Abstract
Drug residues, including various antibiotics, are being increasingly detected in aqueous environments. Ofloxacin (OFX) is one such antibiotic that is widely used in the treatment of several bacterial infections; however, chronic exposure to this antibiotic can have adverse impacts on human health. Hence, the identification of an effective OFX degradation method is essential. Thus, in this study, the degradation performance of OFX using potassium ferrate (Fe(VI)) under the influence of different initial concentrations, pH, temperature, and common ions in water was investigated. OFX degradation by Fe(VI) was directly proportional to the concentration of Fe(VI) and temperature and inversely proportional to the pH. Among the common ions in water, Fe3+ and NH4+ could significantly promote the degradation of OFX by Fe(IV), while humic acid (HA) significantly inhibited it. Under the conditions of [Fe(VI)]:[OFX] = 15:1, T = 25℃, and pH = 7.0, the removal efficiency of 8 μM OFX reached more than 90% in 4 min. Seven intermediates were identified by quadrupole time-of-flight tandem ultra-performance liquid chromatography mass spectrometry (Q-TOF LC/MS), and two possible pathways for the degradation of OFX by Fe(VI) were proposed. Overall, the results suggest that advanced oxidation technology using Fe(VI) is effective for treating wastewater containing OFX.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the increasing awareness of people’s environmental protection and increasing attention to health, drug residues in the environment have received increasing attention from researchers as well as the public. With the increasing use of antibiotics, high antibiotic concentrations have been detected in wastewater, as well as on the surface waters, drinking water, soil, and other environments now (Huang et al. 2019; Sanganyado and Gwenzi 2019; Cerqueira et al. 2019; Tong et al. 2019). In addition to ecotoxicity, they may cause increased bacterial resistance (Zhang et al. 2015; Hu et al. 2007). It has been reported that the proportion of drug-resistant bacteria in various poultry and livestock has also increased greatly in recent years (Van Boeckel et al. 2019).
Fluoroquinolones (FQs) have been widely used to treat a broad spectrum of bacterial infections over the past few decades (Zhang et al. 2015; Liu et al. 2016; Zhao et al. 2016). Ofloxacin (OFX), a popular FQ antibiotic, is a third-generation quinolone, which is mainly used in the treatment of acute and chronic infections of the respiratory tract, throat, and tonsils caused by gram-negative bacteria (Watanabe et al. 2001). OFX is discharged in non-metabolic form mainly through wastewater produced by the industries such as pharmaceuticals and waste generated by humans and livestock, enter into municipal wastewater treatment plants through sewer networks, and finally enter into the natural environment (Zhang et al. 2015; Jara et al. 2020; Radjenovic et al. 2007). However, existing sewage treatment facilities have low processing efficiency with regard to antibiotics (Jia et al. 2012). According to reports, various antibiotic residues have been detected in sewage treatment plants around the world, such as the average content of OFX in the effluent of sewage treatment plants has reached an astonishing 97.0 ng/L (Wang et al. 2020a). Hence, finding an effective antibiotics degradation method is essential.
Various technologies have been developed for the removal of various antibiotics from the water now, such as adsorption, advanced oxidation processes (AOPs), biodegradation/photodegradation/sonic degradation, and membrane separation (Peng et al. 2012; Jiang et al. 2016; Gorito et al. 2018; Elmolla and Chaudhuri 2010; Wang and Wang 2019a; 2018a). However, they have not been widely applied due to their low removal efficiency and high operational cost. By contrast, advanced oxidation technology mainly generates highly active oxidation species through a series of chemical oxidation processes for the degradation of antibiotics or converts them to small molecule substances, which could enhance their biodegradability and the removal rate (Zhou et al. 2021; Sharma et al. 2016; Wilde et al. 2013; Wang and Wang 2019b; 2018b). AOPs use strong oxidation agents to degrade organic pollutants. According to the different ways used to produce oxidation agents, AOPs can be classified into many different types (Wang and Zhuan 2020; Wang and Chen 2020). Among them, Fe(VI) advanced oxidation is a promising technology for the degradation of organic pollutants in wastewater (Pi et al. 2021; Shin et al. 2018; Wang et al. 2020b). It is characterized by rapid degradation of pollutants and high pollutant removal efficiency; hence, it can be regarded as green technology and has been widely used in wastewater treatment (Gong et al. 2020; Li et al. 2005).
