Abstract
The use of jackfruit peel as a source for natural and fully biodegradable “nanocellulose” (NC) for the production of bioplastics with Azadirachta indica (A. indica) extracts and polyethylene glycol (PEG) for the antibacterial properties is investigated. The characterization of the biocomposite using FT-IR and WXRD was reported. The physicochemical properties including thickness, moisture content, water holding capacity, swelling, porosity, and biodegradability in soil were investigated. The incorporation of A. indica extract revealed an increased shelf life due to the strong antibacterial activity, and these biocomposites were degraded in soil within 60 days after the end use without any harm to the environment. Jackfruit-derived nanocellulose film blended with A. indica extract exhibited strong antibacterial activity against gram-positive and gram-negative food spoilage bacteria. Disc diffusion assay, live/dead assay, and CFU analysis confirmed the antibacterial property of the synthesized film. Moreover, the films clearly prevented the biofilm formation in bacteria. Thus, the developed bioplastics can be utilized as appropriate substitutes to food packaging materials and also for biomedical applications such as wound dressings.
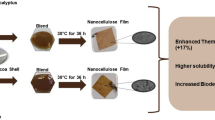
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Agricultural waste materials are of growing interest nowadays to produce value-added biocomposites for various industry applications (Bilo et al. 2018; Dilamian and Noroozi 2019; Reshmy et al. 2020a, 2020b, 2021a). Among them, an interesting one is jackfruit peels. These agroresidues are composed of mainly three constituent such as cellulose, hemicellulose, and lignin (Reshmy et al. 2021b). Among them, cellulose as well as hemicellulose is carbohydrate derivatives that can be easily broken down by enzymes and/or chemical treatments followed by fermentation to produce valuable products such as bioelectricity, biofuels, biomass-derived platform chemicals, and other useful products. Recently, both the research and industrial sectors are focusing immensely on the development of bioproducts in a cheaper and easier route because of the demand for renewable resources and ecological concerns. Some cellulose derivatives are already being implemented in pharmaceuticals and scaffolds, food additives, constructions, and textiles (El-Bakry et al. 2015; Arantes et al. 2020; Maraveas 2020).
Nanocellulose (NC) can be extracted from jackfruit (scientific name: Artocarpus heterophyllus Lam.) agroresidue as reported by several recent studies (Dutta et al. 2011; Retnowati et al. 2015; Raj and Ranganathan 2018; Trilokesh and Uppuluri 2019). The peel was processed by chemical extraction routes to produce pure nanocellulose in support with steam explosion and homogenization (Reshmy et al. 2021b). NC is an extremely smart nanobiomaterial for biomedical applications due to its high porosity and suitable water retention properties, as well as its ability to mold into any form and manipulate with various bioagents. Neem extract (NM) (scientific name: Azadirachta indica) are used in the present study because of their activities including antibacterial, antiviral, and antifungal properties (Sharma and Bhardwaj 2020; Oyekanmi et al. 2021). Biopolymers with several antimicrobial functionalities are significant in diversifying its use in various applications such as bioactive packagings, wound dressings, specialized nanopapers, and antimicrobial apparel for the biomedical industry. The use of bioagents in scientific study is attaining traction in a variety of technical fields. However, research on the use of bio-extracts especially neem extract to impart antimicrobial properties to NC is limited. Herbal agents derived from plants are very easy to get, have low mammalian cytotoxic effects, and degrade easily in the environment, making them comparatively straightforward to exploit as environmentally acceptable alternatives to antibiotics that induce biological system and environmental adverse effects. In this context, this paper aims to report the effect of A. indica extract on jackfruit-derived NC along with poly ethylene glycol (PEG) as scaffold for both food industry and biomedical application.
