Abstract
The present study aims to extract a natural reddish brown colorant from Peepal (Ficus religiosa) for silk dyeing using the microwave radiation process (MW). The colorant was isolated in aqueous and acidic media, and MW treatment for 1, 2, 3, 4, and 5 min has been given to both fabric and extract to observe changes in color intensity. The dye variables have been optimized, and for sustainable shade making process with good fastness, 1.0–5.0 g/100 mL of sustainable chemical and bio-mordants has been employed. It has been found that after microwave treatment for 3 min, under selected conditions, the irradiated aqueous extract has given high color intensity onto silk fabric. The utilization of 3% of Al, 4% of Fe, and 2% of tannic acid (T.A.) as pre chemical mordant whereas 4% of Al, 4% of Fe, and 3% of tannic acid as post chemical mordant have given good color characteristics. In comparison, 4% of acacia and 3% of turmeric and pomegranate while 3% of acacia and turmeric and 4% of pomegranate extracts as post-bio-mordant have given excellent color characteristics. It is concluded that MW treatment has an excellent sustainable efficacy to isolate colorant from Peepal bark for silk dyeing, whereas the inclusion of bio-mordants has not only made the process more sustainable and environmental friendly but also best K/S, and L*a*b* values have been acquired.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
One of the major causes of global pollution is the widespread utilization of synthetic dyes (Fadil et al. 2021). About 8000 metric tons of dye-stuff per annum is released worldwide, where approximately, in every processing, 10–50% of their effluent is released into water bodies without purification. This concentration of dye-stuff causes hindrance in the way of light penetrating deep in water bodies and disturbing the water ecosystem (Yılmaz and Bahtiyari 2020). Ultimately their presence lowers the aquatic photosynthetic process and destroys water quality parameters which in turn become harmful for agri-land and aquatic life (dos Santos Silva et al. 2020a). These wastes have carcinogens that are non-biodegradable and take a lot of time, money, and labor for their treatment (Haji and Vadood 2021). Many world-renowned associations such as ETAD (Ecological and Toxicological Associations of Dyes and Pigments), GOTS (Global Organic Testing Standards), EPA (Environmental Protection Agency), UNESCO (United Nations Educational Scientific and Cultural Organization), FAO (Food and Agriculture Organization), World Health Organization (WHO have seriously taken this matter under observation. These global institutes are forcing all industrialists to replace such stuff with green, sustainable, and eco-friendly products in all fields particularly textiles, cosmetics, food and flavor, and pharmaceutical industries (Hosseinnezhad et al. 2020; Salauddin et al. 2021). Among green products, particularly natural dyes have been now revived as the art of cultural heritage in textiles. Natural dyes are the dyes obtained from natural sources such as plants, animals, and minerals without any change in their morphology (Chen et al. 2020; Sk et al. 2021). In the current times, with the gradual increase in awareness among people related to eco-friendly and less polluted products, researchers are shifting towards natural dye as the possible substitute for synthetic dyes, and the researches on their possible isolation, standardization, and application in all fields are growing on (Viera et al. 2019; Zheng et al. 2021).
Natural dyes are facing two major problems, i.e., low extraction yield and poor color fastness ratings. A group of researchers is working to overcome these issues for decades. In the natural dyeing process, extraction of colorant is the basic step (Borrás-Enríquez et al. 2021). It is the process in which the cell wall is ruptured and the colorant is extracted out from the plant into solvent media at employed condition. Soaking, stirring, heating, and reflux are some of the important conventional methods, which are used for the extraction of the bio-potent molecule (Hasan et al. 2021; Kovačević et al. 2021). Due to low cost and simplicity, the solvent extraction method is considered as one of the good choices for the extraction of the colorant, but their conventional methods are not energy, time, and labor effective (Handayani et al. 2019). For better extraction, such methods are needed to be replaced with modern methods, which are sustainable, eco-friendly, cheap, energy-efficient, rapid, and effective in color yield of colorant (Chai et al. 2021; Naebe et al. 2021; Kumar et al. 2020). Various radiation techniques such as ultraviolet radiations, gamma radiations, ultrasonic radiations, microwave radiations, and plasma radiations have been widely used for extraction purposes (Haji and Naebe 2020; Mia et al. 2021; del Pilar Sánchez-Camargo et al. 2021).
