Abstract
Microalgal biomass is an emerging source of renewable energy and health-related compounds. However, harvesting of microalgae is a techno-economic hinder. In this research, chitosan and polyacrylamide were optimized harvesting condition for Chlorella vulgaris. Stirring at 300 rpm for 2 min is optimum for chitosan and polyacrylamide. Low-dose (10 mg/L) chitosan (flocculation efficiency (FE), 98.10 ± 1.06%) is more efficient than high-dose (25 mg/L) polyacrylamide (FE 94.57 ± 0.55%) for harvesting C. vulgaris. Chitosan resulted flocs settled more quickly than polyacrylamide, while polyacrylamide keep > 90% FE in a wider pH range (7–10) than chitosan (7–8). Chitosan and polyacrylamide both have no negative effect on biomass composition, including protein, carbohydrate, and carotenoid. C. vulgaris in flocs could successfully regrow in fresh culture media. The residual culture media was recycled with little impact on cell growth. All the results suggested that chitosan and polyacrylamide could harvest high-quality microalgal biomass.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microalgae, with their ability to produce large amounts of oils and biomass, are increasingly applied in biofuels and value-added products such as food, cosmetics, and pharmaceuticals. However, commercializing microalgae production is limited by expensive harvesting costs due to their dilute microalgae culture, colloidal stability, small cells size, etc. (Muhammad et al. 2021). Indeed, the cost of microalgae harvesting is estimated to be ~ 30% of the total biomass production cost (Singh and Patidar 2018).
Flocculation technology is widely used for harvesting different strains of microalgae from dilute liquid suspension, due to its efficient, environmental-friendly, and effective (Muhammad et al. 2021). During flocculation process, the addition of flocculants could neutralize the negative charge at the surface of microalgal cells or bridge the microalgal cells, leading them to coalesce into larger aggregates and agglomerate the suspended particles, accelerating of the settle rate and promotion of the harvesting efficiency. Various flocculants have been studied in biomass recovery, including inorganic and organic flocculants. Flocculant type determined the harvesting efficiency and downstream operation. Inorganic flocculants, especially metal flocculants, are widely used recently. However, using metal flocculants has two important bad effects: more (toxic) sludge production and higher metal concentration in the finally released water which may harmful to human health (Renault et al. 2009). Recently, organic flocculants have been increasingly used to harvest microalgae due to their effectiveness. Chitosan and polyacrylamide are successfully used to harvest different strains of microalgae, including the freshwater cyanobacteria Synechocystis sp. PCC6803 (Labeeuw et al. 2021), the freshwater green alga Chlorella vulgaris CS-41 (Labeeuw et al. 2021; Vu et al. 2020), the marine diatom P. tricornutum CCMP 632 (Labeeuw et al. 2021), and Porphyridium purpureum (Vu et al. 2021), However, there has been an inconsistency with flocculant concentration used, sedimentation time, working pH, stirring speed, and time for comparing commercially. The optimal flocculation dosage and conditions depended on the type of microalgae, cell density, growth conditions, etc. Therefore, further studies are necessary to clarify the best conditions.
In addition, media recycling can reduce the overall cost of production and downstream operation. It has been reported that recycling the used growth media can save up to 80% of the water requirements and 44% of nutrient requirement of culture medium (de Carvalho et al. 2019; Fret et al. 2017). However, flocculants added to the algal suspension is difficult to remove from the growth media and be carried over to next culture cycle. Some flocculants, accumulated in the media and algal cells, will be harmful to the recycling of the spent media and contaminate the final applications of the algal biomass (e.g., biofuel, food, feed, or fertilizer). A previous study showed that neither chitosan nor polyacrylamide impacted the regrowth of flocculated Scenedesmus cells, nor did the reused media have any negative effect on algal groups (Wu et al. 2015), while another research showed that polyacrylamide may leave traces of toxic acrylamide (Vandamme et al. 2013) and cationic polymers may have a long-term toxic effect on ecosystems (Beim and Beim 1994). There is no agreement on the toxic potential for microalgae by chitosan and polyacrylamide.
Besides the successful flocculation and sedimentation of microalgal cells, the growth ability of cells in settled flocs is worthy of attention, which can analyze the growth inhibition of residual flocculants. However, there is lack of related research in this area.