Materials and methods
The chemicals consumed in the present study were of high purity. Jackfruit peel utilized in the present work was taken from a local market at Alappuzha, Kerala, India and A. indica was from a nearby biodiversity spot in Kerala, India. The extraction of high-purity NC was carried out according to the previous reports using steam explosion–aided chemical treatment followed by mechanical processing (Reshmy et al. 2021b). The high-purity NC suspensions were kept at 4 °C for the production of different formulations. The NM extract used in the present study was prepared by soxhlet extraction of 20 g A. indica using 95% pure ethanol, and the two different compositions of this natural resin was added to impart targeted properties to NC + PEG formulations.
Thin-film production
Thin films of NC, NM, and PEG systems were developed using solvent cast method. The control film was produced by the direct evaporation of 100 mL stock NC suspension using the reported conditions. Polyethylene glycol 600 (PEG) is used in the present method as a plasticizer, and this thin films were developed by casting the formulation including 1 g of PEG-600 dispersed in very minimum quantity of distilled water and 100 mL NC suspension. Similarly, other novel biocomposite scaffolds were developed by adding two different compositions such as 6% and 8% of A. indica extract to 100 mL of NC suspension containing 1 g PEG-600 ( NC + PEG + 6% NM and NC + PEG + 8% NM).
Characterization of thin films
The developed films were characterized by sophisticated instruments, namely, Fourier transform infrared (FT-IR) and wide-angle X-ray diffraction (WXRD). FT-IR (instrument: Perkin-Elmer spectrophotometer) spectra were recorded in the wave number range 400–4000 cm−1 with high precision. WXRD analysis (instrument: Rigaku Miniflex 600) were conducted in a step-scan mode by scanning samples under a monochromatic light with wave length 0.154 nm (scanning time of 5.0 min. and 2θ of 0–50°). The presence of characteristic peaks and functional groups was confirmed using the spectra.
Physical properties of thin films
The physical properties such as thickness, moisture content, water holding, swelling nature, porosity, and biodegradability in soil were also investigated to study the effectiveness of new formulations in food packaging and biomedical industry. The thicknesses of the developed NC films were measured by digital micrometer (Model: 2K706). The thickness of film at different points was measured, and the average value of about 10 measurements was reported as the thickness of the sample. Moisture content of the 3 × 3 cm2 thin-film samples was analyzed using digital moisture analyzer (Model: BTS 110). The water holding capacity of the thin-film samples (2.5 × 2.5 cm2) was calculated using the equation (Mathew et al. 2019):
[W2 wet weight of thin film, W1 initial dry weight of thin film].
Water swelling nature of the thin films was analyzed according to ASTM D2765-95C. The % swelling was calculated as shown in Eq. (2).
[W2 sample weight after swelling, W1 weight of the dry sample].
Porosity of the thin-film samples was determined as per ASTM standard ASTM C20-00(2015). The porosity was determined by immersing the oven-dried sample in distilled water for 48 h and was calculated using Eq. (3) shown below.
[Wi initial weight of dry sample, Wf weight after immersing in distilled water, V volume of the film before immersion in distilled water, ρ density of cellulose (standard value 1.5 g/cm3)] (Fittipaldi et al. 2017; Sharma and Bhardwaj 2020).
Biodegradability is determined by soil burial route using ASTM D 2216. The analysis was carried out by placing the specimens (1 cm × 1 cm) in the soil in a pot with tiny holes and was buried approximately 10 cm in depth. Note the physical changes at regular intervals after taking out the specimens carefully and dried in oven at 65 °C for 24 h and weighed. The % weight loss was determined using Eq. (4):
[W1 dry sample weight, W2 weight of the buried sample].
Colony-forming units
The antimicrobial activity of neem-incorporated NC composite was analyzed by CFU analysis. The assay was performed as described earlier (Reshmy et al. 2022). In short, the bacterial cultures were grown and diluted to maintain an optical density of 0.05 units. The nanocellulose composite films (NC/NC + PEG/NC + PEG + NM[6%]/NC + PEG + NM[8%]) were positioned on culture and incubated for 24 h at 37 °C. Followed by incubation, 1 mL of culture from each group was serially diluted and plated on agar plates to count the colonies.