One of these modern methods is the microwave-assisted extraction (MAE) technique which yields better quality extracts in higher amounts with less consumption of time, energy, and solvent (Xu et al. 2021; Jafari et al. 2019). These rays are volumetrically selective, permit the system to be heated rapidly, and modify the process for better action (Abdelileh et al. 2021; Yeong et al. 2021). The abridged size of equipment and waste, the controlled healing process, and no direct interaction between the source and materials occurred are another gains of this tool (Li et al. 2017). Microwave extraction depends upon the nature of the solvent, volume, extraction temperature, time, microwave power, and properties of the product. Hence, these rays are an environment-friendly source of radiation which can enhance the dye uptake ability of fabrics also by tunning the surface of fabric fibers (Arain et al. 2021).
For improving color quality parameters (fastness and lab), salts of metals called mordants are used. Mordants are the essential component needed for the dyeing of the fabric for achieving the broad spectrum of different shades on natural and synthetic textile materials (Hosseinnezhad et al. 2021a; Hosen et al. 2021). The type and nature of the mordant greatly affect the nature of color and shades of the dyes’ textile materials (Yaqub et al. 2020). Most commonly used chemical mordants include salts of aluminum (Al)3+, Cr3+ (chromium), Cu2+ (copper sulfate), Fe2+ (ferrous sulfate), Sn2+ (stannous chloride) (Hosseinnezhad et al. 2018; İşmal and Yıldırım 2019), but some mordants (salt of Cu, Cr, Co, W) are carcinogenic and make dye effluent much more toxic (Yusuf et al. 2017). Keeping in view the unfriendly nature of the chemical mordants, researchers are now moving towards the biological mordants because bio-mordants are biodegradable and ecosystem friendly and can give new shades with improved color characteristics (Islam et al. 2019; Rather et al. 2019).
Peepal (Ficus religiosa) is an evergreen herbal plant that belongs to the Moraceae family (Priya and Singh 2017). Its bark contains several components such as flavonoids, tannins, steroids, and saponins (Sharma et al. 2016; Singh and Jaiswal 2014). This plant extract also shows antiprotozoal, antibacterial, antiviral, and analgesic anti-diabetic characteristics. Its extract is also used to cure wounds, fertilization, diarrhea, asthma, anti-amnesic, antiulcer, anthelmintic, jaundice, etc. (Kumar et al. 2018; Sharma et al. 2019; Tiwari et al. 2019). Its bark contains which when after extraction is used for coloration of fabrics (Sharma et al. 2016; Singh and Jaiswal 2014). Silk is the natural fabric that has fibroin which plays the role of functional moiety (amide linkage) for bonding with colorant. Keeping in the view of the advantages of MW rays for high colorant yield and bio-molecules for getting fast shade, the purpose of current study is to
-
a)
Extract the colorant in a suitable medium using the selected MW ray level
-
b)
Study physiochemical nature of fabrics before and after dyeing
-
c)
Select the dyeing variable for mordanting
-
d)
Select mordanting level (%) for colorfast shade with better fastness ratings
Material and method
The crude powder of periderm (bark) of Peepal (Ficus religiosa) has been collected and sieved (20 meshes). Plant powders acting as the source of biological mordants have been ground, sieved, and stored. Silk fabric (20 = GSM) after pretreatment with neutral soap has also been stored for the coloration process. The rest of the chemicals used for isolation, dyeing, mordanting, and fastness procured was commercial (Pakistan-made).
Dye extraction and irradiation process
Aqueous (water solubilized) and acidic (acid solubilized = 1% HCl v/v) extracts have been prepared by taking 4 g of powder with 100 mL of media at boiling. Extract and soaped silk fabrics were given MW ray treatment up to 5.0 min, with the interval of 1 min. After the irradiation process, the dyeing process has been adopted as discussed in Table 1. After dyeing, the data were analyzed statistically using a two-way ANOVA design.
Mordanting treatment
Using optimum irradiation and extraction conditions, the set of experiments was designed to optimized extract pH (2.0–7.0), salt amount for exhaustion (1.0–6.0 g/100 mL), dyeing temperature (35–95 °C), and dyeing time (25–75 min.). For shade production, 1.0–5.0 g of three bio-mordants and 1.0–5.0 g of three sustainable chemical mordants were used. For the application of these mordants, the solutions were boiled in water using given amount and employed before and after dyeing by following already given method of Habib et al. (2021).
Fabric assessment
The undyed silk fabric before and after microwave treatment for 3 min have been subjected for physicochemical analysis (SEM & FTIR) (Adeel et al. 2021a). For analysis of dyed fabrics, data color, SF 600 equipped with the light source (D 6510° observer), was used to get color characteristics. For observing shade fastness, ISO standards for light washing and rubbing were used, and the results were compared at grey scale (Habib et al. 2021).
