Abstract
The toxicity of carbaryl, tebufenpyrad, cypermethrin and permethrin was evaluated in European sea bass Dicentrarchus labrax during the embryonic and larval development using six different concentrations per chemical. The order of the toxicity effectiveness was carbaryl > tebufenpyrad > cypermethrin > permethrin. The larvae were more sensitive to all tested chemicals than embryos. The LC50 of carbaryl, tebufenpyrad, cypermethrin and permethrin was determined as 13.88, 43.96, 92 and 142 ppm and 9.27, 25.67, 48.4 and 72.7 ppm in embryo and larvae, respectively. Furthermore, the tested pesticides exhibited teratogenic effects on D. labrax embryo-larval stages. The observed malformations were coagulation, no spherical egg, unhatched egg, pericardial oedemata, yolk oedemata, lordosis, kyphosis, scoliosis, no eye, cranial deformation and body atrophy. Malformations were induced with 0.5 ppm carbaryl, 10 ppm tebufenpyrad and 50 ppm cypermethrin and permethrin; the highest rates of malformation were noted with 16 ppm carbaryl, 160 ppm tebufenpyrad, 400 ppm cypermethrin and 400 ppm permethrin as 34.5%, 28%, 17.5% and 16%, respectively. A positive correlation between the incidence of malformation and the increase of pesticide concentration was established.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pesticide usage has considerably increased during the last decade specially with the development of the agriculture; in fact, the worldwide use of those compounds was estimated to be 3 billion kilograms each year (Hayes et al. 2017). Definitely, the use of pesticides has become required in order to avoid losses of agricultural products, which were estimated to exceed 70% for fruit, 50% for vegetable and 30% for cereal (Tudi et al. 2021). Pesticides were also used in urban and industrial activities, which contributes to the continual increase of pesticide occurrence in the aquatic ecosystems (Syafrudin et al. 2021). This is not without consequences on non-target species, particularly fish (Hernández-Moreno et al. 2011; Syafrudin et al. 2021).
Carbaryl or Sevin was commercialized for the first time in 1958. It is commonly used in agriculture for the control of pests, and as a molluscicide, its mechanism of action was documented to act as a reversible inhibitor of acetylcholinesterase (Hodgson 2012). Carbaryl toxicity was investigated in freshwater fish species as Poecilia reticulata, Oncorhynchus mykiss, Catla calta, Mystus vittatus, M. cavasius, Anabas testutus, Danio rerio, Cirrhinus mrigala, Labeo rohita and Channa punctatus (Boran et al. 2007; Mahboob et al. 2015; Mustafa et al. 2014; Sambasiva Rao et al. 1985; Tilak et al. 1981; von Hellfeld et al. 2020; Zinkl et al. 1987). Comparative carbaryl toxicity with its degradation product (1-naphthol) was conducted for five freshwater fish species (Tilak et al. 1981). Carbaryl is among the most detected pesticides in streams and groundwater (Gilliom et al. 2006). It was also found in surface water and groundwater in Southern Malawi at concentrations 0.083–0.254 mg/l and 0.165–0.492 mg/l, respectively (Kanyika-Mbewe et al. 2020). In Tunisia, carbaryl was detected in drinking water and in Medjerda River, Mellègue River, Siliana River and Sidi Salem dam (Latrous El Atrache et al. 2016).
The second pesticide chosen is tebufenpyrad also known as Masai, Pyranica or Shirudo®, a pyrazole insecticide, and acaricide was mostly used for the treatment of citrus fruit against Tetranychus urticae, Panonychus ulmi and P. citri (Tomlin 1997). It was recorded as one of the most toxic pesticides for fish (EFSA 2008). Tebufenpyrad acts like a mitochondrial complex I electron transport inhibitor (Charli et al. 2016). Studies on the occurrence and ecotoxicology of this pesticide for fish are very limited; according to Kapsi et al. (2019), tebufenpyrad was detected in the Louros River (north-western Greece) at concentrations ranging from 0.127 to 0.337 ppm. In Tunisia, it was found in chili pepper at a concentration of 0.186 mg/kg (Toumi et al. 2018).
The third and fourth insecticides selected are two pyrethroids cypermethrin and permethrin; both were reported as toxic to non-target species, mainly to fish (Başer et al. 2003). Cypermethrin and permethrin are both synthetic pyrethroids type II and I, respectively (WHO 1989). Cypermethrin was synthesized in 1974 and commercialized for the first time in 1977; it is mostly used for the control of many pests especially Lepidoptera (WHO 1989). Cypermethrin affects the nervous system by disrupting the transport of sodium ion through the cell membrane (WHO 1989). Its toxicity was reported in various fish species like the Indian major carp Labeo rohita (Marigoudar et al. 2013), the common carp Cyprinus carpio (Suvetha et al. 2010), guppy fish Poecilia reticulata (Salako et al. 2020), the Nile tilapia Oreochromis niloticus (Majumder and Kaviraj 2021), the freshwater carp Catla catla (Sharma and Jindal 2020) and zebrafish Danio rerio (Guo et al. 2021).
