Abstract
The protective efficacy of dietary naringenin (NG) has been investigated against the toxicity caused by cadmium chloride (CdCl2) using biomarkers of oxidative stress in the liver, gills and kidney of Labeo rohita. The fish were exposed to environmentally relevant concentrations of CdCl2 (0.37 and 0.62 mg/L) and simultaneously orally administered with NG (50 mg/kg bw/day) for 60 days. Tissue (gills, liver and kidney) samples were collected on days 15, 30 and 60 of the experiment and analysed for endogenous antioxidants and oxidative stress biomarkers. CdCl2 exposure for 15 and 30 days induced the development of adaptive mechanism as demonstrated by the enhanced activities of superoxide dismutase, catalase, glutathione peroxidase and glutathione-S-transferase in all three tissues. However, on the 60th day, CdCl2-induced oxidative damage was stipulated by a decline in the enzyme activities and reduced glutathione (GSH) content significantly (p < 0.05) below control levels along with enhanced levels of lipid peroxidation. Oral administration of NG in toxicant exposed fish significantly restored the altered levels of antioxidants, oxidative enzymes and lipid peroxidation. Besides, integrated biomarker response (IBR) analysis was applied by combining all the biomarkers to indicate the overall stress response index. IBR analysis confirmed the altered levels of biomarkers, the oxidative stress induced by CdCl2 exposure and the ameliorative potential of NG. The present study suggested that NG might have protective role against Cd-induced oxidative insult which might be ascribed to the ability of NG to chelate metals and scavenge free radicals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The protective antioxidant defence system of aquatic organisms continuously experience many challenges as a consequence of exposure to diverse natural and anthropogenic factors capable of inducing redox imbalance (Danabas et al. 2015; Wen et al. 2018). Reactive oxygen species (ROS) are produced continuously as typical by-products of cellular metabolic processes and, at low concentrations, possess dualistic role; i.e. they are not only involved in cell signalling and biosynthetic reactions but also provide defence against pathogens and have deleterious effects on macromolecules (Rajendran et al. 2014). However, overproduction of ROS alters the redox homeostasis, overpowers the antioxidant defence system of the cell and subsequently results in oxidative damage, often termed as oxidative stress (Pisoschi and Pop 2015). Oxidative stress instigates deleterious alterations in cellular macromolecules like DNA, lipid and proteins (Naik et al. 2020). Alterations in levels of endogenous free radical scavengers can be applied as potent biomarkers for studying the oxidative damage caused by pollutants (Ziech et al. 2010; Poletta et al. 2016). Literature is replete with evidences suggesting that numerous xenobiotics, and amongst them heavy metals, may enhance ROS production which is considered an important mechanism of toxicity (Waisberg et al. 2003; Cobbina et al. 2015). Amongst heavy metals, Cd is of major concern as a consequence of its considerable toxic effects to aquatic animals at relatively low concentrations and long elimination half-life (Jindal and Verma 2015; Prabu et al. 2011a; McRae et al. 2019). The extensive use of Cd in modern industries and agricultural fertilisers has resulted in increased concentrations of Cd in the environment which contributes to the entry of Cd in food chain and exerts adverse effects in organisms (Gad 2014; Naik et al. 2020; Bhardwaj et al. 2021). Many studies have reported higher levels of Cd in underground water, soil, sewage, sediments, vegetation and animal tissues (El-Ghasham et al. 2008; Aulakh et al. 2009; Kumar and Singh 2010; Idrees et al. 2018; Abougabal et al. 2020; Tamele and Vázquez Loureiro 2020). Elevated Cd levels such as 0.04 mg/L in fresh water fish farm at Qassim Region, K.S.A. (El-Ghasham et al. 2008), and 0.01 mg/L in water samples from Koekemoerspruit, Africa (Fernández-Luqueño et al. 2013), have been recorded in aquatic ecosystem. Bhardwaj et al. (2017) reported the variation in the level of Cd from 0.006 mg/L (post-monsoon) to 0.11 mg/L (pre-monsoon) in Yamuna River during 2013 to 2015. Cd induces deleterious effects in animals such as nephrotoxicity, hepatotoxicity, cytotoxicity, genotoxicity, immunotoxicity, cancer and disrupted reproductive processes thus causing infertility (Kumar and Singh 2010; Bhardwaj et al. 2021). Cd is considered to augment ROS production which in turn leads to lipid peroxidation (McRae et al. 2018; Verma et al. 2020), protein modifications and altered gene expression as well as DNA damage (Valko et al. 2005).
Recently, great emphasis has laid on intervening Cd-induced oxidative stress by using naturally occurring antioxidants. In this context, a variety of compounds has been evaluated for their efficacy to reduce the detrimental effects of Cd by inhibiting lipid peroxidation or chelating the metal ions thus, easing their elimination (Karaytug et al. 2014; Bhardwaj and Panchal 2021). Flavonoids (a group of polyphenols) are prevalent in numerous fruits and vegetables, and display remarkable array of pharmacological properties. Many researchers have investigated the potential of flavanoids to alleviate oxidative damage (Cheng and Breen 2000; Prabu et al. 2011a). Naringenin (4,5,7-trihydroxy flavonone), a naturally occurring flavonoid present in citrus fruits, has attracted considerable attention because of its widespread biological applications including antiatherogenic, hepatoprotective, neuroprotective, anti-inflammatory, anticancer and antimutagenic (Choi et al. 1994; Lee et al. 2001, 2004; Amaro et al. 2009; Zhang et al. 2019; Zeng et al. 2020). In the recent years, NG as an antioxidant has been the focus of research owing to its potential to chelate metal ions and scavenge free radicals (Kapoor and Kakkar 2014; Priscilla et al. 2015; Huang et al. 2019).
Fish, being poikilothermic and sensitive, are the most susceptible to metal contamination in aquatic environment and, therefore, are extensively employed in biomonitoring studies (Çavaş and Ergene-Gözükara 2005). L. rohita (Indian major carp), have high growth potential, consumer preference and are widely used as an ecological indicator (Prusty et al. 2011). Taking into consideration ROS production as the key investigated mechanisms of Cd toxicity and the antioxidative property of NG, the present study hypothesised that NG possess a protective role against CdCl2-induced oxidative stress in L. rohita. To elucidate Cd-induced toxicity and protective efficacy of NG, oxidative stress biomarkers of gills, liver and kidney were chosen because they are the most vulnerable to toxicity caused by pollutants in aquatic environment and, thus, considered an efficient tool for biomonitoring of Cd-mediated oxidative damage in aquatic fauna. The major objectives of the present study were (1) to explicate the antioxidative defence response of L. rohita to short- (15 days) and long-term exposure (60 days) of environmentally relevant concentrations of CdCl2, (2) to elucidate the extent of toxicity caused to three different organs (gills, liver and kidney) using integrated biomarker response and (3) to evaluate the ability of NG in mitigating the toxicity induced by CdCl2 through oxidative stress biomarkers.
Materials and methods
Experimental fish
The live specimens of L. rohita (12.05 ± 0.19 cm long and 18.05 ± 0.98 g, mean ± SE) were obtained from Sultan Fish Seed Farm, Haryana, India. After disinfection (0.01% KMnO4 solution for 1 min), the specimens were shifted to glass aquaria (300-L capacity). The fish were then acclimatised for 15 days in dechlorinated tap water at 24–25 °C temperature with dissolved oxygen content of 8.07 ± 0.50 mg/L, pH 6.9–7.1 and hardness 92.675 as CaCO3 mg/L.