Results and discussion
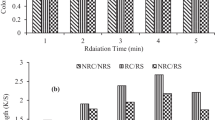
The uniform and leveled action of microwave rays by consuming less time, energy, and solvent are the unique properties that have attracted the attention of people working in the field of natural products (Suktham et al. 2021; Buyukakinci et al. 2021). In this study, its role has been studied for the isolation of the natural brown colorant (tannin) from Peepal bark in aqueous and acidic media for silk dyeing. The results given in Fig. 1 show that irradiation of aqueous extract (RE) and fabric (RS) for 3 min has given better color depth (K/S up to 12.173). On changing medium, the results displayed in Fig. 2 show that irradiation of extract (RE) and fabric (RS) for 4 min has given excellent results (K/S up to 10.412). In comparison, the un-irradiated aqueous extract (NRE) has given low color depth (K/S up to 3.8561), and un-irradiated acidic extract (NRE) also has given good color depth (K/S up to 3.626) onto un-irradiated fabric (NRS). The color coordinates given in Table 2 show that most dyed fabrics are darker in shade and reddish yellow in hue but irradiated aqueous extract (R.E. = 3 min), when employed onto irradiated silk (RS = 3 min), has given darker shades (L* = 43.71) with more reddish yellow hue (a* = 29.14; b* = 33.65). Similarly, on using acidic extract (R.E. = 4 min) for dyeing of irradiated silk (R.S. = 4 min), the shade obtained is less darker (L* = 47.19) in shade and less reddish but yellower in hue (a* = 27.15; b* = 36.24).
This excellent yield is due to the effect of MW rays at the molecular level, where the rays penetrate the matrix, rupture its cell walls, and make the solid–liquid (colorant-solvent) interaction through mass transfer kinetics (Pal and Jadeja 2020; Zghaibi et al. 2019; Wang et al. 2021). The other factor running side by side is the irradiation of the fabric. It has been observed that MW ray treatment didn’t alter the functional nature of silk which has been verified by FTIR spectra. The stretching peaks of -NH2 (3100–3200 cm−1), -C = O (1717 cm−1), and CH3 (3190 cm−1) have not altered their positions after exposure to MW rays up to 3 min (Figs. 3 and 4). However, the SEM analysis (Figs. 5 and 6) shows that these rays have produced scaling on the surface of the fabrics. The scratching produced after MW rays has modified the coloring behavior of silk which has been observed through the rise of color strength (K/S = 12.173) in an aqueous medium. Hence, it is recommended that both extract and fabrics should be MW irradiated for 3 min. to get excellent yield. The statistical analysis given in Table 3 reveals that selection of treatment ether onto fabric or extract is significant (0.000). The role of dyeing variable, i.e., application of extract at given dyeing conditions, is also significant (P = 0.001).
In the bio-coloration of silk, optimum dyeing levels are necessary to select for getting a high yield of fast shades. The results show in Fig. 6a–d that irradiation of acidic extract (4.0 pH) for 3 min containing 3.0 g/100 mL of table salt as an exhausting agent when employed at 55 °C for 55 min has given good results onto irradiated silk fabric. The role of pH of the extract is very essential because silk fabric contains both acidic (-COOH) and basic group (-NH2), which require an acidic medium to sorb colorant promisingly (Adeel et al. 2021b). Due to low pH, dye molecules (tannin) interact with fabric functional site (amide linkage) via H-bonding to give firm shades of high strength (K/S up to 6.7701). Hence acidic extract of pH 4.0 after MW treatment for 3 min should be used to dye the irradiated silk fabric (RS = 4 min). The color coordinates given in Table 4 show that the other factor is the role of electrolyte (table salt) for achieving excellent exhaustion of colorant from medium towards surface-modified silk fabric. The utilization of salt amount (< 3.0 g/100 mL) can’t shift the dye from the bath toward the fiber promisingly, whereas the salt amount (> 3.0 g/100 mL) exhausts the molecules significantly, and colorant molecules are sorbed onto fabric in form of aggregates due to over-exhaustion (Adeel et al. 2021c). In both cases, the colorant molecules cannot fill the voids of fibers, and upon washing shades of low color, strength is observed. But the utilization of 3.0 g/100 mL of leveling agent in a selected extract of 4.0 pH onto surface modified silk has given excellent tint strength (K/S up to 4.7763). The color coordinates given in Table 4 show that using 3.0 g/100 ml of salt, the shade is darker (L* = 51.11) and reddish yellow in hue (a* = 17.50; b* = 21.93). Irradiation for 3 min has imparted good strength with a darker shade (L* = 48.18) but reddish yellow hue (a* = 13.53) (b* = 22.29).
