Abstract
The present study used multivariate techniques, to analyze the fish species diversity and distribution patterns in order to determine the possible role of environmental parameters as drivers of fish community structure and composition in the Yangtze River Estuary (YRE). This analysis was conducted using data obtained in the YRE from February 2012 to December 2014. Analysis of the catch data showed that species composition, total density, and total biomass varied significantly between stations and seasons. Thirty-eight species belonging to 18 families were collected. Sciaenidae was the most dominant family accounting for 40.8% of total captured specimens. In descending order, Collichthys lucidus, Cynoglossus gracilis, Chaeturichthys stigmatias, and Lophiogobius ocellicauda dominated catches in the YRE. These four species constituted 64.2% of the total catches and showed average dissimilarities of 74.19% between stations and 81.3% between months. The highest number of fish specimens captured was recorded in August 2012 while the highest species richness was observed in December 2013. The mean fish density and biomass for the YRE was 0.35 individuals/m2 and 2.5 g/m2, respectively. The mean density and biomass for the most important and dominant species changed significantly between stations and seasons. Canonical correspondence analysis indicated that salinity and chlorophyll-a were the key variables that structured the fish assemblage in the YRE. High total species density and biomass were recorded in high saline stations (North Branch) of the YRE. This study confirms that most species captured in the YRE needs estuarine conditions to complete their growth and development. Hence, the findings in this study are important to understanding and developing suitable conservation plans for the management of fish resources in the YRE.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fisheries populations in estuaries are very much dynamic in both temporal and spatial spectrums. Quite a lot of authors highlighted the importance of estuaries for marine fisheries, by indicating that more than half of the world’s landings are comprised of species that spend part of their lives in estuaries (Pauly 1988; Barletta et al. 1998, 2005, 2010, 2016). Estuaries, interfaces between land, freshwaters, and the sea, are locations where hydrological (e.g., river discharge), oceanographic, and anthropogenic processes interact (Merigot et al. 2016). Estuaries are dynamic habitats characterized by large variations in hydrological conditions (Hossain et al. 2012). Estuarine milieus in tropical, sub-tropics, and temperate regions create more organic matter on a yearly basis, making them the most productive on earth when compared to forests or grasslands. Estuarine zones also have products (such as fish) of high socioeconomic value that serves particularly as a source of income and food to the neighboring populations. Fish species proving tolerant toward hydrological changes use estuaries as their breeding grounds, migratory pathways, hibernating areas, feeding, and as refuge zones (Barletta-Bergan et al. 2002a, b; Barletta et al. 2003; Kamrani et al. 2015; Islam et al. 2017).
Dominance, evenness, Margalef, and Shannon-Weiner are biodiversity indicators that are often used as reference to determine the assortment status of aquatic populations (Vyas et al. 2012). Nevertheless, relationships between fish species and their habited environments play vital roles in the preservation and management of estuarine species. The concentration of environmental factors such as water quality variables has been noted to be highly influential to fish assemblages and distributions in both marine and inland waters (Islam et al. 2017). Some fish species are forced to stay in intertidal zones or move to the sub-littoral on receding tides when environmental conditions change (Barletta et al. 2000, 2003). Salinity change causes a good number of fish species to migrate, moving up and down the estuary while some few fish species either move from less deep to deeper waters, or move straight toward less variable water states such as seas due to changes in temperature (Barletta et al. 2003, 2005, 2016).
Seasonal changes in species assemblage, species number, biomass, density, and their importance as nursery areas have been discussed in some estuarine main channels worldwide, such as, in south Florida, USA (Thayer et al. 1987), in Australia (Gibbs and Matthews 1982; Laegdsgaard and Johnson 1995), and in some South Western Atlantic Estuaries (Brazil; Barletta et al. 2003, 2005, 2010, 2016). These studies differed in estimating the importance of salinity gradients to the distribution of fish assemblages in estuaries. These disagreements have been attributed to differences in seasonal fluctuations in large-scale salinity gradients, and the integration of sequential recruitment of species throughout years in these estuaries (Ramos et al. 2016). Ecological studies of estuaries through their fish communities is known to be fundamental in understanding the functioning of their entire ecosystems (Barletta-Bergan et al. 2002a, b; Barletta et al. 2005, 2008; Barletta and Blaber 2007; Barletta and Barletta-Bergan 2009; Dantas et al. 2010, 2015; Lima et al. 2015, 2016; Ramos et al. 2016). Many past studies have emphasized on the importance of estuarine ecosystem for marine, estuarine, and freshwater fish species at each phase of their lives. Some of these fishes have socioeconomic importance for the local populations (Barletta and Costa 2009; Barletta et al. 2010).
The Yangtze River Estuary (YRE) is the largest estuary in China, and the Yangtze River, the third largest river in the world. The former is a critical system where geological, biological, and socioeconomic processes interact, and the latter deposits about five billion tons per year of fine sediments into the East China Sea with more than half of these sediments deposited in the estuarine area (Yu and Xian 2009; Quan et al. 2009; Shan et al. 2010). This YRE is economically important thanks to these deposited sediments which provide advantageous living conditions for many fish species (Yu and Xian 2009). The ecological importance of the YRE is a result of high biodiversity level and wide habitats range its offers (Zhang et al. 2015). For the past years, completed projects such as the Three Gorges Dam, South to North Water Diversion, and Yangtze–Taihu Water Diversion reduced the rate of freshwater inflow into the YRE which expansively modified and threatened the its ecosystem, and probably bringing heavy contamination to the estuarine habitation, and deteriorating water quality (Yu et al. 2007; Yu and Xian 2009; Quan et al. 2009; Shan et al. 2010).
