpur.
The various types of anthropogenic disturbances have modified the aquatic environment by the way of change in land use pattern, industrialization and urbanization, dam construction and deforestation etc. which disturbs the ecological integrity. The multimetric approach of IBI assessment through integration of ecological information can provide biological criteria for restoration of aquatic ecosystems (Royer et al. 2001). The utilization of fish assemblage data for establishing multimetric index by using reference condition approach has been used widely (Bailey et al. 1998; Pont et al. 2007). Studies based on multimetric indices comprising 8 to 12 metrics representing species richness, composition, tolerance, and trophic measures were developed earlier (Boonsoong et al., 2009). In India, the research based on IBI was first conducted by Ganasan and Hughes (1998), where they incorporated seven new metrics and five adopted metrics from Karr et al. (1986). More works related to IBI in Indian contexts were conducted by Das and Samanta (2006) in Hooghly estuary, Das et al. (2013) in River Ganga, Das et al. (2014) in River Churni, Das et al. (2016) in River Brahmani, and Sajina et al. (2022) in Mahanadi and Kathajodi-Devi. In the present scenario, where the water quality is highly deteriorated due to human perturbations, IBI-based ecological health assessment is invariably required for management and restoration purposes. The previous works are the explicit examples signifying their reliability in assessing the river health status.
Metrics performance
Twelve metrics used in the present study were appraised in terms of their applicability and sensitivity and were found satisfactory for the development of IBI. The metric selection was performed on the basis that less taxa richness and abundance is predominant in impaired sites rather than the reference sites as anthropogenic influences enhance unsuitable habitats (Barbour et al. 1996). The twelve metrics used in the present study and their implications are discussed below.
Species richness concept is widely used for evaluating the environment quality (Roth et al. 2000) and it increases with lower levels of impairment and acceptable ecosystem health (Barbour et al. 1996). If species richness decreases along with the dominance of few species, it indicates the degraded environment (Casatti et al., 2006) whereas the few dominant species coupled with presence of rare species represent the slightly degraded environments (Ferreira and Casatti 2006). Among the various metrics incorporated in IBI, metrics representing taxonomic richness are efficient indicators which show high responsiveness to anthropogenic disturbance and the proportional abundance of taxon to the whole community is considered as a measure of community balance (Barbour et al. 1996). The number of native species, originally proposed by Karr (1981), can discriminate sites with good condition as some species can be lost due to the loss of habitat quality (Karr et al. 1986). Araujo et al. (2003) utilized native and non-native species as an indication of fish species richness.
Here, we used the three components to examine the species richness, viz., number of native fish species, number of native families, and % of individuals as non-native. Literature survey on River Tapti revealed that scanty data on fish biodiversity and its distribution are available except the study of Karamchandani and Pisolkar (1967) and Patole (2013). The historic data represented by the work of Karamchandani and Pisolkar (1967) reported 52 species belonging to 30 genera under 14 families. However, the present study reported 80 species, of which five species, Chanda nama, Mystus cavasius, Salmostoma bacaila, Pethia ticto, Osteobrama cotio, and Notopterus notopterus, occurred in all the sites. The high number of species reported in the present study is due to increased sampling effort, duration of sampling, and site coverage. The presence of native species and native families indicate the biodiversity, of which their measure indicates the level of anthropogenic disturbances (Karr 1981).
In the present study, maximum number (48) of native species and families were found in S1, S2, and S6 which showed a declining trend towards downstream. The higher species richness reported in these three sites may be due to the presence of deep pools and the suitable ecological conditions which helps to sustain the population of fish species. The loss of native species over a period is considered as the warning signal of environmental degradation; other chemical and biological factors become important along with the study on physical degradation while examining the biological integrity (Casatti et al. 2009).
The non-native species indicates the biological pollution (Whittier et al. 2007) which can alter the fish assemblage pattern by predation or competition thereby facilitating the exotics to survive in the disturbed systems; thus, Ganasan and Hughes (1998) included this metric in their study. In India, most of the exotic species were introduced for the purpose of aquaculture and many of them established in natural waters like rivers and lakes. Of the five exotic species reported, O. mossambicus (in 1952), C. carpio (in 1939), H. molitrix (in 1959), and O. niloticus (in 1987) were officially introduced to India for broadening the species spectrum in aquaculture whereas P. hypophthalmus was introduced clandestinely in 1990s and became popular as a candidate species for aquaculture in the country (Lakra and Singh 2010). Although these species were introduced for aquaculture purpose, most of them have paved their way into inland open waters through escape from captivity (Joshi et al. 2022) or even deliberate release (Lévêque, 1996) and established as breeding and self-recruiting populations (Lawson and Hill 2021). The dominance of exotics was found to be higher in Multai (S1), where O. mossambicus, C. carpio, and H. molitrix altogether contributed 18.84% of the total fishes caught. The stocking of O. mossambicus and C. carpio was observed in Maa Tapti Temple Pond in Multai for religious activities during the study period. Notably, the abundance of herbivores was quite low in Multai which might be due to competitive feeding behavior with the well-established exotic species. High numbers of P. hypophthalmus in the lower stretch might be associated with the cage farming of the species in the Ukai reservoir situated in the lower stretch of Tapti.