In the bio-dyeing of silk contact of heat for a particular time play a significant contribution in giving high strength (Khan et al. 2021). The results show that dyeing of irradiated silk (RS) for 55 min at 55 °C using irradiated extract of 4.0 pH containing 3.0 g/100 mL of electrolyte (T.S.) has given promising results. The bio-coloration of fabric for a low time (55 min) at a low heat level (55 °C) does significantly not accelerate the colorant molecules. Due to this poor effect, the kinetic energy of dye becomes low, and during dyeing, molecules remain unfixed onto the fabric surface. For high contact levels (55 min) at high temperature (> 55 °C), the dyeing rate becomes recessive, and the rate of stripping becomes dominant, and again molecules face desorption problems which give low yield. But dyeing of fabric at 55 °C for 65 min gives leveled shade of high color strength which might be due to equilibrium of dye bath with fabric. The shade parameters (Table 4) reveal that the dyeing of fabric for 65 min has given a darker tone (L* = 43.54) with a reddish yellow hue (a* = 15.59; b* = 18.78). The fabric dyed at 55 °C has given a darker shade (L* = 51.02) with a less reddish yellow hue (a* = 14.80; b* = 21.61). Overall, at selected levels, the shades are darker in appearance having a reddish yellow tone. Hence MW rays have not only reduced salt amount but also save time and energy for dyeing of silk with Peepal bark extract at selected conditions.
In bio-dyeing of fabric, the problem of poor fastness is tried to overcome either by utilization of electrolytes (Al3+, Fe2+, Cu2+, Co2+, Ni2+) or by tannic acid (T.A.) called chemical mordants. But the salts of Cu2+, Ni2+, Cr3+, Co2+, etc. are toxic and need replacement by some sustainable anchors. In this study, 1.0–5.0 g/100 mL of electrolytes of Al+3, Fe+2, and tannic acid (T.A.) before and after dyeing of irradiated silk at 55 °C for 65 min has been utilized. The results show in Fig. 7a–c that 3.0 g/100 mL of salt of Al+3 and 4.0 g/100 mL of salt of Fe+2 and 2.0 g/100 mL of tannic acid (T.A.) before dyeing (pre-mordanting) has given good results. The application of salt of Al+3 (4.0 g/100 mL) and of Fe+2 (4.0 g/100 mL) and tannic acid (T.A. = 3.0 g/100 mL) after dyeing (post mordanting) has given good results.
The color coordinates given in Table 5 reveal that utilization of Al3+ salt (3%) before dyeing has given darker shade (L* = 67.94) with the less red (a* = 7.60) but yellow hue (b* = 25.69) on using the salt of Al3+ after dyeing (4% post); the shade becomes brighter (L* = 73.64) having less-redder (a* = 5.71) but the more yellower tone (b* = 22.92). Similarly, utilization (4.0 g/100 mL) of iron salt (Fe2+) before dyeing (pre) has given darker shade with more reddish yellow hue (L* = 58.69; a* = 10.39; b* = 25.27), whereas after dyeing (post) has given brighter shade with more reddish yellow hue (L* = 65.09; a* = 9.45; b* = 24.92). Tannic acid is a good ecofriendly chemical mordant, where its application before dyeing (2% pre) has given a brighter shade (L* = 58.53) with a reddish yellow hue (a* = 10.98; b* = 20.03). However, its application after dyeing (3% post) has given a darker shade (L* = 53.51) with a more reddish yellow hue (a* = 12.30; b* = 20.72). The possible interaction of chemical mordant, by the functional site (-OH of tannin) and amide linkage of silk, has been demonstrated in Fig. 9a.
The role of plant-based biological molecules as bio-mordants has been observed for the last five years (Hosseinnezhad et al. 2021b). These functional biomolecules have gained a special place as an alternative to toxic chemicals as well as for making dyeing processing more eco-friendly and pollution-free (Thakker 2020). In this study, extracts of acacia, turmeric, and pomegranate obtained by employing 1.0–5.0 g with 100 mL water have been utilized before (pre) and after (post) dyeing of silk with Peepal bark extract at selected conditions. It has been found in Fig. 8a–c that 4.0 g/100 mL of acacia extract (K/S = 6.438) and 3.0 g/100 mL of turmeric (K/S = 15.583) and pomegranate extract (K/S = 16.592) before dyeing (pre-mordanting) have given good shades with acceptable fastness ratings, whereas 3 g/100 mL of acacia extract (K/S = 2.2998) and turmeric extract (K/S = 16.985) and 4 g/100 mL of pomegranate extract (K/S = 14.08) after dyeing (post mordanting) have also developed good shades with good to excellent fastness characteristics. The color coordinates and fastness grades are given in Table 5 and show that using the optimal amount of mordants (chemical and bio) has improved these characteristics. The proposed bio-mordant, colorant (tannin), and fabric (amide linkage) interactions have been displayed in Fig. 9b.