Previous studies carried out in the YRE focused mainly on ichthyoplankton assemblage compositions, species distribution (Jiang and Shen 2006), and few studies on relationships between assemblage structure of ichthyoplankton and environmental factors (Yang et al. 1990; Jiang and Shen 2006; Yu and Xian 2009; Zhang et al. 2015, 2016). However, these past studies used data from the last decades for their analyses; given the fact that the world is facing some drastic climatic changes, we deemed it necessary to effectuate a study in the YRE using recently collected fisheries and environmental data. For that reason, the spatio-temporal changes of environmental variables in relation to fish assemblage, diversity, density, and biomass was analyzed in the YRE using data from 2012 to 2014.
Materials and methods
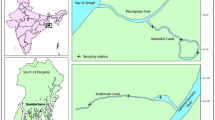
Data on fish assemblage and environmental conditions were collected from bottom trawl surveys done in the YRE from 2012 to 2014. The surveys were carried out seasonally (winter, spring, summer, and autumn) using a fixed-station sampling design with a total of 19 stations located around southeast Chongming Island (Fig. 1). However, only samples obtained in 18 stations were used in this study, as insignificant sample numbers were observed in the omitted station. Sampling area was divided into three locations as per the fishermen; North Branch (NP), Open Sea (EP), and South Branch (SP). Fin fish samples were collected during the day. The actual number and period of survey months changed among seasons and years due to weather conditions (2012: February, May, June, August, November, December), in 2013 (March, May, August, September, November, December), and in 2014 (March, May, August, November). Sampling was conducted using a 6-m-wide beam trawl with a 20-mm cod end; width (6 m) and height (2 m) of the trawl mouth opening. The beam trawl was towed once at a constant speed (0.56 m s−1) for 30 min during each sampling process at each survey station. Collected specimens were identified to the lowest taxonomic level possible then measured (length), counted and weighed (g). Few species of interest were later preserved in 10% formalin and transported to the Shanghai Ocean University laboratory for further analysis.
A GPS was used to record the boat’s position before and after sampling and was later used to estimate the swept area. For each haul sample, the swept area (A) was calculated from:
where D is the length of the path, h is the length of the head-rope, and X2 is the fraction of the head-rope (hX2) which is equal to the width of the path swept by the trawl, the wing spread (Sparre and Venema 1995; Barletta et al. 2005). The fraction of the head-rope which was close to the width of the swept area by the net during a haul was assumed to be X2 = 0.5 as in Barletta et al. (2005). Density (D) and biomass (B) were estimated by dividing the Catch per unit area (CPUA) by the swept area, A (ha):
where CN is the catch in numbers and CM is the catch in mass of fish. The total mean density (DT) and biomass (BT) was estimated from DT = DaX1 and BT = BaX1, where D and B are the mean catch, in number and in mass respectively, per unit area of all hauls, a is the total sampled area, and X1 is the catchability coefficient (in this study X1 = 1).
Environmental parameters were measured after hauling at each sampling station during monthly surveys. A CTD (SEADIRD SBE-19) rosette apparatus and a depth sampler were used to measure these environmental variables that included water depth, temperature, salinity, dissolved oxygen (DO), PH, chlorophyll-a (Chla), and chemical oxygen demand (COD).
Data analyses
Four major biodiversity indices namely Shannon-Wiener diversity index (H′; Shannon and Weaver 1949), Simpson index of diversity (D; Simpson 1949), and species richness (d; Margalef 1968) were used for species diversity; Pielou’s evenness index (J′; Pielou 1966) was used for evenness. High values of M, H′, and J′ were assumed to represent high ecological quality status. High values of Simpson’s index were indicative of low ecological quality status. These indices are usually applied to evaluate the discrepancies of aquatic communities or populations. Hence, for us to evaluate the status of fish community structure and assemblage in the YRE, data was collected seasonally throughout the 3-year survey period. Diversity indices were calculated for sampled months and then yearly; the following expressions below were used for the analyses.
Shannon Weiner diversity index (Shannon and Weaver 1949) considers both the number of species and the distribution of individuals among species and is described by the following formula:
where ni is the number of individuals of each species (the ith species), N is the total number of all the individuals for captured species at that station, and Ln is the natural logarithm (Shannon and Weaver 1949).
The dominance or Simpson’s dominance index D (Simpson 1949) is measured to determine whether or not particular species dominate in a particular aquatic system, and is calculated using the equation:
where n is the number of individuals of each specie and N is the overall number of all sampled individuals.
The species evenness J′ (Pielou 1966) was determined using the formula:
where H′ is the number derived from Shannon and Wiener diversity index, S is the total number of species available in the community during the survey period, and Ln is the natural Logarithm.
The species richness (d) was calculated using Margalef’s diversity Index (d) with the help of the formula:
where Ln is the natural Logarithm (Margalef 1968).
Kruskal-Wallis test and one-way analysis of variance (ANOVA) were used to analyze the differences in the observed diversity indices among sampling months and years. Kruskal-Wallis test was also used to test temporal changes among environmental variables. All multivariate statistical analyses were done on the relative abundances of fish species caught.