Disc diffusion assay
The antimicrobial effect of neem-incorporated NC composite was analyzed against the pathogenic E. coli bacteria by disc diffusion method. Discs having 5-mm diameter were made from neem-incorporated NC composite and was sterilized by autoclaving. Fresh cultures of pathogenic bacteria were spread on agar plates, and the sterilized discs were positioned on them. The plates were incubated for 24 h at 37 °C. Following incubation, the zone of inhibition was imaged and measured.
Live/dead assay
The antimicrobial efficacy of NM incorporated NC composite were also confirmed by live-dead assay. The bacterial cells were treated with corresponding NC composites. After 24-h growth in incubator, the bacteria were stained (SYTO9 + propidium iodide (PI)) according to manufacturer’s (L7007, LIVE/DEAD kit, Thermo Fisher Scientific, USA) instructions and the fluorescence intensity was read at 485 and 530 nm (green) and 485/630 nm (red) and green to red intensity ratio was calculated (Mah et al. 2003). Fluorescence images were captured using Nikon confocal microscope.
Inhibition of biofilm formation
Nanocellulose discs (NC/NC + PEG, NC + PEG + NM[6%], NC + PEG + NM[8%]) with diameter 50 mm was placed on the appropriate wells of 24-well plate containing 2 mL 7H9 media inoculated with 20 µL of 1% M. smegmatis culture (~ 1.5 OD at 600 nm). The plate was kept for incubation at 30 °C and was monitored at 4th day for biofilm formation. The images were taken at 4th day (Thibeaux et al. 2020).
Result and discussions
Characteristics of nanocellulose scaffolds from jackfruit peel and A. indica extract
The FT-IR spectrum of the developed NC and their composites confirmed peaks attributed to O–H and C-H stretching vibrations at an absorption region of 3406 cm−1 and 2903 cm−1, respectively. The C–O–C stretching vibration due to the presence of pyranose ring was attributed to a characteristic peak at 1056 cm−1. The peaks correspond to -CH2 wagging vibrations, C-H bending, and C–O–C asymmetric stretching of the polysaccharide moieties of the cellulose were observed at 1311 cm−1, 1370 cm−1, and 1160 cm−1 respectively. The presence of β-(1 → 4)-glycosidic linkage was confirmed by the stretching peak at 898 cm−1. The WXRD spectral patterns of NC and its biocomposites showed characteristic peaks 16.4°, 22.7°, and 25.4° corresponding to the crystalline structure of the NC. The FT-IR and XRD spectra of the biocomposites are depicted in Fig. 1a and b.
The developments of novel thin films using A. indica leaf extract was chosen in this study because of their prominent antibacterial and antiviral activities. Thin films using two different compositions, namely, 6% and 8% of A. indica extract, NC and PEG were cast to produce biocomposites and investigated for their activities as a scaffold for food packaging applications.
Physical properties of thin films
Thicknesses of the thin films were obtained in the range of 0.06 to 0.08 mm. Analysis of moisture content of the films revealed a reduction in moisture absorption due to the incorporation of A. indica extract and a direct relationship with the A. indica extract content and moisture reduction was observed. The water holding capacity of thin films were found to be high for pure cellulose, and the result showed a reduction in water holding and moisture absorption that could facilitate for the production of effective food packaging. A higher value of water holding capacity for pure NC thin films reveals the hydrophilicity of NC. The strong hydrogen bonding present between the nanofibrils of NC allows water molecules to rapidly entrap well within NC framework. The mechanical properties of jackfruit peel–derived NC/PEG biocomposite were already reported by the same research group and found that the Young’s modulus (2959.92 Mpa), tensile strength (88.69 MPa), and elongation at break (8.25%) were improved due to the compatibility of PEG with NC (Reshmy et al. 2021a, b). These improved mechanical properties along with neem extract for inducing antibacterial and anti-biofilm formation activities will make it more suitable for food packaging applications. The comparably good values of water holding, moisture content, and porosity of NC thin films also point to the application of these NC thin films in adsorption applications such as wound dressing, drug delivery, scaffold fabrication, and facial biomasks. The above-described physical characteristics of NC biocomposites are depicted in Table 1.
