Abstract
Mangroves are regarded as important settlement grounds and nurseries for fishes due to the sheltered nature of these ecosystems. To identify the drivers of temporal variations in fish assemblages, seasonal variations of fish assemblages and environmental variables were investigated in Nanliu River Estuary, China, from July 2019 to May 2020. In total, 17,680 individuals weighing 24,724.73 g belonging to 23 families and 45 species were collected. The dominant species were Ambassis urotaenia, Mugil cephalus, Coptodon zillii, Gambusia affinis, and Bostrychus sinensis. Fish assemblages were significantly different in seasonal periodicity according to the results of nonmetric multidimensional scaling (NMDS) and permutational multivariate analysis of variance (PERMANOVA). Kruskal–Wallis test results revealed that species richness, fish abundance, and biomass were significantly different, seasonally. The highest number of fish specimens captured was recorded in winter and summer, while the highest biomass was observed in autumn. Different ecological types of fish utilized mangroves in different ways. Hence, the findings in this study are instrumental in understanding and the conservation of mangroves even coastal wetland and fish resources in the process of mangrove restorations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Located between the land and the sea on tropical and subtropical coastlines, mangroves are essential habitats for a variety of fish, which support and enhance fishery resources and species diversity (Nagelkerken et al. 2008; Igulu et al. 2013). Mangrove ecosystems contain complex creek systems which expand the scope of mangrove areas and promote the migration of fish within the shallow coastal zone (Mwandya et al. 2010). Fish resident species and transient species enter mangroves through tidal creeks for foraging, refuge, and rearing (Rehage and Loftus 2007; Vaslet et al. 2012).
Numerous studies have been carried out about seasonal differences of fish assemblages in estuarine mangroves (Ikejima et al. 2003; Wainaina et al. 2013; Huang et al. 2016). Seasonal variations of freshwater discharge and tidal changes throughout a year may cause huge fluctuations in salinity and other physicochemical conditions so that seasonal changes in the immigration and emigration of freshwater and marine species in estuarine mangroves affected fish assemblage structures (Barletta et al. 2008; Mandal et al. 2013; Shahraki et al. 2016). In the mangrove creeks of Qeshm Island, higher numbers of resident species existed during the wet season when the salinity was very low, and fish abundance and biomass were the highest in winter due to the migration of dominant marine transient species at high salinity (Shahraki et al. 2016). It is also found that the seasonal pattern of the fish assemblages was caused by the reproductive characteristics of dominant species (Tsai et al. 2015). Ikejima et al. (2003) showed that fish abundance and species richness in the rainy season was significantly higher than that in the dry season, and this change may be caused by the breeding patterns of fishes and seasonal changes in food availability. In addition, Barletta’s view was that seasonal changes of fish assemblages may be determined by a combination of temporal fluctuations in the fish assemblages induced by rainfall, reproduction, recruitment of resident estuarine species, and recruitment of marine or freshwater species in tidal mangrove creeks of the lower Caeté Estuary (Barletta et al. 2003). Therefore, it is very important to explore the temporal variation characteristics and causes of the fish assemblages in estuarine mangroves for the protection and utilization of fish resources (Desmond et al. 2002; de Azevedo et al. 2016).
Nanliu River is the largest independent river flowing into the sea in Guangxi province, and its estuary is a complex subtropical estuary ecosystem that integrates freshwater ecosystems, seawater ecosystems, brackish water-freshwater mixed ecosystems, tidal flat wetland ecosystems, estuary islands, and sandbar wetland ecosystems. Many scholars have conducted extensive studies on the fish community in mangrove forests listed as nature reserves (Wang et al. 2009; Wu et al. 2018; Zhang et al. 2019). Fish data from mangroves in Nanliu River Estuary (NRE), as a nonnatural reserve, were rarely reported. Therefore, based on the investigation of fish assemblage structures in mangrove creeks on different seasons, we analyzed the differences of fish utilization patterns in mangroves and the relationship between fish assemblages and environmental factors. These will clarify the temporal variation characteristics and main driving factors of the fish assemblages in estuarine mangroves and provide the basis for the protection and utilization of fish resources.