Analysis of similarities (ANOSIM) is usually used for taxa-in-samples data, where groups of samples are to be compared (Clarke and Warwick 2001). Based on a Bray-Curtis similarity matrix obtained using ln (x + 1) transformed data (Clarke and Warwick 2001), a non-parametric ANOSIM was performed to test the inter-annual significant differences of fish assemblages and environmental variables in the YRE. To determine the inter-annual dissimilarity among parameters, R-statistical values for pair-wise comparisons provided by ANOSIM were used. R values close to 1 indicated a very high different composition, and values close to 0 represented slight differences among parameters (Clarke and Warwick 2001).
Similarity percentages (SIMPER) analysis was used to analyze the dissimilarity of fish communities and identify those fish species that contributed most to the average dissimilarity (months and stations; and inter-annual) among groups and determined the percentage contribution of each fish species to the overall group dissimilarity (Clarke and Warwick 2001). Cluster analysis based on Bray-Curtis similarity (Clarke and Warwick 1994) was calculated to produce a dendrogram for investigating fish abundance similarities presented as groups among stations. Groups observed in the cluster analysis were later used to observe the structure of fish assemblages in each station (Hammer et al. 2001, PAST 3.19).
Canonical correspondence analysis (CCA) is an analysis which considers unimodal relations among dependent and independent parameters. CCA was used to investigate associations between fish species’ abundance and environmental variables (Lepš and Šmilauer 2003). Monte Carlo permutation test (no. of permutations = 999) was used to assess the statistical significance of fish abundance and environmental variables. Seven environmental variables were used for the CCA analysis and fish species were ordinated to indicate the relative strengths among those associations. Species located near the origin of the CCA plot observed little associations with environmental variables tested or presents no particular preference. Those proximal to the distal portion of each vector and beyond indicate strong positive relationships with the environmental parameters. Species located directly in line, in the opposite direction from each vector, indicate strong negative relationships with that parameter. A significance difference of a minimum of P < 0.05 was considered in all test procedures. Species that represented less than 1% of the total catch were excluded from multivariate analyses. Analyses were performed using MS excel 2016 and a statistical package PAleontological STatistics (PAST) version 3.19 (Hammer et al. 2001).
Results
Spatio-temporal variation of diversity indices
The values of Shannon-Wiener diversity index (H′), Simpson index of diversity (D), species richness (Margalef: d), and Pielou’s evenness index (J′) were determined spatially and temporally (Table 1). The monthly values of H′ ranged from 0 to 2.25 (Table 1); values of H′, d, and J were zero for the month of December 2012, as just one specie was observed for that month. Highest values of H′, d, J′, and D were observed in December (winter). Inter-annual species diversity and richness indices were also checked using ANOSIM; significant differences occurred for species richness (d) among years; the other indices did not show significant inter-annual variations (Table 1).
Spatio-temporal variation of environmental parameters
All environmental variables measured in this study showed a seasonal trend. Maximum water depth (20 m) was recorded during November 2012 at stations Z1 and Z3, while the minimum water depth (1.5 m) was observed in June in 2012 at station Z6. Water temperature ranged from 5.6 °C (February 2012, Z12) to 30.1 °C (stations Z5 and Z9, August 2014). Maximum pH (9.03) was observed during September 2013 at Z9 and minimum pH (6.62) at Z16 in May in 2012. Maximum DO was recorded at Z10 (29.8 mg/l) and lowest (Z4; 6.6 mg/l) during August 2012. A high concentration of COD was registered at Z16 (3.12 mg/l) during February 2012, with a minimum concentration (0.16 mg/l) observed at Z12 (March, November 2013). A peak value for chlorophyll-a was recorded at Z15 (11.25 mg/m3) in August in 2012, and a minimum value (0.086 mg/m3) at Z16 (August 2014). The North Branch of the YRE was the highest saline zone, with the maximum value obtained at Z15 (29.8 ppt) during February 2012 (Table 2). Inter-annual changes of environmental factors were also analyzed (Table 3). Chla varied significantly throughout sampling years (P < 0.05). COD did not show inter-annual (2012 vs. 2014) changes (P > 0.05). Significant changes were observed for pH (2013 vs. 2014; P < 0.05). Although, salinity did not vary significantly among years; however, the lower values of salinity recorded in this study were from sampling stations found in the South Branch and Open Sea areas of the YRE.
Composition of the fish fauna
During the survey period, a total of 7984 individuals weighing 56,970 g belonging to 18 families, comprising 38 finfish species were harvested in the YRE, representing a total swept sampled area of 22,680 m2 (Table 4). The absolute mean density and biomass estimated from all collected samples was 0.35 individuals/m2 and 2.5 g/m2, respectively, with the North Branch having the highest mean values of density and biomass. The most abundant fish species was Collichthys lucidus (2888 individuals and represented 36.2% of the total individuals) and the less abundant registered for Paraplagusia japonica, Rhinogobius cliffordpopei, Platycephalus indicus, Zebrias zebra, Takifugu obscurus, and Takifugu xanthopterus. In this study, four families were highly represented notably, Gobiidae, Cynoglossidae, Engraulidae, and Sciaenidae. The Sciaenidae family (four species) recorded the highest fish abundance and represented 40.8% of the total individuals harvested; this high abundance was greatly influenced by the presence of the marine species Collichthys lucidus. Collichthys lucidus had the highest sampled density and biomass throughout the study period, and Lateolabrax japonicus (Japanese seabass) was the heaviest species (427.5 g) recorded. The highest number of species and individuals were harvested in summer in 2012, notably in the months of May and August (Fig. 2). The highest number of individuals (2038) was observed at station Z7 in the North Branch of the YRE, and a peak of 2673 individuals was recorded in August (2012). Fourteen fish species (species codes on Table 4; sp2, sp4, sp5, sp9, sp10, sp13, sp14, sp16, sp17, sp20, sp29, sp31, sp32, and sp35) mainly dominated by marine species constituted 96.5% (7705 individuals) of the total individuals captured during this study. Inter-annual-wise, more individuals per m2 were observed in the year, 2012 while in 2013, the total mean biomass of individuals was the highest among years (Table 4).