In this study, the flocculation condition of chitosan and polyacrylamide were optimized to harvest C. vulgaris. The formed flocs and the harvested biomass quality were analyzed after chitosan and polyacrylamide flocculation. To assess the influence of residual flocculant on microalgal regrowth and spent media recycle, the growth of C. vulgaris in formed flocs was analyzed, as well as the spent media recycled to regrow the fresh C. vulgaris.
Materials and methods
Microalgae and growth conditions
C. vulgaris (FACHB-275) was obtained from Freshwater Algae Culture Collection at the Institute of Hydrobiology (Wuhan, China), which was grown in 3 L BG11 medium in the illumination incubator (HNGZ-250 Honour, China) at pH of 7.00 ± 0.2 and kept at 25 ± 1 °C under continuous illumination of 65 ± 5 μmol/m2.s (Miao et al. 2016; Pandey et al. 2020).
Flocculation experiments
Before flocculation, the C. vulgaris suspension was diluted with BG11 medium to approximately 708 mg dry weight/L to obtain comparable initial conditions between different runs. Different experiments were designed to optimize flocculation process. For comparison, the effects of flocculant types (chitosan and polyacrylamide (Tianjin Kwangfu Fine Chemical Industry Research Institute)), flocculant dose (6–35 mg/L), stir speed (100–500 rpm), stir time (1–5 min), and pH values (4–10) on flocculation efficiencies of C. vulgaris were studied through one-factor method by varying one parameter and retaining the other factors as constant. Chitosan (purchased from Solarbio, product nr.: C8320-25 g) was dissolved overnight in 0.1% (v/v) acetic acid after which the pH was adjusted to pH 7.0 ± 0.2. Flocculants were stored at 4 °C in a dark environment and used for flocculation microalgae within 7 days. After adding the flocculant, C. vulgaris cells were mixed using a magnetic stirrer to ensure complete dispersal of the flocculants.
Growth measurement
After flocculation, flocs were filtered using a filter paper (Beimu, China) to separate the settled flocs and culture medium. The flocs with microalgal cells remained on the filter paper were re-cultured in fresh BG11 medium. Fresh algal culture was used as control. The growth of the recovered C. vulgaris was compared with the control.
The residual culture media after removing flocs was used as “spent medium.” The nutrients were added in the same concentration as the fresh medium. The spent medium’s pH was also adjusted to standard BG11 medium, and fresh algal cells were inoculated into the spent medium. The control comprised fresh BG11 medium, and growth of the algal cells in the spent medium and fresh medium was compared. The growth conditions for C. vulgaris were the same as the “Microalgae and growth conditions” section.
Analysis methods
Microscopic observation was performed utilizing a light microscope (H550S Nikon, Japan). Scanning electron microscopy (SEM) (JSM-6380LV, Agilent, USA) was used to observe the microstructure differences between fresh cells and harvested cells.
Microalgal cell dry weight was measured as follows: 10–20 mL of culture sample was filtered through 0.45-μm pore filter paper and dried to constant weight at 60 °C. The lipid content of was measured using solvent extraction method and determined gravimetrically (Bligh and Dyer 1959). Protein concentration was determined by Coomassie Brilliant Blue method (Sedmak and Grossberg 1977). Carbohydrate content was determined by phenol sulfuric acid method (Haldar et al. 2017). Total carotenoids content was measured by phenol–sulfuric acid colorimetric method (Wellburn 1994). The flocculation efficiency (FE) was calculated as the variation value of OD680nm of upper liquid divided by the original suspension OD680nm and multiplied by 100. Experiments were done in triplicate, and data were expressed as mean ± standard deviation. Statistical differences were acquired by Tukey test through one-way analysis of variance (ANOVA) (p < 0.05).
Results and discussion
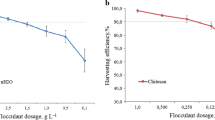
Flocculant dosage
Flocculant dosage has important influence on microalgae harvesting. Optimizing flocculant concentration and achieving harvesting at low dosage could decrease microalgae production cost and deleterious effect on microalgae biomass. Both chitosan and polyacrylamide show high C. vulgaris harvest efficiency (Fig. 1a). FE of 74.10 ± 2.36% and 74.03 ± 1.72% was observed for low doses of chitosan (6 mg/L) and polyacrylamide (15 mg/L), respectively. FE was 98.07 ± 1.27% and 94.60 ± 0.62% for both chitosan (10 mg/L) and polyacrylamide (25 mg/L) at optimal dose, respectively. Further increasement in flocculant concentrations has no significant increase in FE of C. vulgaris.