The experiments carried out on fish and the protocols included in the study followed the IAEC guidelines (Panjab University, Chandigarh, IAEC/527).
Chemicals
The anhydrous CdCl2 and NG were of technical-grade (Sigma-Aldrich, USA). Other reagents used in the study were of analytical grade (Merck, Mumbai, India, and Hi Media India Ltd.).
Feed preparation
The basal diet was prepared by thoroughly mixing locally available ingredients (see Table S1 in supplementary material). Water was added to prepare thick dough and pellets were formed. The pellets were sun-dried and stored in air tight container at − 20 °C. The fish were fed (two times/day) with the basal diet at 2% of body weight (bw).
Determination of LD50 of NG
To determine LD50 of NG in L. rohita, ‘Acute toxic class method’ (Fig. 1) was used according to OECD guidelines (Organization for Economic Cooperation and Development 2001). It is a sequential method involving use of three animals in each group at each step with a particular dose level. All the experiments were performed in triplicates to ensure reproducibility of results. As there is no information available on the toxicity of NG to fish, 5 mg/kg bw was selected as the initial dose to carry out the study. Three fish in a plastic tank (40-L capacity) were fed with a basal diet of 5 mg/kg bw of NG at 2% of body weight. The remnants of feed were removed after 2 h by siphoning. The fish were observed every half an hour for 4 h and then after 24 h for any signs of mortality. According to Acute Toxic Class method, if the death of 2 or 3 fish occurs then, the experiment has to be stopped immediately and LD50 falls in level 1 (GHS 2013). On the other hand, if there is no mortality or only 1 fish dies, the above step is repeated with the identical dose. Since no fish died at this step, then the next step was conducted with 50 mg/kg bw dose. Further, the above procedure was repeated with a 300 and 2000 mg/kg bw dose and the range of 24 h LD50 was obtained (Fig. 1).
Estimation of LD50 of NG to L. rohita by oral administration (in diet) through acute toxic class method based on OECD guidelines 423 (Organization for Economic Cooperation and Development 2001). Dotted lines represent the procedure given in the guidelines, while bold lines show the results obtained during the study. Oval boxes represent the no. of fish died during the experiment
Exposure protocol
Fish were randomly distributed into six groups (n = 6) as depicted in Table 1 and maintained in the plastic tanks (40-L capacity) filled with dechlorinated water at experimental conditions as mentioned in the “Experimental fish” section. All the experiments were conducted in triplicates with the same no. of fish in each (n = 6, total 108), and experimental tanks (capacity 50 L) were provided with aerators and heaters (to maintain temperature).
The CdCl2 exposure concentrations were selected based on our previous study (Jindal and Verma 2015), while the dose of NG (50 mg/kg bw/day) was chosen according to the effective dose used by earlier workers for amelioration of metal-induced toxicity in experimental models (Wang et al. 2012; Mershiba et al. 2013). Test media was replaced every 2 days and the concentration of CdCl2 was examined using ICP-AES (Thermo Electron Corporation, iCAP 6000 series) on random basis during the experimental period.
Sample collection and preparation
On days 15, 30 and 60 of the experiment, 2 fish from each replicate (6 fish/group) were selected at random and sacrificed by cervical dislocation. Liver, gills and kidney were immediately removed using sterile forceps on ice-cold plates, rinsed (with chilled 0.9% NaCl), dried in filter paper and weighed separately. The 10% (W/V) homogenate of tissues was prepared in 100 mM potassium phosphate buffer (pH 7.4) using Potter-Elvejhem homogeniser. Then centrifugation of homogenate was done at 4 °C (10,000 × g for 30 min.). Afterwards, the supernatant was carefully separated and stored at − 30 °C. All the antioxidant levels and enzyme activities were analysed within 3 days of sampling.
Biochemical analysis
The superoxide dismutase (SOD) activity was determined following the method of Kono (1978). Briefly, the reaction mixture containing 1.2 ml of 50 mM sodium carbonate in 0.1 mM EDTA (pH 10.8), 0.5 ml of 96 µM nitro blue tetrazolium (NBT) and 0.1 ml of 0.6% Triton X-100 were incubated at 37 °C for 10 min. Reaction was initiated by adding 0.1 ml of 20 mM hydroxylamine hydrochloride. The rate of NBT dye reduction by superoxide radical generated due to photoactivation of hydroxylamine hydrochloride was recorded at 560 nm for 3 min for blank. Then immediately 0.1 ml supernatant was added to it. After mixing thoroughly, 50% inhibition in the rate of NBT reduction by SOD present in the enzyme source was recorded at 560 nm for 3 min. The enzyme activity was expressed as U/mg protein.
The activity of catalase (CAT) was assessed as change in the absorbance due to H2O2 decomposition as described by Luck (1965). The reaction mixture consisted of 2.9 ml of H2O2-phosphate buffer and 0.1 ml of supernatant. The decrease in absorbance/30 s at 240 nm was read for 3 min using double-distilled water as blank. The enzyme activity was expressed as µmole H2O2 decomposed/min/mg protein.
The activity of glutathione peroxidase (GPx) was assayed at 340 nm following the method of Mohandas et al. (1984), which involves the oxidation of NADPH to NADP. A total of 2 ml reaction volume consisted of 0.1 ml EDTA (1 mM), 0.1 ml sodium azide (1 mM), 1.49 ml phosphate buffer (0.1 M, pH 7.4), 0.05 ml glutathione reductase (1 IU/ml), 0.05 ml reduced glutathione (1 mM), 0.1 ml NADPH (0.2 mM) and 0.01 ml H2O2 (0.25 mM) and 0.1 ml supernatant. The depletion of NADPH at 340 nm was recorded. The enzyme activity was represented as µmol NADPH oxidised/min/mg protein.
The activity of glutathione-S-transferase (GST) was estimated according to Habig et al. (1974) by measuring the change in absorbance due to formation of GST catalysed CDNB-GSH conjugates at 340 nm. The reaction mixture contained 0.1 ml of 30 mM GSH, 0.1 ml of 30 mM CDNB, 2.7 ml of potassium phosphate buffer (pH 6.5) and 0.1 ml of supernatant. The enzyme activity was expressed as µmol conjugate formed/min/mg protein.
The reduced glutathione (GSH) level was estimated spectrophotometrically at 412 nm according to Moron et al. (1979) by monitoring the reaction of GSH and 5,5′-dithiobis-2-nitrobenzoic acid (DTNB) resulting in yellow-coloured complex formation. The GSH content was expressed as µmol conjugate formed/g protein.
Malondialdehyde (MDA) level as marker of lipid peroxidation was estimated following the method of Buege and Aust (1978) and the results were expressed as µmol/mg protein. 0.25 ml supernatant was incubated in 0.25 ml each of 150 mM Tris–HCl (pH 7.4), 1.5 mM ascorbic acid and 1.0 mM ferrous sulphate in a final volume of 1.0 ml at 37 °C for 15 min. Then 1.0 ml of 10% TCA and 2.0 ml of 0.375% TBA was added to it, and kept in boiling water bath for 15 min. The contents were then centrifuged at 3,000 rpm for 10 min. The absorbance of the clear supernatant was measured against reference blank at 532 nm.
The protein content quantification was carried out according to Lowry et al. (1951) using BSA as a standard.
Statistical analysis
SPSS 18.0 statistical software was used for the analysis. The data was analysed by Kolmogorov–Smirnov test and was found to be for normally distributed. The data was then subjected to one-way ANOVA followed by Tukey’s post hoc test to analyse the statistical differences amongst the groups. P value < 0.05 was considered statistically significant.