Multivariate analyses of the fish fauna and environmental parameters
Based on SIMPER analysis, 74.19% and 81.1% average dissimilarity were found among stations and months, respectively (Table 5). The four fish species that greatly contributed to this dissimilarity were Collichthys lucidus (25.8% and 25.29%), Lophiogobius ocellicauda (12.4% and 13.95%), Cynoglossus gracilis (11.36% and 11.91%), and Chaeturichthys stigmatias (7.05% and 6.9%) for stations and months, respectively. There was no significant difference in species occurrence for stations in the South Branch (SP) and Open Seas (EP); significant difference was observed in species occurrence among stations in the North (NP) and South Branches of the YRE. ANOSIM analysis showed significant differences in the structure of the fish assemblages among the three parts of the YRE (Table 6). Fish community differed among different parts in the YRE, confirming ecological segregation in this Estuary (ANOSIM, P < 0.05, global R = 0.38). Also, species assemblage varied significantly between 2012 and 2013 (P < 0.05, R = 0.23; Table 6); these years showed a more diverse species composition as compared to the other inter-annual interactions (highest R value; Table 6).
As shown by the Bray-Curtis’s cluster analysis, two distinct assemblage structures via stations similarity were observed in the YRE (Fig. 3). The first major cluster showed 32% similarity between stations mostly comprised of those found in the SP and Open Seas of the YRE. Stations Z14/Z16 presented high similarity patterns between them. Principal fish species in the first cluster were comprised of freshwater and marine species (Pelteobagrus nitidus, Cynoglossus gracilis, Coilia mystus, Coilia nasus, Lophiogobius ocellicauda, Synechogobius ommaturus). The second cluster was merely formed by stations in the NP presenting 45% similarity between stations, with Z4/Z5 showing highest resemblance between them while dissimilarity was observed among stations Z3, Z7, and Z18. Dominant species in this cluster were comprised of marine species notably Chaeturichthys stigmatias, Cynoglossus joyneri, Collichthys lucidus, and Nibea albiflora.
The importance of the CCA axes is shown by eigenvalues varying between 0 and 1. For this study, the CCA was performed following Monte-Carlo permutations (999 iterations) based on the first two axes (axis 1: eigenvalue = 0.42 and axis 2: eigenvalue = 0.24) which expressed 79.53% of the cumulative percentage variance of the species data (Table 7). Salinity was highly associated to species denoted sp13, 17, 20, 32, and sp35; and chlorophyll-a was associated to species such as sp13, 29, and sp31 (Fig. 4). Salinity and chlorophyll-a had the highest influence on the fish species composition and structure in the YRE, while water depth had an insignificant effect on species composition. The family Sciaenidae, particularly Collichthys lucidus, had the highest captured individuals in the YRE, with most individuals occurring at stations in the North Branch and Open Seas.
CCA ordination diagram based on species abundances, with environmental variables for species codes, see Table 4
Discussion
Due to changes in sampling methods and efforts, as well as differences in geomorphology of estuaries, much care is needed when comparing the fish biomass, density, and fish species assemblages in estuarine ecosystems. Therefore, the present results are mostly compared with those studies that used similar sampling methods in the same habitats, though we also compared changes in environmental parameters with estuaries in other localities.
As one of the basic concepts of ecology, species diversity has gained grounds in ecosystems and communities’ characterizations (DeJong 1975; Kamrani et al. 2015). The Shannon-Weaver biodiversity index (H′) obtained in this study shows an intermediate biodiversity for the YRE, since the values obtained are neither high nor low (Table 1; Kamrani et al. 2015). Variations of H′ were not observed among stations in the region; according to Hossain et al. (2012), this might be due to the selective nature of the trawl gear used during sampling. However, as reported by other studies, the Shannon-Weaver diversity may have a tendency to miscalculate the condition of fish communities in situations where certain species are dominating, as is the case in this study (Salas et al. 2006; Kamrani et al. 2015). Therefore, the value obtained for the Shannon-Weaver diversity may have been underestimated because of the overall dominance of Collichthys lucidus in the YRE. Species richness number peaked during cold seasons (autumn and winter), with the highest in December in 2013. This result corroborates partly with reports by Zhang et al. (2015, 2016), confirming fish richness in autumn for the YRE. Our study also showed that the highest species richness occurred in the North branch (NP) of the YRE which doubled as the most saline zone. Changes in evenness observed in this study might be related to the fact that the YRE is a spawning ground (as many juvenile specimens were collected during our study) to many fisheries in this region. So, the highest values of evenness obtained in this study could be attributed to the period when many fish species are recruited to different fisheries. This result corroborates with past studies that observed captures of many small individuals in the YRE, and attributed this area as a spawning ground to many species (Yang et al. 1990; Jiang and Shen 2006; Yu and Xian 2009).