On comparison analysis, as pre-chemical mordant, salt of iron (Fe2+ = 4.0 g/100 mL) has given fast shade with a reddish yellow hue, and as pre-bio-mordant, the extract of pomegranate (3.0 g/100 mL) has given fast shades with the reddish yellow hue. As post chemical mordant, 3.0 g/100 mL of T.A. (tannic acid) has given good color characteristics, whereas post-bio-mordant, the turmeric extract (3.0 g/100 mL) has given a high yield with a more reddish yellow hue. Bio-mordants have given darker shades with reddish but yellow hue depending upon the nature of plant parts. The results given in Table 5 show that turmeric and pomegranate extract has given darker shades as compared to acacia extract. During pre-mordanting, the utilization of 3% of pomegranate extract has given darker shade (L* = 41.92) with reddish yellow hue (a* = 14.18) (b* = 31.76). But the application of 3% extract of turmeric after dyeing has given a brighter shade (L* = 54.31) with a reddish (a* = 18.34) yellow hue (b* = 53.77).
This excellent color strength with improved fastness grading is due to the formation of a metal dye complex onto the surface tunned silk fabric (dos Santos Silva et al. 2020b; Zhao et al. 2020). A low amount cannot form a stable complex, whereas in the above selected mordant, the over amount may form complex and sorb onto fabrics inform of aggregates. Upon soaping, the overcrowded complex is stripped, and low tint strength with poor fastness rating is observed.. On using bio-mordants, the colorant functional site (-OH) interacts with amide linkage (-NH, COO-) of silk and -OH of bio-mordants through additional H-bonding to form firm shades (Sadeghi-Kiakhani et al. 2020; Habib et al. 2021). Additionally, the benzenoid ring present in colorant and bio-mordant also plays their role, where extra conjugation adds value in the bio-coloration of fabrics to develop fast shades (Boruah et al. 2021; Islam et al. 2021). Upon washing, crocking (rubbing) and heat exposure, the bio-mordanted dyed fabrics show maximum resistance to fade Table 6. Hence after assessment of selected chemical and bio-mordanted fabrics, as per ISO standards, the good to excellent fastness at grayscale has been observed by chemical and bio-mordanted dyed fabrics. It is concluded that the made dyeing process of silk is not only more attractive, colorfast, and ecofriendly with Peepal bark-based tannin natural reddish brown dye.
Conclusion
The current era is the age of sustainability wherein the current worsening pandemic scenario that is getting worse is now in need of treatment with nature rather than chemical products. Natural products with exceptional biological properties, especially of an antioxidant antibacterial and antiviral in nature, are gaining widespread fame to be utilized in all walks of life. Among these products, Peepal (Ficus religiosa) is a great plant with advanced coloring potential for textiles. The addition of MW radiations as an extraction method for natural biological components (tannin) from Peepal for silk coloration and bio-molecules as bio-mordants for getting fast shades has made the method more eco-friendly as well as gorgeous for the global community. The researchers found that Peepal bark extract of 4 pH containing 3 g/100 ml of table salt after MW treatment up to 3 min at 55 °C for 55 min has given excellent shade strength when employed onto MW-treated chemical and bio-mordanted silk before and after dyeing. It is concluded that MW radiation not only has the potential to extract the colorants but also is helpful in exploring new dye yielding plant with high yield of colorants. Also the use of bio-mordants has made the process more sustainable, cleaner, and environmentally friendly.
Data availability
Not applicable.