Permethrin was synthesized in 1973, and was then classified as “Restricted use pesticides” by the US Environmental Protection Agency (EPA) based on its high toxicity to aquatic organisms (Başer et al. (2003); among the studied fish species, we cite the narrow-clawed crayfish Astacus leptodactylus (Günal et al. 2021).
The European sea bass Dicentrarchus labrax was chosen for many reasons; it has been found effective for the biomonitoring of the marine ecosystem, a suitable model of laboratory bioassays (Hernández-Moreno et al. 2011; Mhadhbi et al. 2020; Varo et al. 2003), a highly consumed species with a broad range of distribution (FAO 2021). Indeed, the global farming production of this species has increased from 60,000 t in 2003 to 235,537 t in 2018, with Mediterranean countries being the most important producer (FAO 2021). Besides this, D. labrax is relatively easy in handling, and is characterized by high relative fecundity ranging from 350,000 to 542,000 eggs/kg (Forniés et al. 2001). D. labrax is a euryhaline species with an interesting cycle of life, including an embryonic and a larval stage whose development occurs in the marine environment; juvenile development may occur in marine and/or freshwater environments (Jennings and Pawson 1992). This capacity to tolerate strong variation in salinity allowed for this fish species the colonization of various habitats, which make it an interesting model for ecotoxicological studies in both marine and freshwater ecosystems. As stated above, data reporting the toxicity of carbaryl, cypermethrin and permethrin are available for some fish species; however, there is no report of their effects on Dicentrarchus labrax. Also, investigations of tebufenpyrad toxicity on fish are very rare.
This study focuses on the assessment of carbaryl, tebufenpyrad, cypermethrin and permethrin toxicity on the European sea bass (Dicentrarchus labrax) early life stages. The lethal and sublethal effects of the selected pesticides were determined using six increasing concentrations. The results provided here could serve as useful baseline information, in the future, for the monitoring of contaminant in the aquatic system using Dicentrarchus labrax early life stages as a model.
Materials and methods
Sampling and experimental conditions
Eggs of the European sea bass Dicentrarchus labrax (diameter ~ 1.5 mm) were obtained from a broodstock maintained in captivity at the company SUD AQUACULTURE TUNISIE (SAT), located in the Djorf region of the governorate of Medenine, Southern Tunisia. An egg collector was used for the collection of the floating one, then they were transported to the laboratory in plastic bags put in portable ice boxes. At the laboratory, embryos were acclimated to laboratory conditions during 24 h; the temperature was maintained around 14.0 ± 1.0 °C, pH = 7.80 ± 0.10, and a photoperiod of 12 h light: 12 h dark, and the salinity was measured to around 34‰.
Carbaryl (purity ≥ 98%), tebufenpyrad (purity ≥ 98%), cypermethrin (purity ≥ 98%) and permethrin (purity ≥ 98%) were purchased from Sigma-Aldrich, Co. (St. Louis, MO, USA). The physiochemical proprieties are summarized in Table S1. Stock solutions were made in 1% dimethyl sulfoxide (DMSO, Sigma-Aldrich), and were maintained at 4 °C until use. Effects of the selected pesticides were tested using six increasing concentrations chosen based on results from previous studies: 0.5, 1, 2, 4, 8 and 16 ppm for carbaryl (Toumi et al. 2016); 10, 20, 40, 80, 120 and 160 ppm for tebufenpyrad (Kapsi et al. 2019); and 12.5, 25, 50, 100, 200 and 400 ppm for cypermethrin and permethrin (Ali et al. 2018; Günal et al. 2021).
Acute toxicity test
The acute toxicity test was realized according to OECD Guidelines for the testing of chemicals No. 203 (OECD 2019) and No. 236 (OCDE 2013). The bioassays were conducted on floating fertilized eggs being examined using a dissection microscope. The non-floating eggs and those exhibiting an abnormal development at the stages blastula were eliminated. Two hundred eggs were considered of each concentration, and 50 eggs (× 4 replicates) were carefully distributed into 500-ml glass beakers filled with filtered seawater. Two controls were considered, one without pesticide and the other with DMSO added in volumes equal to that of the tested pesticide. The percentage of mortality was calculated as the following: number of dead embryos / total number for each concentration * 100.