Many studies have reported that temperate estuarine fish assemblages are dominated seasonally by estuarine spawners, including species in the Clupeidae and Engraulidae families (Monteleone 1992; Harris and Cyrus 2000; Strydom et al. 2003; Ramos et al. 2006). Four species from these two families dominated throughout our study; they represented 64.2% (Collichthys lucidus (36.17%), Cynoglossus gracilis (12.16%), Chaeturichthys stigmatias (8.19%), and Lophiogobius ocellicauda (7.6%)) of the total species number captured in the YRE. The present study show a small number of species composition in the YRE as compared to surveys done in 1998–2001 (48 species, 28 families were collected) reported by Yu and Xian (2009), and higher numbers as compared to the report by Quan et al. (2009) in the 2006–2007 survey (26 species, 15 families). The species composition differences observed in these studies could mainly have been caused by seasonality, as shown in this study. Seasonality is responsible for changes in environmental factors and spawning actions, and can hugely influence fish assemblages in estuaries (McErlean et al. 1973; Whitfield 1989; Loneragan and Potter 1990; Young and Potter 2003). Nonetheless, we reported many rare species and four dominant and abundant species. This result corroborates with many studies that the fish fauna in estuaries are mostly composed of few dominant species and a large number of rare species (Harris and Cyrus 1995; Whitfield 1999; Zhang et al. 2015, 2016). Collichthys lucidus was abundant throughout our study period, be it seasonally or yearly although their percentage of contribution differed (Tables 5 and 6).
This study indicates that the total mean density (0.35 individuals/m2) and biomass (2.5 g/m2) of fish species in the YRE were higher than reports (total mean density (0.25 individuals/m2) and biomass (0.9 g/m2)) presented by Barletta et al. (2005) in the Caete River Estuary, but lower than the report by Barletta et al. (2016) of 2 individuals/m2 and 104 g/m2 after dredging was done in the Paranaguá Estuary (south Brazil). The differences in density and biomass observed from various studies could be attributed to the sampling frequency and dissimilarities in geographical locations. However, the high density and biomass values obtained in the Paranaguá Estuary could be attributed to the fact that dredging operations were done in this estuary, which apparently attracted some species as food supply increased thanks to disposals from dredged materials (Barletta et al. 2016). In our study, total mean density and biomass was regulated mainly by the presence of Collichthys lucidus as this species registered the highest individuals seasonally as well as annually.
Fish assemblage composition in estuaries is formed by both abiotic and biotic ecological components (Marshall and Elliott 1998; Elliott and Hemingway 2008; Garcia et al. 2012; Kamrani et al. 2015). The idea that abiotic factors play a role in the estuarine fish assemblage was established in this study. A strong spatial variation of fish assemblage was observed throughout our study period. The high number of individuals collected at station Z7 in the NP of the YRE could be as a result of the presence of dominant species and favorable environmental conditions with high salinity and slow water currents, whereas the low number of individuals observed at Z18 in the EP could be due to extreme human interference in this area of the studied site. High catches were recorded in May and August of 2012 (Fig. 2) representing periods of high temperatures; this corroborates with reports by Zhang et al. (2016) that species abundance in the YRE was high during summer. This could be explained by the fact that most fish species particularly marine species enter the YRE during this period for spawning thereby increasing the catch rates. Moreover, high salinities trigger the influx of most marine species (Sciaenidae) running from predators into the YRE and also due to the availability of food in the YRE.
As presented by the CCA analysis, salinity and chlorophyll-a influenced the most the fish community structure in the YRE. These environmental variables were associated with axes 1 and 2 (Table 7). Salinity and chlorophyll-a had the greatest effect on fish species distribution and abundance, consistent with findings of fish communities from other estuaries (Eick and Thiel 2014; Roux et al. 2015; Kamrani et al. 2015; Liu et al. 2015). Salinity is the most important environmental variable for estuarine animals, influencing not only growth development and reproduction of living organisms but also the temporal and spatial distribution of larval fish assemblages (Zhang et al. 2015). Salinity has a considerable influence on the species distribution within the YRE as it is the key factor affecting fish assemblage structure. Nevertheless, in many cases, the range of salinity values at which most fish species are habitually found are much narrower than their tolerance range. Hence, the response of many species to salinity may vary with different life stages. Seasonal fluctuation in fish composition, density, and biomass in the Caete River estuary (eastern Amazon), Goiana Estuary (eastern Amazon), and the Paranaguá Estuary (south Brazil) were positively correlated with salinity (Barletta et al. 2005; Barletta et al. 2008; Barletta et al. 2010; Dantas et al. 2010; Barletta et al. 2016). These reports corroborate with our result confirming salinity as the most influential parameter causing changes of biomass, density, and species composition in the YRE.