The swelling in water (Fig. 2) and the porosity (Figs. 3 and 4) of the thin films were also analyzed to confirm the suitability of these NC scaffolds for various applications. The swelling nature of NC control film is found to be high because of their strong hydrophilicity. The incorporation of PEG with NC results in the reduction of swelling nature due to its plasticizing nature which reduces the water uptake. In various biomedical applications, porosity is a significant factor for promoting cell migration and enables diffusion of nutrients and oxygen within tissue. The water holding capacity and swelling are in direct relationship with porosity.
Biodegradability of different NC biocomposites and pure NC in soil analyzed by soil burial method (Reshmy et al. 2020b)
Biodegradability analysis
Biodegradation of these thin films were analyzed by soil burial method. The degradation were analyzed at regular intervals by removing the strips and dried in an oven at 65 °C for 24 h and weighed. It was seen that the degradation of pure NC film is completed within 35 days, that of plasticized films were happened in 45 days, and the A. indica extract–incorporated thin films were degraded completely only after 60 days due to the antibacterial effect of the resin.
Antibacterial action of jackfruit-derived NC blended with A. indica extract
Packaging of food is vital in preserving the safety and quality of foodstuffs, from process and manufacture to handling and storage, until the foodstuff reaches the customers. Active food packing guarantees the quality and safeness of the food commodity through the contact between the packing film, food items, and inner and outer environments (Realini and Marcos, 2014). The technique denotes to the integration of active chemicals or natural chemicals into packaging material. The antimicrobial active packing is a form of packing that comprises antibacterial agents that can interact with the headspace and packed food with the intention to stop bacterial infection. The authentication of the antibacterial nature of jackfruit-derived NC blended with A. indica was performed by determining the growth inhibition of gram-positive (Bacillus cereus) and gram-negative (Escherichia. coli, Pseudomonas sp.) bacteria, which are key microorganisms responsible for food spoilage in food industry (Alzohairy Mohammad 2016, Ali et al. 2021). A. indica extract displayed antibacterial action by in vitro and in vivo experiments due to the occurrence of several natural chemical constituents, namely, presence of bioactives like desactylimbin, triterpenes or the limonoids like desactylimpin, meliantriol, quercetin, nimbin, nimbosterol, nimbinin, and margisine. Other chemical bioactives like azadiradione, nimonol, and epoxy azadradione were also reported to have antimicrobial properties (Dai et al. 2001). Therefore, we introduced A. indica to NC derived from jackfruit. The antimicrobial action of the NC composite was analyzed towards Escherichia coli, Bacillus cereus, and Pseudomonas sp. Jackfruit-derived NC with A. indica extract displayed high antimicrobial activity towards both gram-positive and gram-negative bacteria. A highly substantial reduction of CFU of Escherichia coli, Bacillus cereus, and Pseudomonas sp. (approximately 40%) after treatment with NC neem–blended composite related to the un-treated bacteria (Fig. 5a, b, c). To reinforce our finding, we have further demonstrated the live/dead assay of E. coli bacteria treated with NC blended with A. indica extract using SYTO9 and PI stain. The live bacterial cells were appeared green by the dye SYTO9 as this dye could attach to genomic DNA after entering through the intact cellular membrane. However, the dead bacterial cells were appeared red as the as the dye PI enter the bacterial cells via the injured cell membrane and attach to DNA after replacing SYTO9. Thus, the live/dead assay using the propidium iodide and SYTO9 demonstrated the antibacterial action of A. indica against E. coli (Fig. 6a and b). The disc diffusion assay further demonstrated that the antimicrobial effect was dose-dependent. The diameter of the inhibition zone for the E. coli strain was measured to be 10 mm and 15 mm for 6% and 8% neem extract–blended NC