In the control groups, the percentages of mortality were all below 10%, which is required for the validity of the test. Food and oxygen were not provided during the test period. Pesticides’ effects on D. labrax embryos and larvae were monitored daily during 6 days; the first 2 days correspond to the embryo stage after which the larval stage occurs, which corresponds to 96 h of exposure to the tested compounds. The number of dead eggs and embryos was noted. The survival and malformations were daily recorded after hatching; successful hatching was marked by the rupture of the egg membrane. Mortality was recognized by a coagulation of the embryos, a missing of heartbeat, a failure in the development of somites and a non-detached tail.
Teratogenic assessment
Examinations were made every 24 h immediately after hatching; malformed embryo was considered when a coagulated fertilized egg, an abnormal egg shape and an unhatched egg were observed. Larval malformations were reported when a larva with yolk sac and pericardial oedemata is observed; a lordosis, kyphosis, scoliosis, absence of eye, cranial deformity and general atrophy of the body were detected (von Hellfeld et al. 2020). The observations were made under a binocular dissection microscope using a thick slide with a concave chamber filled with seawater (Table 1).
Statistical analyses
Lethal concentrations (LC10–50) and their 95% confidence intervals were calculated according to the Probit method (Finney 1971) using SPSS software, version 19. The no observed effect concentration (NOEC) and the lowest observed effect concentration (LOEC) were calculated through ANOVA with Dunnett’s post hoc test using the SPSS application, version 19.0. Data that did not meet the assumptions for ANOVA were tested using non-parametric tests (Kruskal–Wallis and Mann–Whitney U. Significant differences were considered for (p < 0.05).
Results
Acute toxicity
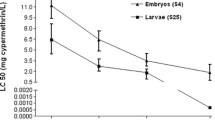
Results from the exposure of Dicentrarchus labrax embryos and larvae to six increasing concentrations of carbaryl, tebufenpyrad, cypermethrin and permethrin showed an increase lethally associated with the received dose of pesticides; this was expressed by a clear dose–effect relationship (Fig. 1). In embryos and larvae, mortality begins to occur after 24 h of exposure to 2 ppm of carbaryl, 10 ppm of tebufenpyrad, 25 ppm of cypermethrin and 25 ppm of permethrin (Table 2). Embryo and larval mortality was time and dose dependent (Table 2). The percentage of hatching success decreased with the increase in pesticide concentration, and a significant difference was detected for a concentration greater than 8 ppm carbaryl, 20 ppm tebufenpyrad, 100 ppm cypermethrin and 100 ppm permethrin (Table 2). LC50 were calculated as 13.88, 43.96, 92 and 142 ppm and 9.27, 25.67, 48.4 and 72.7 ppm for embryos and larvae treated with carbaryl, tebufenpyrad, cypermethrin and permethrin, respectively (Table 3). Also, NOEC and LOEC were higher in embryos than in larvae (Table 3). D. labrax larvae were found more sensitive than embryos to chemicals. Carbaryl was the most toxic pesticide to D. labrax early life stages followed by tebufenpyrad, cypermethrin and permethrin.
Teratogenic effects
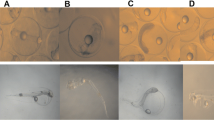
The assessment of the effect of carbaryl, tebufenpyrad, cypermethrin and permethrin during the embryonic development of the European sea bass revealed the occurrence of several malformations (Figs. 2 and 3). Coagulation, unhatched egg, pericardial oedemata, yolk oedemata, lordosis, kyphosis and scoliosis were induced with carbaryl and tebufenpyrad (Fig. 4). One specimen was found without eye following the exposure to 16 ppm carbaryl; also for the same concentration, a cranial deformity was detected (Figs. 3 and 4). A no spherical egg was observed with 120 and 160 ppm (Figs. 2 and 4). A delay in egg hatching was noted in the groups exposed to carbaryl and tebufenpyrad; the longer period (~ 5 h) was recorded in specimens exposed to 8 and 16 ppm carbaryl against (~ 3 h) in those exposed to 120 and 160 ppm tebufenpyrad. A positive correlation between the incidence of malformation and the concentration of the tested pesticide was detected; r is > 0.92 for all the tested pesticides. In fact, the highest rates of malformation were observed with 16 ppm carbaryl (34.5%), 160 ppm tebufenpyrad (28%), 400 ppm cypermethrin (17.5%) and 400 ppm permethrin (16%) (Table 2). No malformations were observed in D. labrax embryos and larvae exposed to cypermethrin and permethrin concentrations ≤ 25 ppm (Fig. 3). Malformations observed in embryos were mainly coagulation (Fig. 2 and 4). In larvae, the most observed malformations were yolk oedemata, pericardial oedemata, lordosis and kyphosis (Figs. 3 and 4).