In this work, K2FeO4 (Fe(VI)) was selected as the oxidant and OFX was selected as the target contaminant. The degradation efficiency and kinetics of OFX by Fe(VI) under different reaction conditions were investigated. In addition, on the basis of the intermediate products identified, a possible degradation pathway of OFX by Fe(VI) was proposed. This research provides theoretical support for the treatment of OFX-containing wastewater using an Fe(VI) advanced oxidation process.
Materials and methods
Reagents
All standards of the pharmaceuticals used (sodium hydroxide (NaOH), hydrochloric acid (HCl), sodium thiosulfate (Na2S2O3), ammonium acetate (CH3COONH4), phosphoric acid (H3PO4), tert-butanol (TBA), isopropanol (IPA), sodium bicarbonate (NaHCO3), sodium nitrate (NaNO3), sodium sulfate (Na2SO4), sodium chloride (NaCl), ammonium chloride (NH4Cl), ferric chloride (FeCl3), potassium chloride (KCl), and humic acid (HA)) were of analytical purity grade and purchased from Sinopharm Chemical Reagents (Shanghai, China). The solvent (methanol) was HPLC grade and was purchased from Merck (Darmstadt, Germany). OFX was obtained from Aladdin (Shanghai, China). The water was produced using a UPH purification system (ULUP-I, Ulpure, China, resistivity ≥ 18.2 MΩcm).
Experimental procedures
Fe(VI) was prepared using a hypochlorite oxidation method (Li et al. 2005). The reaction was carried out in a 250 mL glass conical flask under a constant stirring rate of 400 rpm at 25℃ in a water bath using a thermostatic magnetic stirrer. The reaction between OFX (8.0 μM) and Fe(VI) (40.0–160.0 μM) was initiated by mixing them in equal volumes of 100.0 mL; the pH of the reaction mixtures was adjusted using 1.0 mM HCl or NaOH buffer. The reaction was quenched completely at certain reaction times (i.e., 0–5 min) using 200.0 μL of 1.0 mM Na2S2O3 solution. Samples were filtered using 0.45 μm polytetrafluoroethylene syringe filters (Fisherbrand, Fisher Scientific) and transferred into 2.0 mL high-performance liquid chromatography (HPLC) vials for analysis. All experiments took an average of three parallel samples.
Analytical methods
The concentrations of OFX in the samples were measured by HPLC (LC-2030PLUS, Shimadzu, Japan) equipped with a SinChrom ODS-B column (5 μm, 4.6 mm × 250 mm), and the detection was performed using a G1365MWD UV detector at 293 nm. The mobile phase was composed of 60% ammonium acetate (1% phosphoric acid adjusted to pH 2.7) and 40% methanol. Samples were analyzed at a flow rate of 1.0 mL/min, the injection volume was set to 20 μL, and the column temperature was set at 25℃.
The intermediates of OFX were measured using a quadrupole time-of-flight (Q-TOF) liquid chromatography/mass spectrometry (LC/MS) (6545Q-TOF, Agilent, USA). Isocratic elution was performed at a flow rate of 0.3 mL/min with 0.1% (v/v) ammonium acetate (A) and methanol (B). The mass spectra with electrospray ionization (ESI) source were recorded across the range of 50–500 m/z in positive scan mode.
Results and discussion
Influence of initial oxidant concentration and degradation kinetics
The effect of the initial Fe(VI) concentration on OFX degradation is shown in Fig. 1a. It can be observed that the pseudo-first-order rate constants for OFX degradation (Kobs) increased from 0.0031 to 0.0125 s−1 when the Fe(VI) concentration increased from 40 to 160 μM. Then, Ln[Fe(VI)]0 and LnKobs were fitted by linear regression, and the results are shown in Fig. 1b. The correlation coefficient R2 was greater than 0.99, which means that the degradation of OFX by Fe(VI) follows the second-order reaction kinetics equation. The rate equations under different initial concentrations of Fe(VI) are shown in Table 1.