References
Abdelileh M, Ticha MB, Meksi N (2021) Reviving of cotton dyeing by saxon blue: application of microwave irradiation to enhance dyeing performances. Fibers Polym 22(7):1863–1873
Adeel S, Rehman FU, Zuber M, Batool F, Habib N, Hussaan M, Amin N (2021a) Environmental friendly application of ultrasonic rays for extraction of natural colorant from Harmal (P. harmala) for dyeing of bio-mordanted silk. J Eng Fiber Fabr 16:1–8
Adeel S, Habib N, Batool F, Rahman A, Ahmad T, Amin N (2021b) Eco-friendly approach towards isolation of colorant from Esfand for bio-mordanted silk dyeing. Environ Sci Pollut Res 1–11
Adeel S, Rehamn FU, Hussaan M, Amin N, Majeed A, Pervaiz M, Rehman HU (2021c) Microwave assisted green isolation of laccaic acid from lac insect (Kerria laca) for wool dyeing. Prog Color Color Coat 14(4):293–299
Arain RA, Ahmad F, Khatri Z, Peerzada MH (2021) Microwave assisted henna organic dyeing of polyester fabric: a green, economical and energy proficient substitute. Nat Prod Res 35(2):327–330
Borrás-Enríquez AJ, Reyes-Ventura E, Villanueva-Rodríguez SJ, Moreno-Vilet L (2021) Effect of ultrasound-assisted extraction parameters on total polyphenols and its antioxidant activity from mango residues (Mangifera indica L. var. Manililla). Separations 8(7):94
Boruah G, Phukan AR, Kalita BB, Pandit P, Jose S (2021) Dyeing of mulberry silk using binary combination of henna leaves and monkey jack bark. J Nat Fibers 18(2):229–237
Buyukakinci YB, Guzel ET, Karadag R (2021) Organic cotton fabric dyed with dyer’s oak and barberry dye by microwave irradiation and conventional methods. Indust Text 72(1):30–38
Chai YH, Yusup S, Kadir WNA, Wong CY, Rosli SS, Ruslan MSH, Yiin CL (2021) Valorization of tropical biomass waste by supercritical fluid extraction technology. Sustain 13(1):233
Chen J, Liao C, Ouyang X, Kahramanoğlu I, Gan Y, Li M (2020) Antimicrobial activity of pomegranate peel and its applications on food preservation. J Food Quality 2020:1–8
del Pilar Sánchez-Camargo A, Ballesteros-Vivas D, Buelvas-Puello LM, Martinez-Correa HA, Parada-Alfonso F, Cifuentes A, Ferreira Sandra RS, Gutiérrez LF (2021) Microwave-assisted extraction of phenolic compounds with antioxidant and anti-proliferative activities from supercritical CO2 pre-extracted mango peel as valorization strategy. LWT 137:110414
dos Santos Silva PM, Fiaschitello TR, de Queiroz RS, Freeman HS, da Costa SA, Leo P, da Costa SM (2020a) Natural dye from Croton urucurana Baill. bark: extraction, physicochemical characterization, textile dyeing and color fastness properties. Dyes Pigm 173:107953
dos Santos Silva PM, Fiaschitello TR, Queiroz RS, Freeman HS, Costa SA, Leo P, Montemor AF, Costa SM (2020b) Natural dye from Croton urucurana Baill. bark: extraction, physicochemical characterization, textile dyeing and color fastness properties. Dyes Pigm 173:1–14
Fadil M, Bousta D, El OualiLalami A, Lachkar M, Farah A (2021) Ultrasound-assisted extraction of phenolic compounds from Moroccan Lavandula stoechas L.: optimization using response surface methodology. J Chem 2021:1–11
Habib N, Adeel S, Ali F, Amin N, Khan SR (2021) Environmentally friendly sustainable application of plant-based mordants for cotton dyeing using Arjun bark-based natural colorant. Environ Sci Pollut Res 28(38):54041–54047
Haji A, Naebe M (2020) Cleaner dyeing of textiles using plasma treatment and natural dyes: a review. J Cleaner Prod 265:121866
Haji A, Vadood M (2021) Environmentally benign dyeing of polyester fabric with madder: modelling by artificial neural network and fuzzy logic optimized by genetic algorithm. Fibers Polym 22(12):3351–3357
Handayani PA, Chafidz A, Ramadani NS, Kartika D (2019) Microwave assisted extraction (MAE) process of tannin from mangrove propagules waste as natural dye for coloring Batik tulis. Key Eng Mater 805:128–133
Hasan MU, Adeel S, Batool F, Ahmad T, Tang RC, Amin N, Khan SR (2021) Sustainable application of Cassia obovata–based chrysophanic acid as a potential source of yellow natural colorant for textile dyeing. Environ Sci Pollut Res 1-14 https://doi.org/10.1007/s11356-021-16447-0
Hosen MD, Rabbi MF, Raihan MA, Al Mamun MA (2021) Effect of turmeric dye and biomordants on knitted cotton fabric coloration: a promising alternative to metallic mordanting. Clean Eng Technol 3:100124
Hosseinnezhad M, Gharanjig K, Belbasi S, Saadati SHS, Saeb MR (2018) The use of sumac as a natural mordant in green production of Iranian carpet. Fibers Polym 19(9):1908–1912