Morphological abnormalities observed in Dicentrarchus labrax embryos exposed to carbaryl, tebufenpyrad, cypermethrin and permethrin. Coagulation caused by carbaryl (a–o), no spherical egg (p–q) and unhatched egg (r, t). Malformation observed with 8 ppm carbaryl (a–r), 16 ppm carbaryl (d), 80 ppm tebufenpyrad (b), 120 ppm tebufenpyrad (c, l, p), 160 ppm tebufenpyrad (s, q), 400 ppm cypermethrin (e–g, t), 100 ppm permethrin (h–k) and 400 ppm permethrin (m–o). Scale bars 300 µm
Morphological abnormalities reported in Dicentrarchus labrax larvae exposed to carbaryl, tebufenpyrad, cypermethrin and permethrin. Normal larvae (a), lordosis (b–d), kyphosis (e–h), scoliosis (i–r), yolk oedemata (g, i, k, m, n, q, r), pericardial oedemata (p), pericardial and yolk oedemata (h, j), no eye (r), body atrophy (s) and cranial deformity (t). YO yolk oedemata, PO pericardial oedemata, NE no eye. Malformation observed with 4 ppm carbaryl (i), 16 ppm carbaryl (b, d, h, j, r, t), 160 ppm tebufenpyrad (c, g, m, p, q, s), 400 ppm cypermethrin (e) and 400 ppm permethrin (f, k, l, n, o). Scale bars 500 µm
Discussion
Decades of research on ecotoxicology have allowed the assessment of insecticide effects on non-target organisms in both terrestrial and aquatic environments (Antwi and Reddy 2015; Hill 1985). Toxicity of several insecticides like deltamethrin, carbofuran, fenitrothion, chlorpyrifos, dichlorvos, fipronil and trichlorfon was investigated on the European sea bass Dicentrarchus labrax (Almeida et al. 2010; Banni et al. 2011; Dallarés et al. 2020; Hernández-Moreno et al. 2011; Varo et al. 2003). However, most of the conducted studies were focused on the assessment of pesticide toxicity on adult sea bass. Non-target organisms were frequently subjected to pesticide pollution through two ways, directly following spray deposition and drift and indirectly during surface runoff and erosion (Antwi and Reddy 2015). Pesticides were also used in aquaculture for the control of parasite in farmed Dicentrarchus labrax; trichlorfon and teflubenzuron were used for the treatment of Diplectanum aequans infestations (Tokşen et al. 2010, 2013). Moreover, deltamethrin is used for the treatment of Ceratothoa oestroides infestations (Čolak et al. 2019).
Data from the studies of pesticide toxicity on fish species showed a difference in their effects among species and the same time, even in the same species between live stages (Hernández-Moreno et al. 2011). The results for this study showed that the four tested insecticides (carbaryl, tebufenpyrad, cypermethrin and permethrin) exhibited different effects on D. labrax early life stages. Carbaryl was found more toxic than tebufenpyrad, cypermethrin and permethrin. LC50 calculated for D. labrax exposed to carbaryl were 13.88 ppm, 48 h, and 9.27 ppm, 96 h. Those values were lower than those of LC50 reported for other fish species (Table 1). Sublethal concentrations from the literature were in milligrams per litre level and ranged between 0.52 mg/l for O. mykiss (Boran et al. 2007) and 12.2 mg/l for zebrafish embryos (Danio rerio) (von Hellfeld et al. 2020). Carbaryl was found to induce several morphological malformations in zebrafish embryo at concentration < 6.6 mg/l (von Hellfeld et al. 2020). Malformations were induced in D. labrax early life stages with 0.5 ppm carbaryl, and the occurrence of malformed embryos and larvae increased with the increase in the concentration. The results of the bioassay conducted on D. labrax early life stages have proven the toxicity of tebufenpyrad; values of LC50 estimated for embryos 43.96 ppm and larvae 25.67 ppm were approximately similar to those calculated for O. mykiss 23 and 30 ppm (EFSA 2008), while they were higher than the LC50 value, 10.98 ppm 48 h, reported for Daphnia pulex (Asselman et al. 2014). Tebufenpyrad teratogenicity was not reported previously; the results from this study revealed the occurrence of malformed embryos and larvae with 10 and 20 ppm, respectively. Teratogenic effects of pyrazole carboxamides as bixafen and isopyrazam were demonstrated on D. rerio and Xenopus tropicalis during embryonic development (Li et al. 2016; Xiao et al. 2021).