Materials and methods
Study area
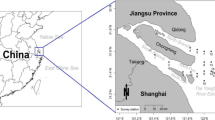
This study was carried out in the NRE, which was divided into four main branches and debouched into Beibu Gulf, the northwestern part of the South China Sea (Fig. 1). The NRE is influenced by irregular diurnal tide, ranging in height from −2.15 during neap tide to 3.37 m during spring tide. Mangroves are distributed within the estuary and fringe the river banks up to 7 km upstream. These forests are dominated by dwarf Aegiceras corniculatum intermixed with patches of Kandelia candel, and the total mangrove area increased significantly in recent decades (Liu et al. 2017, 2020). NRE belongs to a subtropical monsoon climate with a dry season in winter-spring and a wet season in summer-autumn (Wu et al. 2008). Human activities in the estuary are mainly aquaculture ponds. Ponds on both sides of the estuary area cover more than 6500 ha, and pond water emissions are restricted during the two short harvesting periods in summer and autumn (Kaiser et al. 2013). The land-based pollutants carried by rivers and the discharge of aquaculture water lead to serious eutrophication in the estuary (Yang et al. 2015).
Sampling methods
Sampling sites were set up according to the length of each creek, that is, more sampling sites were set up in the longer creek, and fewer sites were arranged in the shorter creek. A total of eight sites were set up in three creeks, five were in the longest creek into which the shrimp pond water was directly discharged, and three were in two shorter creeks (Fig. 1). The width of the longest creek varies from about 3.8 upstream to about 20.7 m downstream. The width of the shorter creeks varies from 2.5 to 9.3 m.
Fishes were collected from July 2019 (summer), October 2019 (autumn), January 2020 (winter), and May 2020 (spring) using a fyke net (mesh 5 mm; high 1 m; length about 5–20 m) and one trap (mesh 8.5 mm; 35 cm2 × 10 m for 1 unit). For every season, samples were taken at day tides and night tides. For everyday tides and night tides, samplings lasted for three consecutive tidal cycles. Day and night tides were defined as those in which the flood occurred primarily during day or night, respectively. For each tidal cycle, we set the nets before flowing tide, and fish samples were then collected after ebbing tide at all sites. Therefore, a total of 192 fish samplings were conducted during the study. All fish were removed from the net, stored in 10% formalin solution, and taken back to the laboratory for identifying, weighing, counting, and then were subsequently stored in 5% formalin solution.
Environmental parameters were measured at each site during sampling. Water temperature (WT), pH, dissolved oxygen (DO), and salinity were measured using a handheld multiparameter meter provides (YSI Professional Plus). The measurement of turbidity was applying a portable turbidimeter (HACH 2100Q). Chlorophyll-a (Chl-a) was measured using a portable algal detector (ALGAE-Wader). At the same time, surface water samples (0.5 m) were collected and brought back to the laboratory to measure chemical indices including chemical oxygen demand (COD), total nitrogen (TN), total organic carbon (TOC), and total phosphorus (TP) (General Administration of Quality Supervision and Administration 2007). Besides, tidal ranges were the difference between the maximum and minimum tide levels of the day, and tidal level data was obtained from the State Oceanic Administration People’s Republic of China (http://ocean.cnss.com.cn/).
Species classification
The identification and biological characteristics of fishes were determined according to reference books (Lai and He 2016; Liu et al. 2017) and Fisbase (www.fishbase.org) (Froese and Pauly 2020). Fish species were categorized into bioecological groupings depending on their utilization of mangroves/estuary following Wainaina (Wainaina et al. 2013) and Potter (Potter et al. 2015). The functional three groups were as follows:
Mangrove/estuary-transient species (MT): those fish species that may use the estuary as transit routes between the marine and freshwater environments.
Mangrove/estuary-resident species (MR): those fish species that may use the estuary as foraging habitats, and their life history are associated with the estuary.
Mangrove/estuary-occasional species (MO): Those fish species that inhabit freshwater and marine and their life history are not related to the estuary.
Data analyses
All the fish captured on the day tides or night tides at each site for each season were collected as the catch per unit effort (Jin et al. 2007). Therefore, a total of 64 samplings participated in the analysis. Index of relative importance (IRI) was used to determine the dominant species in this region (Pinkas et al. 1971). IRI was calculated based on abundance, biomass, and frequency of occurrence as follows: IRI = (N% + W%) × F%, where N%, W%, F% are percentage contribution of abundance, the contribution of biomass, and frequency of occurrence. When the IRI value of a species was greater than 1000, it is recognized as the dominant species. Kruskal–Wallis test was carried out for fish abundance, species richness, biomass, as well as the environmental variables with the R package agricolae due to these parameters running counter to assumptions of ANOVA, even after the log transformation of data.
PERMANOVA was performed by the adonis function in R package vegan to test the difference of fish assemblages among seasons based on log-transformed fish abundance data (Anderson 2001; Ruppert et al. 2018). NMDS was used to visualize temporal patterns revealed by PERMANOVA. Then, similarity percentages (SIMPER) analysis was used by PAST3.0 to analyze the dissimilarity of fish assemblages and identify those fish species that contributed most to the average dissimilarity of the seasonal period determined the percentage contribution of each fish species to the overall dissimilarity (Clarke 1993; Kindong et al. 2020).