Hosseinnezhad M, Gharanjig K, Razani N, Imani H (2020) Green dyeing of wool fibers with madder: study of combination of two biomordant on K/S and fastness. Fibers Polym 21(9):2036–2041
Hosseinnezhad M, Gharanjig K, Jafari R, Imani H (2021a) Green dyeing of woolen yarns with weld and madder natural dyes in the presences of biomordant. Prog Color Color Coat 14(1):35–45
Hosseinnezhad M, Gharanjig K, Jafari R, Imani H, Razani N (2021b) Cleaner colorant extraction and environmentally wool dyeing using oak as eco-friendly mordant. Environ Sci Pollut Res 28(6):7249–7260
Islam SU, Rather LJ, Shabbir M, Sheikh J, Bukhari MN, Khan MA, Mohammad F (2019) Exploiting the potential of polyphenolic biomordants in environmentally friendly coloration of wool with natural dye from Butea monosperma flower extract. J Nat Fibers 16(4):512–523
Islam M, Molla M, Hossain T, Bashar M, Chandra D, Ahsan M (2021) Ultrasound-assisted extraction of natural dye from Swietenia mahagoni and its application on silk fabric. Indian J Fibre Text Res 46:69–77
İşmal ÖE, Yıldırım L (2019) Metal mordants and biomordants. In The impact and prospects of green chemistry for textile technology. Woodhead Publishing 57–82
Jafari SM, Mahdavee Khazaei K, Assadpour E (2019) Production of a natural color through microwave-assisted extraction of saffron tepal’s anthocyanins. Food Sci Nutri 7(4):1438–1445
Khan AA, Adeel S, Azeem M, Iqbal N (2021) Exploring natural colorant behavior of husk of durum (Triticum durum Desf.) and bread (Triticum aestivum L.) wheat species for sustainable cotton fabric dyeing. Environ Sci Pollut Res 28:51632–51641
Kovačević Z, Sutlović A, Matin A, Bischof S (2021) Natural dyeing of cellulose and protein fibers with the flower extract of Spartium junceum l. plant. Materials 14(15):4091
Kumar A, Sandeep D, Tomer V, Gat Y, Kumar V (2018) Ficus religiosa: a wholesome medicinal tree. J Pharmacog Phytochem 7(4):32–37
Kumar K, Srivastav S, Sharanagat VS (2020) Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultras Sonochem 70:105325
Li Y, Li S, Lin SJ, Zhang JJ, Zhao CN, Li HB (2017) Microwave-assisted extraction of natural antioxidants from the exotic Gordonia axillaris fruit: optimization and identification of phenolic compounds. Molecules 22(9):1481
Mia R, Sk MS, Oli ZBS, Ahmed T, Kabir S, Waqar MA (2021) Functionalizing cotton fabrics through herbally synthesized nanosilver. Clean Eng Technol 4:100227
Naebe M, Haque ANMA, Haji A (2021) The effect of plasma treatment on dyeing of natural fibers. Innova Emerg Technol Text Dye Finish 191–212
Pal CBT, Jadeja GC (2020) Microwave-assisted extraction for recovery of polyphenolic antioxidants from ripe mango (Mangifera indica L.) peel using lactic acid/sodium acetate deep eutectic mixtures. Food Sci Technol Inter 26(1):78–92. https://doi.org/10.1177/1082013219870010
Priya DS, Singh DRA (2017) Pharmaco therapeutic study of Ficus religosa (peepal) and Cinnamomum zeylanicum (Dalchini) w.s.r to uti caused by e coli. World J Pharma Medical Res 3(9):329–330
Rather LJ, Khan MA, Mohammad F (2019) Biomordanting potential of acacia nilotica (Babul) in conjunction with Kerria lacca and Rheum emodi natural dyes. J Nat Fibers 16(2):275–286
Sadeghi-Kiakhani M, Tehrani-Bagha AR, Safapour S, Eshaghloo-Galugahi S, Etezad SM (2020) Ultrasound-assisted extraction of natural dyes from Hawthorn fruits for dyeing polyamide fabric and study its fastness, antimicrobial, and antioxidant properties. Environ Develop Sustain 114:1–18
Salauddin Sk M, Mia R, Haque MA, Shamim AM (2021) Review on extraction and application of natural dyes. Text Leather Rev 4(4):218–233
Sharma D, Dangi CBS, Kaur MA (2016) Review on pharmacological activities and therapeutic potentials of Ficus religiosa (Pipal). Ind J App Res 6(1):623–626
Sharma V, Mishra S, Yesudas R, Rajput RS (2019) A review on Ficus religiosa (Sacred Fig) 6(2)
Singh S, Jaiswal S (2014) Therapeutic properties of Ficus religiosa. Int J Eng Res Gen Sci 2(5):149–158
Sk MS, Mia R, Ahmed B, Rahman A, Palash MMR (2021) Effect of neutralizers and silicone softeners on phenolic yellowing phenomenon of OBA treated cotton knitted fabric. Heliyon 7(11):E08320
Suktham K, Daisuk P, Shotipruk A (2021) Microwave-assisted extraction of antioxidative anthraquinones from roots of Morinda citrifolia L (Rubiaceae): errata and review of technological development and prospects. Separ Purif Technol 256:117844
Thakker AM (2020) Sustainable processing of cotton fabrics with plant-based biomaterials Sapindus mukorossi and Acacia concinna for health-care applications. J Text Inst 19 https://www.tandfonline.com/doi/abs/10.1080/00405000.2020.1776537.