Benthic and water column species are sensitive to various physical and chemical water quality parameters such as silt load, turbidity, toxic contaminants, and reduced dissolved oxygen (Ganasan and Hughes 1998). The number of benthic and column species was found high in S2, the reference site, indicating the good health of the ecosystem. Authorized and unauthorized sand mining activities were observed in some sampling locations in the lower stretch of River Tapti and the number of benthic species was also found relatively less in those sites. Meng et al. (2018) reported that commercial sand mining activities caused substantial decline of benthic animals. The dredging-related activities can decrease the dissolved oxygen in the sediment water interface and inhibit the survival of oxygen-sensitive organisms (Ilarri et al. 2011).
Fishes inhabiting rivers will migrate in search of spawning areas or for other reasons and the constructions of dams across the rivers may halt their migratory behavior (Elsen 1977). Most of the sites were having good score for metrics based on migratory behavior, as common fishes of River Tapti were either potamodromous or amphidromous in nature. The unexpected good score of this metric at site S1 with low water level is attributed to the abundance of O. mossambicus in the site, which was included in migratory species category owing to its amphidromous trait as per descriptions given in FishBase.
Rheophilic fishes indicate the integrity of riverine habitats and its ecological quality since they need a variety of specific habitats in their entire life period in broad spatio-temporal aspects (Copp 1989; Schiemer 2000). Many works have clearly depicted the decline of rheophilic fish population subsequent to fragmentation of rivers (Birnie-Gauvin et al. 2017; van Puijenbroek et al. 2019). Tapti is a significantly fragmented river with a number of dams, weirs, and barrages and these structural modifications have intensively altered the natural flow regime of the river (Sharma et al. 2019). The major river projects altering the river flow are the Hatnur Dam and Lower Tapi Dam in Jalgaon district, Maharashtra, and Ukai Dam, Tapi district, Gujarat, besides a number of small dams and barrages. The steep decline in the rheophilic fish populations observed in the middle and lower stretches (S6 to S11) with the poorest metric score indicated the effectiveness of this metrics in highlighting the impacts of river fragmentation in the IBI score.
The population size of intolerant species may get reduced when environmental degradation increased and this metrics differentiate moderate and high habitat quality (Karr et al. 1986). The intolerant species may take longer time for their recovery and thus number of tolerant species and percent of intolerant individuals can be used in the multi-metrics (Terra and Araújo, 2011). The intolerant species reported from the study are Notopterus notopterus, Clupisoma garua, Chitala chitala, Ophichthys cuchia, Sperata aor, S. seenghala, Wallago attu, Lates calcarifer, Channa marulius, Labeo dyocheilus, Labeo calbasu, Amblypharyngodon mola, Hypophthalmichthys molitrix, Tor tor, T. khudree, and Mystus cavasius. The lowest score for metric with intolerant species was at S1, which can be accounted for the very low water level in the site with great variation in seasonal water availability.
Tolerant species are those having high flexibility in water quality and habitat requirements (Verneaux 1981; Grandmottet 1983) and are adapted to live in less oxygenated conditions (Bozzetti and Schulz 2004). They are last to disappear when disturbances come and first to reappear when the ecosystem is recovered (Ganasan and Hughes 1998). The poorest score for the metric based on tolerant species was for S11, Kamrej, which is a fully urbanized industrial area and greatly affected by domestic and industrial effluents (Jha et al. 2012; Chander et al. 2018).
Species resilience is an indirect measure of the recovering capacity of a species from environmental changes (Musick 1999) as increased anthropogenic pressure tends to have species with higher vulnerability and lower resilience (Vasconcelos et al. 2017). The metric based on PDT of fishes works with the concept that species with high PDT needs more time to recover even though the stress factor is relieved owing to longer generation time (Myers and Worm 2005). The six native species whose high PDT (low resilience) targeted in this metric were Tor tor, T. khudree, Labeo dyocheilus, Clupisoma garua, Ophichthys cuchia, and Oryzias dancena.
Trophic composition can provide baseline information about the trophic status, functional feeding groups, and the potential food sources available in an ecosystem (Cummins 1988). The aggregation of particular food sources may increase the higher abundance of functional feeding groups in a particular habitat type (Merritt and Cummins 1996). The carnivores indicate the diverse trophic web supporting top pyramid species (Pinto and Araújo, 2007) and they often play the role of keystone species and are integral components of a healthy ecosystem (Miller et al. 2001). In the present study, decrease in the percentage of individuals as carnivores and herbivores was recorded towards downstream of the river. The structural changes in trophic composition may be attributed to alterations in habitat and water quality accelerated by human interventions causing potential changes in the availability of food resources (Karr et al. 1986). The least score for metric based on carnivores at S10 may be attributed to the habitat alterations in the downstream of the largest dam in the river, Vallabh Sagar or Ukai Dam.
Influence of environmental parameters and hydromorphological features on biotic integrity
The Tapi basin experiences three dissimilar climate regimes based on temperature and precipitation information; the upper basin exhibits tropical dry savanna (approximate area of 47.1%), middle basin exhibits hot semi-arid climate (37.7%), and lower basin exhibits tropical wet savanna climate (15.2%) (Sharma et al. 2019). River Tapti heavily depends on the rainfall and is subjected to wide seasonal variations in the water flow and availability. It is well evident that heterogeneity in stream flow is a major issue in this river, as explained by Sharma et al. (2019) and also the chronic water shortage observed in the present study during pre-monsoon at some sites especially S1. The discontinuity of water flow during summer season resulted in fragmentation and formation of multiple water pools in the middle stretch of river leading to subsequent degradation in water quality and associated higher value of specific conductivity. These deteriorations might have had impact on biotic integrity of the ecosystem as reflected by the overall IBI scores falling under moderately impaired condition from S5 downwards.