Redundancy analysis (RDA) was used to investigate the associations between fish species’ abundance and environmental variables (Ruppert et al. 2018; Huang et al. 2019). Firstly, rare species were removed with an occurrence frequency lower than 10%. Then, detrended correspondence analysis (DCA) on the log-transformed species abundance was carried out, and the longest gradient length of 2.38 along the first axis was obtained, which suggested that RDA was effective (Šmilauer and Lepš 2014). The significance of fish abundance and environmental variables was tested by using the Monte Carlo permutation test (permutations = 999). Only if it was significant, a forward stepwise selection procedure of environmental variables with a permutation test (999 permutations) was used to obtain the reduced environmental variables (Huang et al. 2019). The DCA and the RDA analysis were performed in the Canoco 5.
Results
Fish assemblage composition
A total of 45 species (17,680 individuals weighing 24,724.73 g) belonging to 23 families were caught in the NRE (Table 1). Ambassis urotaenia, Mugil cephalus, Coptodon zillii, Gambusia affinis, and Bostrychus sinensis were dominant species throughout the year (IRI > 1000). The most numerically abundant species were A. urotaenia (39.86%), M. cephalus (15.34%), G. affinis (14.41%), and Pseudogobius javanicus (11.50%), while the most fish species in biomass were C. zillii (20.46%), M. cephalus (16.08%), B. sinensis (13.96%), and Pisodonophis boro (11.47%).
The majority of fish surveyed were mangrove/estuary-associated species (28 species) and mangrove/estuary-occasional species (14 species) (Table 1). The remaining species were mangrove/estuary-transient species (3 species). Throughout the year, we found a seasonal variation in the abundance and relative abundance of mangrove/estuary-associated species, which were more abundant in autumn and winter (Fig. 2). The maximum abundance and relative abundance of mangrove/estuary-occasional species were observed in summer (Fig. 2). The peak abundance of mangrove/estuary-transient species appeared in summer and winter (Fig. 2). Maximum relative abundance occurred in summer, and the increasing trend of relative abundance from autumn to spring of the second year existed on these guilds (Fig. 2).
Seasonal variation of fish assemblages
Kruskal–Wallis test results showed that species richness, fish abundance, and biomass were significantly different seasonally (Fig. 3). There were significant differences in fish abundance among the three seasons (spring, autumn, and winter) (p < 0.05) (Fig. 3), and the higher fish abundance captured was recorded in winter, followed by autumn and the lowest in spring. The fish abundance in summer was significantly higher than that in spring, but there was no significant difference with that in autumn and winter. The seasonal significant difference of species richness only existed between summer and autumn, and the higher species richness captured was recorded in summer than that in winter (Fig. 3). The seasonal significant difference of fish biomass only existed between autumn and other seasons, and the fish biomass in autumn was the highest (Fig. 3).
A clear separation of fish samples with a clockwise direction was found for different seasons (Fig. 4), which indicated a gradual change in fish assemblages over the seasons. PERMANOVA analysis further revealed a highly significant effect of the time of seasons (R2 = 0.4512, p = 0.001) on fish assemblages. A 79.61% average dissimilarity was found among seasons based on SIMPER analysis, and A. urotaenia, M. cephalus, G. affinis, T. jarbua, and C. zillii were the major fish species that contributed to seasonal dissimilarity, those which contributed 27.96%, 17.68%, 14.20%, 8.992%, and 7.380% of the difference, respectively (Table 2).
Relationship between fish assemblages and environmental variables
All the environmental variables were significantly different between seasons (Fig. 5). For water temperature, significantly higher values were observed in summer compared with other seasons, whereas significantly lower values were found in winter (p < 0.05); conversely, salinity in winter was the highest, and that in summer was the lowest (p < 0.05). TOC, COD, and turbidity in summer and autumn were greater than that in spring and winter (p < 0.05). The peak concentrations for TN recorded in autumn were significantly higher than those in other seasons (p < 0.05). Tidal ranges in spring were significantly higher than those in other seasons (p < 0.05).