As shown in Table 1, the average value of the secondary reaction rate constant (Kapp) for the degradation of OFX by Fe(VI) was 78.28 M−1 s−1. As the initial concentration of Fe(VI) increased from 40 to 160 μM, the half-life t1/2 decreased from 224 to 55 s. The results showed that with an increase in the initial concentration of [Fe(VI)], the degradation rate of OFX gradually increased; similar results have been reported by Wang et al. (2019) and Liu et al. (2019).
Influence of pH and the corresponding kinetics
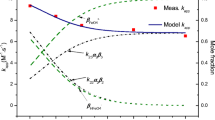
The degradation of OFX by Fe(VI) under different pH conditions was measured, and the results are shown in Fig. 2a. When the pH increased from 5 to 10, the Kobs decreased from 0.0168 to 0.0039 s, implying that the degradation rate of OFX by Fe(VI) increased with a decrease in the pH value; similar results have been reported by Zheng et al. (2020) and Han et al. (2018).
Because OFX and Fe(VI) have various ionisable groups, their final ionization morphologies can be determined based on different pH values (Huang et al. 2017; Peterson et al. 2015). Therefore, the kinetic equations of the pH and Kapp values were simulated using Eq. (1)
where αi and βj represent the distribution coefficients of OFX and Fe(VI), respectively. When the OFX dissociation constants pKa1 and pKa2 are 6.11 and 8.18, respectively, α1, α2, and α3 refer to the fractions of OFX+, OFX0, and OFX−, respectively (Peterson et al. 2015). For Fe(VI) (pKa = 7.23, pH ≥ 5 in the experiment), β1 and β2 refer to the fractions of HFeO4− and FeO42−, respectively (Han et al. 2018; Zajicek et al. 2015). Under alkaline conditions, the reaction between FeO42− and OFX− was very slow (Zheng et al. 2020; Han et al. 2018; Huang et al. 2017); therefore, the reaction could be ignored in the model calculation. In addition, because it was difficult for HFeO4− and OFX− (or FeO42− and OFX+) to exist at the same time under certain pH conditions, their reaction could also be ignored in the model calculation; therefore, the effect of pH on the Kapp value of Fe(VI) can be further simplified as Eq. (2):
The experimental values of Kapp (Meas Kapp) under different pH conditions were fitted with Eq. (2), and the results are shown in Fig. 2b; it can be seen that they exhibited a significant curve correlation (R2 ≥ 0.99).
The results showed that K11, K21, and K22 were 150.23 M−1S−1, 94.7 M−1S−1, and 45.1 M−1S−1, respectively. Among them, K11 or K21 was > K22, indicating that HFeO4− was more oxidizing than FeO42−, and with the gradual decrease in pH (when PH ≥ 5), HFeO4− would become more dominant in the system. Moreover, K11 > K21 or K22 implied that HFeO4− showed higher reactivity toward OFX+ than OFX0 or OFX−. In conclusion, the degradation rate of OFX gradually increased with a continuous decrease in pH.
Influence of temperature and the corresponding kinetics
The degradation of OFX was measured as the temperature increased from 10 to 30℃; Kobs correspondingly increased from 0.0055 to 0.0110 s (Fig. 3a). This illustrates that the degradation rate of OFX gradually increases with an increase in temperature (Han et al. 2018). Figure 3b shows the results of fitting LnKapp and 1000/T, and a good linear relationship (R2 ≥ 0.99) can be observed. This shows that the degradation of OFX with temperature change satisfies the Arrhenius equation as follows (Luo et al. 2015; Han et al. 2018):
Here, the molar gas constant R is 8.314 J·mol−1·k−1, and hence, the reaction activation energy Ea can be calculated to be 24.2 kJ·mol−1. The results suggest that the reaction between Fe(VI) and OFX could occur even when the activation energy was relatively low.