There were pronounced seasonal variation in the concentration of nutrients at some sites, especially phosphate. Phosphate (PO4−3)-phosphorus being an essential nutrient and is a major component of many of the fertilizers. Therefore, erosion and runoff from fields with extensive application of fertilizers can result in phosphate accumulation in water bodies and lead to eutrophication and associated oxygen depletion, besides other types of water pollution (Yang et al. 2008). Comparison of inorganic phosphate in Tapti River during different seasons revealed that concentration of PO4-P increased during post-monsoon in most of the stations. Most probable reason was that excess part of phosphatic fertilizers used in agricultural fields in the surrounding areas were washed with rainwater and drained into the river, but its concentration did not increase in monsoon due to dilution effects. However, the concentration increased during post-monsoon when the influx and water flow were reduced. The phosphate concentrations were high at stations like Sarangkheda, Singalkhanch, and Kamrej (S9–S11) during pre-monsoon.
The IBI functional groups showed meaningful interactions with some of the environmental variables. pH, nitrate-N, and dissolved oxygen were the most deterministic environmental parameters influencing the IBI functional groups. Non-native species are often seen to thrive well and flourish in deteriorated and unfavorable environments, as observed by the positive gradient of non-natives towards total hardness of water. Similarly, intolerant species are sensitive to deteriorations in habitat quality (Lyons et al. 2000), as they showed negative gradient towards pH and positive towards dissolved oxygen.
There are many biotic and abiotic factors that do not alter the water quality parameters but can greatly influence the overall biotic integrity of the ecosystem. WQI, an index based on physico-chemical parameters expressing the status of river contamination, cannot reflect the biological responses towards various ecological stressors such as abridged stream flow, sand mining, and dominance of exotics. The deviation between IBI and WQI scores at S1 is due to the lean availability of water, where it almost gets dried up in peak summer season critically impacts the biotic integrity. The prevalence of sand mining in the lower stretch (S8–S10) can be the underlying reason for the poor IBI scores of the stretch, despite excellent water quality as per WQI. The increased turbidity and sediment suspension arises from sand mining practices can affect the breeding and feeding sites of fish population and alter the community composition (Affandi and Ishak 2019).
Hydromorphological conditions are the primary control on the biotic elements of the river ecosystems as they influence species richness of river communities (Elosegi et al. 2010). Studies have indicated that channel regulation is the major cause of the hydromorphological deterioration of rivers (Hajdukiewicz et al. 2017) and fish communities undergo substantial degradation in channelized rivers (Schmutz et al. 2015). Sharma et al. (2019) investigated the impact of anthropogenic activities on stream flow changes and water stress conditions of River Tapti and their study comprising four-decade data analysis (1973–2013) showed that the imbalance in stream flow and water scarcity is a prime concern across the Tapti River basin. Ukai Dam (the second largest reservoir in Gujarat after Sardar Sarovar) construction (1972) for catering the irrigation, industrial and municipal needs, flood control, and hydropower generation has pronounced effect on the decreased stream flow in lower Tapti stretch. The hydromorphological alterations of the river have impacted the ecosystem health and fish communities in the downstream stretches, as reflected the moderate impairments in sites S6 to S11.
Tapti, one of the major peninsular rivers in India, attracted less attention even though it supports millions of peoples. The water scarcity being a major crisis in Tapti River, more conservation measures are necessary for the river to recover from extreme stresses. The loss of river network connectivity is an ever-present threat globally (Grill et al. 2015) and the booming of hydropower projects for meeting human demands (Zarfl et al. 2015) has altered the riverine habitats drastically. These modifications can disrupt the nutrient and sediment transport which further affects aquatic organisms and ecosystem processes and functions (Pitlick and Wilcock 2001). Damming and river fragmentation lead to alterations in the fish species diversity with implications on ecological strategies of fishes (Merona and VigourouxR, 2005). The disappearance of rheophilic species such as Barilius barila, B. evezardi, and cave fish Indoreonectes evezardi, which were reported from River Tapti (Karamchandani and Pisolkar 1967), may be consequential to the habitat alterations in the river. As declining trends of stream flow with deteriorating water quality had been highlighted as the major issue in River Tapti (Sharma et al. 2019), more attention is to be given to determine the required minimum environmental flow and to maintain the same considering the river topography and its location in the semi-arid geographical belt, so that the river can restore the ecological functions and well sustain its biota. Indiscriminate sand mining practices need to be curbed.
Conserving aquatic biodiversity including fishes and their habitats is a great challenge in the present scenario of increasing anthropogenic stress. As rivers are the natural gene-pool reserves for fishes, measures are to be taken towards conservation of native fish diversity in Tapti. Biosecurity measures have to be strengthened to prevent entry of cultured non-native fish species through accidental escaped from culture systems including cages in reservoirs as evident in the lower stretch.