Seasonal comparisons for environmental variables from the mangrove creek in the NRE. Different letters (a, b, c, d) indicate significant differences (Kruskal–Wallis test, p-values < 0.05). (WT, water temperature; Chl-a, chlorophyll-a; COD, chemical oxygen demand; TN, total nitrogen; TOC, total organic carbon)
A total of 26 fish species were selected for analysis with occurrence frequency higher than 10% (Table 1), and 6 environmental variables were selected after a forward stepwise selection including salinity, TN, COD, water temperature, TOC, and tidal ranges (Table 3). In the RDA ordination biplot, two axes totally explain 42.0% of species-environment variations. Among the axes, the first axis explains 20.11% and the second axis 8.44% of species-environment variations. T. jarbua, Butis butis, Ambassis interrupta, Scorpaenopsis gibbose, and Selenotoca multifasciata were positively related to water temperature but negatively related to high salinity (Fig. 6). While A. urotaenia, Acanthopagrus latus, Lagocephalus spadiceus, Trypauchen vagina, and M. cephalus were positively associated with salinity. In addition, Trypauchen vagina, Acentrogobius viridipunctatus, Oreochromis niloticus, and Glossogobius olivaceus were positively associated with TN. Moreover, C. zillii, Synechogobius ommaturus, Sillago sihama, and P. boro were positively associated with COD and TOC. Solea ovata and P. javanicus were positively associated with tidal ranges.
RDA ordination of fish assemblages and environmental variables from the mangrove creek in the NRE. (Notes: species names were used by generic acronym and the first three letters of the species name in Table 1. WT, water temperature; COD, chemical oxygen demand; TN: total nitrogen; TOC, total organic carbon)
Discussion
Seasonal variations on fish assemblages
Fish assemblage structures in mangrove creeks in this study were seasonally different, and this temporal distribution pattern, generally, occurs in mangroves in other regions all over the world (Wainaina et al. 2013; Castellanos-Galindo and Krumme 2015; Mahesh and Saravanakumar 2015). The seasonal variation of the contributing species abundance was the reason for the seasonal difference of fish assemblages (Table 2), which may be related to the breeding dates of some species (M. cephalus, G. affinis, and T. jarbua ) as found in estuary mangroves in Taiwan (Tsai et al. 2015). Firstly, this difference was reflected in the reproductive cycles to anadromous species (M. cephalus) and catadromous species (T. jarbua). The spawning period of the M. cephalus occurred from November to February, reaching the highest intensity in January (Ibáñez Aguirre and Gallardo-Cabello 2004); other studies have also found that M. cephalus reproduced from December to January in China and India (Hsu et al. 2007; Rekha et al. 2021). The breeding season of T. jarbua is from April to October every year (Miu et al. 1990). In their own breeding season, the individuals of these fish were higher than that in other seasons (Table 2), which supported our guess that the seasonal difference was reflected in the reproductive cycles of contributing species. The annual reproductive cycles with a distinct breeding season for G. affinis extends from about mid-spring until mid-autumn with a peak in summer, meanwhile, its foraging time also varied seasonally, with a peak in summer and a trough in winter (Pyke 2005). Overall, it may explain the high abundance of G. affinis in summer (Table 2).
Influence of environmental variables in fish assemblages
The seasonal pattern of fish assemblages was also related to the change of environment and fish-specific tolerance (Rousseau et al. 2018). The most influential environmental variables were salinity, water temperature, and COD (Fig. 6). Higher species richness captured were recorded in summer than that in winter in this study (Fig. 3) and might be explained by the comprehensive effects of seasonal variation in salinity and water temperature (Fig. 3). As a response to high salinity, low species richness has been recognized in tropical estuaries (Sloterdijk et al. 2017). With the increase of salinity in autumn and winter, only marine species (A. latus, L. spadiceus) with high salinity preference and some resident species (A. urotaenia, T. vagina) were suitable for the environmental characteristics of mangroves (Fig. 6), resulting in the absolute predominance of a few species of fish and lower species richness (Fig. 3). Meanwhile, the increase of primary and secondary productivity from freshwater inflows and supplementing this productivity in the case of warmer water temperatures in the wet season facilitated faster juvenile fish growth (Pichler et al. 2015). T. jarbua and G. affinis preferred high temperature and low salinity environments (Fig. 6). So, these species, together with the resident species, entered with every flood tide to exploit mangroves, resulting in higher species richness in summer (Fig. 3). C. zillii had a positive correlation with TOC and COD (Fig. 6). The sources of TOC and COD in mangroves were the combination of degradation and release of mangrove litter, benthic microalgae, and plant detritus imported with tides, which provided ample food for C. zillii (Tsutsumi et al. 2006; Kristensen et al. 2008). It was worth noting that the input of emissions from shrimp ponds in summer and autumn increased organic nutrition for C. zillii, which was consistent with the higher TOC and COD in summer and autumn (Fig. 5). Nevertheless, C. zillii abundance peaked in the autumn rather than in the breeding peak season (summer) (Table 2) (El-Sayed, H.K.A.; Moharram 2007), which was caused by the maximum values of feeding activity that was during autumn before overwintering (Shehata et al. 2017; Gu et al. 2018). The most fish species in biomass were C. zillii (20.46%), and this was the reason that fish biomass in autumn was the highest (Fig. 3).