In Fig. 3c, the results of fitting Ln(Kapp/T) and 1/T are shown; a good linear relationship (R2 ≥ 0.99) is observed. This implies that the degradation of OFX with temperature change satisfies the Eying equation as follows (Luo et al. 2015):
where the Boltzmann constant kB is 1.38 × 10−23 J·k−1 and the Planck constant h is 6.626 × 10−34 J·s; therefore, ΔH and ΔS can be calculated as 21.319 kJ·mol−1 and − 135.62 J·mol−1·k−1, respectively. The results show that the degradation of OFX by Fe(VI) was an endothermic reaction. With an increase in temperature, the number of effective collisions between the polymers increased, resulting in an acceleration of the reaction rate.
Influence of free radicals in reaction system
Studies have shown that the degradation of organic pollutants by Fe(VI) includes the direct oxidation by Fe(VI) and the indirect oxidation by generated free radicals (Zhang et al. 2012). And Fe(VI) and its intermediates (Fe(V) and Fe(IV)) can generate highly reactive hydroxyl radicals (OH•) during their self-decomposition process (Zhang et al. 2012; Noorhasan et al. 2010). In traditional advanced oxidation process experiments, OH• plays a significant role (Wang et al. 2018; Chen et al. 2020, 2019; Wang and Wang 2020). Therefore, in order to verify the contribution of ROS, TBA and IPA were used as free radical scavengers of OH•. As shown in Fig. 4, the OFX degradation rate decreased by 4.57% and 5.82% with the addition of TBA and IPA to the reaction system, respectively. Thus, it can be concluded that Fe(VI) does exist OH• in the experiments of degrading OFX, and OH• contributed little to the degradation of OFX. Therefore, it can be considered that the degradation of OFX is mainly due to the oxidation by high-valent iron-based. Similar results have been reported by Shao et al. (2019) and Jin et al. (2021).
Influence of anions, cations, and organic matter
The common anions, cations, and organics in water were selected to investigate their influence on the degradation of OFX by Fe(IV). K+, Na+, Cl−, SO42−, and NO3− had almost no effect; Fe3+ and NH4+ greatly promoted OFX degradation; while HCO3− only had a small inhibitory effect on OFX degradation in the reaction system (Fig. 5). In addition, organic matter, represented by HA, significantly inhibited the degradation of OFX by Fe(IV) (Fig. 5).
In water, HCO3− can slightly inhibit the degradation of OFX by Fe(VI). On the one hand, the presence of bicarbonate can increase the solution pH, which in turn affects the stability of the oxidant. As shown in Fig. 2, the degradation rate of OFX by Fe(VI) decreases with increasing pH. The reason is that Fe(VI) is more present in the form of FeO42− under alkaline conditions, and the reaction between FeO42− and OFX− is very slow (Zheng et al. 2020; Han et al. 2018; Huang et al. 2017). On the other hand, the HCO3− will react with OH•, thereby depleting free radicals in solution and reducing the reaction rate (Wang and Wang 2021).
However, the pH of the solution raised after bicarbonate hydrolysis is limited, and Fig. 4 also proves that the contribution of OH• is small. Therefore, HCO3− has only a small inhibitory effect on OFX degradation in the reaction system.
Fe3+ enhanced the OFX removal by Fe(VI) mostly via self-catalysis of Fe(VI) to generate more Fe(IV) or Fe(V) (Jiang et al. 2016; Ma et al. 2016; Zhao et al. 2018a). Moreover, NH4+ was reductive, which could promote the generation of Fe(IV) or Fe(V) from Fe(VI), thus improving the removal rate of OFX (Zhao et al. 2018b). Since HA and OFX compete with Fe(VI), HA has a significant inhibitory effect on the Fe(VI) degradation of OFX (Sharma et al. 2016; Horst et al. 2013).