film discs, respectively (Figs. 6c, 7). Thus, we proved that the synthesized nano-composite is efficient for sterile packing of food items to control bacteria triggering food contamination. A functionalized nano-crystalline cellulose modified with aldehyde group and immobilized with nisin and lysozyme displayed antibacterial action against Bacillus. subtilis and Staphylococcus aureus was reported (Tavakolian et al. 2018). A nanocellulose Ag-NP nanocomposite was developed and evaluated for antibacterial effect against Staphylococcus aureus and E. coli (Sarwar et al. 2018). Panaitescu et al. (2018) had constructed a PHB-based composite with zinc oxide–coated nanocellulose and nanoparticles for antibacterial action against E. coli and S. aureus. This resulted in complete inhibition of S. aureus growth (Panaitescu et al. 2018). Tyagi et al. (2019) developed a combination of CNC and chitosan for coatings for antimicrobial and superabsorbent tissue papers. These modification of nanocellulose could inhibit 99% of the survival of E. coli (Tyagi et al. 2019). In comparison with the above-mentioned composites of nanocellulose, our nanocellulose composite showed wide range of antibacterial action against both gram-positive and gram-negative bacteria and the antimicrobial property is plant derived instead of direct usage of a synthetic chemical compound. Thus, our NC combined with A. indica extract might be a good candidate to make food packing films.
Determination of antimicrobial action of nanocellulose derived from jackfruit residue incorporated with A. indica extract against various pathogenic bacterial strains by colony counting method. a E. coli, b B. cereus, and c Pseudomonas sp. #Differ significantly (p value ≤ 0.05) from control, NC and NC + PEG groups
Determination of antimicrobial efficacy of nanocellulose derived from jackfruit residue incorporated with A. indica extract via live/dead and disc diffusion methods. a Fluorescent images of treated bacteria. The live and dead bacteria are stained green and red, respectively. b Ratio of green to red fluorescence was measured in triplicates to determine the relative viability of bacteria. c Representative image showing the zone of inhibition produced by neem extract–incorporated nanocellulose against E. coli
Nanocellulose derived from jack fruit residue incorporated with A. indica extract inhibits biofilm formation in M. smegmatis. M. smegmatis treated with (a) nanocellulose (NC), (b) NC with polyethylene glycol (PEG), (c) NC + PEG with 6% A. indica extract (NM) and (d) NC + PEG + NM (8%) (Reshmy et al. 2022)
Jackfruit NC blended with A. indica extract showed anti-biofilm property against Mycobacterium smegmatis
Biofilms are highly multifaceted bacterial ecosystems created by one or more bacterial species immersed in an extracellular matrix of different compositions depending on the type of biomedical device or food manufacturing environment or and the colonizing species. Bacterial colonization and following biofilm formation significantly hamper surface activity in a plethora of situations including food packaging and medical devices (Koo et al. 2017). As a result, the growth of surfaces by bacteria has been a rigorous research aiming at finding anti-biofilm agents for the benefit of humans.
Nanocellulose derived from jackfruit blended with A. indica extract inhibited biofilm formation in M. smegmatis at the tested concentration. The 8% A. indica extract blended with NC had significant anti-biofilm activities compared to 6% extract; however, both extracts failed to completely inhibit M. smegmatis biofilm formation. However, control nanocellulose films did not inhibit biofilm formation. Recently Hassan et al. (2020) have developed antibacterial nanocellulose films by modification of the surface with dehydroabietic acid derivatives. Their film reduces the number of drug-resistant Staphylococcus aureus and also prevented the biofilm formation in chronic wounds. Lead extracts of neem was highly effective in disrupting formation and structure of biofilms in Pseudomonas aeruginosa (Harjaj et al. 2013; Jack et al. 2017). Thus, neem extract–blended jackfruit NC can be a better candidate for biomedical application as wound dressing material.