When the dosage of flocculant is insufficient, the flocculant polymer could attach few cells, and other cells might be free from the attachment. Therefore, the FE is low at low flocculants concentrations. With the increasing of flocculants dosage, the attached microalgal cells increased and FE increased. Excessive polymers may cause a flocculants attach fully on cell surface and form a steric layer on cell surface which made cell colloidally stable again and settlement of microalgal cell became more difficult (Nguyen et al. 2019; Vu et al. 2020). Excessive flocculants could not only result in FE reduction, but also the flocculant residual increased in the suspension.
The excellent performance of chitosan and polyacrylamide for other microalgae species has been reported in literatures (Correa et al. 2019). Twenty milligrams/liter of chitosan resulted about 99% FE of Desmodesmus subspicatus at pH 9 (Correa et al. 2019). The optimal polyacrylamide doses to achieve 75% FE for C. vulgaris was 10 mg/L (You et al. 2019). In this study, 10 mg/L chitosan were required to obtain 98.07 ± 1.06% FE for C. vulgaris. However, there have some reports that a very high dosage (200 mg/L) of chitosan only resulted in low FE (62%) of C. vulgaris (CS-41) (Vu et al. 2020). The molecular weight of polymer flocculants (chitosan and polyacrylamide) influenced microalgae FE greatly. For example, using the same dosage of chitosan, high molecular weight chitosan (600,000–800,000 Da) gave high FE of 97%, while low molecular weight chitosan (50,000–190,000 Da) can only achieve 49% FE (Low and Lau 2017). Another reason maybe is that the variation in the microalgal culture and growth conditions might be accountable for the difference in optimal doses among these studies. In this test, the FE of low dosage (10 mg/L) of chitosan was more efficient than that of high dosage (25 mg/L) of polyacrylamide.
Settling time
Chitosan resulted flocs settled more quickly than polyacrylamide (Fig. 1b). For chitosan, after only 2-min settlement, the flocs settled and resulted a high FE (96.77 ± 1.02%). Further increase settlement time from 2 to 12 min, FE did not further increase, while, for polyacrylamide, short settlement time resulted low FE. When settlement time is 10 min, the FE achieves the maximum value (94.37 ± 0.61). Further increase settlement time to 12 min, there was no significant FE increase. Some researcher utilized sulfate or chloride salts to harvest microalgae and achieved a maximum FE of 80% after 3–4 h (Papazi et al. 2010), which is much longer than this test. Shorting the settlement time can greatly improve flocculation performance.
Some research found that the settling velocity of flocs was nearly linear with the size of resulted flocs (Wei et al. 2020). The formed flocs of chitosan were much larger than that of polyacrylamide (Fig. 2), which can explain the quick sedimentation of chitosan resulted flocs.
Stirring speed
With low stirring speed (100–200 rpm), chitosan and polyacrylamide cannot achieve good mixing with C. vulgaris, and FE was low (Fig. 1c). When the stirring speed increased to 300 rpm, the chitosan and polyacrylamide achieved the highest FE. For chitosan, further increase stirring speed, FE did not significantly increase. For polyacrylamide, when stirring speed increased to 500 rpm, FE decreased. These results suggested that flocs formed by chitosan are more anti-destructive. From Fig. 2, the formed flocs by chitosan were more compact than that of polyacrylamide, which made them more resistant to shearing force resulted from stirring. The more stirring speed, the more energy required. Thus, in this test, stirring speed 300 rpm is the optimal stirring speed for chitosan and polyacrylamide.
When stirring speed was low, the connection between the added flocculant and microalgae cells was also low, therefore, leading to less floc formation and low FE (Wang et al. 2018a, b). With increasing stirring speed, the collision opportunity between the algae cells and flocculants increased accordingly, and FE resulted by flocculants increased (Yeon et al. 2018). When stirring speed exceed certain values, the formed flocs will be easily destroyed and resulted in the FE decreasing (Tran et al. 2017).
Stirring time
Flocculation is a slow process, unlike biochemical reactions such as acid–base neutralization, so it takes some time. Stirring can make the components in the reaction system mix evenly and fully. Figure 1d shows the effect of stirring time on FE of C. vulgaris. For both chitosan and polyacrylamide, with the extension of stirring time, the recovery rate increased rapidly. When the stirring time reached 2 min, the recovery rate reached the highest, 98.00 ± 1.68% and 94.57 ± 0.50% for chitosan and polyacrylamide, respectively. And the recovery rate decreased when the stirring time continues to increase.