In India, the assessment and maintenance of river water quality is being carried out through National Water Quality Monitoring Programme by Central and State Pollution Control Boards. These assessments are mainly based on physico-chemical parameters and presence of pollutants, and the biomonitoring component limited to specific locations. For sustainable river management, periodic river monitoring programs have to be conducted for assessing the river health and its biotic communities using innovative methodologies and tools for efficient mitigation and restoration. Globally, IBI has been proficiently used as an effective and competent tool for environmental assessment, restoration, and conservation of aquatic ecosystems (Aura et al. 2017; Kim et al. 2019; Zhu et al. 2021) as it provides profound understanding of ecosystem degradation processes.
Conclusion
Rivers are the repositories of fish and aquatic biodiversity, but facing various anthropogenic threats in association with inevitable climate change. Despite these, importance of clean water for human health, food security, and livelihood has recognized globally by United Nations Sustainable Development Goal 6 (UN SDG). The health of an aquatic ecosystem is of prime importance as it essentially requires well maintained and balanced biological communities. Hence, it is apposite to measure the river health status to prevent further worsening of water quality and quantity and to avert the endangerment of aquatic life and spread of water borne diseases. IBI is an effective tool for assessing the environmental quality of water bodies by utilizing the fish assemblage pattern. The multimetric approach of IBI based on quantitative expectations gives an overall view of the biological integrity of fish communities residing in a particular habitat. The IBI-based approach for monitoring the river health status can assist the management authorities to revive, rehabilitate, and restore the impaired sites for ensuring ecosystem sustainability. In the present study, the multimetric index developed could reflect the degradation and cumulative impacts at the ecosystem level, exposing the various levels of ecosystem impairment of stretches of River Tapti with only one of the eleven sampled sites under acceptable integrity class. We therefore suggest that biological integrity of the fish assemblages of River Tapti need further attention and proper management measures to revert the riverine stretches, ecosystems, and associated biota.
Data availability
The study is a result of institutional project of the ICAR-CIFRI, and the data would be available on legitimate request.
References
Achieng AO, Masese FO, Coffey TJ, Raburu PO, Agembe SW, Febria CM, Kaunda-Arara B (2021) Assessment of the ecological health of Afrotropical rivers using fish assemblages: a case study of selected rivers in the Lake Victoria Basin, Kenya. Front Water 2:620704
Affandi FA, Ishak MY (2019) Impacts of suspended sediment and metal pollution from mining activities on riverine fish population-a review. Environ Sci Pollut Res 26:16939–16951
Aparicio E, Carmona-Catot G, Moyle PB, García-Berthou E (2011) Development and evaluation of a fish-based index to assess biological integrity of Mediterranean streams. Aquatic Conser: Mar Freshw Ecosyst 21(4):324–337
APHA (2017) Standard methods for the examination of water and wastewater, 23rd edn. APHA, AWWA and WEF, Washington DC
Araujo FG (1998) Adaptation of the index of biotic integrity based on fish assemblages in the Paraíba do Sul River, RJ, Brazil. Braz J Biol 58:547–558
Araujo FG, Fichberg I, Pinto BCT, Peixoto MG (2003) A preliminary index of biotic integrity for monitoring the condition of the Rio Paraíba do Sul, southeast Brazil. Environ Manage 32:516–526
Araujo FG, Pinto BCT, Teixeira TP (2009) Longitudinal patterns of fish assemblages in a large tropical river in southeastern Brazil: evaluating environmental influences and some concepts in river ecology. Hydrobiologia 618:89–107
Atique U, An KG (2018) Stream health evaluation using a combined approach of multi-metric chemical pollution and biological integrity models. Water 10(5):661
Aura CM, Kimani E, Musa S, Kundu R, Njiru JM (2017) Spatio-temporal macroinvertebrate multi-index of biotic integrity (MMiBI) for a coastal river basin: a case study of River Tana, Kenya. Ecohydrol Hydrobiol 17:113–124
Ayyappan S, Usha M, Gopalakrishnan A, Jena JK, Pandey AK (2011) Handbook of fisheries and aquaculture. Chandu press, New Delhi
Bailey RC, Kennedy MG, Dervish MZ, Taylor ARM (1998) Biological assessment of freshwater ecosystems using a reference condition approach: comparing predicted and actual benthic invertebrate communities in Yukon streams. Freshw Biol 39(4):765–774