Integrated biomarker response analysis
Integrated biomarker response (IBR) analysis was applied to evaluate an integrated response of L. rohita towards treatment with CdCl2 and NG by combining the results obtained from the biomarkers’ analysis (described in the “Oxidative stress biomarkers” section) into general stress index. IBR was calculated for each experimental group at various exposure periods according to Beliaeff and Burgeot (2002) and Guerlet et al. (2010).
For IBR calculation, the hierarchy of biological organisation of biomarkers from the subcellular to individual level, i.e. SOD, CAT, GPx, GSH and MDA, was used for their clockwise arrangement (Serafim et al. 2012).
Results
Determination of LD50 of NG
Twenty-four-hour LD50 of NG on L. rohita was found to be more than 5000 mg/kg based on the ‘Acute toxic class method’ (Fig. 1) performed according to OECD toxicological protocols. Accordingly, NG was considered to be safe up to 5000-mg/kg dose for the fish and ranked at level five (Organization for Economic Cooperation and Development 2001).
Oxidative stress biomarkers
No mortality was observed during the course of chronic toxicity tests. Furthermore, in general, non-significant differences in the activities of antioxidant enzymes and levels of GSH and MDA were observed in the three tissues of fish administered with NG alone in comparison to control. The numerical data of all the biomarkers in the form of mean ± SE is provided in the supplementary material (Table S2-S7).
SOD activity
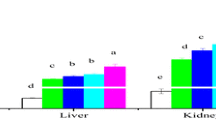
The SOD activity in the liver, gills and kidney of L. rohita upon treatment with CdCl2 and NG is depicted in Fig. 2(A). A concentration-dependent enhancement in SOD activity in the gills, liver and kidney was noticed in CdCl2-exposed groups on the 15th day in comparison to corresponding control. Afterwards, the decreasing trend in SOD activity in all tissues exposed to both the concentrations was observed and on 60th day, SOD activity reached below control. However on the 30th day, fish exposed to lower concentration of CdCl2 revealed increase in activity of hepatic SOD. The results also showed the maximum inhibition of the SOD activity (45.79%) in the gills of fish exposed to CdCl2 (at higher concentration) on the 60th day of exposure (Table S2). Co-administration of NG to fish exposed to CdCl2 (at both concentrations) revealed significant (p < 0.05) reduction in the activity of SOD in the three tissues at the 15th day as compared with CdCl2 alone treated groups. Further on the 30th and 60th day, treatment with NG and CdCl2 (at both concentrations) provided a marked normalisation (p < 0.05) of SOD activity in the three tissues. However, gills of fish co-treated with NG and higher concentration of the CdCl2 on the 60th day showed significant (p < 0.05) lesser enzyme activity in comparison to the control. Thus, indicating that NG supplementation in fish intoxicated with higher concentration of CdCl2 could not completely normalise the SOD activity in the gills on the 60th day.
Activity of SOD (A) and CAT (B) in the gills, liver and kidney of L. rohita exposed to CdCl2 and orally administered with NG at different sampling days. Data are presented as mean ± SE on six individual estimations. Statistical significance is considered at p < 0.05 determined by one-way ANOVA followed by Tukey’s post hoc test. ‘a’: significant difference with respect to control; ‘b’: significant difference CdCl2 (0.37 mg/L) + NG with respect to CdCl2 (0.37 mg/L); ‘c’: significant difference CdCl2 (0. 62 mg/L) + NG with respect to CdCl2 (0.62 mg/L)
CAT activity
The effects of CdCl2 and NG treatment alone and in combination, on CAT activity in the gills, liver and kidney of L. rohita, are displayed in Fig. 2(B). Exposure to low concentration of CdCl2 resulted in increased CAT activity in the gills and kidney on the 15th and 30th day followed by a sharp decline on the 60th day. However, the liver showed increase in CAT activity throughout the experiment. Administration of NG along with CdCl2 exposure restored the CAT activity in the three tissues.
Upon exposure to higher concentration of CdCl2, CAT activity revealed significant (p < 0.05) elevation in the gills (103.61%), liver (88.64%) and kidney (71.91%) on the 15th day; afterwards, a decreasing trend in its activity was observed with maximum decrease of 40.06% in the gills on the 60th day (Table S3). On the contrary, NG treatment along with CdCl2 restored the altered levels of CAT activity in the three tissues but these significantly differ from the control levels.
GST activity
The GST activity in the gills, liver and kidney of the fish treated with CdCl2 and NG is shown in Fig. 3(A). Non-significant alteration in GST activity was noted in both the gills and kidney throughout the experiment. On the other hand, GST activity in the liver was significantly altered by both the concentrations of CdCl2. Exposure to lower concentration of CdCl2 showed significant (p < 0.05) time-dependent increase in GST activity by 29.93% (15th day) to 44.37% (60th day) in comparison to control. Co-treatment of NG and CdCl2 (low concentration) significantly (p < 0.05) reduced the GST activity at each sampling day (Table S4). Exposure to CdCl2 at higher concentration significantly (p < 0.05) elevated hepatic GST activity by 48.24% and 72.27% on 15th and 30th day, respectively. Subsequently, marked inhibition of 30.98% in hepatic GST activity over control was seen on 60th day of exposure. However, the combined treatment with NG and CdCl2 (higher concentration) resulted in significant reduction in GST activity on the 15th and 30th day, as compared to the CdCl2-treated group thereby normalising the enzyme activity near the control, while on the 60th day NG supplementation increased the CdCl2 (0.62 mg/L)-induced fall in hepatic GST activity.
Activity of GST (A) and GPx (B) in the gills, liver and kidney of L. rohita exposed to CdCl2 and orally administered with NG at different sampling days. Data are presented as mean ± SE on six individual estimations. Statistical significance is considered at p < 0.05 determined by one-way ANOVA followed by Tukey’s post hoc test. ‘a’: significant difference with respect to control. ‘b’: significant difference CdCl2 (0.37 mg/L) + NG with respect to CdCl2 (0.37 mg/L). ‘c’: significant difference CdCl2 (0. 62 mg/L) + NG with respect to CdCl2 (0.62 mg/L)
GPx activity
Figure 3(B) depicts the effects of CdCl2 and NG treatment alone and in combination on GPx activity in the gills, liver and kidney of L. rohita. At lower concentration of CdCl2, elevation in GPx activity over control in all three tissues was observed with maximum increase of 35% in the gills followed by 31.97% in the kidney and 20.93% in the liver on the 60th day (Fig. 3(B)). However, the combined treatment of NG with CdCl2 (lower concentration) significantly (p < 0.05) decreased the GPx activity to near control levels. Conversely, groups exposed to higher concentration of CdCl2 revealed enhancement in GPx activity by 43.71%, 43.98% and 39.98% in the gills, liver and kidney, on the 15th day, respectively (Table S5). Afterwards, rapid depletion in GPx activity was observed in the gills and kidney whereas the liver showed increased GPx activity on the 30th day over control. On the other hand, combined treatment of NG and CdCl2 (higher concentration) provided significant (p < 0.05) restoration of altered GPx activity in the three tissues.