If stirring time is too short, it will lead to incomplete flocculation and unsatisfactory recovery. The results showed that the recovery reached the highest point at stirring 2 min for chitosan and polyacrylamide, respectively. And the recovery decreased when the stirring time was prolonged. This showed that the internal structure of the flocculating mass is stable with the completion of flocculating in the proper shearing force range. The results showed that C. vulgaris could be harvested by using chitosan and polyacrylamide, and the highest recovery could be achieved by stirring 2 min.
Flocculation pH value
Microalgae suspension colloids and surface charge of flocculants are pH dependent, and their behavior has great influence on flocculation of microalgal biomass. The flocculation resulted by chitosan is more sensitive to pH change than polyacrylamide (Fig. 1e). For chitosan, FE obtained at pH 4 was only 52.87 ± 0.55% and increased to 96.23 ± 0.96% and 98.10 ± 1.06% at pH 7 and pH 8. A significant reduction in FE (39.87 ± 1.81–16.83 ± 1.45%) was observed with further increase in pH to 9 and 10, while for polyacrylamide, FE keeps more than 90% in a wide pH range (7–10).
At acidic environment, the negative charge on the microalgae surface was neutralized and positively charged H+ (Sun et al. 2019). Chitosan and polyacrylamide were also positively charged. Electrostatic repulsion between the positively charged microalgae and flocculants (chitosan and polyacrylamide) leads to low microalgae FE. At neutral and weak alkaline condition, with the increasing of OH− in the raw water that caused the algal cell particles to be negatively charged, which was effectively absorbed by the positively flocculants, and FE was increased (Wang et al. 2018a, 2018b; Zhang et al. 2018). When pH further increased to strong alkaline conditions, the added flocculant cannot neutralize all the negative charges in microalgae. In addition, high pH environment causes hydrolysis of flocculant, thereby leading to FE reduce (Rao et al. 2018).
Morphology of flocculated cells
During flocculation process, preventing cell surface damage could effectively avoid the leakage of the compounds and assuring overall yield and quality of the biomass harvested. The surface structure of C. vulgaris was studied by microscopic and SEM technology. Fresh cells were separated from each other and suspended in water solution (Fig. 2a), while addition of chitosan and polyacrylamide to the culture resulted in the formation of flocs of algal cells, and minute changes in cell surface (Fig. 2b and c ). Cationic polyelectrolyte has a negligible effect on the surface structure of Nannochloropsis oculata (Sales et al. 2019). Polyacrylamide has negative effect on surface structure of cyanobacteria Synechocystis sp. but only minor effect on eukaryotic microalgae Phaeodactylum purpureum and C. vulgaris at dosage of 31.3 mg/g (Labeeuw et al. 2021). The difference in cell surface property could account for the differences in cellular surface structure, including microalgal species, growth stage, flocculants dosage, and culture medium. Eukaryotic microalgae have carbohydrate rich cell walls, which can affect their resilience to various stressors (Popper et al. 2011; Popper and Tuohy 2010).
Biomass quality after flocculation
Chitosan and polyacrylamide both have no negative effect on cellular protein, carbohydrate, and carotenoid contents (Table 1). Chitosan (10 mg/L) and polyacrylamide (25 mg/L) had no or limited (decreased 4.52%) effect on lipid content in C. vulgaris, respectively, compared with the control (natural settlement). These findings demonstrated that chitosan has no adverse influence on the quality of the harvested biomass. And, polyacrylamide only has limited influence on lipid content.
Flocculant type can cause different changes of cell composition, which may in turn affect the overall production cost and downstream processing. Chitosan did not affect the lipid content of C. vulgaris (Zhu et al. 2018). Another research showed that chitosan decreased protein and carbohydrate content in Scenedesmus, but it did not influence lipid content in Scenedesmus (Kumar Gupta et al. 2018). Polyacrylamide greatly decreased the total lipid, total carbohydrates, and total protein in S. obliquus, but it has no effect on total carbohydrates in Scenedesmus sp. compared with the control (Wu et al. 2015). The choice of flocculant can impact the quality of the product differently. In this test, chitosan and polyacrylamide have little influence on biomass quality.