Mangrove utilization of different ecological guild
Mangrove/estuary-resident species are composed of 28 species, mainly Gobiidae (16 species), Leotridae (3 species), Ambassidae (2 species), Pisodonophis boro, Thryssa vitrirostris, Encrasicholina heteroloba, Sillago sihama, Acanthopagrus latus, Elops machnata, and Selenotoca multifasciata. This group was considered euryhaline due to their adaptability to variable salinities and also since most species appeared in all four seasons, indicating that mangrove ecosystems provide habitats and/or food sources for these species (Krumme et al. 2015; Wu et al. 2018). Estuarine resident species had higher relative abundances in winter at a small estuary of the Portuguese coast and was more abundant during the wet season in Indian estuaries (Cardoso et al. 2011; GB et al. 2020), which indicated seasonal variations were found in the abundances of estuarine-resident species. In our study, the abundance of mangrove-resident species in autumn and winter was higher than that in spring and summer, which was the result of a large number of A. urotaenia and P. javanicus moving into mangroves (Fig. 2; Table 2). The reasons for the remarkably larger-sized individuals of A. urotaenia and P. javanicus in the mangrove creeks in autumn and winter are unclear and deserve further study. Functions such as feeding, rearing, or predator avoidance may have been involved.
Mangrove/estuary-transient species were formed by diadromous species (those that migrate between freshwater and marine habitats), including M. cephalus, T. jarbua, and Salanx ariakensis. From last winter to the next summer, this group occupied a certain proportion in mangrove creeks, and the number of individuals was the most in summer and winter (Fig. 2). During their own spawning migration season, the abundance of M. cephalus and T. jarbua was higher than that in the other three seasons (Table 2) (Miu et al. 1990; Ibanez et al. 2012). These species stayed in mangroves and regulated osmotic pressure to adapt to salinity changes after moving into seawater/freshwater (Kültz 2015). Thus, the mangrove creeks were used as a breeding migration transit area for these two species.
The mangrove/estuary-occasional species consists of 14 species, including 6 freshwater species (G. affinis, Poecilia latipinna, Hypophthalmichthys nobilis, C. zillii, Oreochromis niloticus, and Macropodus opercularis) and 8 marine species (Sardinella aurita, Sardinella gibbose, Hypoatherina tsurugae, Scorpaenopsis gibbose, Epinephelus fuscoguttatus, Branchiostegus argentatus, Lagocephalus spadiceus, and Solea ovata). Except for G. affinis and C. zillii, the frequency and the abundance of these species were low in this category (Table 1), which was washing into mangroves by tides or freshwater runoff accidentally. Besides, G. affinis moved into mangroves for foraging and avoiding the pressure of high temperature in the shade of mangrove creeks (Hendy et al. 2020). C. zillii took mangrove creeks as a temporary transit place for overwintering. Hence, the mangrove creeks were used as a refuge for these two species.
Conclusion
This study was conducted in an estuarine mangrove of an unnatural reserve. Our findings emphasized that fish assemblages had seasonal variations in the mangrove creeks system in NRE. Mangrove creeks provided habitats for mangrove/estuary-resident species. It also was a breeding migration transit area for mangrove/estuary-transient species and provided refuges for mangrove/estuary-occasional species that migrated to mangroves intentionally. Mangrove restoration has been currently carried out in China, and the mixed forests to increase habitat complexity of the mangrove should be taken into account in the restoration process to ensure good connectivity between mangroves and other types of habitats.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecol 26:32–46. https://doi.org/10.1046/j.1442-9993.2001.01070.x
Barletta M, Amaral CS, Correa MFM et al (2008) Factors affecting seasonal variations in demersal fish assemblages at an ecocline in a tropical-subtropical estuary. J Fish Biol 73:1314–1336. https://doi.org/10.1111/j.1095-8649.2008.02005.x