Conclusion
This is the first report on modification of jackfruit peel–derived NC with the A. indica extract and PEG as a plasticizer for inducing antibacterial properties to pure NC. These properties showed a considerable improvement for application in food packagings by reducing the high hydrophilic tendencies of NC films that will normally lead to spoil the preserved food commodities. All the developed biocomposites have a fully degradation ability within 60 days in soil. These films showed remarkable antibacterial and anti-biofilm formation properties due to the incorporation of 8% A. indica extract compared to 6% extract. These characteristics will contribute to the design of new bioplastics for both food packagings and also for developing wound dressings biomaterial for biomedical applications.
Data availability
This article presents an original research work executed by the authors, so all the data presented are depending on their findings and analysis techniques. The datasets used in this article are available from the corresponding author on reasonable request.
References
Ali E, Islam MS, Hossen MI, Khatun MM, Islam MA (2021) Extract of neem (Azadirachta indica) leaf exhibits bactericidal effect against multidrug resistant pathogenic bacteria of poultry. Vet Med Sci. 7(5):1921–1927. https://doi.org/10.1002/vms3.511
Alzohairy M A (2016) “Therapeutics Role of Azadirachta indica (Neem) and Their Active Constituents in Diseases Prevention and Treatment.” Evidence-based complementaryand alternative medicine : eCAM vol 2016, 7382506. https://doi.org/10.1155/2016/7382506
Arantes V, Dias IKR, Berto GL, Pereira B, Marotti BS, Nogueira CFO (2020) The current status of the enzyme-mediated isolation and functionalization of nanocelluloses: production, properties, techno-economics, and opportunities. Cellulose 27:10571–10630
Bilo F, Pandini S, Sartore L, Depero LE, Gargiulo G, Bonassi A, Federici S, Bontempi E (2018) A sustainable bioplastic obtained from rice straw. J Clean Prod 200:357–368. https://doi.org/10.1016/J.JCLEPRO.2018.07.252
Dai J, Yaylayan VA, Vijaya Raghavan GS, Parè JR, Liu Z (2001) Multivariate Calibration for the Determination of Total Azadirachtin-Related Limonoids and Simple Terpenoids in Neem Extracts Using Vanillin Assay. J Agric Food Chem 49 (3):1169–1174. https://doi.org/10.1021/jf001141n
Dilamian M, Noroozi B (2019) A combined homogenization-high intensity ultrasonication process for individualizaion of cellulose micro-nano fibers from rice straw. Cellulose 26:5831–5849. https://doi.org/10.1007/s10570-019-02469-y
Dutta H, Kumar S, Kalita D, Lata C (2011) Effect of acid concentration and treatment time on acid – alcohol modified jackfruit seed starch properties. Food Chem 128:284–291. https://doi.org/10.1016/j.foodchem.2011.03.016
El-Bakry M, Abraham J, Cerda A, Barrena R, Ponsá S, Gea T, Sánchez A (2015) From wastes to high value added products : novel aspects of ssf in the production of enzymes. Crit Rev Environ Sci Technol 45:1999–2042. https://doi.org/10.1080/10643389.2015.1010423
Fittipaldi N, Pessoa J, Feitosa A, Miguel F, Paulo J, Morais S, Karine F, De SM, De SM, De FM (2017) Bacterial cellulose nanocrystals produced under different hydrolysis conditions : properties and morphological features. Carbohyd Polym 155:425–431. https://doi.org/10.1016/j.carbpol.2016.08.090
Harjaj K, Bala A, Gupta RK, Sharma R (2013) Leaf extract of Azadirachta indica (neem): a potential antibiofilm agent for Pseudomonas aeruginosa. Pathog Dis 69(1):62–65. https://doi.org/10.1111/2049-632X.12050