Growth of flocculated C. vulgaris in fresh medium
The growth of flocculated cells was comparable to that of fresh C. vulgaris cells (Fig. 3a). These results suggested the flocculated process resulted by chitosan, and polyacrylamide have little influence on cell re-growth, which can be explained by the little effect on surface structure (Fig. 2). As far as we know, there is no related research.
Growth of fresh C. vulgaris in spent medium
A large volume of water is needed for cultivation of microalgae. The recycled culture medium could effectively reduce production cost, save water resources, and protect the environment. In this test, culture medium, after flocculated by chitosan and polyacrylamide, was used to re-culture fresh C. vulgaris cells. The growth curves of C. vulgaris cultured in spent medium of chitosan and polyacrylamide and fresh medium were shown in Fig. 3b. The growth curves of C. vulgaris cells in chitosan and polyacrylamide flocculated medium were close to that in the fresh growth medium, indicating that the tested flocculated media have limited adverse effect on cell growth and the spent media after chitosan and polyacrylamide flocculation could be potentially recycled for the re-cultivation of microalgae. In similar study, the recycled medium could sustain microalgae growth, including Scenedesmus sp., S. acuminatus, S. obliquus, Chlorella sp., Chlamydomonas reinhardtii, and C. pyrenoidosa (Bleeke et al. 2015; Mehta and Chakraborty 2021; Wu et al. 2015). Furthermore, improved microalgae regrowth was found after flocculation (Farooq et al. 2015; Morocho-Jácome et al. 2016), while some other researches showed that microalgal growth slowed down or reduced the biomass yield, which is probably due to the toxicity of the residual flocculants or the chemical stress imposed by chemical flocculants (Depraetere et al. 2015; Kim et al. 2011). These results indicate that the types of flocculants must be chosen wisely for successful reuse of the spent medium.
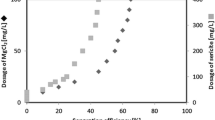
In addition to the flocculation efficiency and the effects on the microalgae cells, the processing cost is also one of the important factors in the microalgae production. Table 2 summarized the cost analysis of C. vulgaris flocculation by chitosan and polyacrylamide. It can be noted from the table that flocculation of 1 ton dry biomass of microalgae can be accomplished using the flocculant chitosan for a cost of 274.4 USD and polyacrylamide for a cost of 151.7 USD. Considering the processing cost of microalgae, chitosan and polyacrylamide are two cost-effective flocculants.
Conclusion
Flocculation experiments demonstrated that both chitosan and polyacrylamide successfully harvested C. vulgaris. Chitosan had a lower dosage requirement than polyacrylamide and which was more effective. Chitosan and polyacrylamide have no or little adverse effect on biomass contents. The cells in flocculated flocs successfully regrow and the spent medium re-culture microalgal cells after flocculated by chitosan and polyacrylamide. All the results suggested that chitosan and polyacrylamide could harvest high-quality C. vulgaris biomass. The information generated in this study can contribute to making the microalgae industry more competitive.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Beim AA, Beim AM (1994) Comparative ecological – toxicological data on determination of maximum permissible concentrations (MPC) for several flocculants. Environ Technol 15(2):195–198
Bleeke F, Milas M, Winckelmann D, Klöck G (2015) Optimization of freshwater microalgal biomass harvest using polymeric flocculants. Int Aquat Res 7(3):235–244
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Phys 37(8):911–917
Correa DdO, Rabello Duarte ME, Noseda MD (2019) Biomass production and harvesting of Desmodesmus subspicatus cultivated in flat plate photobioreactor using chitosan as flocculant agent. J Appl Phycol 31(2):857–866
de Carvalho JC, Sydney EB, Assú Tessari LF, Soccol CR (2019) Chapter 2 - Culture media for mass production of microalgae. In: Pandey A, Chang J-S, Soccol CR, Lee D-J (eds) Biofuels from Algae, 2nd edn. Elsevier, Y. Chisti, pp 33–50
