Abstract
The present study, with the aid of GIS, utilizes high-density groundwater (GW) sampling data (1398 samples) to analyze the spatial variation characteristics of GW fluoride in Weifang City (WFC), and evaluate the health risks associated with drinking water routes. The concentration of fluoride in the GW of WFC is observed to be between 0.08 and 9.16 mg/L, with a mean value of 0.62 mg/L. The fluoride concentration of a total of 192 GW samples exceeded the limit of China’s GW quality standards (1 mg/L), accounting for 14.74%. The GW fluoride concentration in most areas of WFC is less than 1 mg/L. However, the relatively high-value zones are mostly concentrated in the upper reaches of Wen River, the east of Shouguang, the southeast of Anqiu, the east of Qingzhou, the east of Fangzi, and the southeast and northwest of Gaomi. The hydrochemical types of GW in WFC are mostly HCO3-Ca·Mg and SO4·Cl-Ca·Mg, while GW samples with hydrochemical types HCO3-Na and SO4·Cl-Na are characterized by high fluoride content. The hydrochemical characteristics of GW in WFC are mostly dominated by rock weathering. In addition, the northern coastal plain is evidently influenced by seawater intrusion. The concentration of fluoride in GW is affected by the dissolution of fluorine-containing minerals, cation exchange, and alkaline environmental factors. The effect of GW by seawater intrusion and very high content of Na+ will decrease the fluoride content of the GW through cation exchange. Health risk assessment demonstrated that the mean values of non-carcinogenic hazard quotient (HQ) for infants, children, teenagers, and adults were 0.52, 0.35, 0.31, and 0.30, respectively. In addition, the distribution characteristics of GW fluoride in high health risk areas (HQ > 1) in WFC are further consistent with the spatial variation of GW fluoride content. Overall, the health risk distribution area of GW fluoride in WFC is decreasing in the following order: infants > children > teenagers > adults.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Globally, groundwater (GW) is an important source of water for various purposes such as drinking, industrial usage, and agriculture, especially in arid or semi-arid regions with scarce surface water resources (Houatmia et al. 2016; Liu et al. 2018). However, with rapid industrialization, acceleration of urbanization, rapid growth of population, and extensive development of agricultural activities, the quality of GW is increasingly threatened (Gao et al. 2020, 2019).
Fluoride is one of the most essential trace elements for the human body (Zuo et al. 2019; Aghapour et al. 2018). Appropriate amount of fluoride is beneficial to accelerate the formation of bones and enhance the ability of anti caries of the teeth. However, lack of fluoride is harmful for the human body, and long-term intake of excessive fluoride GW is prone to fluorosis and skeletal fluorosis (Egor and Birungi 2020; Raja and Neelakantan 2021; Yousefi et al. 2018). If GW containing high fluoride is the major source of water supply, then the excessive fluoride content will directly harm human health. Around the world, millions of people suffer from fluorosis due to the high intake of fluoride through drinking water (Hanse et al. 2019; Yadav et al. 2020). Fluoride pollution in GW is a major challenge affecting the health of local residents in several countries including India (Hanse et al. 2019; Yadav et al. 2019), Thailand (Sawangjang et al. 2019), China (Li et al. 2020; Liu et al. 2021a), and Ghana (Ganyaglo et al. 2019; Zango et al. 2019). Therefore, conducting regional GW fluoride surveys and investigation, and analyzing the distribution characteristics of GW fluoride content, along with the distribution rules and causes of high-fluoride water, is imperative for ensuring safe drinking water for public. In addition, surveys are also helpful for detecting safe water sources, and carrying out endemic fluorosis prevention and treatment.