Intermediate products and degradation pathways
Through a study of relevant references and Q-TOF LC/MS analysis of intermediate products of OFX degradation (Michael et al. 2013; Bi et al. 2019; Xue et al. 2017; Meng et al. 2021; Jin et al. 2021), and possible degradation pathways were proposed (Fig. 6). There are two main ways in which Fe(IV) degrades OFX. One of the degradation pathways is as follows: Firstly, the methyl group on the benzoxazine ring of OFX was carboxylated to form P1 (m/z = 391.1). P1 was then converted to P3 (m/z = 303.1) through decarboxylation. After the opening of the piperazine and oxazine rings, P3 was transformed into P5 (m/z = 178.1), and finally, P7 (m/z = 110.0) was formed through pyridine ring opening, defluorination, deamination, and hydroxylation. The other degradation pathway is as follows: OFX was converted into P2 (m/z = 337.1) through pyridine ring opening, and then P4 (m/z = 218.1) was formed by the opening of the piperazine and oxazine rings. P4 was finally transformed into P6 (m/z = 122.0) by deamination, defluorination, and dehydroxylation. P6 and P7 were further oxidized to form small molecular intermediates, which were finally decomposed into small molecular acids, CO32−, H2O, NO3−, and minerals (Zhou et al. 2021).
Conclusion
This study showed that Fe(VI) is an effective oxidant for the degradation of OFX. The degradation rate of OFX by Fe(VI) was in accordance with the second-order kinetic equation. OFX degradation by Fe(VI) was directly proportional to the initial concentration of Fe(VI) and temperature and inversely proportional to the pH. Under the conditions of [Fe(VI)]:[OFX] = 15:1, T = 25℃, and pH = 7.0, the removal efficiency of 8 μM OFX reached more than 90% in 4 min. Seven intermediates were identified by Q-TOF LC/MS, and the opening loop, decarboxylation, breaking the C-N bond, and deamination were the main reaction processes identified in their formation. In conclusion, Fe(VI) advanced oxidation technology has obvious advantages in the treatment of wastewater containing OFX.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Bi W, Jin Y, Wang H (2019) Migration and transformation of ofloxacin by free chlorine in water distribution system. Water 11(4):817
Cerqueira F, Matamoros V, Bayona J, Elsinga G, Hornstra LM, Pina B (2019) Distribution of antibiotic resistance genes in soils and crops. A field study in legume plants (Vicia faba L.) grown under different watering regimes. Environ Res 170:16–25
Chen P, Blaney L, Cagnetta G, Huang J, Wang B, Wang Y, Deng S, Yu G (2019) Degradation of ofloxacin by perylene diimide supramolecular nanofiber sunlight-driven photocatalysis. Environ Sci Technol 53(3):1564–1575
Chen X, Yao J, Xia B, Gao N, Zhang Z (2020) Influence of pH and DO on the ofloxacin degradation in water by UVA-LED/TiO2 nanotube arrays photocatalytic fuel cell: mechanism, ROSs contribution and power generation. J Hazard Mater 383(Feb.5):121220.1-121220.11
Elmolla ES, Chaudhuri M (2010) Degradation of amoxicillin, ampicillin and cloxacillin antibiotics in aqueous solution by the UV/ZnO photocatalytic process. J Hazard Mater 173:445–449
Gong S, Sun Y, Zheng K, Jiang G, Li L, Feng J (2020) Degradation of levofloxacin in aqueous solution by non-thermal plasma combined with Ag3PO4/activated carbon fibers: mechanism and degradation pathways. Sep Purif Technol 250:117264
Gorito AM, Ribeiro AR, Gomes CR, Almeida CMR, Silva AMT (2018) Constructed wetland microcosms for the removal of organic micropollutants from freshwater aquaculture effluents. Sci Total Environ 644:1171–1180