GSH level
The effects of CdCl2 and NG treatment on GSH levels of the gills, liver and kidney of L. rohita are displayed in Fig. 4(A). Exposure to CdCl2 revealed significant (p < 0.05) concentration-dependent depletion in GSH levels in the three tissues with respect to control. Gills showed some stability in GSH content at lower concentration of CdCl2 which was significantly restored to control levels by NG administration. At higher CdCl2 concentration, a sharp decline in GSH content was observed overtime. The maximum decrease of 48.50% was recorded on the 60th day in the gills of fish exposed to higher concentration of CdCl2 over corresponding control (Fig. 4(A)). On the contrary, co-treatment of CdCl2 and NG showed increase in GSH content but the levels were not completely restored to near control groups. A much slower non-significant decrease in GSH content was observed in the liver at lower Cd concentration, whereas at higher concentration it decreased initially followed by an increase on the 30th day and then again got depleted (by 10.37%) on the 60th day (Table S6). NG administration completely rescued GSH content in the liver. The kidney showed significant depletion of GSH content at both concentrations in comparison to the respective control groups which was softened by NG.
GSH (A) and MDA (B) content in the gills, liver and kidney of L. rohita exposed to CdCl2 and orally administered with NG at different sampling days. Data are presented as mean ± SE on six individual estimations. Statistical significance is considered at p < 0.05 determined by one-way ANOVA followed by Tukey’s post hoc test. ‘a’: significant difference with respect to control. ‘b’: significant difference CdCl2 (0.37 mg/L) + NG with respect to CdCl2 (0.37 mg/L). ‘c’: significant difference CdCl2 (0. 62 mg/L) + NG with respect to CdCl2 (0.62 mg/L)
MDA levels
Figure 4(B) shows the exposure to CdCl2 resulted in concentration and duration-dependent augmentation of MDA levels in three tissues of fish. The maximum MDA level (82.47%) was recorded in the gills followed by kidney (71.87%) and liver (68%) of fish exposed to higher concentration of CdCl2 on the 60th day. Co-treatment of NG and CdCl2 significantly (p < 0.05) depleted MDA levels in the three tissues (Table S7). However, in the kidney, although co-treatment of NG with higher CdCl2 concentration showed reduction in MDA levels, these could not be restored near the control groups.
Integrated biomarker response
The IBR index was applied to the six investigated biomarkers in the gills, liver and kidney of L. rohita and the findings are displayed as star plots in Fig. 5. The IBR value shows that higher concentration of CdCl2 caused severe toxicity in L. rohita accompanied with higher values of IBR on the 60th day in all tissues. A concentration-dependent elevation in the IBR values was registered in the three tissues of CdCl2-exposed fish on the 15th day, which further decreased on the 30th day of the exposure except in the liver. However, on the 60th day of exposure to CdCl2 (both concentrations), a marked increase in IBR values in the gills (Fig. 5A) and kidney (Fig. 5C) was noted while the liver showed slight decrease in IBR values. Meanwhile, a remarkable decrease in IBR values was detected in the three tissues of fish treated with NG along with CdCl2 exposure. Based on the IBR values (Fig. 5D), it is apparent that the most impacted organ due to Cd toxicity is the gills with a maximum IBR value of 37.62, on the 60th day of exposure to CdCl2 (at higher concentration). On this basis, the organs can be ranked in terms of impact by Cd toxicity as gills > kidney > liver.
Discussion
In this study, protective efficacy of NG on CdCl2-mediated oxidative stress has been assessed in different tissues of L. rohita. Several researchers have documented the beneficial effects of flavonoids against the toxicity induced by various heavy metals in experimental animals. However, there is paucity of available scientific literature related to the effects of NG against the deleterious effects instigated due to Cd intoxication in fish. The present study is the first attempt to analyse the potential of NG to alleviate the toxicity caused by Cd in L. rohita through integrated response of oxidative stress biomarkers. These biomarkers such as GST, GPx, SOD and CAT form an integral components of antioxidant defence system of an organism to provide protection against oxidative stress induced by xenobiotics and are often employed as indicators of Cd toxicity (Asagba et al. 2008; Ural 2013; McRae et al. 2018). SOD, a group of metalloenzymes, catalyses scavenging of superoxide anion radicals and converts them into molecular oxygen and H2O2 (Liu et al. 2020) while CAT metabolises H2O2 into oxygen and water (Pandey et al. 2008). GPx is a selenoenzyme that scavenges the H2O2 by catalysing the oxidation of GSH to GSSG (oxidised glutathione), while GST belongs to a family of detoxifying enzymes involved in cellular detoxification. GST catalyses conjugation of GSH with electrophilic compounds (both endogenous and exogenous), thereby facilitating their removal from the organism (Dringen 2000).
In our study, all the three tissues showed significant concentration-dependent increase in SOD and CAT activity up to 15th day of CdCl2 exposure. These findings agree with the previous observations of Asagba et al. (2008), Abdel-Rahim et al. (2014), McRae et al. (2019) and Naik et al. (2020). The enhanced SOD and CAT activity might be an adaptive mechanism of Cd-exposed animals in order to reduce oxygen radicals. Furthermore, increased exposure to CdCl2, i.e. up to 60 days, caused inhibition in the both SOD and CAT activities of three tissues. Our results are supported by Basha and Rani (2003) who reported initial increase and then later inhibition of SOD and CAT activity in Oreochromis mossambicus treated with sublethal concentration of CdCl2. This reduction in SOD and CAT activity might be attributed to overproduction of ROS by long-term Cd exposure (El-Boshy et al. 2015). SOD exists in the form of CuZnSOD (mainly present in cytosol, nucleus and peroxisomes) and MnSOD (present in mitochondria) (Arroyo et al. 2012). Alternately, Cd causes structural instability in the enzyme by substituting manganese in MnSOD (Casalino et al. 2002) and zinc in CuZnSOD either by direct interaction between Cd and Zn via competitive inhibition or by alternative biological pathway (Huang et al. 2006). Cd-induced inhibition in CAT activity might also be attributed to peroxidative damage in the tissues, as indicated by increased lipid peroxidation observed in our study. Alternately, the metal ions may directly bind to thiol groups of CAT and thus inhibit its activity (Atli and Canli 2010).
Significant decline in SOD and CAT activities of the investigated tissues of fish co-treated with CdCl2 and NG on the 15th day may be ascribed to the potential of NG to scavenge free radical (van Acker et al. 2000). However on the 60th day, fish exposed to CdCl2 along with NG administration showed appreciable enhancement in the activities of SOD and CAT in relation to the metal-exposed groups. This restoration of enzyme activities might be attributed to the potential of NG to limit the accretion of free radicals generated by Cd exposure (Gnanasoundari and Pari 2006; Wang et al. 2012). Our results are supported by the findings of other authors who also observed alleviation of hepatic SOD and CAT activities by NG in Cd (Renugadevi and Prabu 2010)- and arsenic (Mershiba et al. 2013)-intoxicated animals.
GPx also increased in the three tissues of Cd-intoxicated fish (15th day) which might be to compensate the higher level of H2O2. Similar increase in GPx activity has also been reported in Cyprinus carpio treated with heavy metals, thereby suggesting the enhanced GPx activity as an indicative of its role in preventing the metal-induced lipid peroxidation and oxidative damage (Vinodhini and Narayanan 2009). Enhanced activity of hepatic GST was observed in CdCl2-exposed fish due to the pivotal involvement of liver in detoxification of xenobiotics. Our results substantiate the suitability of liver as indicator in determining the response of GST to metal intoxication in studies related to environmental monitoring. Raised levels of GST may reveal that the fish attempts to reduce metal-induced stress by conjugating GSH to metals, hence decreasing their concentration (Vieira et al. 2009). Conversely, decline in GPx and GST activity in the tissues upon prolonged exposure (60th day) to CdCl2 may be due to the inefficiency of tissues in neutralising the impact of peroxides, and also due to the glutathione depletion upon prolonged exposure as noticed in our study. Similar reduction in GPx activity has also been observed by Ognjanovic et al. (2008), Messaoudi et al. (2009) and Jamakala and Rani (2015) in the kidneys and liver of Cd-intoxicated animals. Treatment of NG in CdCl2-exposed fish significantly restored altered levels of GPx and GST, suggesting the antioxidative potential of NG. This may be explained by the potential of NG to scavenge free radicals and hence alleviates the excess consumption of endogenous non-enzymatic antioxidants by the metal. These findings are also corroborated by Gnanasoundari and Pari (2006) and Ozkaya et al. (2016).