Fluorine-bearing minerals are a major source of fluoride in GW. Fluorine-rich rocks and minerals, after long-term geological processes, release fluorine ions which are leached into GW to form high fluoride GW (Aravinthasamy et al. 2020; Jadhav et al. 2015; Rondano Gómez et al. 2020). In addition, the accumulation of fluoride in GW requires certain hydrochemical conditions, such as pH, hydrochemical medium concentration, and salinity and hydraulic gradient, which are important factors affecting the enrichment of fluoride in GW (Li et al. 2020; Liu et al. 2021a; Marghade et al. 2020; Zango et al. 2019; Zhang et al. 2020). The high concentration of fluoride in GW is a global concern. Accordingly, several scholars have conducted research on the distribution characteristics, laws, and influencing factors of fluoride in GW. Li et al. (2020) discussed the influencing factors predominating the migration and enrichment of fluoride in GW in North China Plain (NCP). They observed that the GW flow path from mountainous areas to coastal areas, cation exchange, carbonate precipitation/dissolution, fluorite dissolution, and seawater intrusion affect the characteristics of fluoride in GW. Aravinthasamy et al. (2020) evaluated the geochemical mechanism of fluoride pollution in GW and its impact on public health in the Shanmuganadhi River Basin in Tamil Nadu, India. It was indicated that geological background and application of synthetic fertilizers in agricultural fields have a considerable impact on fluoride concentration in GW, and rising GW level further reduces fluoride concentration. Zango et al. 2019) analyzed the source and influencing factors of GW fluoride in northeast Ghana. Their study indicated that the geological background along with semi-arid climate conditions, water–rock interaction, mineral dissolution, and precipitation were important factors for GW fluoride formation.
GW is an essential source of water for industrial and agricultural production and the common public in Weifang City (WFC). However, its GW resources are relatively poor, resulting in being one of the severely water-scarce cities in the Shandong Province. In recent years, with the continuous progress of society, the degree of development and utilization of GW has increased considerably. Xu et al. (2011) analyzed the nitrate content and pollution sources of GW in WFC. Liu et al. (2021b) assessed the hydrochemical characteristics of GW in the Weibei Plain and the health risks associated with GW nitrate. However, very few studies exist on GW fluoride in Weifang City. Therefore, implementing high-density GW sampling and testing in WFC, the distribution characteristics and influencing factors of GW fluorine were studied. In addition, the health risk assessment of GW fluoride was conducted. Thus, this study provides a scientific basis for local fluorosis prevention, treatment of high-fluorine GW, protection of drinking water safety, and rational development and utilization of GW resources.
Study area
WFC is located in the western part of Shandong Peninsula, and its geographical position consists of longitude 118° 10′ 00″ ~ 120° 01′ 00 ″ and latitude 35° 42′ 33″ ~ 37° 26′ 00 ″ (Fig. 1). WFC is bordered by the Taiyi Mountains in the south and the Laizhou Bay in the Bohai Sea towards the north. It is 168.0 km wide from east to west and 180.0 km long from north to south, with a total area of 15,859.3 km2. WFC falls within the northern temperate monsoon climate zone. Furthermore, the northern coast is affected by the common influence of Eurasia and the Pacific, manifesting it into a warm-zone monsoon, semi-humid continental climate. There are four distinct seasons in this region, with an average annual precipitation of 611.94 mm (1986–2017), and a maximum annual precipitation of 948.24 mm (Xu et al. 2011). WFC is rich in surface water resources with distributed streams. The natural rivers originate in the southern hilly areas, originating from intermittent rain source rivers. The major rivers are Wei River, Mi River, Bailang River, and Jiaolai River, which flows into the Bohai Sea in the north. The terrain of WFC is higher in the south and lowers in the north, and gradually leans into the Laizhou Bay of Bohai Sea, forming several conspicuous steps. The southern part consists of low mountain and hilly terrain, with an altitude of more than 100 m, accounting for 35.6% of the total area of the city. The central part of the city is an inclined plain with an altitude of 7–100 m, accounting for 41.6% of the total area of the city. From the north to Laizhou Bay, alluvial plains, proluvial plains, and marine plains with wide beach and saline-alkali land exist. The terrain is flat, and the altitude is below 7 m, accounting for 22.8% of the total area of the city.
GW is mostly recharged by atmospheric precipitation. In addition, it is recharged by riverside leakage and fissure water runoff in the southern mountainous area. GW is abundantly recharged, and its flow direction is generally consistent with the topographic slope, from south to north. The type of GW in the area can be roughly divided into Quaternary pore water, carbonate fracture karst water, and bedrock fracture water. Quaternary pore water is mostly distributed in the northern Weifang Plain. The major source of recharge is atmospheric precipitation, followed by runoff recharge in the southern mountainous areas, and lateral infiltration recharge by rivers. Carbonate-fractured karst water is distributed in the hilly area of middle and low mountains in southwest WFC. Furthermore, the aquifer lithology consists of Cambrian and Ordovician limestone. The bedrock fissure water is distributed in the middle and low hills and southeast hilly areas in the southwest of WFC.