Depraetere O, Pierre G, Noppe W, Vandamme D, Foubert I, Michaud P, Muylaert K (2015) Influence of culture medium recycling on the performance of Arthrospira platensis cultures. Algal Res 10:48–54
Farooq W, Moon M, Ryu B-g, Suh WI, Shrivastav A, Park MS, Mishra SK, Yang J-W (2015) Effect of harvesting methods on the reusability of water for cultivation of Chlorella vulgaris, its lipid productivity and biodiesel quality. Algal Res 8:1–7
Fret J, Roef L, Blust R, Diels L, Tavernier S, Vyverman W, Michiels M (2017) Reuse of rejuvenated media during laboratory and pilot scale cultivation of Nannochloropsis sp. Algal Res 27:265–273
Haldar D, Sen D, Gayen K (2017) Development of spectrophotometric method for the analysis of multi-component carbohydrate mixture of different moieties. Appl Biochem Biotechnol 181(4):1416–1434
Kim D-G, La H-J, Ahn C-Y, Park Y-H, Oh H-M (2011) Harvest of Scenedesmus sp. with bioflocculant and reuse of culture medium for subsequent high-density cultures. Bioresource Technol 102(3):3163–3168
Kumar Gupta S, Kumar NM, Guldhe A, Ahmad Ansari F, Rawat I, Nasr M, Bux F (2018) Wastewater to biofuels: comprehensive evaluation of various flocculants on biochemical composition and yield of microalgae. Ecol Eng 117:62–68
Labeeuw L, Commault AS, Kuzhiumparambil U, Emmerton B, Nguyen LN, Nghiem LD, Ralph PJ (2021) A comprehensive analysis of an effective flocculation method for high quality microalgal biomass harvesting. Sci Total Environ 752:141708
Low YJ, Lau SW (2017) Effective flocculation of Chlorella vulgaris using chitosan with zeta potential measurement. In: 29th Symposium of Malaysian Chemical Engineers (eds) Saptoro A, Khur WS, Wei LS, Ng WPQ, Anwar M, Yeo C, Huey KE, Vol. 206
Mehta AK, Chakraborty S (2021) Multiscale integration of mixotrophic microalgal cultivation, lipid synthesis, rapid biomass harvesting, and nutrient recycling in pilot-scale photobioreactors. Algal Res 53:102146
Miao M-s, Yao X-d, Shu L, Yan Y-j, Wang Z, Li N, Cui X-t, Lin Y-m, Kong Q (2016) Mixotrophic growth and biochemical analysis of Chlorella vulgaris cultivated with synthetic domestic wastewater. Int Biodeter Biodegr 113:120–125
Morocho-Jácome AL, Mascioli GF, Sato S, de Carvalho JCM (2016) Evaluation of physicochemical treatment conditions for the reuse of a spent growth medium in Arthrospira platensis cultivation. Algal Res 13:159–166
Muhammad G, Alam MA, Mofijur M, Jahirul MI, Lv Y, Xiong W, Ong HC, Xu J (2021) Modern developmental aspects in the field of economical harvesting and biodiesel production from microalgae biomass. Renew Sust Energ Rev 135:110209
Nguyen LN, Labeeuw L, Commault AS, Emmerton B, Ralph PJ, Johir MAH, Guo W, Ngo HH, Nghiem LD (2019) Validation of a cationic polyacrylamide flocculant for the harvesting fresh and seawater microalgal biomass. Environ Technol Innov 16:100466
Pandey A, Shah R, Yadav P, Verma R, Srivastava S (2020) Harvesting of freshwater microalgae Scenedesmus sp. by electro–coagulation–flocculation for biofuel production: effects on spent medium recycling and lipid extraction. Environ Sci Pollut Res 27(3):3497–3507
Papazi A, Makridis P, Divanach P (2010) Harvesting Chlorella minutissima using cell coagulants. J Appl Phycol 22(3):349–355
Popper ZA, Michel G, Hervé C, Domozych DS, Willats WGT, Tuohy MG, Kloareg B, Stengel DB (2011) Evolution and diversity of plant cell walls: from algae to flowering plants. Annu Rev Plant Biol 62(1):567–590
Popper ZA, Tuohy MG (2010) Beyond the green: understanding the evolutionary puzzle of plant and algal cell walls. Plant Phy 153(2):373–383
Rao NRH, Yap R, Whittaker M, Stuetz RM, Jefferson B, Peirson WL, Granville AM, Henderson RK (2018) The role of algal organic matter in the separation of algae and cyanobacteria using the novel “Posi” - dissolved air flotation process. Water Res 130:20–30
Renault F, Sancey B, Charles J, Morin-Crini N, Badot P-M, Winterton P, Crini G (2009) Chitosan flocculation of cardboard-mill secondary biological wastewater. Chem Eng J 155(3):775–783