Despite the increased number of studies on the assessment of cypermethrin toxicity, data about its toxicity on the European sea bass D. labrax especially early life stages lacked. Cypermethrin was reported as very toxic for fish based on results conducted on laboratory, with 96 h LC50s being generally within the range of 0.4 and 2.8 ppm (WHO 1989). Sublethal concentrations of 92 ppm 48 h and 48.4 ppm 96 h measured for D. labrax early life stages were higher than values measured for Labeo rohita, Mystus cavasius and Cnesterodon decemmaculatus early life stages of 8.43, 6.12 and 0.48 ppm, respectively (Ali et al. 2018; Carriquiriborde et al. 2007; Dawar et al. 2016). Malformation was observed in D. labrax embryos and larvae exposed to cypermethrin for concentrations ≥ 50 ppm, while it was reported to induce malformations in Mystus cavasius embryos and larvae with 16 and 32 ppm (Ali et al. 2018).
Concerning permethrin, it was classified as toxic to fish since LC50 values were in micrograms per litre level (Başer et al. 2003). Indeed, LC50 ranged between 2 ppm, 24 h in Channa striatus and > 75 mg/l, 48 h in Pimephales promelas (Milam et al. 2000, Singh &Agarwal 1994). Sublethal concentrations measured for D. labrax were 142 ppm, 48 h and 72.7 ppm, 96 h; those values were higher compared to the LC50 estimated for other Actinopterygii (Başer et al., 2003 and reference therein). The teratogenic effect of permethrin was reported for Danio rerio (Dach et al. 2019). Also, the results for this study showed that permethrin has teratogenic effects on D. labrax early life stages; the majority of the malformed specimens were detected for 100 and 200 ppm.
Dicentrarchus labrax larvae were found more sensitive than embryos; Mhadhbi et al. (2012) reported similar results in Psetta maxima exposed to the pesticides (alachlor, atrazine, diuron). The low sensitivity of fish embryos was attributed to the protective role of the external envelope, chorion (Groot and Alderdice 1985). However, it seems that pesticides affected the structure of this envelope by blocking the canal pore, which inhibits the egg permeability (Groot and Alderdice 1985). This could explain the increased rate of coagulated D. labrax embryos. In addition, it was found that pesticides induce a delay and even an inhibition in embryo hatching as a consequence to a disturbance in the morphology and function of the hatching gland cells; therefore, the secretion of the hatching enzyme is reduced Xiao et al. (2021).
Conclusion
Carbaryl, tebufenpyrad, cypermethrin and permethrin were found toxic to the European sea bass, Dicentrarchus labrax, early life stages. The toxicity followed the order carbaryl > tebufenpyrad > cypermethrin > permethrin. Embryo and larval mortality was time and dose dependent. Larvae were found more sensitive than embryos to the studied pesticides. Teratogenic effects of the studied pesticides were recognized of D. labrax early life stages. Results from the current survey provided baseline information that could be used in the future for the risk assessment of pesticides in the aquatic system using Dicentrarchus labrax early life stages as a model.
Data availability
All data are mentioned in the body of manuscript, tables, and figures.
References
Ali MH, Sumon KA, Sultana M, Rashid H (2018) Toxicity of cypermethrin on the embryo and larvae of Gangetic mystus, Mystus cavasius. Environ Sci Pollut Res 25:3193–3199
Almeida JR, Oliveira C, Gravato C, Guilhermino L (2010) Linking behavioural alterations with biomarkers responses in the European seabass Dicentrarchus labrax L. exposed to the organophosphate pesticide fenitrothion. Ecotoxicology 19:1369–1381
Antwi FB, Reddy GVP (2015): Toxicological effects of pyrethroids on non-target aquatic insects. Frank B. Antwi∗, Gadi V.P. Reddy∗ 40, 915–923
Asselman J, Janssena CR, Smagghe G, De Schamphelaere KAC, Silva Hilsdorf AW (2014) Ecotoxicity of binary mixtures of Microcystis aeruginosa and insecticides to Daphnia pulex. Environ Pollut 188:56–63