Materials and methods
GW sampling
In 2016, a total of 1398 wells (Fig. 1) in WFC were investigated and sampled, and the depth of most wells was less than 100 m. Clean and dry plastic bottles are utilized for GW sampling, and each well is pumped for about 3 min before sampling, to collect fresh GW. Before GW sampling, the sampling bottles are cleaned with fresh GW more than three times. After sampling, the samples are refrigerated and sent to the laboratory for GW quality determination, within seven days.
Analysis of the GW chemical parameters
The total soluble solids (TDS) are measured by an electronic balance (SARTORIUS), and the main cations including potassium (K+), sodium (Na+), calcium (Ca2+), and magnesium (Mg2+) are determined by an inductively coupled plasma emission spectrometer (iCAP 7400). For anions, chloride ion (Cl−) and fluoride (F−) are measured by ion chromatography (ICS-600). Sulfate (SO42−) and bicarbonate (HCO3−) are measured by titration with a stoppered burette (500 mL), and nitrate (NO3−) is determined by a spectrophotometer (iCE 3400). The pH is measured by the acidity meter (PHS-3C). In addition, in order to ensure the accuracy of the data, all GW samples have been calculated for the charge balance error (%CBE). The settlement results exhibit that the %CBE of more than 95% of the GW samples is within ± 5, indicating the reliability of the GW quality data in WFC.
Spatial interpolation
Inverse distance weighting (IDW) is a common and simple spatial interpolation method, which is based on the similarity of the sample points in the interpolation area. It calculates the weighted average of the sample points in the neighboring area to estimate the value of the cell, and further interpolates to obtain a surface (Qu et al. 2019; Liu et al. 2021a). The IDW method assumes that each measurement point has a local influence, and this influence decreases as the distance increases. Since this method assigns a larger weight to the point closest to the predicted position, but the weight decreases as a function of distance, it is called the inverse distance weight method.
For n points, the plane coordinates are (x, y), the vertical height is zi, i = 1, 2, ···, n, and the function of inverse distance weighted interpolation is:
where, dj is the horizontal distance from point (x, y) to point (xj, yj), j = 1, 2,…,n, and p is a constant greater than 0, which is termed as weighted power exponent.
Health risk assessment (HRA)
GW health risk assessment can be roughly divided into two categories: genetic toxic substances (carcinogens) and body toxic substances (non-carcinogens). This study adopted the USEPA health risk assessment model, which included four steps: hazard identification, dose–effect analysis, exposure assessment, and risk characterization (Nawale et al. 2021; USEPA 1989). Fluoride is a somatic toxic substance and a non-carcinogen, and its evaluation model adopts a non-carcinogen risk assessment model.
where HQ is the hazard quotient. E and RfD are the exposure dose and the reference dose (0.06 mg·kg−1·d−1), respectively. C and IR are the measured concentration of fluoride and the daily water consumption (infants, children, teenagers, and adults are 0.5 L·days−1, 1.0 L·days−1, 1.5 L·days−1, and 2 L·days−1, respectively); EF and ED are the exposure frequency (365 days·a−1) and exposure duration (infants, children, teenagers, and adults are 6a, 6a, 6a, and 30a, respectively); BW and AT are the average weight (infants, children, teenagers, and adults are 10 kg, 30 kg, 50 kg, and 70 kg, respectively) and average time (365 × ED a), respectively. When HQ < 1, the human health risk caused by pollutants is permissible, while HQ > 1 exhibits that the HRA is unacceptable.
Results and discussion
Descriptive statistics of fluoride in GW
The statistical results of fluorine content in GW in WFC are listed in Table 1. The maximum and minimum concentrations of fluoride in GW are 9.16 mg/L and 0.08 mg/L, respectively, with a mean value of 0.62 mg/L. The coefficient of variation is 151.87, indicating that the variability of the spatial variation of GW fluoride in WFC is relatively unstable. According to the WHO standard (1.5 mg/L), the fluoride in GW of 107 water samples exceeded 1.5 mg/l, accounting for 7.65%. However, according to China’s permissible limit (1 mg/L), the fluoride in 192 GW samples exceeded 1 mg/L, accounting for 14.74%. Overall, the fluoride concentration of most GW samples in WFC is within the permissible range, but few GW samples cannot be directly used as drinking water due to its high fluoride content.