Tiwari AK, Chaudhary IJ, Pandey AK (2019) Indian traditional trees and their scientific relevance. J Med Plants 7(3):29–32
Viera I, Pérez-Gálvez A, Roca M (2019) Green natural colorants. Molecules 24(1):154
Wang X, Yi F, Zhang W, Guo X (2021) Optimization of the application of walnut green peel pigment in wool fiber dying and fixing process under microwave-assisted condition. J Nat Fibers 1–14 https://wwwtandfonlinecom/doi/abs/10.1080/15440478.2020.1870632
Xu K, Gao X, Chi M, Chen K, Zhang Y, Kong W, Qin K (2021) Microwave-assisted extraction coupled with mass spectrometry for determining five volatile compounds from soy sauces. J Analy Methods Chem 2021:1–8
Yaqub A, Iqbal Z, Toyota T, Chaudhary N, Altaf A, Ahmad SR (2020) Ultrasonic extraction of onion (Allium cepa) peel dye, its applications on silk fabric with bio-mordants and its antibacterial activity. Clinical Med Biochem 8:1–9
Yeong YL, Pang SF, Putranto A, Gimbun J (2021) Optimisation of microwave-assisted extraction (MAE) of anthraquinone and flavonoids from Senna alata (L.) Roxb. Nat Prod Res 1–5
Yılmaz F, Bahtiyari Mİ (2020) Antibacterial finishing of cotton fabrics by dyeing with olive tree leaves fallen during olive harvesting. J Cleaner Prod 270:122068
Yusuf M, Mohammad F, Shabbir M, Khan MA (2017) Eco-dyeing of wool with Rubia cordifolia root extract: assessment of the effect of acacia catechu as bio-mordant on color and fastness properties. Text Cloth Sustain 2(1):1–9
Zghaibi N, Omar R, Mustapa Kamal SM, Awang Biak DR, Harun R (2019) Microwave-assisted brine extraction for enhancement of the quantity and quality of lipid production from microalgae nannochloropsis sp. Molecules 24(19):3581
Zhao Z, Zhang M, Hurren C, Zhou L, Wu J, Sun L (2020) Effects of UV absorbers and reducing agents on light fastness of cotton fabrics pre-dyed with sodium copper chlorophyllin and gardenia yellow. Text Res J 90(19–20):2245–2257
Zheng J, Wang Y, Zhang Q, Wang D, Wang S, Jiao M (2021) Study on structure and properties of natural indigo spun-dyed viscose. Fiber Polym 1(1):327–335
Author information
Authors and Affiliations
Contributions
Dr Shahid Adeel is supervisor of the work in whose lab practical work has been done, whereas Mr. Waseem Akram and Ms. Nimra Amin jointly conducted experiments. Dr Noman Habib and Dr Mozhgan have guided the group technically and scientifically, whereas Mr. Ehsan ul Haq has helped in physiochemical analysis of fabrics.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate and publish
We give consent to publish the work of M.Phil studies and is jointly contributed by all authors.
Competing interests
The authors declare no competing interests.
Additional information
Responsible editor: Philippe Garrigues
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Habib, N., Akram, W., Adeel, S. et al. Environmental-friendly extraction of Peepal (Ficus Religiosa) bark-based reddish brown tannin natural dye for silk coloration. Environ Sci Pollut Res 29, 35048–35060 (2022). https://doi.org/10.1007/s11356-022-18507-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-18507-5