Hassan G, Forsman N, Wan X, Keurulainen L, Bimbo LM, Stehl S, Chrubasik FVCM, Prakash AS, Johansson LS, Mullen DC, Johnston BF, Zimmermann R, Werner C, Yli-Kauhaluoma J, Coenye T, Saris PEJ, Österberg M, Moreira VM (2020) "Nonleaching,highly biocompatible nanocellulose surfaces that efficiently resist fouling by bacteria in an artificial dermis model." ACS Applied Bio Materials 3,(7): 4095–4108
Jack AA, Nordli HR, Powell LC, Powell KA, Kishnani H, Johnsen PO, Pukstad B, Thomas DW, Chinga-Carrasco G, Hill KE (2017) The interaction of wood nanocellulose dressings and the wound pathogen P. aeruginosa. Carbohydr Polym 157:1955–1962. https://doi.org/10.1016/j.carbpol.2016.11.080
Koo H, Allan RN, Howlin RP, Stoodley P, Hall-Stoodley L (2017) Targeting microbial biofilms: current and prospective therapeutic strategies. Nat Rev Microbiol 15:740−755
Mah TF, Pitts B, Pellock B, Walker GC, Stewart PS, O’Toole GA (2003) A genetic basis for Pseudomonas aeruginosa biofilm antibiotic resistance. Nature 426:306–310. https://doi.org/10.1038/nature02122
Maraveas C (2020) Production of sustainable construction materials using agro-wastes. Materials 13:262–291
Mathew S, Snigdha S, Mathew J, Radhakrishnan EK (2019) Biodegradable and active nanocomposite pouches reinforced with silver nanoparticles for improved packaging of chicken sausages. Food Packag Shelf Life 19:155–166. https://doi.org/10.1016/j.fpsl.2018.12.009
Oyekanmi AA, Kumar USU, Abdul Khalil HPS, Olaiya NG, Amirul AA, Rahman AA, Nuryawan A, Abdullah CK, Rizal S (2021) Functional properties of antimicrobial neem leaves extract based macroalgae biofilms for potential use as active dry packaging applications. Polymers 13:1–22. https://doi.org/10.3390/polym13101664
Panaitescu DM, Ionita ER, Nicolae CA, Gabor AR, Ionita MD, Trusca R, Lixandru BE, Codita I, Dinescu G (2018) Poly(3-hydroxybutyrate) modified by nanocellulose and plasma treatment for packaging applications. Polymers 10:1–24. https://doi.org/10.3390/polym10111249
Raj AAS (2018) Thottiam Vasudevan Ranganathan (2018) Characterization of cellulose from jackfruit ( Artocarpus integer ) peel. J Pharm Res 6:1–6. https://doi.org/10.18006/2018.6(2).414.424
Reshmy R, Madhavan A, Philip E, Paul SA (2021a) Sugarcane bagasse derived nanocellulose reinforced with frankincense ( Boswellia serrata ): physicochemical properties, biodegradability and antimicrobial effect for controlling microbial growth for food packaging ap. Environ Technol Innov 21:101335. https://doi.org/10.1016/j.eti.2020.101335
Reshmy R, Philip E, Paul S, Madhavan A, Raveendran S, Binod P, Pandey A, Sirohi R (2020a) Nanocellulose-based products for sustainable applications-recent trends and possibilities. Rev Environ Sci Biotechnol 19:779–806. https://doi.org/10.1007/s11157-020-09551-z
Reshmy R, Philip E, Paul S, Madhavan A, Raveendran S, Parameswaran B, Pandey A (2020b) A green biorefinery platform for cost-effective nanocellulose production: investigation of hydrodynamic properties and biodegradability of thin films. Biomass Convers Biorefin 9:1–10. https://doi.org/10.1007/s13399-020-00961-1
Reshmy R, Aravind Madhavan, Arun K B, Philip E, Sindhu R, Binod P, Anoop Puthiyamadam, Mukesh Kumar Awasthi, Ashok Pandey. (2022). Chili post-harvest residue-derived nanocellulose composite as a matrix for in vitro cell culture and Hemigraphis colorata blended nanocellulose extends antimicrobial potential. Sustain Chem Pharm 25, 100584. https://doi.org/10.1016/j.scp.2021.100584