Banerjee US, Gupta S (2013) Impact of industrial waste effluents on river Damodar adjacent to Durgapur industrial complex, West Bengal, India. Environ Monit Assess 185(3):2083–2094
Barbour MT, Gerritsen J, Griffith GE, Frydenborg R, McCarron E, White JS, Bastian ML (1996) A framework for biological criteria for Florida streams using benthic macroinvertebrates. J North Am Benthol Soc 15:185–211
Belpaire C, Smolders R, Auweele IV, Ercken D, Breine J, Van Thuyne G, Ollevier F (2000) An index of biotic integrity characterizing fish populations and the ecological quality of Flandrian water bodies. Hydrobiologia 434(1):17–33
Bhat A (2003) Diversity and composition of freshwater fishes in river systems of Central Western Ghats, India. Environ Biol Fishes 68(1):25–38
Birnie-Gauvin K, Aarestrup K, Riis TM, Jepsen N, Koed A (2017) Shining a light on the loss of rheophilic fish habitat in lowland rivers as a forgotten consequence of barriers, and its implications for management. Aquat Conserv 27(6):1345–1349
Bock K, Polt R, Schulting L (2018) Ecosystem services in river landscapes. In: Riverine ecosystem management, science for governing towards a sustainable future. Springer, Cham, pp 413–433
Boonsoong B, Sangpradub N, Barbour MT (2009) Development of rapid bioassessment approaches using benthic macroinvertebrates for Thai streams. Environ Monit Assess 155:129–147
Bozzetti M, Schulz UH (2004) An index of biotic integrity based on fish assemblages for subtropical streams in southern Brazil. Hydrobiologia 529:133–144
Brauman KA, Van Der Meulen S, Brils J (2014) Ecosystem services and river basin management. In: Risk-informed management of European river basins. Springer, Berlin, Heidelberg, pp 265–294
Brook BW, Traill LW, Bradshaw CJ (2006) Minimum viable population sizes and global extinction risk are unrelated. Ecol Lett 9:375–382
Casatti L, Ferreira CP, Langeani F (2009) A fish-based biotic integrity index for assessment of lowland streams in southeastern Brazil. Hydrobiologia 623:173–189
Casatti L, Langeani F, Silva AM, Castro RMC (2006) Stream fish, water and habitat quality in a pasture dominated basin, southeastern Brazil. Braz J Biol 66:681–696
Chander S, Pompapathi V, Gujrati A, Singh RP, Chaplot N, Patel UD (2018) Growth of invasive aquatic macrophytes over Tapi river. International archives of the photogrammetry, remote sensing and spatial information sciences
Chovanec A, Hofer R, Schiemer F (2003) Fish as bioindicators. In: Trace metals and other contaminants in the environment. Elsevier, pp 639–676
Copp GH (1989) The habitat diversity and fish reproductive function of floodplain ecosystem. Environ Biol Fishes 26(1):1–27
Cummins KW (1988) The study of stream ecosystems: a functional view. In: Pomeroy LR, Albetrs JJ (eds) Concepts of ecosystem ecology: a comparative view. Springer, New York, pp 247–262
Das MK, Samanta S, Sajina AM, Sudheesan D, Naskar M, Bandopadhyay MK, Paul SK, Bhowmick S, Srivastava PK (2016) Fish diversity, community structure and ecological integrity of river Brahmani. J Inland Fish Soc India 48:1–13
Das MK, Samanta S (2006) Application of an index of biotic integrity (IBI) to fish assemblage of the tropical Hooghly estuary. Indian J Fish 53:47–57
Das MK, Samanta S, Mitra K, Bandyopadhyay MK, Bhowmick S (2014) Assessment of fishery and ecological integrity of the anthropogenically stressed River Churni, India. J Inland Fish Soc India 46:9–28
Das MK, Sharma AP, Vass KK, Tyagi RK, Suresh VR, Naskar M, Akolkar AB (2013) Fish diversity, community structure and ecological integrity of the tropical River Ganges, India. Aquat Ecosyst Health Manag 16:395–407
Dubey M, Ujjania NC (2013) Water quality and pollution status of Tapi river, Gujarat, India. Int J Pure Appl Zool 1:261–266
EFI+ CONSORTIUM (2009). Manual for the application of the new European fish index – EFI+. A fish-based method to assess the ecological status of European running waters in support of the Water Framework Directive.
Elosegi A, Díez J, Mutz M (2010) Effects of hydromorphological integrity on biodiversity and functioning of river ecosystems. Hydrobiologia 657(1):199–215
Elsen DS (1977) Distribution of fishes in the James River in North Dakota and South Dakota prior to Garrison and Oahe diversion projects. Master’s thesis. University of North Dakota, Grand Forks, North Dakota, USA
Fausch KD, Karr JR, Yant PR (1984) Regional application of an index of biotic integrity based on stream fish communities. Trans Am Fish Soc 13:39–55
Ferreira CDP, Casatti L (2006) Integridadebiótica de um córregonabacia do Alto Rio Paraná avaliadapormeio da comunidade de peixes. Biota Neotrop 6:1–25
Fricke R, Eschmeyer WN, R. van der Laan (eds) (2022) Eschmeyer’s catalog of fishes: genera, species, references. (http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp).