Barletta M, Barletta-Bergan A, Saint-Paul U, Hubold G (2003) Seasonal changes in density, biomass, and diversity of estuarine fishes in tidal mangrove creeks of the lower Caeté Estuary (northern Brazilian coast, east Amazon). Mar Ecol Prog Ser 256:217–228. https://doi.org/10.3354/meps256217
Cardoso I, França S, Pais MP et al (2011) Fish assemblages of small estuaries of the Portuguese coast: a functional approach. Estuar Coast Shelf Sci 93:40–46. https://doi.org/10.1016/j.ecss.2011.03.016
Castellanos-Galindo GA, Krumme U (2015) Tides, salinity, and biogeography affect fish assemblage structure and function in macrotidal mangroves of the neotropics. Ecosystems 18:1165–1178. https://doi.org/10.1007/s10021-015-9887-4
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Aust J Ecol 18:117–143. https://doi.org/10.1111/j.1442-9993.1993.tb00438.x
de Azevedo MCC, da Cruz AG, Araujo FG (2016) Mangrove habitat use by fishes in Southeastern Brazil: are there temporal changes in the structure of the community? Mar Ecol Evol Perspect 37:1223–1238. https://doi.org/10.1111/maec.12302
Desmond JS, Deutschman DH, Zedler JB (2002) Spatial and temporal variation in estuarine fish and invertebrate assemblages: analysis of an 11-year data set. ESTUARIES 25:552–569. https://doi.org/10.1007/BF02804890
El-Sayed HKA, Moharram SG (2007) Reproductive biology of Tilapia zilli (Gerv, 1848) from Abu Qir Bay. Egypt. Egypt J Aquat Res 33:379–393
Froese R, Pauly D (2020) FishBase. World Wide Web electronic publication
GB S, AK J, HB S et al (2020) Fish composition and assemblage structure in tropical monsoonal estuaries: estuarine use and feeding guild approach. Estuar Coast Shelf Sci 244:106911. https://doi.org/10.1016/j.ecss.2020.106911
Gu DE, Yu FD, Xu M et al (2018) Temperature effects on the distribution of two invasive tilapia species (Tilapia zillii and Oreochromis niloticus) in the rivers of South China. J Freshw Ecol 33:511–524. https://doi.org/10.1080/02705060.2018.1517698
Hendy IW, Burt O, Clough S et al (2020) Mosquitofish avoid thermal stress by moving from open water to the shade of the mangrove Rhizophora mangle. Mar Ecol Prog Ser 637:103–116. https://doi.org/10.3354/meps13253
Hsu C, Han Y, Tzeng W (2007) Evidence of flathead mullet Mugil cephalus L . spawning in waters northeast of Taiwan. Zool Stud 46:717–725
Huang J, Huang L, Wu Z et al (2019) Correlation of fish assemblages with habitat and environmental variables in a headwater stream section of Lijiang River, China. Sustain 11:1–14. https://doi.org/10.3390/su11041135
Huang LL, Huang DL, Wu ZQ et al (2016) Temporal variation of fish diversity and assemblages and their associations to environmental variables in the mangrove of Qinzhou Harbor, Guangxi Province, China. Turkish J Fish Aquat Sci 16:297–310. https://doi.org/10.4194/1303-2712-v16_2_10
Ibáñez Aguirre AL, Gallardo-Cabello M (2004) Reproduction of Mugil cephalus and M. curema (Pisces: Mugilidae) from a coastal lagoon in the Gulf of Mexico. Bull Mar Sci 75:37–49
Ibanez AL, Chang CW, Hsu CC et al (2012) Diversity of migratory environmental history of the mullets Mugil cephalus and M. curema in Mexican coastal waters as indicated by otolith Sr:Ca ratios. CIENCIAS Mar 38:73–87. https://doi.org/10.7773/cm.v38i1A.1905
Igulu MM, Nagelkerken I, van der Velde G, Mgaya YD (2013) Mangrove fish production is largely fuelled by external food sources: a stable isotope analysis of fishes at the individual, species, and community levels from across the globe. Ecosystems 16:1336–1352. https://doi.org/10.1007/s10021-013-9687-7
Ikejima K, Tongnunui P, Medej T, Taniuchi T (2003) Juvenile and small fishes in a mangrove estuary in Trang province, Thailand: seasonal and habitat differences. Estuar Coast SHELF Sci 56:447–457. https://doi.org/10.1016/S0272-7714(02)00194-4
Jin B, Fu C, Zhong J et al (2007) Fish utilization of a salt marsh intertidal creek in the Yangtze River Estuary, China. Estuar Coast Shelf Sci 73:844–852. https://doi.org/10.1016/j.ecss.2007.03.025
Kaiser D, Unger D, Qiu G et al (2013) Natural and human influences on nutrient transport through a small subtropical Chinese estuary. Sci Total Environ 450:92–107. https://doi.org/10.1016/j.scitotenv.2013.01.096
Kindong R, Wu J, Gao C et al (2020) Seasonal changes in fish diversity, density, biomass, and assemblage alongside environmental variables in the Yangtze River Estuary. Environ Sci Pollut Res 27:25461–25474. https://doi.org/10.1007/s11356-020-08674-8