Sales R, Derner RB, Tsuzuki MY (2019) Effects of different harvesting and processing methods on Nannochloropsis oculata concentrates and their application on rotifer Brachionus sp. cultures. J Appl Phycol 31(6):3607–3615
Sedmak JJ, Grossberg SE (1977) A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal Biochem 79(1–2):544–552
Singh G, Patidar SK (2018) Microalgae harvesting techniques: a review. J Environ Manage 217:499–508
Sun Y, Sun W, Shah KJ, Chiang P-C, Zheng H (2019) Characterization and flocculation evaluation of a novel carboxylated chitosan modified flocculant by UV initiated polymerization. Carbohyd Polym 208:213–220
Tran N-AT, Seymour JR, Siboni N, Evenhuis CR, Tamburic B (2017) Photosynthetic carbon uptake induces autoflocculation of the marine microalga Nannochloropsis oculata. Algal Res 26:302–311
Vandamme D, Foubert I, Muylaert K (2013) Flocculation as a low-cost method for harvesting microalgae for bulk biomass production. Trends Biotechnol 31(4):233–239
Vu HP, Nguyen LN, Lesage G, Nghiem LD (2020) Synergistic effect of dual flocculation between inorganic salts and chitosan on harvesting microalgae Chlorella vulgaris. Environ Technol Innov 17:100622
Vu HP, Nguyen LN, Vu MT, Labeeuw L, Emmerton B, Commault AS, Ralph PJ, Mahlia TMI, Nghiem LD (2021) Harvesting Porphyridium purpureum using polyacrylamide polymers and alkaline bases and their impact on biomass quality. Sci Total Environ 755:142412
Wang W, Sha J, Lu Z, Shao S, Sun P, Hu Q, Zhang X (2018a) Implementation of UV-based advanced oxidation processes in algal medium recycling. Sci Total Environ 634:243–250
Wang Y-S, Tong Z-H, Wang L-F, Sheng G-P, Yu H-Q (2018b) Effective flocculation of Microcystis aeruginosa with simultaneous nutrient precipitation from hydrolyzed human urine. Chemosphere 193:472–478
Wei C, Liao Q, Huang Y, Zhu X, Xia A, Zhu X (2020) Simultaneous enhancing the sedimentation and adsorption performance of Chlorella vulgaris with montmorillonite modified cationic starch. Biochem Eng J 164:107785
Wellburn AR (1994) The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Phy 144(3):307–313
Wu J, Liu J, Lin L, Zhang C, Li A, Zhu Y, Zhang Y (2015) Evaluation of several flocculants for flocculating microalgae. Bioresource Technol 197:495–501
Yeon HH, June SJ, Kim Mi S, Kim D-Y (2018) A Study on efficiency of wastewater treatment using microalgae: focusing on nutrients and flocculation. J Korean Soc Environ Technol 19(6):563–569
You Y, Sun X, Yang W, Dai L, He L, Wang H, Zhang J, Xiang W (2019) A high-performance and low-cost strategy to harvest saltwater Chlorella vulgaris using cationic polyacrylamide coupled with bentonite. Algal Res 41:101579
Zhang W, Song R, Cao B, Yang X, Wang D, Fu X, Song Y (2018) Variations of floc morphology and extracellular organic matters (EOM) in relation to floc filterability under algae flocculation harvesting using polymeric titanium coagulants (PTCs). Bioresource Technol 256:350–357
Zhu L, Li Z, Hiltunen E (2018) Microalgae Chlorella vulgaris biomass harvesting by natural flocculant: effects on biomass sedimentation, spent medium recycling and lipid extraction. Biotechnol Biofuels 11
Funding
This work was supported by the Chinese Scholarship Council of the Ministry of Education (201908120094), the National Training Programs of Innovation and Entrepreneurship for undergraduates (202010057158), and the Open Project Program of Key Laboratory of Marine Resource Chemistry and Food Technology (TUST), Ministry of Education (EMTUST-21-07).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Yu Wang, Juan Wang, Chenchen Feng, Jinyang Li, and Naike Wang. The first draft of the manuscript was written by Yu Wang, Jinyang Li, and Jinling Cai, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, Y., Wang, J., Feng, C. et al. High-quality Chlorella vulgaris biomass harvesting through chitosan and polyacrylamid2e. Environ Sci Pollut Res 29, 34651–34658 (2022). https://doi.org/10.1007/s11356-021-17847-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-17847-y