Froese R, Pauly D (eds) (2022) FishBase. World Wide Web electronic publication www.fishbase.org, version (02/2022)
Ganasan V, Hughes RM (1998) Application of an index of biological integrity to fish assemblages of the rivers Kahn and Kshipra (Madhya Pradesh). Fresh Water Biol 40:367–383
Grandmottet JP (1983) Principales exigences des téléostéensdulcicoles vis-à-vis de l’habitataquatique. In Annales scientifiques de l’Université de Franche-Comté, pp 3–32
Grill G, Lehner B, Lumsdon AE, MacDonald GK, Zarfl C, Liermann CR (2015) An index-based framework for assessing patterns and trends in river fragmentation and flow regulation by global dams at multiple scales. Environ Res Lett 10(1):015001
Hajdukiewicz H, Wyżga B, Zawiejska J, Amirowicz A, Oglęcki P, Radecki-Pawlik A (2017) Assessment of river hydromorphological quality for restoration purposes: an example of the application of RHQ method to a Polish Carpathian river. Acta Geophy 65(3):423–440
HaRa J, Mamun M, An KG (2019) Ecological river health assessments using chemical parameter model and the index of biological integrity model. Water 11(8):1729
Harris JH (1995) The use of fish in ecological assessments. Austral J Ecol 20:65–80
Hocutt C, Johnson P, Hay C, Van Zyl B (1994) Biological basis of water quality assessment: Okavango River, Namibia. Rev Hydrobiol trop 27(4):361–384
Hughes RM, Burch-Johnson C, Dixit SS, Herlihy AT, Kaufmann PR, Kinney WL, Larsen DP, Lewis PA, McMullen DM, Moors AK, Connor RJO (1993) Development of lake condition indicators for EMAP-1991 pilot. EMAP surface waters 1991 pilot report EPA/620/R-93/003. U.S.EnvironmentalProtection Agency, Corvallis
Hughes RM, Noss RF (1992) Biological diversity and biological integrity: current concerns for lakes and streams. Fisheries 17:11–19
Hughes RM, Kaufmann PR, Herlihy AT, Kincaid TM, Reynolds L, Larsen DP (1998) A process for developing and evaluating indices of fish assemblage integrity. Can J Fish Aquat Sci 55:1618–1631
Ilarri MI, Antunes C, Guilhermino L, Sousa R (2011) Massive mortality of the Asian clam Corbicula fluminea in a highly invaded area. Biol Invasions 13(2):277–280
Jain CK, Singh S (2020) Impact of climate change on the hydrological dynamics of River Ganga, India. J Water Clim Change 11(1):274–290
Jayaram KC (1991) Revision of the genus Puntius Hamilton from Indian region. Rec ZoolSurv India, occasional paper no.13, Zoological Survey of India, Kolkata, pp. 113-123
Jayaram KC (1999) The freshwater fishes of the Indian region. Narendra publishing house, New Delhi, India
Jayaram KC (2010) The freshwater fishes of the Indian region. Narendra Publishing House, New Delhi, India
Jha MM, Patra AK, Gadhia M, Ravi PM, Hegde AG, Sarkar PK (2012) Multivariate statistical interpretation of physico-chemical and radiological parameters of Tapi River water due to the operation of Kakrapar Atomic Power Station. Int J Environ Prot 2(11):22–29
Jhingran VG (1991) Fish and fisheries of India. Hindustan Pub. Co., New Delhi
Joshi KD, Kumar A, Srivastava SM, Sahu V, Lal KK (2022) Mounting threat of invasive alien fish species in Ganga river system and open water resources of the basin. Indian J Anim Sci 92(2). https://doi.org/10.56093/ijans.v92i2.122109
Joy MK, Death RG (2004) Application of the index of biotic integrity methodology to New Zealand freshwater fish communities. Environ manage 34:415–428
Jumani S, Deitch MJ, Valle D, Machado S, Lecours V, Kaplan D, Krishnaswamy J, Howard J (2022) A new index to quantify longitudinal river fragmentation: conservation and management implications. Ecol Indic 136:108680
Jun YC, Won DH, Lee SH, Kong DS, Hwang SJ (2012) A multimetric benthic macroinvertebrate index for the assessment of stream biotic integrity in Korea. Int J Environ Res Public Health 9:3599–3628
Karamchandani SJ, Pisolkar MD (1967) Survey of the fish and fisheries of the Tapti river. Survey report no. 4,. Central Inland Fisheries Research Institute, Barrackpore
Karr JR (1981) Assessment of biotic integrity using fish communities. Fisheries 6:21–27
Karr JR (1991) Biological integrity: a long-neglected aspect of water resource management. Ecol appl 1:66–84
Karr JR, Dudley DR (1981) Ecological perspective on water quality goals. Environ manage 5:55–68
Karr JR, Fausch KD, Angermeier PL, Yant PR, Schlosser IJ (1986) Assessing biological integrity in running waters. A method and its rationale, vol 5. Illinois Natural History Survey, Champaign, Special Publication, pp 1–28
Khade RN, Dabhade DS, Chondekar RP, Tayade SN (2017) Ichthyofaunal diversity of Wan River, tributary of Tapi River. Int J Appl Res 73-75
Kim JJ, Atique U, An KG (2019) Long-term ecological health assessment of a restored urban stream based on chemical water quality, physical habitat conditions and biological integrity. Water 11(1):114. https://doi.org/10.3390/w11010114
Kim JY, An KG (2015) Integrated ecological river health assessments, based on water chemistry, physical habitat quality and biological integrity. Water 7(11):6378–6403
Lakra WS, Singh AK (2010) Risk analysis and sustainability of Pangasianodon hypophthalmus culture in India. Aquac Asia 15(1):34–37
Lawson KM, Hill JE (2021) Predicting successful reproduction and establishment of non-native freshwater fish in peninsular Florida using life history traits. J Vertebr Biol 70(4):21041.1-17. https://doi.org/10.25225/jvb.21041