Spatial distribution characteristics of fluoride in GW
In this study, the ArcGIS 10.2 software and IDW interpolation method were implemented to obtain the spatial distribution map of GW fluoride in WFC, as displayed in Fig. 2. It can be observed that the fluoride content in GW in most areas is less than 1 mg/L, and the distribution characteristics have no obvious regularity. The relatively high fluoride GW are mostly located in the upper reaches of Wen River, the east of Shouguang, the southeast of Anqiu, the east of Qingzhou, the east of Fangzi, and the southeast and northwest of Gaomi. However, others are mostly distributed in WFC with dotted characteristics. The highest value of fluoride in GW appears in the upper reaches of the Wen River.
Water type
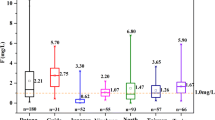
Piper diagram is widely utilized in the classification of water chemistry, and is considered to be a simple and effective tool (Liu et al. 2020, 2019; Piper 1944). Figure 3 illustrates the hydrochemical type of GW in WFC and its relationship with fluorine concentration. It can be observed that the GW samples are mostly concentrated in zone ① and zone ③, which infers that the GW chemistry types in WFC are mostly HCO3-Ca·Mg and SO4·Cl-Ca·Mg types. In addition, it can be clearly observed that the GW sample points with higher fluoride concentration (F− > 3 mg/L) mostly fall in zone ② and zone ④, indicating that the fluoride concentration in type HCO3-Na and SO4·Cl-Na groundwater is more likely to be enriched, which is similar to the results of few previous studies (Liu et al. 2021a; Ganyaglo et al. 2019; Li et al. 2020). Combined with the spatial distribution map (Fig. 2) of fluoride concentration in groundwater, it can be inferred that the water chemistry type of high-fluoride groundwater areas such as the upper reaches of Wen River, the east of Shouguang, the southeast of Anqiu, the east of Qingzhou, the east of Fangzi, and the southeast and northwest of Gaomi is mostly Na type.
Influencing factors of fluoride in GW
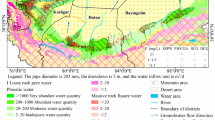
Gibb’s diagram (Gibbs 1970) is widely utilized in identifying the controlling factors of GW water chemistry (Gao et al. 2020; Liu et al. 2020, 2021c). The Gibbs diagram is mostly divided into three large regions (Fig. 4), representing rock weathering, atmospheric precipitation, and evaporative crystallization. In addition, the influence of seawater can be represented in the upper right corner of the Gibbs diagram (Liu et al. 2020; Wang et al. 2021). Figure 4 illustrates that the main effect of controlling the hydrochemical characteristics of GW in WFC is rock weathering. In addition, few water samples were affected by evaporation and seawater intrusion. Combined with the location of the GW samples, the GW obviously influenced by seawater intrusion is mostly distributed in the coastal plain towards the northern part of WFC. In Fig. 4a, several GW sample points are distributed in the region of Na+/(Na+ + Ca2+) > 0.5, indicating that these GW samples may be affected by cation exchange. However, the GW samples with higher fluoride concentration (F− > 2 mg/L) in these water samples are distributed in the zone of Na+/(Na+ + Ca2+) > 0.5. This indicates that the concentration of fluoride in GW is likely to be related to cation exchange.
Binary scatter graph of Na+-K+-Cl− and HCO3− + SO42−-Mg2+-Ca2+ is widely applied to identify the cation exchange in a GW system (Liu et al. 2021a). When cation exchange is an important process affecting the hydrochemical characteristics of GW, the ratio of Na+-K+-Cl− and HCO3− + SO42−-Mg2+-Ca2+ is close to 1 (Liu et al. 2021b). Figure 5 indicates that the GW samples in WFC are mostly distributed along the y = x line and exhibits significant correlation (y = 1.0926x + 2.2197, R2 = 0.9842). This indicates that cation exchange is an important hydrochemical process that affects the hydrochemical characteristics of GW in WFC.