Han Q, Dong WY, Wang HJ, Liu TZ, Tian Y, Song X (2018) Degradation of tetrabromobisphenol A by ferrate(VI) oxidation: performance, inorganic and organic products, pathway and toxicity control. Chemosphere 198:92–102
Horst C, Sharma VK, Baum JC, Sohn M (2013) Organic matter source discrimination by humic acid characterization: synchronous scan fluorescence spectroscopy and Ferrate(VI). Chemosphere 90:2013–2019
Hu JY, Wang W, Zhu Z, Zhu Z, Chang H, Pan F, Lin BL (2007) Quantitative structure-activity relationship model for prediction of genotoxic potential for quinolone antibacterials. Environ Sci Technol 41:4806–4812
Huang JH, Wang YH, Liu GG, Chen P, Wang FL, Ma JS, Li FH, Liu HJ, Lv WY (2017) Oxidation of indometacin by ferrate(VI): kinetics, degradation pathways, and toxicity assessment. Environ Sci Pollut R 24:10786–10795
Huang YH, Liu Y, Du PP, Zeng LJ, Mo CH, Li YW, Lu HX, Cai QY (2019) Occurrence and distribution of antibiotics and antibiotic resistant genes in water and sediments of urban rivers with black-odor water in Guangzhou, South China. Sci Total Environ 670:170–180
Jara D, Bello-Toledo H, Domínguez M, Cigarroa C, Fernández P, Vergara L, Quezada-Aguiluz MA, Opazo- Capurro AF, Lima GA, González-Rocha G (2020) Antibiotic resistance in bacterial isolates from freshwater samples in Fildes Peninsula, King George Island. Antarctica Sci Rep 10(1):3145
Jia A, Wan Y, Xiao Y, Hu J (2012) Occurrence and fate of quinolone and fluoroquinolone antibiotics in a municipal sewage treatment plant. Water Res 46:387–394
Jiang C, Ji Y, Shi Y, Chen J, Cai T (2016) Sulfate radical-based oxidation of fluoroquinolone antibiotics: kinetics, mechanisms and effects of natural water matrices. Water Res 106:507–517
QY Jin, DY Ji, YH Chen, ZM Tang, YS Fu (2021) Kinetics and pathway of levofloxacin degradation by ferrate(VI) and reaction mechanism of catalytic degradation by copper sulfide. Sep Purif Technol 282
Li C, Li XZ, Graham N (2005) A study of the preparation and reactivity of potassium ferrate. Chemosphere 61:537–543
Liu X, Zhang H, Li L, Fu C, Tu C, Huang Y, Wu L, Tang J, Luo Y, Christie P (2016) Levels, distributions and sources of veterinary antibiotics in the sediments of the Bohai Sea in China and surrounding estuaries. Mar Pollut Bull 109:597–602
Liu HX, Chen J, Wu NN, Xu XX, Qi YM, Jiang LJ, Wang XH, Wang ZY (2019) Oxidative degradation of chlorpyrifos using ferrate(VI): kinetics and reaction mechanism. Ecotox Environ Safe 170:259–266
Luo Z, Li X, Zhai J (2015) Kinetic investigations of quinoline oxidation by ferrate(VI). Environ Technol 37(9–12):1–19
Ma L, Lam WWY ,Lo PK ,Lau KC, Lau TC (2016) Ca2+-Induced Oxygen Generation by FeO42− at pH 9–10. Angewandte Chemie 128(9): 3064-3068 10.1002/ange.201510156
Meng FQ, Wang YL, Chen Z, Hu J, Ma W (2021) Synthesis of CQDs@FeOOH nanoneedles with abundant active edges for efficient electro-catalytic degradation of levofloxacin: degradation mechanism and toxicity assessment. Appl Catal B- Environ 282:119597
Michael I, Hapeshi E, Acena J, Perez S, Petrovic M, Zapata A, Barceló D, Malato S, Fatta-Kassinos D (2013) Light-induced catalytic transformation of ofloxacin by solar Fenton in various water matrices at a pilot plant: mineralization and characterization of major intermediate products. Sci Total Environ 461:39–48
Noorhasan N, Patel B, Sharma VK (2010) Ferrate(VI) oxidation of glycine and glycylglycine: kinetics and products. Water Res 44:927–935
Peng H, Pan B, Wu M, Liu R, Zhang D, Wu D, Xing B (2012) Adsorption of ofloxacin on carbon nanotubes: solubility, pH and cosolvent effects. J Hazard Mater 211–212:342–348