In the present study, significant depletion in GSH levels was recorded throughout the experimental period except elevated hepatic GSH on the 30th day. A possible explanation for the reduction is high consumption of reduced GSH by GST in detoxifying mechanisms or by GPx in reducing lipid hydroperoxides (Banerjee et al. 1999). The binding of Cd tightly to thiol groups may also result in GSH depletion (Pari and Murugavel 2005; Liu et al. 2009). These results agree with Newairy et al. (2007) and Goodarzi et al. (2020) who also found reduction in glutathione level in experimental animals intoxicated with Cd (Newairy et al. 2007; Goodarzi et al. 2020). Oral administration of NG in Cd-exposed fish restored the levels of GSH in the three tissues, which might be due to the role of NG in scavenging the free radicals and thus preventing thiol group oxidation. Moreover, the metal-chelating potential of NG augments Cd removal, inhibits iron-dependent Fenton reaction and thus reduces the consumption of non-enzymatic antioxidants (Cheng and Breen 2000).
It is interesting to note that hepatic GSH level significantly elevated on the 30th day of exposure to higher concentration of CdCl2. Additionally, increased activity of GST and GPx was also found on the same day. These variations showed development of an adaptive mechanism in response to the metal intoxication, by strengthening antioxidative ability of liver with increased rate of GSH synthesis. Consequently, on the 60th day of exposure to CdCl2, depletion in both GSH and GST was observed that indicate progressive weakening and loss of ability of tissue to respond with continuing Cd exposure (Zirong and Shijun 2007).
Lipid peroxidation is an important mechanism of cell injury which involve broad spectrum of alterations in cell triggered by free radicals and intermediate derivatives of peroxidation (Prabu et al. 2011b). MDA, a major product of peroxidation, can actively react with a variety of cellular components, and hence, impair the structural and physiological integrity of membranes (Valavanidis et al. 2006). Enhanced levels of MDA in the liver, gills and kidney of CdCl2-treated fish are in line with the previous findings (Shimada et al. 2004; Dabas et al. 2012; El-Boshy et al. 2015; Verma et al. 2020). Cd imparts inhibitory effects on mitochondrial electron transfer by biding to thiols and thus generates ROS which causes peroxidative damage in the tissue (Dorta et al. 2003; Heyno et al. 2008). Reduction in antioxidant enzymes might be the major cause of enhanced lipid peroxidation as observed in the current study (El-Boshy et al. 2015). Cd intoxication has also been suggested to induce phagocytes for ROS production (Stohs and Bagachi 1995). Alternately, Cd has been believed to displace iron from its binding sites resulting in increased generation of ROS via Fenton reaction, ultimately leading to extensive lipid peroxidation (Casalino et al. 1997). As a result, free radicals and lipid peroxidation cause extensive cellular changes by imparting oxidative damage to intracellular molecules (DNA, proteins and lipids) and cross-linking and polymerisation of membrane components. This leads to destabilisation and disintegration of cell membrane structure and function thereby inducing many pathological and physiological changes in the tissues (Gibson, 2005; El-Boshy et al. 2015). Amongst the investigated tissues, gills being in immediate contact with Cd in ambient environment and possess poor ability to scavenge ROS, and therefore showed maximum MDA level upon Cd exposure (Dabas et al. 2012). Our study showed simultaneous administration of NG in fish exposed to CdCl2 significantly blunted the increased MDA levels in the tissues, which might be attributed to antilipoperoxidative ability of NG (Lee et al. 2004). Similar restoration of MDA level upon administration of NG was also documented in arsenic (Jain et al. 2011) and lead acetate (Ozkaya et al. 2016)–intoxicated animals. Another possible reason for reduction in MDA level by NG may be its lipophilic nature which enables the attachment of NG to phospholipid bilayer, thereby reducing generation of free radicals and stabilising plasma membrane (Honohan et al. 1976).
The IBR analysis which eases in visualising the biological effects of various biomarkers substantiates the above results indicating the induction of an early detoxification mechanism in L. rohita in response to oxidative stress generated by CdCl2 exposure. IBR analysis clearly differentiated the damage with respect to oxidative stress provoked due to long-term Cd exposure to the three tissues viz. gills, liver and kidney. Amongst them, the liver was observed to be the least affected organ by Cd-mediated toxicity which might be due to the tendency of the liver to synthesise metallothioneins (protein that binds to non-essential metals for sequestration) rapidly thereby eliminating Cd efficiently (Capaldo et al. 2016). Contrarily, gills and kidney were more influenced by Cd exposure, which may be due to the fact that gills are the sensitive respiratory organ and the first point of contact with waterborne Cd, while kidneys are inefficiently equipped to metabolise Cd and therefore being the final destination for Cd accumulation (Asagba et al. 2008). The study showed reduction of enhanced IBR values in groups co-treated with NG and CdCl2 as compared to CdCl2 alone. In the light of these findings, IBR analysis further substantiated the protective role of NG. It has been apparent from star plot of IBR that NG is effective against lower concentration of CdCl2 than higher concentration of CdCl2, indicating that NG can be useful to maintain the redox homeostasis under low to moderate oxidative stress but it fails to do so if tissues are under tremendous oxidative stress as in case of higher concentration of CdCl2. These findings reinforce that integrated biomarker response is a valuable tool for visualising general stress response of fish exposed to Cd than individual stress biomarkers (Qu et al. 2014) and surveying the toxicological effects of CdCl2 on fish.
The current study revealed that the short-term exposure (15 days) to CdCl2 at relatively low concentrations resulted in escalation of SOD, CAT and GPx activities, thus reflecting the development of compensatory response against Cd-mediated oxidative stress. Further increase in exposure to CdCl2 (up to 30th day) showed decrease in their activity which may reflect the acclimatisation of the fish to the pollutant in its environment. Conversely, with further increase in exposure period (60 days), the results showed a decline in antioxidant enzyme activities below the control level, revealing the inhibition of these enzymes by CdCl2 or inability of the organism to produce these enzymes which may be due to tissue damage. This is validated with increased lipid peroxidation in all three tissues and also supported by findings of IBR analysis.
Conclusions
Exposure to CdCl2 in the present study showed biphasic profile of antioxidant defence system in L. rohita, i.e. induction in SOD, CAT, GPx and GST activities in the first phase (short-term exposure) and then progressive loss due to the oxidative stress in the second phase (long-term exposure), which was further supported by integrated biomarker response analysis. Consequently, oral administration of NG along with CdCl2 exposure appreciably alleviated Cd-induced alterations in enzymatic and non-enzymatic antioxidants levels, thereby restoring the oxidative stress biomarker levels to near normal. Gills were found to be the most affected organ by Cd-induced toxicity, as indicated by IBR analysis. All these findings together confirmed the hypothetical framework, i.e. NG possesses the ability to alleviate Cd-mediated oxidative stress and facilitates restoration of oxidant-antioxidant status in L. rohita. The data concerning the long-term toxic effects of CdCl2 to L. rohita, thus generated, could be useful for the environmental risk assessment of this metal. Further, the key findings related to the protective efficacy of NG could add to the development of NG as therapeutic option against Cd-induced toxicity. However, the study shows that at higher concentration of CdCl2, NG could not completely restore the parameters to the levels of the control group overtime, thus indicating its inefficiency to maintain redox homeostasis under tremendous oxidative stress. Therefore, further studies are required with different concentrations of the metal and NG doses before using NG as dietary therapeutic molecule for fish and human consumption.