The Chlor-Alkali Index (CAI) can further identify the direction of cation exchange, that is, whether it is a direct cation exchange or reverse cation exchange (Gao et al. 2020, 2019). When CAI > 0, reverse cation exchange is inferred (Eq. 8) and the Na+ in the GW replaces the Ca2+ in the aquifer, causing the Na+ in the GW to decrease and Ca2+ to increase. When CAI < 0, direct cation exchange is inferred (Eq. 9) and the Ca2+ in the GW replaces the Na+ in the aquifer, which increases the Na+ in the GW and reduces the Ca2+. As displayed in Fig. 5, the GW samples with relatively high fluoride concentration (F− > 1 mg/L) are mostly distributed in the region of CAI < 0. Furthermore, as the CAI decreases, the fluoride concentration increases, indicating that cation exchange is an important hydrogeochemical process that affects the concentration of fluoride in GW of WFC.
Correlation analysis between fluoride and other chemical composition in GW is useful to determine the related factors affecting the fluoride characteristics of GW (Zango et al. 2019; Liu et al. 2021a). Figure 6a indicates that the concentration of F− in GW of WFC decreases with the increase of Ca2+ concentration, because the increase of Ca concentration will inhibit the dissolution of calcium fluoride (CaF2) (Eq. 10). This further explains the reason why cation exchange may affect the concentration of F− in GW. Theoretically, the increase of Na concentration in GW will promote cation exchange, and indirectly increase the fluoride concentration in GW. Figure 6b illustrates the binary scatter plot of F− and Na+ in GW. It can be observed that the Na+ concentration in the GW sample points affected by seawater intrusion is very high, but with the increase of Na+ content in these GW samples, the F− in GW exhibits a downward trend. This indicates that very high Na+ concentration will not increase the F− concentration in the GW, but will further reduce it.
According to the scatter diagram of F− and pH in GW as displayed in Fig. 6c, it can be observed that, to a certain extent, with the increase of pH value, the concentration of F− in GW exhibits an upward trend, especially when the concentration of F− is relatively high (F− > 2 mg/L). This is because fluorine-containing minerals such as biotite [KMg3(AlSi3O10)F2], muscovite [KAl2(AlSi3O10)F2], and fluoro apatite [Ca5(PO4)3F] in GW are more likely to release F− into GW in alkaline environment (Eqs. 11 ~ 13). As displayed in Fig. 6d, there is a certain degree of positive correlation between F− and HCO3−, because the increase in HCO3− content will promote the dissolution of CaF2.
Health risk assessment
Health risk assessment (HRA) is a method of assessing possible harmful human health effects on different population (Zhang et al. 2020). The human body absorbs harmful substances in GW mostly through drinking water and bathing. However, previous studies (Adimalla and Qian 2019; Chen et al. 2016) have inferred that several factors leading to health risks through bathing have a relatively high degree of uncertainty. Thus, this study solely considers the impact of drinking water routes on the health of the different populations in WFC.
Figure 7 displays a histogram of the calculated HQ values of health risk assessments for different populations through drinking water pathway in WFC. The results of health risk assessment based on USEPA recommended model demonstrated that the infant’s HQ ranged from 0.07 to 7.36, with an average of 0.52. HQ values ranged from 0.04 to 5.09 for children, with an average of 0.35. For teenagers and adults, the HQ values ranged from 0.04 to 4.58, and 0.30 to 4.36, with a mean of 0.31, and 0.30, respectively. As far as infants are concerned, the calculation results of human health risks indicated that 150 GW samples exceeded the permissible limit (HQ > 1), accounting for 10.73% of the total GW samples. For children, teenagers, and adults, 92, 84, and 81 GW samples exceeded the acceptable limit, accounting for 6.58%, 6.0%, and 5.79% of the total GW samples, respectively. Overall, calculated HQ indicated that the non-carcinogenic health risks of WFC are in the order of infants > children > teenagers > adults.
For an improved understanding of the spatial variation characteristics of the health risk of GW fluoride in the entire WFC, the ArcGIS software was utilized to perform inverse distance weight interpolation, as displayed in Fig. 8. Consistent with the spatial variation of fluoride content in GW in WFC (Fig. 2), the high non-carcinogenic health risk of infants was mostly distributed in the upper reaches of Wen River, east of Shouguang, southeast of Anqiu, east of Qingzhou, east of Fangzi, and towards the southeast and northwest of Gaomi. In addition, several small areas with relatively high HQ value were observed to be scattered in the study area. As for children, teenagers, and adults, the spatial variation of HQ is consistent with that of infants, but the range of HQ decreases in turn, that is, the non-carcinogenic health risk distribution area of GW fluoride in WFC is as follows: infants > children > teenagers > adults.