Banni M, Jebali J, Guerbej H, Dondero F, Boussetta H, Viarengo A (2011) Mixture toxicity assessment of nickel and chlorpyrifos in the sea bass Dicentrarchus labrax. Arch Environ Contam Toxicol 60:124–131
Başer S, Erkoç F, Selvi M, Koçak O (2003) Investigation of acute toxicity of permethrin on guppies Poecilia reticulata. Chemosphere 51:469–474
Boran M, Altinok I, Çapkin E, Karaçam H, Biçer V (2007) Acute toxicity of carbaryl, methiocarb, and carbosulfan to the rainbow trout (Oncorhynchus mykiss) and guppy (Poecilia reticulata). Turk J Vet Anim Sci 31:39–45
Carriquiriborde P, Díaz J, Mugni H, Bonetto C, Ronco AE (2007) Impact of cypermethrin on stream fish populations under field-use in biotech-soybean production. Chemosphere 68:613–621
Charli A, Jin H, Anantharam V, Kanthasamy A, Kanthasamy AG (2016) Alterations in mitochondrial dynamics induced by tebufenpyrad and pyridaben in a dopaminergic neuronal cell culture model. Neurotoxicology 53:302–313
Čolak S, Barić R, Kolega M, Mejdandžić D, Mustać B, Petani B, Župan I, Šarić T (2019) Effect of the pesticide deltamethrin as a treatment of Ceratothoa oestroides infestations of farmed sea bass Dicentrarchus labrax. Aquaculture 500:322–326
Dach K, Yaghoobi B, Schmuck MR, Carty DR, Morales KM, Lein PJ (2019) Teratological and behavioral screening of the National Toxicology Program 91-Compound Library in Zebrafish (Danio rerio). Toxicol Sci 167:77–91
Dallarés S, Dourado P, Sanahuja I, Solovyev M, Gisbert E, Montemurro N, Torreblanca A, Blázquez M, Solé M (2020) Multibiomarker approach to fipronil exposure in the fish Dicentrarchus labrax under two temperature regimes. Aquat Toxicol 219:105378
Dawar FU, Zuberi A, Azizullah A, Khan Khattak MN (2016) Effects of cypermethrin on survival, morphological and biochemical aspects of rohu (Labeo rohita) during early development. Chemosphere 144:697–705
EFSA (2008) Scientific Report. Conclusion on the Peer Rev Tebufenpyrad 192:1–100
FAO (2021): Fishery and aquaculture statistics. Global aquaculture production 1950–2019 (FishstatJ). In: FAO Fisheries Division [online]. Rome. Updated 2021. www.fao.org/fishery/statistics/software/fishstatj/en
Finney DJ (1971): Probit analysis, Cambridge University, London, UK, 3rd edition.
Forniés MA, Mañanós E, Carrillo M, Rocha A, Laureau S, Mylonas CC, Zohar Y, Zanuy S (2001) Spawning induction of individual European sea bass females (Dicentrarchus labrax) using different GnRHa-delivery systems. Aquaculture 202:221–234
Gilliom RJ, Barbash JE, Crawford CG, Hamilton PA, Martin JD, Nakagaki N, Nowell LH, Scott JC, Stackelberg PE, Thelin GP, Wolock DM 2006: Pesticides in the nation’s streams and ground water, 1992–2001. 1291, Reston, VA
Groot EP, Alderdice DF (1985) Fine structure of the external egg membrane of five species of Pacific salmon and steelhead trout. Can J Zool 63:552–566
Günal AÇ, Tunca SK, Arslan P, Göktuğ Gül G, Dinçel AS (2021): How does sublethal permethrin effect non-target aquatic organisms? Environ Sci Pollut Res
Guo D, Liu W, Yao T, Ma M, Wang Q, Qiu J, Qian Y (2021) Combined endocrine disruptive toxicity of malathion and cypermethrin to gene transcription and hormones of the HPG axis of male zebrafish (Danio rerio). Chemosphere 267:128864
Hayes TB, Hansen M, Kapuscinski AR, Locke KA, Barnosky A (2017) From silent spring to silent night: agrochemicals and the anthropocene. Elem Sci Anth 5:1–24
Hernández-Moreno D, Pérez-López M, Soler F, Gravato C, Guilhermino L (2011) Effects of carbofuran on the sea bass (Dicentrarchus labrax L.): study of biomarkers and behaviour alterations. Ecotoxicol Environ Saf 74:1905–1912
Hill IR (1985) Effects on non-target organisms in terrestrial and aquatic environments. In: Leahey JP (ed) The Pyrethroid Insecticides. Taylor and Francis, Philadelphia, pp 151–262
Hodgson E (2012) Chapter 9 - biotransformation of individual pesticides: some examples. In: Hodgson E (ed) Pesticide biotransformation and disposition. Academic Press, Boston, pp 195–208