Credit author contribution statement
Sakshi Verma: conceptualization; investigation; methodology; formal analysis; writing—original draft preparation; visualisation. Rajinder Jindal: supervision, resources, methodology, conceptualization, project administration. Smriti Batoye: methodology, investigation.
Data availability
All data generated or analysed during this study are included in this published article.
References
Abdel-Rahim EA, Abdel-Mobdy YE, Ali RF, Mahmoud HA (2014) Hepatoprotective effects of Solanum nigrum Linn fruits against cadmium chloride toxicity in albino rats. Biol Trace Elem Res 160(3):400–408
Abougabal K, Moselhy WA, Korni FM (2020) The effect of cadmium toxicity on Oreochromis niloticus and human health. Afr J Aquat Sci 45(3):303–309
Amaro MI, Rocha J, Vila-Real H, Eduardo-Figueira M, Mota-Filipe H, Sepodes B, Ribeiro MH (2009) Anti-inflammatory activity of naringin and the biosynthesised naringenin by naringinase immobilized in microstructured materials in a model of DSS-induced colitis in mice. Food Res Int 42(8):1010–1017
Arroyo VS, Flores KM, Ortiz LB, Gómez-Quiroz LE, Gutiérrez-Ruiz MC (2012) Liver and cadmium toxicity. J Drug Metab Toxicol S5:1–7
Asagba SO, Eriyamremu GE, Igberaese ME (2008) Bioaccumulation of cadmium and its biochemical effect on selected tissues of the catfish (Clarias gariepinus). Fish Physiol Biochem 34(1):61–69
Atli G, Canli M (2010) Response of antioxidant system of freshwater fish Oreochromis niloticus to acute and chronic metal (Cd, Cu, Cr, Zn, Fe) exposures. Ecotoxicol Environ Saf 73:1884–1889
Aulakh MS, Khurana MP, Singh D (2009) Water pollution related to agricultural, industrial, and urban activities, and its effects on the food chain: case studies from Punjab. J New Seeds 10(2):112–137
Banerjee BD, Seth V, Bhattacharya A, Pasha ST, Chakraborty AK (1999) Biochemical effects of some pesticides on lipid peroxidation and free-radical scavengers. Toxicol Lett 107(1–3):33–47
Basha PS, Rani AU (2003) Cadmium-induced antioxidant defense mechanism in freshwater teleost Oreochromis mossambicus (Tilapia). Ecotoxicol Environ Saf 56(2):218–221
Beliaeff B, Burgeot T (2002) Integrated biomarker response: a useful tool for ecological risk assessment. Environ Toxicol Chem 21(6):1316–1322
Bhardwaj JK, Panchal H (2021) Quercetin mediated attenuation of cadmium-induced oxidative toxicity and apoptosis of spermatogenic cells in caprine testes in vitro. Environ Mol Mutagen 62(6):374–384
Bhardwaj JK, Panchal H, Saraf P (2021) Cadmium as a testicular toxicant: a review. J Appl Toxicol 41(1):105–117
Bhardwaj R, Gupta A, Garg JK (2017) Evaluation of heavy metal contamination using environmetrics and indexing approach for River Yamuna, Delhi stretch. India Water Sci 31(1):52–66
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. In: Fleischer S, Packer L (eds) Methods in enzymology. Academic Press, pp 302–310
Capaldo A, Gay F, Scudiero R, Trinchella F, Caputo I, Lepretti M, Marabotti A, Esposito C, Laforgia V (2016) Histological changes, apoptosis and metallothionein levels in Triturus carnifex (Amphibia, Urodela) exposed to environmental cadmium concentrations. Aquat Toxicol 173:63–73
Casalino E, Calzaretti G, Sblano C, Landriscina C (2002) Molecular inhibitory mechanisms of antioxidant enzymes in rat liver and kidney by cadmium. Toxicology 179(1–2):37–50
Casalino E, Cesare S, Clemente L (1997) Enzyme activity alteration by cadmium administration to rats: the possibility of iron involvement in lipid peroxidation. Arch Biochem Biophys 346(2):171–179
Çavaş T, Ergene-Gözükara S (2005) Induction of micronuclei and nuclear abnormalities in Oreochromis niloticus following exposure to petroleum refinery and chromium processing plant effluents. Aquat Toxicol 74(3):264–271
Cheng IF, Breen K (2000) On the ability of four flavonoids, baicilein, luteolin, naringenin, and quercetin, to suppress the Fenton reaction of the iron-ATP complex. Biometals 13(1):77–83
Choi JS, Park KY, Moon SH, Rhee SH, Young HS (1994) Antimutagenic effect of plant flavonoids in the Salmonella assay system. Arch Pharm Res 17(2):71–75
Cobbina SJ, Chen Y, Zhou Z, Wu X, Zhao T, Zhang Z, Feng W, Wang W, Li Q, Wu X, Yang L (2015) Toxicity assessment due to sub-chronic exposure to individual and mixtures of four toxic heavy metals. J Hazard Mater 294:109–120
Dabas A, Nagpure NS, Kumar R, Kushwaha B, Kumar P, Lakra WS (2012) Assessment of tissue-specific effect of cadmium on antioxidant defense system and lipid peroxidation in freshwater murrel Channa Punctatus. Fish Physiol Biochem 38(2):469–482
Danabas D, Yildirim NC, Yildirim N, Onal AO, Uslu G, Unlu E, Danabas S, Ergin C, Tayhan N (2015) Changes in antioxidant defense system in gills of Capoeta umbla caught from Uzuncayir Dam Lake Turkey. Biochem Syst Ecol 63:72–79
Dorta DJ, Leite S, DeMarco KC, Prado IM, Rodrigues T, Mingatto FE, Uyemura SA, Santos AC, Curti C (2003) A proposed sequence of events for cadmium-induced mitochondrial impairment. J Inorg Biochem 97(3):251–257
Dringen R (2000) Glutathione metabolism and oxidative stress in neurodegeneration. Eur J Biochem 267(16):4903–4903
El-Boshy ME, Risha EF, Abdelhamid FM, Mubarak MS, Hadda TB (2015) Protective effects of selenium against cadmium induced hematological disturbances, immunosuppressive, oxidative stress and hepatorenal damage in rats. J Trace Elem Med Biol 29:104–110
El-Ghasham A, Mehana EE, Abdel-Reheem M (2008) Evaluation of lead and cadmium levels in freshwater fish farms at Qassim region KSA. J Agric Vet Sci 1(2):59–69
Fernández-Luqueño F, López-Valdez F, Gamero-Melo P, Luna-Suárez S, Aguilera-González EN, Martínez AI, García-Guillermo MD, Hernández-Martínez G, Herrera-Mendoza R, Álvarez-Garza MA, Pérez-Velázquez IR (2013) Heavy metal pollution in drinking water-a global risk for human health: a review. Afr J Environ Sci Technol 7(7):567–584
Gad SC (2014) Cadmium. In: Wexler P (ed) Encyclopedia of toxicology. Academic Press, Oxford, pp 613–616
GHS (2013) Globally harmonized system of classification and labelling of chemicals (GHS), Fifth Revised Edition, UN New York and Geneva. http://www.unece.org/trans/danger/publi/ghs/ghs_rev05/05files_e.html. Accessed 10 Feb 2020
Gibson BW (2005) The human mitochondrial proteome: oxidative stress protein modifications and oxidative phosphorylation. J Biochem Cellbiol 37:927–934