Suggestions on the control of high fluoride water hazards
China is one of the few countries with the most widespread endemic fluorosis of drinking water in the world with considerable suffering. Therefore, the treatment of high-fluoride water is urgent. Therefore, the following suggestions are implemented as the control measures:
-
(1)
For areas with high fluoride GW formed by natural causes, the regional environmental hydrogeological conditions should be ascertained. High-fluoride water is mostly distributed in shallow GW; therefore, deep wells can be drilled in high-fluoride areas to extract GW.
-
(2)
For areas where industrial pollution forms high-fluoride GW, the treatment of industrial “three wastes” should be strengthened, and the discharge of fluoride-containing industrial “three wastes” into the environment should be strictly prohibited.
-
(3)
Establish water supply stations in high-fluorine GW areas to remove fluoride and purify water, centralize water supply and unified management. In addition, high-quality GW or surface water from other nearby areas can be utilized.
-
(4)
Protect the natural environment, vigorously plant trees leading to afforestation, reduce soil erosion, and the weathering and leaching of fluorine-containing minerals in the bedrock mountainous area.
-
(5)
Strengthen GW dynamic monitoring and increase publicity to increase local residents’ awareness regarding the hazards associated with high-fluorine GW. Explore new technologies and methods for reducing and treating regional high-fluorine GW.
Conclusions
Based on high-density sampling, this study evaluated the hydrogeochemical characteristics of GW fluoride in WFC and the potential health risks associated with drinking water. The main conclusions are as follows:
-
(1)
The concentration of fluorine in GW in WFC was in the range of 0.08 to 9.16, with an average value of 0.62. A total of 14.74% of the GW samples (192 samples) displayed a fluoride concentration that exceeded China’s GW quality standard limit (1 mg/L). The high fluoride concentration is mainly distributed in the upper reaches of Wen River, east of Shouguang, the southeast of Anqiu, east of Qingzhou, east of Fangzi, and towards the southeast and northwest of Gaomi.
-
(2)
The hydrochemical types of GW in WFC are mostly HCO3-Ca·Mg and SO4·Cl-Ca·Mg. However, GW with water type of HCO3-Na and SO4·Cl-Na is characterized by high fluoride content. The hydrochemical composition characteristics of GW in WFC are mainly dominated by rock weathering, while the coastal plain GW in the northern part of WFC is significantly affected by seawater intrusion.
-
(3)
The concentration of fluorine in GW in WFC is affected by the dissolution of fluorine-containing minerals, cation exchange, and alkaline environment. In addition, very high concentration of Na+ (affected by seawater intrusion) cannot promote cation exchange to increase the fluorine content in GW, but instead reduces it.
-
(4)
The results of HRA indicated that the distribution characteristics of GW fluoride in high health risk areas (HQ > 1) in WFC are more consistent with the spatial variation of GW fluoride content. Overall, the health risk of GW fluoride is as follows: infants > children > teenagers > adults. Therefore, water management departments should pay more attention to high-fluoride GW and take relevant countermeasures to ensure water safety.
Availability of data and materials
The datasets utilized and analyzed during the current study are available from the corresponding author on reasonable request.