Reshmy R, Philip E, Vaisakh PH, Raj S, Annie S, Madhavan A, Sindhu R, Binod P, Sirohi R, Pugazhendhi A, Pandey A (2021b) Development of an eco-friendly biodegradable plastic from jack fruit peel cellulose with different plasticizers and Boswellia serrata as filler. Sci Total Environ 767:144285. https://doi.org/10.1016/j.scitotenv.2020.144285
Retnowati DS, Ratnawati R, Purbasari A (2015) A biodegradable film from jackfruit (Artocarpus Heterophyllus) AND Durian( Durio Zibethinus) seed flours. Sci Study Res Chemistry Chem Eng Biotechnol Food Ind 16:395–404
Sharma C, Bhardwaj NK (2020) Fabrication of natural-origin antibacterial nanocellulose films using bio-extracts for potential use in biomedical industry. Int J Biol Macromol 145:914–925. https://doi.org/10.1016/j.ijbiomac.2019.09.182
Sarwar MS, Niazi MBK, Jahan Z, Ahmad T, Hussain A (2018) Preparation and characterization of PVA/nanocellulose/Ag nanocomposite films for antimicrobial food packaging. Carbohydr Polym 184:453–464. https://doi.org/10.1016/j.carbpol.2017.12.068
Tavakolian M, Okshevsky M, Ven TGM, Van De, Tufenkji N (2018) Developing antibacterial nanocrystalline cellulose using natural antibacterial agents. ACS Appl. Mater. Interfaces 10, 33827–33838
Thibeaux R, Kainiu M, Goarant C (2020) Biofilm formation and quantification using the 96-microtiter plate. Methods in Molecular Biology. Humana, New York, pp 207–214
Trilokesh C, Uppuluri KB (2019) Isolation and characterization of cellulose nanocrystals from jackfruit peel. Sci Rep 9:1–8. https://doi.org/10.1038/s41598-019-53412-x
Tyagi P, Mathew R, Opperman C, Jameel H, Gonzalez R, Lucia L, Hubbe M, Pal L (2019) High-strength antibacterial chitosan-cellulose nanocrystal composite tissue paper. Langmuir 35:104–112. https://doi.org/10.1021/acs.langmuir.8b02655
Acknowledgements
The authors are grateful to all researchers that contributed to the data collection required for this study. The authors Reshmy Rajasekharan and Raveendran Sindhu are thankful to the Department of Science and Technology (DST), Government of India, for providing the funding through Women Scientist Scheme (WOS-B), vide project grant nos: SR/WOS-B/587/2016 and SR/WOS-B/740/2016 for this research work.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, and writing original draft, Reshmy Rajasekharan; data collection and analysis, Arun Karthika Bahuleyan; analysis and writing original draft, Aravind Madhavan; literature survey, Eapen Philip; conceptualization, Raveendran Sindhu; revision, Parameswaran Binod; reviewing, Mukesh Kumar Awasthi; and editing, Ashok Pandey. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
“Not applicable”, research does not report on or involve the use of any animal or human data or tissue.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Jackfruit peel derived nanocellulose (NC) incorporated with neem extract revealed new possibilities for innovative food packagings.
• The physical and biodegradability studies showed remarkable improvements in NC biocomposites compared to pure NC.
• Neem extracts are found to be a cost-effective material for imparting antibacterial and anti-biofilm activities.
Rights and permissions
About this article
Cite this article
Rajasekharan, R., Bahuleyan, A.K., Madhavan, A. et al. Neem extract–blended nanocellulose derived from jackfruit peel for antibacterial packagings. Environ Sci Pollut Res 30, 8977–8986 (2023). https://doi.org/10.1007/s11356-022-20382-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-20382-z