Kristensen E, Bouillon S, Dittmar T, Marchand C (2008) Organic carbon dynamics in mangrove ecosystems: a review. Aquat Bot 89:201–219. https://doi.org/10.1016/j.aquabot.2007.12.005
Krumme U, Grinvalds K, Zagars M et al (2015) Tidal, diel and lunar patterns in intertidal and subtidal mangrove creek fish assemblages from southwest Thailand. Environ Biol Fishes 85:208–216. https://doi.org/10.1007/s10641-015-0393-5
Kültz D (2015) Physiological mechanisms used by fish to cope with salinity stress. J Exp Biol 218:1907–1914. https://doi.org/10.1242/jeb.118695
Lai T, He B (2016) Marine osteichthyes fishes in Guangxi Beibu Gulf of China. Science Press, Beijing
Jing L, Wu R, Kang B, Ma L (2016) Fishes of Beibu Gulf. Science Press, Beijing
Liu T, Liu SF, Wu B et al (2020) Increase of organic carbon burial response to mangrove expansion in the Nanliu River estuary, South China Sea. Prog EARTH Planet Sci 7. https://doi.org/10.1186/s40645-020-00387-3
Liu T, Tao Y, Liu Y (2017) Mangrove swamp expansion controlled by climate since 1988: a case study in the Nanliu River Estuary, Guangxi, Southwest China. Acta Oceanol Sin 36:11–17. https://doi.org/10.1007/s13131-017-1097-9
Mahesh R, Saravanakumar A (2015) Temporal and spatial variability of fin fish assemblage structure in relation to their environmental parameters in Pichavaram mangrove ecosystem, India. Indian J Geo-Marine Sci 44:910–923
Mandal B, Mukherjee A, Banerjee S (2013) A review on the ichthyofaunal diversity in mangrove based estuary of Sundarbans. Rev Fish Biol Fish 23:365–374. https://doi.org/10.1007/s11160-012-9300-8
Miu T-C, Lee S-C, Tzeng W-N (1990) Reproductive biology of Terapon jarbua from the estuary of Tamshui River. J Fish Soc Taiwan 17:9–20. 10.29822/JFST.199003.0002
Mwandya AW, Gullstrom M, Andersson MH et al (2010) Spatial and seasonal variations of fish assemblages in mangrove creek systems in Zanzibar (Tanzania). Estuar Coast Shelf Sci 89:277–286. https://doi.org/10.1016/j.ecss.2010.08.002
Nagelkerken I, Blaber SJM, Bouillon S et al (2008) The habitat function of mangroves for terrestrial and marine fauna: a review. Aquat Bot 89:155–185. https://doi.org/10.1016/j.aquabot.2007.12.007
Pichler HA, Spach HL, Gray CA et al (2015) Environmental influences on resident and transient fishes across shallow estuarine beaches and tidal flats in a Brazilian World Heritage area. Estuar Coast Shelf Sci 164:482–492. https://doi.org/10.1016/j.ecss.2015.07.041
Pinkas L, Oliphant SM, Iverson L.K.I (1971) Food habits of albacore, bluefin tuna, and bonito in California waters. State of California, Dept.of Fish and Game, State of California
Potter IC, Tweedley JR, Elliott M, Whitfield AK (2015) The ways in which fish use estuaries: a refinement and expansion of the guild approach. Fish Fish 16:230–239. https://doi.org/10.1111/faf.12050
Pyke GH (2005) A review of the biology of Gambusia affinis and G. holbrooki. Rev Fish Biol Fish 15:339–365. https://doi.org/10.1007/s11160-006-6394-x
Rehage JS, Loftus WF (2007) Seasonal fish community variation in headwater mangrove creeks in the southwestern everglades: an examination of their role as dry-down refuges. Bull Mar Sci 80:625–645
Rekha MU, Tomy S, Sukumaran K et al (2021) Comparison of the reproductive biology of two stocks of Indian subcontinental Mugil cephalus (Linnaeus, 1758) with special reference to reproductive isolation and philopatry. Indian J Geo-Marine Sci 50:130–140
Rousseau Y, Blanchard F, Gardel A (2018) Spatiotemporal dynamics of larval fish in a tropical estuarine mangrove: example of the Mahury River Estuary (French Guiana). Can J Fish Aquat Sci 75:235–246. https://doi.org/10.1139/cjfas-2016-0267
Ruppert JLW, Vigliola L, Kulbicki M et al (2018) Human activities as a driver of spatial variation in the trophic structure of fish communities on Pacific coral reefs. Glob Chang Biol 24:E67–E79. https://doi.org/10.1111/gcb.13882
Shahraki M, Saint-Paul U, Krumme U, Fry B (2016) Fish use of intertidal mangrove creeks at Qeshm Island. Iran. Mar Ecol Prog Ser 542:153–166. https://doi.org/10.3354/meps11546
Shehata S, Ghanem M, Ragab A (2017) Effect of some environmental factors on the food and feeding habits of the cichlid fish, Tilapia zillii, inhabiting Lake Qarun, Egypt. Egypt Acad J Biol Sci B Zool 9:55–70. 10.21608/eajbsz.2017.13457