References
Adimalla N, Qian H (2019) Groundwater quality evaluation using water quality index (WQI) for drinking purposes and human health risk (HHR) assessment in an agricultural region of Nanganur, south India. Ecotoxicol Environ Saf 176: 153‒161
Aghapour S, Bina B, Tarrahi MJ, Amiri F, Ebrahimi A (2018) Distribution and health risk assessment of natural fluoride of drinking groundwater resources of Isfahan, Iran, using GIS. Environ Monit Assess 190:3‒137
Aravinthasamy P, Karunanidhi D, Subramani T, Srinivasamoorthy K, Anand B (2020) Geochemical evaluation of fluoride contamination in groundwater from Shanmuganadhi River basin, South India: implication on human health. Environ Geochem Health 42(7):1937‒1963
Chen J, Wu H, Qian H (2016) Groundwater nitrate contamination and associated health risk for the rural communities in an agricultural area of Ningxia Northwest China. Expo Health 8(3):349‒359
Egor M, Birungi G (2020) Fluoride contamination and its optimum upper limit in groundwater from Sukulu Hills, Tororo District Uganda.Sci Afr 7:e00241
Ganyaglo SY, Gibrilla A, Teye EM, Owusu-Ansah EMJ, Tettey S Diabene PY, Asimah S (2019) Groundwater fluoride contamination and probabilistic health risk assessment in fluoride endemic areas of the Upper East Region, Ghana. Chemosphere 233:862‒872
Gao Z, Liu J, Feng J, Wang M, Wu G (2019) Hydrogeochemical characteristics and the suitability of groundwater in the alluvial-diluvial plain of southwest Shandong Province. China. Water 11(8):1577
Gao Y, Qian H, Ren W, Wang H, Liu F, Yang F (2020) Hydrogeochemical characterization and quality assessment of groundwater based on integrated-weight water quality index in a concentrated urban area. J Clean Prod 260:121006
Gibbs RJ (1970) Mechanisms Controlling World Water Chemistry. Science 170:3962‒1088
Hanse A, Chabukdhara M, Gohain Baruah S, Boruah H, Gupta SK (2019) Fluoride contamination in groundwater and associated health risks in Karbi Anglong District, Assam. North India Environ Monitor Assess 191:12‒782
Houatmia F, Azouzi R, Charef A, Bédir M (2016) Assessment of groundwater quality for irrigation and drinking purposes and identification of hydrogeochemical mechanisms evolution in Northeastern. Tunisia Environ Earth Sci 75(9):746
Jadhav SV, Bringas E, Yadav GD, Rathod VK, Ortiz I, Marathe KV (2015) Arsenic and fluoride contaminated groundwaters: a review of current technologies for contaminants removal. J Environ Manage 162:306‒325
Li J, Wang Y, Zhu C, Xue X, Qian K, Xie X, Wang Y (2020) Hydrogeochemical processes controlling the mobilization and enrichment of fluoride in groundwater of the North China Plain. Sci Total Environ 730:138877
Liu J, Gao Z, Wang M, Li Y, Ma Y, Shi M, Zhang H (2018) Study on the dynamic characteristics of groundwater in the valley plain of Lhasa City. Environ Earth Sci 77:18‒646
Liu J, Hao Y, Gao Z, Wang M, Liu M, Wang Z, Wang S (2019) Determining the factors controlling the chemical composition of groundwater using multivariate statistics and geochemical methods in the Xiqu coal mine North China. Environ Earth Sci 78:12‒364
Liu J, Gao Z, Wang Z, Xu X, Su Q, Wang S, Qu W, Xing T (2020) Hydrogeochemical processes and suitability assessment of groundwater in the Jiaodong Peninsula China. Environ Monit Assess 192:6‒384
Liu J, Peng Y, Li C, Gao Z, Chen S (2021) A characterization of groundwater fluoride, influencing factors and risk to human health in the southwest plain of Shandong Province North China. Ecotoxicol Environ Saf 207:111512
Liu J, Peng Y, Li C, Gao Z, Chen S (2021) Characterization of the hydrochemistry of water resources of the Weibei Plain, Northern China, as well as an assessment of the risk of high groundwater nitrate levels to human health. Environ Pollution 268:115947
Liu J, Peng Y, Li C, Gao Z, Chen S (2021) An investigation into the hydrochemistry, quality and risk to human health of groundwater in the central region of Shandong Province, North China. J Clean Prod 282:125416
Marghade D, Malpe DB, Subba Rao N, Sunitha B (2020) Geochemical assessment of fluoride enriched groundwater and health implications from a part of Yavtmal District, India. Hum Ecol Risk Assess Int J 26(3):673–694
Nawale VP, Malpe DB, Marghade D, Yenkie R (2021) Non-carcinogenic health risk assessment with source identification of nitrate and fluoride polluted groundwater of Wardha sub-basin, central India. Ecotoxicol Environ Saf 208:111548
Piper AM (1944) A graphic procedure in the geochemical interpretation of water-analyses. EOS Trans Am Geophys Union 25(6):914‒928