Chlorophyll a also influenced the most species composition in the YRE. This variable indicates the abundance of nutrients (such as standing crop of phytoplankton); the greater the phytoplankton biomass, the higher the primary productivity (Zhang et al. 2015). Chlorophyll-a and nutrients from freshwater flow entering the YRE have a close relationship. Therefore, the higher the nutrient levels, the higher the chlorophyll-a levels in the YRE; thus, abundant food resources necessary for the development and growth for juveniles. Chlorophyll-a and salinity had a major impact on the distribution of Chaeturichthys stigmatias, Collichthys lucidus, and Nibea albiflora particularly at stations found in the North Branch of the YRE. Past studies also reported that salinity and chlorophyll-a had a strong influence in the distribution of phytoplankton in the YRE (Zhang et al. 2015, 2016).
The other environmental parameters recorded during sampling also had some slight impacts on species distribution. Species like Coilia nasus were comfortable in deeper zones, i.e., stations in the South Branch, while Lophiogobius ocellicauda preferred stations with higher COD values such as Z14, Z16, and Z17 in the Open Seas. Stations Z4 and Z5 were perfect habitat for Cynoglossus gracilis, Coilia mystus, Taenioides anguillaris, Trypauchen vagina, Pennahia argentata, and Harpadon nehereus, zones with lower temperatures. High pH zone such as Z3 was favorable for Pelteobagrus nitidus and Synechogobius ommaturus.
Conclusion
The species composition structure of the YRE was composed of four species with high abundance and a large number of rare species, which is a common phenomenon in estuarine milieus. Cluster analysis revealed the formation of two distinct assemblages, which clearly differed in their salinity tolerance. Seasonal and inter-annual changes in fish community structure in the YRE were observed and most of these were associated to salinity and chlorophyll-a. Collichthys lucidus was the most abundant species harvested in this estuary; its composition and distribution were shaped mainly by salinity as they tend to be concentrated in the North Branch of the YRE, branch containing high salinity values. The highest number of specimens collected was recorded during summer while the highest species richness was observed during autumn and winter.
References
Barletta M, Barletta-Bergan A (2009) Endogenous activity rhythms of larval fish assemblages in a mangrove fringed estuary in North Brazil. The Open Fish Sci J 2:15–24
Barletta M, Blaber SJM (2007) Comparison of fish assemblages and guilds in tropical habitats of the Embley (Indo-West Pacifc) and Caeté (Western Atlantic) estuaries. Bull Mar Sci 80:647–680
Barletta M, Costa MF (2009) Living and non-living resources exploitation in a tropical semi-arid estuary. J Coast Res 56:371–375
Barletta M, Barletta-Bergan A, Saint-Paul U (1998) Description of the fishery structure in the mangrove dominated region of Braganca (State of Para – North Brazil). Ecotropica 4:41–53
Barletta M, Saint-Paul U, Barletta-Bergan A, Ekau W, Schories D (2000) Spatial and temporal distribution of Myrophis punctatus (Ophichthidae) and associated fish fauna in a Northern Brazilian intertidal mangrove forest. Hydrobiol 426:65–74
Barletta M, Barletta-Bergan A, Saint-Paul U, Hubold G (2003) Seasonal changes in density, biomass, and diversity of estuarine fishes in tidal mangrove creeks of the lower Caete Estuary (northern Brazilian coast, east Amazon). Mar Ecol Prog Ser 256:217–228
Barletta M, Barletta-Bergan A, Saint-Paul U, Hubold G (2005) The role of salinity in structuring the fish assemblages in a tropical estuary. J Fish Biol 66:45–72
Barletta M, Amaral CS, Corrêa MFM, Guebert F, Dantas DV, Lorenzi L, Saint-Paul U (2008) Factors affecting seasonal variations in demersal fish assemblages at an ecocline in a tropical-subtropical estuary. J Fish Biol 73:1329–1352
Barletta M, Jaureguizar AJ, Baigun C, Fontoura NF, Agostinho AA, Almeida-Val VM, Val AL, Torres RA, Jimenes-Segura LF, Giarrizzo T, Fabré NN, Batista VS, Lasso C, Taphorn DC, Costa MF, Chaves PT, Vieira JP, Corrêa MF (2010) On the way towards fish and aquatic habitats conservation in South America: a continental overview with emphasis on Neotropical systems. J Fish Biol 76:2118–2176
Barletta M, Cysneiros FJA, Lima ARA (2016) Effects of dredging operations on the demersal fish fauna of a South American tropical–subtropical transition estuary. J Fish Biol. https://doi.org/10.1111/jfb.12999
Barletta-Bergan A, Barletta M, Saint-Paul U (2002a) Structure and seasonal dynamics of larval in the Caete River Estuary in North Brazil. Estua Coast Shelf Sci 54:193–206. https://doi.org/10.1006/ecss.2001.0842
Barletta-Bergan A, Barletta M, Saint-Paul U (2002b) Community structure and temporal variability of ichthyoplankton in north Brazilian mangrove creeks. J FISH Biol 61(Suppl. A):33–51. https://doi.org/10.1006/jfbi.2002.2065