Jennings S, Pawson M (1992) The origin and recruitment of bass, Dicentrarchus labrax, larvae to nursery areas. J Mar Biol Assoc UK 72:199–212
Kanyika-Mbewe C, Thole B, Makwinja R, Kaonga CC (2020) Monitoring of carbaryl and cypermethrin concentrations in water and soil in Southern Malawi. Environ Monit Assess 192:595
Kapsi M, Tsoutsi C, Paschalidou A, Albanis T (2019) Environmental monitoring and risk assessment of pesticide residues in surface waters of the Louros River (N.W. Greece). Sci Total Environ 650:2188–2198
Latrous El Atrache L, Hachani M, Kefi BB (2016) Carbon nanotubes as solid-phase extraction sorbents for the extraction of carbamate insecticides from environmental waters. Int J Environ Sci Technol 13:201–208
Li D, Liu M, Yang Y, Shi H, Zhou J, He D (2016) Strong lethality and teratogenicity of strobilurins on Xenopus tropicalis embryos: basing on ten agricultural fungicides. Environ Pollut 208:868–874
Mahboob S, Al-Ghanim K, Sultana S, Al-Balawi HA, Sultana T, Al-Misned F, Ahmed Z (2015): A study on acute toxicity of triazophos, profenofos, carbofuran and carbaryl pesticides on Cirrhinus mrigala. Pak J Zool 47
Majumder R, Kaviraj A (2021) Acute toxicity of cypermethrin to freshwater fish Oreochromis niloticus: influence of aquatic weed and turbidity of water. Natl Acad Sci Lett 44:5–7
Marigoudar SR, Nazeer Ahmed R, David M (2013) Ultrastructural responses and oxidative stress induced by cypermethrin in the liver of Labeo rohita. Chem Ecol 29:296–308
Mhadhbi L, Toumi H, Boumaiza M, Aloui N (2012) Toxicity of three selected pesticides (Alachlor, Atrazine and Diuron) to the marine fish (turbot Psetta maxima). Afr J Biotech 11:11321–11328
Mhadhbi L, El Ayari T, Tir M, Kadri D (2020): Azithromycin effects on the European sea bass (Dicentrarchus labrax) early life stages following acute and chronic exposure: laboratory bioassays. Drug Chem Toxicol 1–7
Milam CD, Farris JL, Wilhide JD (2000) Evaluating mosquito control pesticides for effect on target and non-target organisms. Arch Environ Contam Toxicol 39:324–328
Mustafa G, Mahboob S, Al-Ghanim KA, Sultana S, Al- Balawi HFA, Sultana T, Al-Misned F, Ahmed Z (2014) Acute toxicity I: effect of profenofos and triazophos (organophosphates) and carbofuran and carbaryl (carbamates) to Labeo rohita. Toxicol Environ Chem 96:466–473
OCDE (2013): Test No. 236: Fish Embryo Acute Toxicity (FET) Test
OECD (2019): Test Guideline No. 203. Fish, Acute Toxicity Testing. OECD Guidelines for the Testing of Chemicals. OECD Publishing, Paris
Olufayo M, Alade O (2012) Acute toxicity and histological changes in gills, liver and kidney of catfish, Heterobranchus bidorsalis exposed to cypermethrin concentration. Afr J Agric Res 7:4453–4459
Parent LM, DeLorenzo ME, Fulton MH (2011) Effects of the synthetic pyrethroid insecticide, permethrin, on two estuarine fish species. J Environ Sci Health B 46:615–622
Prashanth MS, Hiragond NC, Nikam KN (2011): The effect of cypermethrin on different tissues of freshwater fish Tilapia mossambica (Perters). 22, 115-119
Rice PJ, Drewes CD, Klubertanz TM, Bradbury SP, Coats JR (1997) Acute toxicity and behavioral effects of chlorpyrifos, permethrin, phenol, strychnine, and 2,4-dinitrophenol to 30-day-old Japanese medaka (Oryzias latipes). Environ Toxicol Chem 16:696–704
Saha S, Kaviraj A (2003) Acute toxicity of synthetic pyrethroid cypermethrin to freshwater catfish Heteropneustes fossilis (Bloch). Int J Toxicol 22:325–328
Saha S, Kaviraj A (2008) Acute toxicity of synthetic pyrethroid cypermethrin to some freshwater organisms. Bull Environ Contam Toxicol 80:49–52
Salako AF, Amaeze NH, Shobajo HM, Osuala FI (2020) Comparative acute toxicity of three pyrethroids (deltamethrin, cypermethrin and lambda-cyhalothrin) on guppy fish (Poecilia reticulata peters, 1859). Sci Afr 9:e00504
Sambasiva Rao KRS, Prasada Rao KS, Ahammad Sahib IK, Ramana Rao KV (1985) Combined action of carbaryl and phenthoate on a freshwater fish (Channa punctatus bloch). Ecotoxicol Environ Saf 10:209–217
Sharma R, Jindal R (2020) Assessment of cypermethrin induced hepatic toxicity in Catla: a multiple biomarker approach. Environ Res 184:109359