Gnanasoundari M, Pari L (2006) Impact of naringenin on oxytetracycline-mediated oxidative damage in kidney of rats. Ren Fail 28:599–605
Goodarzi Z, Karami E, Yousefi S, Dehdashti A, Bandegi AR, Ghanbari A (2020) Hepatoprotective effect of atorvastatin on cadmium chloride induced hepatotoxicity in rats. Life Sci 254:117770
Guerlet E, Vasseur P, Giambérini L (2010) Spatial and temporal variations of biological responses to environmental pollution in the freshwater zebra mussel. Ecotoxicol Environ Saf 73(6):1170–1181
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases the first enzymatic step in mercapturic acid formation. J Biol Chem 249(22):7130–7139
Heyno E, Klose C, Krieger-Liszkay A (2008) Origin of cadmium-induced reactive oxygen species production: mitochondrial electron transfer versus plasma membrane NADPH oxidase. New Phytol 179(3):687–699
Honohan T, Hale RL, Brown JP, Wingard RE (1976) Synthesis and metabolic fate of hesperetin-3-14C. J Agric Food Chem 24(5):906–911
Huang B, Hu P, Hu A, Li Y, Shi W, Huang J, Jiang Q, Xu S, Li L, Wu Q (2019) Naringenin attenuates carotid restenosis in rats after balloon injury through its anti-inflammation and anti-oxidative effects via the RIP1-RIP3-MLKL signaling pathway. Eur J Pharmacol 855:167–174
Huang YH, Shih CM, Huang CJ, Lin CM, Chou CM, Tsai ML, Liu TP, Chiu JF, Chen CT (2006) Effects of cadmium on structure and enzymatic activity of Cu, Zn-SOD and oxidative status in neural cells. J Cell Biochem 98(3):577–589
Idrees N, Tabassum B, Abd Allah EF, Hashem A, Sarah R, Hashim M (2018) Groundwater contamination with cadmium concentrations in some West UP Regions India. Saudi J Biol Sci 25(7):1365–1368
Jain A, Yadav A, Bozhkov AI, Padalko VI, Flora SJS (2011) Therapeutic efficacy of silymarin and naringenin in reducing arsenic–induced hepatic damage in young rats. Ecotoxicol Environ Saf 74(4):607–614
Jamakala O, Rani UA (2015) Amelioration effect of zinc and iron supplementation on selected oxidative stress enzymes in liver and kidney of cadmium-treated male albino rat. Toxicol Int 22(1):1–9
Jindal R, Verma S (2015) In vivo genotoxicity and cytotoxicity assessment of cadmium chloride in peripheral erythrocytes of Labeo rohita (Hamilton). Ecotoxicol Environ Saf 118:1–10
Kapoor R, Kakkar P (2014) Naringenin accords hepatoprotection from streptozotocin induced diabetes in vivo by modulating mitochondrial dysfunction and apoptotic signaling cascade. Toxicol Rep 1:569–581
Karaytug S, Sevgiler Y, Karayakar F (2014) Comparison of the protective effects of antioxidant compounds in the liver and kidney of Cd-and Cr-exposed common carp. Environ Toxicol 29(2):129–137
Kono Y (1978) Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch Biochem Biophys 186(1):189–195
Kumar P, Singh A (2010) Cadmium toxicity in fish: an overview. GERF Bull Biosci 1(1):41–47
Lee CH, Jeong TS, Choi YK, Hyun BH, Oh GT, Kim EH, Kim JR, Han JI, Bok SH (2001) Anti-atherogenic effect of citrus flavonoids, naringin and naringenin, associated with hepatic ACAT and aortic VCAM-1 and MCP-1 in high cholesterol-fed rabbits. Biochem Biophys Res Commun 284(3):681–688
Lee MH, Yoon S, Moon JO (2004) The flavonoid naringenin inhibits dimethylnitrosamine-induced liver damage in rats. Biol Pharm Bull 27(1):72–76
Liu H, Lai W, Liu X, Yang H, Fang Y, Tian L, Li K, Nie H, Zhang W, Shi Y, Bian L (2020) Exposure to copper oxide nanoparticles triggers oxidative stress and endoplasmic reticulum (ER)-stress induced toxicology and apoptosis in male rat liver and BRL-3A cell. J Hazard Mater 401(123349):1–12
Liu J, Qu W, Kadiiska MB (2009) Role of oxidative stress in cadmium toxicity and carcinogenesis. Toxicol Appl Pharmacol 238(3):209–214
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Luck H (1965) Catalase. In: Bergmeyer HU (ed) Methods of enzymatic analysis. Academic Press, New York, pp 885–894
McRae NK, Gaw S, Brooks BW, Glover CN (2019) Oxidative stress in the galaxiid fish, Galaxias maculatus, exposed to binary waterborne mixtures of the pro-oxidant cadmium and the anti-oxidant diclofenac. Environ Pollut 247:638–646
McRae NK, Gaw S, Glover CN (2018) Effects of waterborne cadmium on metabolic rate, oxidative stress, and ion regulation in the freshwater fish, inanga (Galaxias maculatus). Aquat Toxicol 194:1–9
Mershiba SD, Dassprakash MV, Saraswathy SD (2013) Protective effect of naringenin on hepatic and renal dysfunction and oxidative stress in arsenic intoxicated rats. Mol Biol Rep 40(5):3681–3691
Messaoudi I, El Heni J, Hammouda F, Saïd K, Kerkeni A (2009) Protective effects of selenium, zinc, or their combination on cadmium-induced oxidative stress in rat kidney. Biol Trace Elem Res 130(2):152–161
Mohandas J, Marshall JJ, Duggin GG, Horvath JS, Tiller DJ (1984) Low activities of glutathione-related enzymes as factors in the genesis of urinary bladder cancer. Cancer Res 44(11):5086–5091
Moron MS, Depierre JW, Mannervik B (1979) Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta 582(1):67–78
Naik AP, Shyama SK, D’Costa AH (2020) Evaluation of genotoxicity, enzymatic alterations and cadmium accumulation in Mozambique tilapia Oreochromis mossambicus exposed to sub lethal concentrations of cadmium chloride. Environ Chem Ecotoxicol 2:126–131
Newairy AA, El-Sharaky AS, Badreldeen MM, Eweda SM, Sheweita SA (2007) The hepatoprotective effects of selenium against cadmium toxicity in rats. Toxicology 242:23–30
Ognjanovic BI, Markovic SD, Pavlovic SZ, Zikic RV, Stajn AS, Saicic ZS (2008) Effect of chronic cadmium exposure on antioxidant defense system in some tissues of rats: protective effect of selenium. Physiol Res 57(3):403–411
Organization for Economic Cooperation and Development (2001) OECD guidelines for testing of chemicals. Guideline 423: Acute Oral Toxicity-Acute Toxic Class Method. Adopted 17 December 2001, OECD, Paris. https://ntp.niehs.nih.gov/iccvam/suppdocs/feddocs/oecd/oecd_gl423.pdf (accessed 25 October 2019)
Ozkaya A, Sahin Z, Dag U, Ozkaraca M (2016) Effects of naringenin on oxidative stress and histopathological changes in the liver of lead acetate administered rats. J Biochem Mol Toxicol 30:243–248
Pandey S, Parvez S, Ansari RA, Ali M, Kaur M, Hayat F, Ahmad F, Raisuddin S (2008) Effects of exposure to multiple trace metals on biochemical, histological and ultrastructural features of gills of a freshwater fish Channa Punctata Bloch. Chem Biol Interact 174(3):183–192