Clarke KR, Warwick RM (1994) Change in marine communities: an approach to statistical analysis and interpretation. Natural Environment Research Council, Plymouth, p 144
Clarke KR, Warwick RM (2001) Change in marine communities: an approach to statistical analysis and interpretation. 2nd ed. PRIMER-E, Plymouth: Plymouth marine laboratory
Dantas DV, Barletta M, Costa MF, Barbosa-Cintra SCT, Possatto FE et al (2010) Movement patterns of catfshes in a tropical semi-arid estuary. J Fish Biol 76:2540–2567
Dantas DV, Barletta M, Costa MF (2015) Feeding ecology and seasonal diet overlap between Stellifer brasiliensis and Stellifer stellifer in a tropical estuarine ecocline. J Fish Biol 86:707–723
DeJong T (1975) A comparison of three diversity indices based on their components of richness and evenness. Oikos 26:222–227
Eick D, Thiel R (2014) Fish assemblage patterns in the Elbe estuary: guild composition, spatial and temporal structure, and influence of environmental factors. Mar Biodivers 44:559–580
Elliott M, Hemingway KL (2008) Fishes in estuaries. Blackwell Science, London
Garcia A, Vieira J, Winemiller K, Moraes L, Paes E (2012) Factoring scales of spatial and temporal variation in fish abundance in a subtropical estuary. Mar Ecol Prog Ser 461:121–135
Gibbs PJ, Matthews J (1982) Analysis of experimental trawling using a miniature otter trawl to sample demersal fish in shallow estuarine waters. Fish Res 1:235–249
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: Paleontological statistics Software package for education and data analysis. Palaeontologia Electronica:4, 9
Harris SA, Cyrus DP (1995) Occurrence of fish larvae in the St Lucia estuary, KwaZulu-Natal, South Africa. South Afri J Mar Sci 16:333–350. https://doi.org/10.2989/025776195784156601
Harris SA, Cyrus DP (2000) Comparison of larval fish assemblages in three large estuarine systems, KwaZulu-Natal, South Africa. Mar Biol 137:527–541. https://doi.org/10.1007/s002270000356
Hossain MS, Das NG, Sarker S, Rahaman MZ (2012) Fish diversity and habitat relationship with environmental variables at Meghna river estuary, Bangladesh. Egyp J Aqua Res 38:213–226. https://doi.org/10.1016/j.ejar.2012.12.006
Islam MR, Mia MJ, Lithi UJ (2017) Spatial and temporal disparity of fish assemblage relationship with hydrological factors in two rivers Tangon and Kulik, Thakurgaon, Bangladesh. Turk J Fish Aqua Sci 17:1209–1218. https://doi.org/10.4194/1303-2712-v17_6_14
Jiang M, Shen X-Q (2006) Abundance distributions of pelagic fish eggs and larva in the Yangtze River estuary and vicinity waters in summer. Mar Sci 30:92–97 (in Chinese)
Kamrani E, Sharifinia M, Hashemi SH (2015) Analyses of fish community structure changes in three subtropical estuaries from the Iranian coastal waters. Mar Biodivers 46:561–577. https://doi.org/10.1007/s12526-015-0398-5
Laegdsgaard P, Johnson CR (1995) Mangrove habitats as nurseries: unique assemblages of juvenile fish in subtropical mangroves in eastern Australia. Mar Ecol Prog Ser 126:67–81
Lepš J, Šmilauer P (2003) Multivariate analysis of ecological data using CANOCO. Cambridge University Press, Cambridge
Lima ARA, Barletta M, Costa MF (2015) Seasonal distribution and interactions between plankton and microplastics in a tropical estuary. Estua Coast Shelf Sci 161:93–107. https://doi.org/10.1016/j.ecss.2015.05.018
Lima ARA, Barletta M, Costa MF, Ramos JAA, Dantas DV, Melo PA, Justino AKS, Ferreira GV (2016) Changes in the composition of ichthyoplankton assemblage and plastic debris in mangrove creeks relative to moon phases. J Fish Biol 89:619–640. https://doi.org/10.1111/jfb.12838
Liu H, Jiang T, Huang H, Shen X, Zhu J, Yang J (2015) Estuarine dependency in Collichthys lucidus of the Yangtze River Estuary as revealed by the environmental signature of otolith strontium and calcium. Environ Biol Fish 98:165–172. https://doi.org/10.1007/s10641-014-0246-7
Loneragan NR, Potter IC (1990) Factors influencing community structure and distribution of different life-cycle categories of fishes in shallow waters of a large Australian estuary. Mar Biol 106:25–37. https://doi.org/10.1007/BF02114671
Margalef R (1968) Perspectives in ecological theory. University of Chicago Press, Chicago, p 111
Marshall S, Elliott M (1998) Environmental influences on the fish assemblage of the Humber estuary, UK. Estua Coast Shelf Sci 46:175–184. https://doi.org/10.1006/ecss.1997.0268
McErlean AJ, O’Connor SG, Mihursky JA, Gibson CI (1973) Abundance, diversity and seasonal patterns of estuarine fish populations. Estua Coast Shelf Sci 1:19–36. https://doi.org/10.1016/0302-3524(73)90054-6
Merigot B, Lucena-Fredou F, Viana AP, Ferreira BP et al (2016) Fish assemblages in tropical estuaries of northeast Brazil: a multi component diversity approach. Oce Coast Manag 143:175–183. https://doi.org/10.1016/j.ocecoaman.2016.08.004
Monteleone DM (1992) Seasonality and abundance of ichthyoplankton in great south bay, New York. Estuaries 15:230–238. https://doi.org/10.2307/1352697
Pauly D (1988) Fisheries research andthe demersal fisheries of Southeast Asia. In: Gulland JA (ed) Fish population dynamics. John Wiley and Sons, New York, pp 329–348