Singh A, Agarwal RA (1994) Effect of three synthetic pyrethroids to a non-target fish, Channa striatus. Acta Hydrochim Hydrobiol 22:237–240
Suvetha L, Ramesh M, Saravanan M (2010) Influence of cypermethrin toxicity on ionic regulation and gill Na(+)/K(+)-ATPase activity of a freshwater teleost fish Cyprinus carpio. Environ Toxicol Pharmacol 29:44–49
Syafrudin M, Kristanti RA, Yuniarto A, Hadibarata T, Rhee J, Al-onazi WA, Algarni TS, Almarri AH, Al-Mohaimeed AM (2021) Pesticides in drinking water—a review. Int J Environ Res Public Health 18:468
Tilak K, Rao D, Devi A, Murty A (1981) Toxicity of carbaryl and 1-naphthol to four species of freshwater fish. J Biosci 3:457–461
Tokşen E, Değirmenci U, Cankurt M (2010) The effect of Trichlorfon on the control of Lernanthropus kroyeri (van Beneden, 1851)(Lernanthropidae) infestations in cultured sea bass, Dicentrarchus labrax (Linnaeus, 1758). Bull Eur Assoc Fish Pathol 30:205
Tokşen E, Nemli E, Cankurt M (2013) Effect of teflubenzuron on Diplectanum aequans (Monogenea: Diplectanidae) infestations in cultured sea bass, Dicentrarchus labrax. Bul Eur Assoc Fish Pathol 33:91–96
Tomlin CT (1997): The pesticide manual."a Worm Compendium, British Crop Protection Council, Farnham, UK, 11th ed.
Toumi H, Burga-Perez KF, Ferard J-F (2016) Acute and chronic ecotoxicity of carbaryl with a battery of aquatic bioassays. J Environ Sci Health B 51:57–62
Toumi K, Joly L, Tarchoun N, Souabni L, Bouaziz M, Vleminckx C, Schiffers B (2018) Risk assessment of Tunisian consumers and farm workers exposed to residues after pesticide application in chili peppers and tomatoes. Tunis J Plant Prot 13:127–143
Tudi M, Daniel Ruan HD, Li Wang L, Lyu J, Sadler R, Connell D, Chu C, Phung DT (2021): Agriculture development, pesticide application and its impact on the environment. Int J Environ Res Public Health, 1112
Varo I, Navarro JC, Amat F, Guilhermino LE (2003) Effect of dichlorvos on cholinesterase activity of the European sea bass (Dicentrarchus labrax). Pestic Biochem Physiol 75:61–72
von Hellfeld R, Brotzmann K, Baumann L, Strecker R, Braunbeck T (2020): Adverse effects in the fish embryo acute toxicity (FET) test: a catalogue of unspecific morphological changes versus more specific effects in zebrafish (Danio rerio) embryos. Environ Sci Eur 32
WHO (1989): Environmental Health Criteria 82, Cypermethrin. United Nations Environment Programme, the International Labour Organisation, and the World Health Organization p. 156
Xiao P, Li W, Lu J, Liu Y, Luo Q, Zhang H (2021) Effects of embryonic exposure to bixafen on zebrafish (Danio rerio) retinal development. Ecotoxicol Environ Saf 228:113007
Zinkl JG, Shea PJ, Nakamoto RJ, Callman J (1987) Brain cholinesterase activity of rainbow trout poisoned by carbaryl. Bull Environ Contain Toxicol 38:29–35
Acknowledgements
We thank the anonymous reviewers for the comments and suggestions which has considerably improved the quality of the manuscript. We are also grateful to the editor for the effort in compiling and accommodating the reviewers’ comments and suggestions.
Author information
Authors and Affiliations
Contributions
Conceptualization: Lazhar Mhadhbi, M’hamed El Cafsi; methodology: Lazhar Mhadhbi, Tahani El Ayari; formal analysis and investigation: Tahani El Ayari, Lazhar Mhadhbi; writing — original draft preparation: Tahani El Ayari, Najoua Trigui El Menif; writing — review and editing: Tahani El Ayari, Najoua Trigui El Menif, M’hamed El Cafsi.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Bruno Nunes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
El Ayari, T., Mhadhbi, L., Trigui El Menif, N. et al. Acute toxicity and teratogenicity of carbaryl (carbamates), tebufenpyrad (pyrazoles), cypermethrin and permethrin (pyrethroids) on the European sea bass (Dicentrarchus labrax L, 1758) early life stages. Environ Sci Pollut Res 29, 66125–66135 (2022). https://doi.org/10.1007/s11356-022-20421-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-20421-9