Pari L, Murugavel P (2005) Role of diallyl tetrasulfide in ameliorating the cadmium induced biochemical changes in rats. Environ Toxicol Pharmacol 20(3):493–500
Pisoschi AM, Pop A (2015) The role of antioxidants in the chemistry of oxidative stress: a review. Eur J Med Chem 97:55–74
Poletta GL, Simoniello MF, Mudry MD (2016) Biomarkers of oxidative damage and antioxidant defense capacity in Caiman latirostris blood. Comp Biochem Physiol C Toxicol Pharmacol 179:29–36
Prabu SM, Shagirtha K, Renugadevi J (2011a) Quercetin in combination with vitamins (C and E) improve oxidative stress and hepatic injury in cadmium intoxicated rats. Biomed Prev Nutr 1(1):1–7
Prabu SM, Shagirtha K, Renugadevi J (2011b) Naringenin in combination with vitamins C and E potentially protects oxidative stress-mediated hepatic injury in cadmium-intoxicated rats. J Nutr Sci Vitaminol 57(2):177–185
Priscilla DH, Jayakumar M, Thirumurugan K (2015) Flavanone naringenin: An effective antihyperglycemic and antihyperlipidemic nutraceutical agent on high fat diet fed streptozotocin induced type 2 diabetic rats. J Funct Foods 14:363–373
Prusty AK, Kohli MPS, Sahu NP, Pal AK, Saharan N, Mohapatra S, Gupta SK (2011) Effect of short term exposure of fenvalerate on biochemical and haematological responses in Labeo rohita (Hamilton) fingerlings. Pest Biochem Phys 100(2):124–129
Qu R, Wang X, Wang Z, Wei Z, Wang L (2014) Metal accumulation and antioxidant defenses in the freshwater fish Carassius auratus in response to single and combined exposure to cadmium and hydroxylated multi-walled carbon nanotubes. J Hazard Mater 275:89–98
Rajendran P, Nandakumar N, Rengarajan T, Palaniswami R, Gnanadhas EN, Lakshminarasaiah U, Gopas J, Nishigaki I (2014) Antioxidants and human diseases. Clin Chim Acta 436:332–347
Renugadevi J, Prabu SM (2010) Cadmium-induced hepatotoxicity in rats and the protective effect of naringenin. Exp Toxicol Pathol 62(2):171–181
Serafim A, Company R, Lopes B, Fonseca VF, França S, Vasconcelos RP, Bebianno MJ, Cabral HN (2012) Application of an integrated biomarker response index (IBR) to assess temporal variation of environmental quality in two Portuguese aquatic systems. Ecol Indic 19:215–225
Shimada H, Takamure Y, Shimada A, Yasutake A, Waalkes MP, Imamura Y (2004) Strain differences of cadmium-induced hepatotoxicity in Wistar-Imamichi and Fischer 344 rats: involvement of cadmium accumulation. Toxicology 203(1–3):189–197
Stohs SJ, Bagachi D (1995) Oxidative mechanism in the toxicity of metal ions. Free Radic Biol Med 18:321–336
Tamele IJ, Vázquez Loureiro P (2020) Lead, mercury and cadmium in fish and shellfish from the Indian Ocean and Red Sea (African Countries): Public health challenges. J Mar Sci Eng 8(5):1–33
Ural MŞ (2013) Chlorpyrifos-induced changes in oxidant/antioxidant status and haematological parameters of Cyprinus carpio carpio: ameliorative effect of lycopene. Chemosphere 90(7):2059–2064
Valavanidis A, Vlahogianni T, Dassenakis M, Scoullos M (2006) Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants. Ecotoxicol Environ Saf 64(2):178–189
Valko MMHCM, Morris H, Cronin MTD (2005) Metals, toxicity and oxidative stress. Curr Med Chem 12(10):1161–1208
van Acker FA, Schouten O, Haenen GR, van der Vijgh WJ, Bast A (2000) Flavonoids can replace α-tocopherol as an antioxidant. FEBS Lett 473(2):145–148
Verma Y, Rani V, Rana SVS (2020) Assessment of cadmium sulphide nanoparticles toxicity in the gills of a fresh water fish. Environ Nanotechnol Monit Manag 13:1–7
Vieira LR, Gravato C, Soares AMVM, Morgado F, Guilhermino L (2009) Acute effects of copper and mercury on the estuarine fish Pomatoschistus microps: linking biomarkers to behaviour. Chemosphere 76(10):1416–1427
Vinodhini R, Narayanan M (2009) Biochemical changes of antioxidant enzymes in common carp (Cyprinus carpio L.) after heavy metal exposure. Turk J Vet Anim Sci 33(4):273–278
Waisberg M, Joseph P, Hale B, Beyersmann D (2003) Molecular and cellular mechanisms of cadmium carcinogenesis. Toxicology 192(2–3):95–117
Wang J, Yang Z, Lin L, Zhao Z, Liu Z, Liu X (2012) Protective effect of naringenin against lead-induced oxidative stress in rats. Biol Trace Elem Res 146(3):354–359
Wen B, Jin SR, Chen ZZ, Gao JZ, Liu YN, Liu JH, Feng XS (2018) Single and combined effects of microplastics and cadmium on the cadmium accumulation, antioxidant defence and innate immunity of the discus fish (Symphysodon aequifasciatus). Environ Pollut 243:462–471
Zeng H, Shao B, Zhuang J, Peng Y, Chen H, Yu Q, Xu C, Fu X, Zhou H, Cao Y, Yu X (2020) Naringenin reduces early brain injury in subarachnoid hemorrhage (SAH) mice: The role of the AMPK/SIRT3 signaling pathway. J Funct Foods 72(104043):1–13
Zhang B, Wei YZ, Wang GQ, Li DD, Shi JS, Zhang F (2019) Targeting MAPK pathways by naringenin modulates microglia M1/M2 polarization in lipopolysaccharide-stimulated cultures. Front Cell Neurosci 12(531):1–11
Ziech D, Franco R, Georgakilas AG, Georgakila S, Malamou-Mitsi V, Schoneveld O, Pappa A, Panayiotidis MI (2010) The role of reactive oxygen species and oxidative stress in environmental carcinogenesis and biomarker development. Chem Biol Interact 188(2):334–339
Zirong X, Shijun B (2007) Effects of waterborne Cd exposure on glutathione metabolism in Nile tilapia (Oreochromis niloticus) liver. Ecotoxicol Environ Saf 67(1):89–94
Acknowledgements
The authors are thankful to the Chairperson, Department of Zoology, Panjab University, Chandigarh, for providing the necessary research facility, and DST INSPIRE, New Delhi, India, for providing financial assistance to carry out this work.
Funding
The research was funded by DST INSPIRE, New Delhi, India, as SRF to Sakshi Verma (IF120406).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
The experiments were performed according to the guidelines of Institutional Animal Ethics Committee, Panjab University, Chandigarh (PU/ IAEC/527).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Bruno Nunes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Verma, S., Batoye, S. & Jindal, R. Protective efficacy of naringenin against cadmium-induced redox imbalance in Labeo rohita: an integrated biomarker approach. Environ Sci Pollut Res 29, 25591–25604 (2022). https://doi.org/10.1007/s11356-021-17703-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-17703-z