Peterson JW, Gu B, Seymour MD (2015) Surface interactions and degradation of a fluoroquinolone antibiotic in the dark in aqueous TiO2 suspensions. Science of the Total Environment 532(nov.1):398–403
Pi R, Liu H, Sun X, Zhang R, Zhang J, Sharma VK (2021) Strategy of periodic reverse current electrolysis to synthesize ferrate(VI): enhanced yield and removal of sulfachloropyridazine. Sep Purif Technol 263:118420
Radjenovic J, Petrovic M, Barceló D (2007) Analysis of pharmaceuticals in wastewater and removal using a membrane bioreactor. Anal Bioanal Chem 387(4):1365–1377
Sanganyado E, Gwenzi W (2019) Antibiotic resistance in drinking water systems: occurrence, removal, and human health risks. Sci Total Environ 669:785–797
Shao B, Dong H, Sun B, Guan X (2019). Role of ferrate(IV) and ferrate(V) in activating ferrate(VI) by calcium sulfite for enhanced oxidation of organic contaminants. Environ Sci Technol 53: 894-902 10.1021/acs.est.8b04990
Sharma VK, Chen L, Zboril R (2016) Review on high valent FeVI 279 (Ferrate): A sustainable green oxidant in organic chemistry and transformation of pharmaceuticals. ACS Sustain Chem Eng 4:18–34
Shin J, Gunten U, Reckhow D, Allard S, Lee Y (2018) Reactions of ferrate(VI) with iodide and hypoiodous acid: kinetics, pathways, and implications for the fate of iodine during water treatment. Environ Sci Technol 52:7458–7467
Tong X, Wang X, He X, Xu K, Mao F (2019) Effects of ofloxacin on nitrogen removal and microbial community structure in constructed wetland. Sci Total Environ 656:503–511
Van Boeckel TP, Pires J, Silvester R, Zhao C, Song J, Criscuolo NG, Gilbert M, Bonhoeffer S, Laxminarayan R (2019) Global trends in antimicrobial resistance in animals in low-and middle-income countries. Sci 365(6459):eaaw1944
Wang J, Chen H (2020) Catalytic ozonation for water and wastewater treatment: recent advances and perspective. Sci Total Environ 704(feb.20):135249.1-135249.17
Wang JL, Wang SZ (2018) Microbial degradation of sulfamethoxazole in the environment. Appl Microbiol Biotechnol 102:3573–3582
Wang JL, Wang SZ (2018) Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem Eng J 334:1502–1507
Wang JL, Wang SZ (2019) Preparation, modification and environmental application of biochar: a review. J Clean Prod 227:1002–1022
Wang SZ, Wang JL (2019) Activation of peroxymonosulfate by sludge-derived biochar for the degradation of triclosan in water and wastewater. Chem Eng J 356:350–358
Wang JL, Wang SZ (2020) Reactive species in advanced oxidation processes: formation, identification and reaction A. Chem Eng J 401:126158
Wang JL, Wang SZ (2021) Effect of inorganic anions on the performance of advanced oxidation processes for degradation of organic contaminants. Chem Eng J 411(20):128392
Wang JL, Zhuan R (2020) Degradation of antibiotics by advanced oxidation processes: an overview. Sci Total Environ 701:135023
Wang Z, Jiang J, Pang S, Zhou Y, Cuan C, Gao Y, Li J, Yang Y, Qu W, Jiang C (2018) Is sulfate radical really generated from peroxydisulfate activated by iron(II) for environmental decontamination? Environ Sci Technol 52(19):11276–11284
Wang HY, Wang SJ, Jiang JQ, Shu J (2019) Removal of sulfadiazine by ferrate(VI) oxidation and montmorillonite adsorption-synergistic effect and degradation pathways. J Enviro Chem Eng 7:103225
Wang JL, Chu LB, Wojnárovits L, Takács E (2020) Occurrence and fate of antibiotics, antibiotic resistant genes (ARGs) and antibiotic resistant bacteria (ARB) in municipal wastewater treatment plant: an overview. Sci Total Environ 744:140997
Wang S, Shao B, Qiao J, Guan X (2020b) Application of Fe(VI) in abating contaminants in water: state of art and knowledge gaps. Front Env Sci Eng 15:80