Lawton JH, Gaston KJ (2001) Indicator species. Encyclopedia of biodiversity, pp 437–450
Lévêque C (1996) Introduction of fish species in freshwaters: a major threat to aquatic biodiversity? In: Castri F, Younes T (eds) Biodiversity, science and development: towards a new partnership. CAB international, United Kingdom, pp 446–451
Lyons J, Gutiérrez-Hernández A, Díaz-Pardo E, Soto-Galera E, Medina-Nava M, Pineda-López R (2000) Development of a preliminary index of biotic integrity (IBI) based on fish assemblages to assess ecosystem condition in the lakes of central Mexico. Hydrobiologia 418:57–72
Meng X, Jiang X, Li Z, Wang J, Cooper KM, Xie Z (2018) Responses of macroinvertebrates and local environment to short-term commercial sand dredging practices in a flood-plain lake. Sci Total Environ 631:1350–1359
Merona B, VigourouxR T-GFL (2005) Alteration of fish diversity downstream from Petit-Saut Dam in French Guiana. Implication of ecological strategies of fish species. Hydrobiologia 551(1):33–47
Merritt RW, Cummins KW (eds) (1996) An introduction to the aquatic insects of North America. Kendall Hunt Publishing, Dubuque, IA, USA
Miller B, Dugelby B, Foreman D, Rio C, Noss R, Phillips M, Soulé M, Terborgh J, Willcox L (2001) The importance of large carnivores to healthy ecosystems. Endangered Species Update 18(5):202–210
MPCA (2014) Development of a fish-based index of biological integrity for assessment of Minnesota’s rivers and streams. In: Document number wq-bsm2-03. Minnesota Pollution Control Agency, Environmental Analysis and Outcomes Division, St, Paul, MN
Musick JA (1999) Criteria to define extinction risk in marine fishes: the American Fisheries Society initiative. Fisheries 24:6–14
Myers RA, Worm B (2005) Extinction, survival or recovery of large predatory fishes. Philos Trans R Soc Lond B: Biol Sci 360(1453):13–20
Oberdoff T (1996) Reseau Hydrobiologique et Piscicole. Synthess Des Donnees 1995 Sur le Bassin Seine- Normandie. Final report to the Agence de l’Eau Seine-Nor-mandie, Conseil Superieur de la Peche, Compiegne, France
Oberdorff T, Pont D, Hugueny B, Porcher JP (2002) Development and validation of a fish-based index for the assessment of ‘river health’ in France. Freshw Biol 47(9):1720–1734
Patole SS (2013) Ichthyofaunal diversity of Nandurbar District (Northwest Khandesh Region) of Maharashtra (India). Int J Fish Aqu Stud 2:167–172
Pesce SF, Wunderlin DA (2000) Use of water quality indices to verify the impact of Córdoba City (Argentina) on Suquı́a River. Water Res 34(11):2915–2926
Pinto BCT, Araújo FG (2007) Assessing of biotic integrity of the fish community in a heavily impacted segment of a tropical river in Brazil. Braz Arch Biol Technol 50:489–502
Pinto BCT, Araujo FG, Hughes RM (2006) Effects of landscape and riparian condition on a fish index of biotic integrity in a large southeastern Brazil river. Hydrobiologia 556(1):69–83
Pitlick J, Wilcock P (2001) Relations between stream flow, sediment transport, and aquatic habitat in regulated rivers, vol 4. Geomorphic processes and riverine habitat, pp 185–198
Pont D, Hugueny B, Rogers C (2007) Development of a fish-based index for the assessment of river health in Europe: the European fish index. Fish Manag Ecol 14(6):427–439
Qadir A, Malik RN (2009) Assessment of an index of biological integrity (IBI) to quantify the quality of two tributaries of river Chenab, Sialkot, Pakistan. Hydrobiologia 621(1):127–153
Resh VH, Norris RH, Barbour MT (1995) Design and implementation of rapid assessment approaches for water resource monitoring using benthic macroinvertebrates. Austral J Ecol 20:108–121
Roth NE, Southerland M, Chaillou JC, Kazyak PF, Stranko SA (2000) Refinement and validation of a fish index of biotic integrity for Maryland streams. Prepared by Versar Inc. for Maryland Department of Natural Resources, Monitoring and Non-tidal assessment Division, Annapolis, Maryland
Royer TV, Robinson CT, Minshall GW (2001) Development of macroinvertebrate-based index for bioassessment of Idaho rivers. Environ Manage 27:627–636
Ruaro R, Gubiani ÉA (2013) A scientometric assessment of 30 years of the index of biotic integrity in aquatic ecosystems: applications and main flaws. Ecol Indic 29:105–110
Sajina AM, Sudheesan D, Samanta S, Paul SK, Bhowmick S, Nag SK, Kumar V, Das BK (2022) Development and validation of fish-based index of biotic integrity for assessing ecological health of Indian rivers Mahanadi and Kathajodi-Devi. Aquat Ecosyst Health Manag 25(2):25–35
Schiemer F (2000) Fish as indicators for the assessment of the ecological integrity of large rivers. Hydrobiologia 422:271–278
Schmutz S, Cowx IG, Haidvogl G, Pont D (2007) Fish-based methods for assessing European running waters: a synthesis. Fish ManagEcol 14(6):369–380
Schmutz S, Bakken TH, Friedrich T, Greimel F, Harby A, Jungwirth M, Melcher A, Unfer G, Zeiringer B (2015) Response of fish communities to hydrological and morphological alterations in hydropeaking rivers of Austria. River Res Appl 31(8):919–930
Sendzimir J, Schmutz S (2018) Challenges in riverine ecosystem management. In: Riverine ecosystem management, science for governing towards a sustainable future. Springer, Cham, pp 1–16