Pielou E (1966) The measurement of diversity in different types of biological collections. J Theor Biol 13:131–144. https://doi.org/10.1016/0022-5193(66)90013-0
Quan WM, Ni Y, Shi LY, Chen YQ (2009) Composition of fish communities in an intertidal salt marsh creek in the Yangtze River estuary, China. Chin J Oceano Limno 27:806–815. https://doi.org/10.1007/s00343-009-9186-z
Ramos S, Cowen RK, Re P, Bordalo AA (2006) Temporal and spatial distributions of larval fish assemblages in the Lima Estuary (Portugal). Estua Coast Shelf Sci 66:303–314. https://doi.org/10.1016/j.ecss.2005.09.012
Ramos JA, Barletta M, Dantas DV, Costa MF (2016) Seasonal and spatial ontogenetic movement of Gerreidaes in a Brazilian tropical estuarine ecocline and its application for nursery habitat conservation. J Fish Biol 89:696–712. https://doi.org/10.1111/jfb.12872
Roux MJ, Harwood LA, Zhu X, Sparling P (2015) Early summer nearshore fish assemblage and environmental correlates in an Arctic estuary. J Great Lakes Res 42:256–266. https://doi.org/10.1016/j.jglr.2015.04.005
Salas F, Marcos C, Neto J, Patrício J, Pérez-Ruzafa A, Marques J (2006) User-friendly guide for using benthic ecological indicators in coastal and marine quality assessment. Oce Coast Manag 49:308–331. https://doi.org/10.1016/j.ocecoaman.2006.03.001
Shan XJ, Jin XS, Yuan W (2010) Fish assemblage structure in the hypoxic zone in the Yangtze (Yangtze River) estuary and its adjacent waters. Chin J Oceano Limno 28:59–469. https://doi.org/10.1007/s00343-010-9102-6
Shannon CE, Weaver W (1949) The mathematical theory of communication, vol 1. University of Illinois Press, Urbana-Champaign
Simpson EH (1949) Measurement of diversity. Nature 163:688
Sparre P, Venema SC (1995). Introductıon a la evaluacion de recursos pesqueros tropicales, Part 1-Manual. FAO technical paper 306/1, 339–344, Rome
Strydom NA, Whitfield AK, Wooldridge TH (2003) The role of estuarine type in characterizing early stage fish assemblages in warm temperate estuaries, South Africa. Afric Zool 38:29–43
Thayer GW, Colby DR, Hettler WF (1987) Utilization of the red mangrove prop root habitat by fishes in South Florida. Mar Ecol Prog Ser 35:25–38
Vyas V, Damde V, Parashar V (2012) Fish biodiversity of Betwa River in Madhya Pradesh, India with special reference to a sacred Ghat. Inter J Biod Conserv 4:71–77. https://doi.org/10.5897/IJBC10.015
Whitfield AK (1989) Ichthyoplankton in a Douthern African surf zone: nursery area for the postlarvae of estuarine associated fish species? Estua Coast She Sci 29:533–547
Whitfield AK (1999) Ichthyofaunal assemblages in estuaries: a South African case study. Rev Fish Biol Fish 9:15186
Yang D-L, Wu G-Z, Sun J-R (1990) The investigation of pelagic eggs, larvae and juveniles of fishes at the mouth of the Yangtze River and adjacent areas. Oceano Limno Sinica 21:346–355 (in Chinese)
Young GC, Potter IC (2003) Do the characteristics of the ichthyoplankton in an artificial and a natural entrance channel of a large estuary differ? Estua Coast Shelf Sci 56:765–779
Yu HC, Xian WW (2009) The environment effect on fish assemblage structure in waters adjacent to the Yangtze (Yangtze) River estuary (1998–2001). Chin J Oceano Limno 27:443–456. https://doi.org/10.1007/s00343-009-9155-6
Yu W-G, Jiang H-T, Han R-P (2007) The water quality in Yangtze Estuary and the impact about large water project. Gansu Science Technology 23:11–14 (in Chinese)
Zhang H, Xian WW, Liu SD (2015) Ichthyoplankton assemblage structure of springs in the Yangtze Estuary revealed by biological and environmental visions. Peer J. https://doi.org/10.7717/peerj.1186
Zhang H, Xian WW, Liu SD (2016) Autumn ichthyoplankton assemblage in the Yangtze estuary shaped by environmental factors. Peer J. https://doi.org/10.7717/peerj.192216/16
Acknowledgments
We thank the staff and students of the College of Marine Sciences, Shanghai Ocean University and the Shanghai Aquatic Wildlife Conservation Research Center, Shanghai for their vital help during samplings. We are also grateful to the editor and the anonymous reviewers whose comments greatly improved the manuscript.
Funding
This project was supported and financed through the Shanghai Municipal Council by the Ministry of agriculture, China.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed by the authors.
Sampling and field studies
All necessary permits for sampling and observational field studies have been obtained by the authors from the competent authorities and are mentioned in the acknowledgements.
Additional information
Responsible Editor: Thomas Hein
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kindong, R., Wu, J., Gao, C. et al. Seasonal changes in fish diversity, density, biomass, and assemblage alongside environmental variables in the Yangtze River Estuary. Environ Sci Pollut Res 27, 25461–25474 (2020). https://doi.org/10.1007/s11356-020-08674-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-08674-8