Watanabe K, Numata-Watanabe K, Hayasaka S (2001) Methicillin-resistant staphylococci and ofloxacin-resistant bacteria from clinically healthy conjunctivas. Ophthalmic Res 33:136–139
Wilde ML, Mahmoud WMM, Kuemmerer K (2013) Oxidation-coagulation of beta-blockers by K2FeVIO4 in hospital wastewater: assessment of degradation products and biodegradability. Sci Total Environ 452–453:137–147
Xue H, Hu C, Peng J, Wang L, Wang Y, Ji N, Wen X (2017) Degradation of ofloxacin in aqueous solution with UV/H2O2. Desalin Water Treat 72(APR):386–393
Zajicek P, Kolar M, Prucek R, Ranc V, Bednar P, Varma RS, Sharma VK, Zboril R (2015) Oxidative degradation of triazine- and sulfonylurea-based herbicides using Fe(VI): the case study of atrazine and iodosulfuron with kinetics and degradation products. Sep Purif Technol 156:1041–1046
Zhang P, Zhang G, Dong J, Fan M, Zeng G (2012) Bisphenol A oxidative removal by ferrate (Fe(VI)) under a weak acidic condition. Sep Purif Technol 84:46–51
Zhang QQ, Ying GG, Pan CG, Liu YS, Zhao JL (2015) Comprehensive evaluation of antibiotics emission and fate in the river basins of China: source analysis, multimedia modeling, and linkage to bacterial resistance. Environ Sci Technol 49:6772–6782
Zhao S, Liu X, Cheng D, Liu G, Liang B, Cui B, Bai J (2016) Temporal-spatial variation and partitioning prediction of antibiotics in surface water and sediments from the intertidal zones of the Yellow River Delta, China. Sci Total Environ 569–570:1350–1358
Zheng Q, Wu NN, Qu RJ, Albasher G, Cao WM, Li BB, Alsultan N, Wang ZY (2020) Kinetics and reaction pathways for the transformation of 4-tert-butylphenol by ferrate(VI). J Hazard Mater 2020:123405
Zhou Y, Gao Y, Jiang J, Shen Y, Pang S, Song Y, Guo Q (2021) A comparison study of ofloxacin degradation by peroxymonosulfate and permanganate: kinetics, products and effect of quinone group. J Hazard Mater 403:123834
Zhao J, Wang Q, Fu Y, Peng B, Zhou G (2018a) Kinetics and mechanism of diclofenac removal using ferrate(VI): Roles of Fe3+, Fe2+, and Mn2+. Environ Sci Pollut R 25: 22998-23008 10.1007/s11356-018-2375-6
Zhao J, Liu Y, Wang Q, Fu Y, Lu X, Bai XF (2018b) The self-catalysis of ferrate (VI) by its reactive byproducts or reductive substances for the degradation of diclofenac: kinetics, mechanism and transformation products. Sep Purif Technol 192: 412-418 10.1016/j.seppur.2017.10.030
Acknowledgements
We would like to thank the Faculty of Geosciences and Environmental Engineering of Southwest Jiaotong University for their help with the experimental analysis work.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 51803174).
Author information
Authors and Affiliations
Contributions
Yanghan Chen: investigation, resources, visualization, writing – original draft; Qiuye Jin: conceptualization, methodology, formal analysis, data curation, visualization; Zhaomin Tang: validation, supervision, writing – review and editing, funding acquisition, project administration.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This manuscript does not report on or involve the use of any animal or human data or tissue.
Consent for publication
This manuscript does not contain data from any individual person.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Sami Rtimi
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, Y., Jin, Q. & Tang, Z. Degradation of ofloxacin by potassium ferrate: kinetics and degradation pathways. Environ Sci Pollut Res 29, 44504–44512 (2022). https://doi.org/10.1007/s11356-022-18949-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-18949-x