Sharma PJ, Patel PL, Jothiprakash V (2019) Impact of rainfall variability and anthropogenic activities on streamflow changes and water stress conditions across Tapi Basin in India. Sci Total Environ 687:885–897
Singh AK, Kumar D, Srivastava SC, Ansari A, Jena JK, Sarkar UK (2013) Invasion and impacts of alien fish species in the Ganga River, India. Aquat Ecosyst Health Manag 16(4):408–414
Sirota J, Baiser B, Gotelli NJ, Ellison AM (2013) Organic-matter loading determines regime shifts and alternative states in an aquatic ecosystem. Proc Natl Acad Sci 110(19):7742–7747
Stoddard JL, Larsen DP, Hawkins CP, Johnson RK, Norris RH (2006) Setting expectations for the ecological condition of streams: the concept of reference condition. Ecol appl 16(4):1267–1276
Talwar PK, Jhingran AG (1991) Inland fishes of India and adjacent countries Volumes I & II. Oxford & IBH Publishing, New Delhi
Terra BF, Araújo FG (2011) A preliminary fish assemblage index for a transitional river–reservoir system in southeastern Brazil. Ecol Indic 11:874–881
Usha M (2014) Environment and fishery status of major rivers of Indus system within India. Indian J Anim Sci 84:588–595
van Puijenbroek PJ, Buijse AD, Kraak MH, Verdonschot PF (2019) Species and river specific effects of river fragmentation on European anadromous fish species. River Res Appl 35(1):68–77
Vasconcelos RP, Batista MI, Henriques S (2017) Current limitations of global conservation to protect higher vulnerability and lower resilience fish species. Sci Rep 7(1):1–12
Verneaux J (1981) Les poisons et la qualite des coursd’eau. In annals Scientifiques de l’universite de Franche-Comte 2:33–41
Wen Y, Schoups G, Van De Giesen N (2017) Organic pollution of rivers: combined threats of urbanization, livestock farming and global climate change. Sci Rep 7(1):1–9
Whittier TR, Hughes RM, Stoddard JL, Lomnicky GA, Peck DV, Herlihy AT (2007) A structured approach for developing indices of biotic integrity: three examples from streams and rivers in the western USA. Trans Am Fish Soc 136:718–735
Wu J, Mao R, Li M, Xia J, Song J, Cheng D, Sun H (2020) Assessment of aquatic ecological health based on determination of biological community variability of fish and macroinvertebrates in the Weihe River Basin, China. J Environ Manag 267:110651
Wu Z, Wang X, Chen Y, Cai Y, Deng J (2018) Assessing river water quality using water quality index in Lake Taihu Basin, China. Sci Tot Environ 612:914–922
Yang XE, Wu X, Hao HL, He ZL (2008) Mechanisms and assessment of water eutrophication. J Zhejiang Univ Sci B 9(3):197–209
Zarfl C, Lumsdon AE, Berlekamp J, Tydecks L, Tockner K (2015) A global boom in hydropower dam construction. Aquat Sci 77(1):161–170
Zhu H, Hu XD, Wu PP, Chen WM, Wu SS, Li ZQ, Zhu L, Xi YL, Huang R (2021) Development and testing of the phytoplankton biological integrity index (P-IBI) in dry and wet seasons for Lake Gehu. Ecol Indic 129:107
Acknowledgements
The authors are grateful to ICAR, New Delhi and the Director, ICAR-Central Inland Fisheries Research Institute, Barrackpore, for the support to conduct the research work. The work was carried out under institutionally funded project “Habitat characterization, fisheries and socio economics of rivers Cauvery, Tapti, Siang and Chaliyar (REF /17-20/07)” of ICAR-CIFRI, India. The authors express their sincere gratitude to Dr. V.R. Suresh for the immense support and coordination extended for the project. The authors thank the institute staff who assisted during the research. The cooperation rendered by the fishers and local authority during sample collection are gratefully acknowledged. Thanks are owing to Ms. Manisha Bohr for helping to prepare the map of Tapti River.
Funding
This study was funded by the Indian Council of Agricultural Research, New Delhi.
Author information
Authors and Affiliations
Contributions
Sangeetha Mohanachandran Nair: manuscript original draft preparation, data collection and analysis. Sajina Aliyamintakath Muhammadali: manuscript drafting, reviewing, editing and data analysis. Satish Kumar Koushlesh: field data collection and compilation. Thangjam Nirupada Chanu: field data collection and compilation. Sanjoy Kumar Das: estimation of water quality parameters and manuscript editing. Dibakar Bhakta: field data collection and compilation. Vaisakh Gopinatha Pillai: field data collection and compilation. Pranab Gogoi: data analysis. Srikanta Samanta: manuscript editing and guidance. Wakambam Anand Meetei: field data collection and compilation. Raju Baitha: field data collection and compilation. Suhas Prakash Kamble: field data collection and compilation. Vikas Kumar: data analysis. Basanta Kumar Das: overall guidance and supervision.
Corresponding author
Ethics declarations
Ethical approval
The study was conducted as per the guidelines of the institute ethical committee.
Consent to participate
The authors gave their consent to participate in the study.
Consent for publication
The authors consented to publish the study.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Thomas Hein
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nair, S.M., Muhammadali, S.A., Koushlesh, S.K. et al. Probing river health status: a study based on index of biotic integrity (IBI) in Tapti River of Deccan plateau, India. Environ Sci Pollut Res 30, 73185–73201 (2023). https://doi.org/10.1007/s11356-023-27497-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-27497-x