Sloterdijk H, Brehmer P, Sadio O et al (2017) Composition and structure of the larval fish community related to environmental parameters in a tropical estuary impacted by climate change. Estuar Coast Shelf Sci 197:10–26. https://doi.org/10.1016/j.ecss.2017.08.003
Šmilauer P, Lepš J (2014) Multivariate analysis of ecological data using CANOCO 5, 2nd edn. Cambridge Unerversity Press, Cambridge, UK
Tsai CH, Wang YK, Te TS, Wu SH (2015) Seasonal and diel changes of the fish assemblage employing the fyke nets in a subtropical mangrove estuary of Puzih River. Taiwan. J Mar Sci Technol 23:109–116. https://doi.org/10.6119/JMST-014-0110-3
Tsutsumi H, Srithongouthai S, Inoue A et al (2006) Seasonal fluctuations in the flux of particulate organic matter discharged from net pens for fish farming. Fish Sci 72:119–127. https://doi.org/10.1111/j.1444-2906.2006.01125.x
Vaslet A, Phillips DL, France C et al (2012) The relative importance of mangroves and seagrass beds as feeding areas for resident and transient fishes among different mangrove habitats in Florida and Belize: evidence from dietary and stable-isotope analyses. J Exp Mar Bio Ecol 434:81–93. https://doi.org/10.1016/j.jembe.2012.07.024
Wainaina MW, Kaunda-Arara B, Njiru JM (2013) Seasonality in fish assemblage structure in an East African mangrove creek. African J Mar Sci 35:163–173. https://doi.org/10.2989/1814232x.2013.769904
Wang M, Huang Z, Shi F, Wang W (2009) Are vegetated areas of mangroves attractive to juvenile and small fish? The case of Dongzhaigang Bay, Hainan Island, China. Estuar Coast Shelf Sci 85:208–216. https://doi.org/10.1016/j.ecss.2009.08.022
Wu DX, Wang Y, Lin XP, Yang JY (2008) On the mechanism of the cyclonic circulation in the Gulf of Tonkin in the summer. J Geophys Res 113:10. https://doi.org/10.1029/2007jc004208
Wu Z-Q, Zou Q, Chang T et al (2018) Seasonal dynamics of the juvenile fish community structure in the Maowei Sea mangroves. PLoS One 13. https://doi.org/10.1371/journal.pone.0192426
Yang J, Zhang R, Zhao Z, et al (2015) Temporal and spatial distribution characteristics of nutrients in the coastal seawater of Guangxi Beibu Gulf during the past 25 years. Ecol Environ Sci 24:1493–1498. 10.16258/j.cnki.1674-5906.2015.09.011
Zhang Y, Ding Y, Wang W et al (2019) Distribution of fish among Avicennia and Sonneratia microhabitats in a tropical mangrove ecosystem in South China. Ecosphere 10. https://doi.org/10.1002/ecs2.2759
Acknowledgements
We gratefully acknowledge the support of Guangxi Key Laboratory of Environmental Pollution Control Theory and Technology for Science and Education Combined with Science and Technology Innovation Base for their vital help during samplings. Thanks to Dr. Liu Qi for improving this manuscript and thanks to Saeed Rad for helping to polish this manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (Grant Number: U20A2087), Natural Science Foundation of Guangxi Province (2018GXNSFAA281022), the Guangxi “Bagui Scholar” Construction Project (2016A10), and “Chunhui Planning” Project from the Ministry of Education, China.
Author information
Authors and Affiliations
Contributions
L.L. Huang and X. Huang developed the basic idea and designed the investigation. X. Huang, L.L. Huang, H. Liu, J.L. Chen, and L.Y. Gui performed the sampling collection and processing. X. Huang and H. Liu performed the data analyses. X. Huang led the original writing. L.L. Huang reviewed and made corrections to improve the paper. All authors read the full paper and agreed to its publication.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The experimental protocol was established, according to the ethical guidelines of the Council for Laboratory Animal Science of the Guilin University of Technology. Written informed consent was obtained from individual or guardian participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: V.V.S.S. Sarma
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Huang, X., Liu, H., Chen, J. et al. Drivers of temporal variations in fish assemblages from mangrove creeks in Beihai, southern China. Environ Sci Pollut Res 29, 47070–47081 (2022). https://doi.org/10.1007/s11356-022-19029-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-19029-w