Qu R, Xiao K, Hu J, Hu J, Liang S, Hou H, Liu B, Chen F, Xu Q, Wu X, Yang J (2019) Predicting the hormesis and toxicological interaction of mixtures by an improved inverse distance weighted interpolation. Environ Int 130:104892
Raja V, Neelakantan MA (2021) Evaluation of groundwater quality with health risk assessment of fluoride and nitrate in Virudhunagar district, Tamil Nadu India. Arab J Geosci 14:1‒52
Rondano Gómez K, López Pasquali CE, Paniagua González G, Fernández Hernando P, Garcinuño Martínez RM (2020) Statistical evaluation of fluoride contamination in groundwater resources of Santiago del Estero Province Argentina. Geosci Frontiers 11:6‒2197 2205
Sawangjang B, Hashimoto T, Wongrueng A, Wattanachira S, Takizawa S (2019) Assessment of fluoride intake from groundwater and intake reduction from delivering bottled water in Chiang Mai Province Thailand. Heliyon 5(9):e02391
USEPA 1989 Risk Assessment Guidance for Superfund Volume I: Human Health Evaluation Manual (Part A) Office of Emergency and Remedial Response, Washington DC, USA
Wang Z, Gao Z, Wang S, Liu J, Li W, Deng Q, Lv L, Liu Y, Su Q (2021) Hydrochemistry characters and hydrochemical processes under the impact of anthropogenic activity in the Yiyuan city. Northern China Environ Earth Sci 80(2):60
Xu C, Li Y, Li Q, Wang L, Dong Y Jia X (2011) Nitrate contamination and source tracing from NO3-δ15N in groundwater in Weifang. Shandong Province Acta Ecologica Sinica 31(21):6579‒6587
Yadav KK, Kumar V, Gupta N, Kumar S, Rezania S, Singh N (2019) Human health risk assessment: Study of a population exposed to fluoride through groundwater of Agra city, India. Regul Toxicol Pharmacol 106:68‒80
Yadav K, Raphi M, Jagadevan S (2020) Geochemical appraisal of fluoride contaminated groundwater in the vicinity of a coal mining region: Spatial variability and health risk assessment. Geochem 81(1):125684
Yousefi M, Ghoochani M, Hossein Mahvi A (2018) Health risk assessment to fluoride in drinking water of rural residents living in the Poldasht city, Northwest of Iran. Ecotoxicol Environ Saf 148:426‒430
Zango MS, Sunkari ED, Abu M, Lermi A (2019) Hydrogeochemical controls and human health risk assessment of groundwater fluoride and boron in the semi-arid North East region of Ghana. J Geochem Explor 207:106363
Zhang Q, Xu P, Qian H, Yang F (2020) Hydrogeochemistry and fluoride contamination in Jiaokou Irrigation District, Central China: assessment based on multivariate statistical approach and human health risk. Sci Total Environ 741:140460
Zuo R, Liu X, Yang J, Zhang H, Li J, Teng Y, Yue W, Wang J (2019) Distribution, origin and key influencing factors of fluoride groundwater in the coastal area, NE China. Hum Ecol Risk Assess Int J 25(1–2):104‒119
Acknowledgements
We would like to thank the anonymous reviewers and editors for their valuable comments, which helped improve the overall flow of the manuscript.
Funding
This work was financially supported by the general projects of Shandong Natural Science Foundation (ZR2020MD109), the bureau-controlled geological survey and scientific and technological innovation project “Integration and Application of Land Quality Geochemical Survey and Evaluation Results in Weifang City” (202005) of the Shandong Provincial Bureau of Geology and Mineral Resources, “Study on Major Geological Environmental Issues in the Coastal Zone of Shandong Province (KY201911),” and the scientific and technological innovation project “Exploitation of underground brine on the south bank of Laizhou Bay and analysis of resource and environmental effects” (KJ2106) of No. 4 Exploration Institute of Geology and Mineral Resources.
Author information
Authors and Affiliations
Contributions
Jiutan Liu: formal analysis, software, methodology, writing—original draft; Yuanyuan Ma: software, writing—original draft; Zongjun Gao: supervision, conceptualization, writing—review and editing; Yuqi Zhang: software, methodology; Zengbing Sun: investigation, resources; Tianzhu Sun: investigation, resources; Haibin Fan: investigation, resources; Bin Wu: investigation, resources; Mingbo Li: investigation, resources; Lili Qian: software.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Lotfi Aleya
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, J., Ma, Y., Gao, Z. et al. Fluoride contamination, spatial variation, and health risk assessment of groundwater using GIS: a high-density survey sampling in Weifang City, North China. Environ Sci Pollut Res 29, 34302–34313 (2022). https://doi.org/10.1007/s11356-021-18443-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-18443-w