Abstract
Cadmium (Cd) is known for its many toxic effects on male population such as hypogonadism and fertility difficulties, which are oftenly associated with oxidative stress. As beneficial food, Spirulina(Sp) has been proved efficient against the heavy metal toxicity. This capacity can be associated with its phycobiliproteins (PBP). In this study, the capability of PBP and Sp to treat Cd-induced oxidative damage on the testes and spermatozoa was considered. CD-1 strain mice were orally treated with either Sp or PBP for 10 days prior to single-dose Cd challenge. Sperm quality determinations and testicle histology analysis were performed. Testosterone on serum was measured using enzyme-linked immunosorbent assay (ELISA). Oxidative damage was determined. Antioxidant enzyme activity was analyzed by measuring the activity of super oxide dismutase (SOD), catalase (Cat), and glutathione peroxidase (GpX). The motility and viability of sperm decrease with Cd and improve with PBP and Sp, as the acrosomal reaction (AR) is diminished by PBPs. Testosterone levels decrease due to Cd, and only Sp maintains elevated levels. Cd increases the production of malondialdehyde in the spermatozoa, but not in testes; this production of malondialdehyde in the spermatozoa decreases in the presence of PBP. ROS only decreases with Cd, FBP, and Sp at high concentrations. Advanced oxidative protein products (AOPP) decrease with Cd and PBPs. Cat and GpX increase their activity with Cd and are altered by FBP. Cd produces vascular alterations testes. Within the seminiferous tubule, it produces areas of necrosis and apoptosis, which improve with PBPs and Sp. PBPs have a strong antioxidant activity as they show protective properties against Cd oxidative–induced toxicity on testes and sperm.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd) is a heavy metal mainly used for industrial purposes, mainly in plastics, pigmentation, coating, and batteries. Cd has no known physiological functions, but it exerts various toxic effects even at small doses (Bartosiewicz et al. 2001). It can be absorbed by oral, dermal, and even respiratory routes. It binds to metallothioneins (MT), which act as the main detoxificants as they neutralize the heavy metal. However, due to its slow release and excretion, Cd tends to accumulate both in the liver and the kidneys (Waisberg et al. 2003). Yet another frequently affected organ is the testes, where Cd acts aggressively against the testicular tissue, leading to primary hypogonadism and thus altering spermatozoa (Zhao et al. 2017).
Tissue damage occurs by multiple mechanisms, including oxidative stress, further damaging proteins and DNA (Hartwig et al. 2002; Bertin and Averbeck 2006). Three main mechanisms that lead to oxidative stress are (1) impairment of the activity of antioxidant enzymes, mainly by depleting dehydrogenated glutathione (GSH; Asmuss et al. 2000; Hartwig et al. 2002; Matović et al. 2011); (2) interference in the DNA repair mechanism, especially those in excision and mismatch repair system; and (3) severe damage to organelles including mitochondria and endoplasmic reticulum, inducing apoptosis (Yoshida et al. 1998; Andrews 2000; Asmuss et al. 2000; Thévenod and Lee 2013). Moreover, it has recently been claimed that Cd can damage the DNA chains directly due to its affinity for the N7 centers of adenine and guanine, inducing bifunctional adenine-thymine adducts, which in combination with reactive oxygen species (ROS) can cause mutations (Asmuss et al. 2000; Hartwig et al. 2002; Waisberg et al. 2003).
As mentioned before, Cd affects the male reproductive system, particularly the testicular endothelium, where the heavy metal breaks intercellular junctions, thereby disrupting the testes’ vascular barrier and leading to an inflammatory state, which in turn leads to endocrine alterations and low-quality spermatozoa (Benoff et al. 2009; Adamkovicova et al. 2016). This stress, along with ROS, also affects spermatogenesis and the function of the mobility, concentration, and morphology of mature spermatozoa. This makes Cd one of the main causes of male infertility of unknown origin (Monsefi et al. 2009; Adamkovicova et al. 2016).
Spirulina (Arthrospira) maxima(Sp) is a filamentous cyanobacteria, valued not only for its nutrient composition, which includes high biologic value protein, essential fatty acids, cobalamin, and beta-carotene, but also for its multi-organic protective and anti-toxicological properties (Abdel-Daim et al. 2013, 2016, 2020; Gutiérrez-Salmeán et al. 2015; Bin-Jumah et al. 2021). This particular effect is attributed to its high antioxidant activity, as Sp contains C-phycocyanin(C-PC) within the phycobilisome, where it facilitates photosynthesis in poor light conditions. C-PC is known not only for its use as pigment or food additive but also for its beneficial health effects. C-PC has the capacity to scavenge peroxyl, hydroxyl, and alkoxyl radicals, thus preventing lipid peroxidation; nonetheless, less is known if C-PC by itself demonstrates protective properties against heavy metal intoxication in in vivo models as many of these properties has been performed in vitro assays (Romay et al. 1998, 2003; Liu et al. 2016).
The purpose of this study was to determine the protective effect of a C-PC-rich extract from Sp against Cd intoxication on mice’s testes and spermatozoa. This model allows the evaluation of not only direct injuries produced by Cd but also the derivative oxidative stress, as spermatozoa is known to be particularly sensitive to it, at the same time compare against the protective effect of Sp (Chamorro-Cevallos et al. 2014; Mojica-Villegas et al. 2014; Saez and Drevet 2019; Genchi et al. 2020).
Materials and methods
Phycobiliproteins (PBP)
Extracts were obtained using the method described by Guzmán-Gómez (Vázquez-Sánchez et al. 2009; Chamorro-Cevallos2016; Guzmán-Gómez et al. 2018), with some modifications: on centrifugal tubes, 5 g of powdered Sp (AEH Spiral Spring, Mexico City donation from Alimentos Esenciales para la Humanidad S.A. de C.V.) was suspended in 20 mL phosphate buffer at 20 mmol/L (pH7.4) and then frozen at – 70 °C for at least 2 h; it was then heated at 37 °C for 30 min. In dark condition, the heated suspension was centrifuged for 30 min at 9G (18,000 RPM, JA-17 rotor, Beckman Coulte Co., IN, US), and the supernatant was acquired and separated in a new clean centrifugal tube; this step was performed twice to obtain the PBP extract. The PBP extract was lyophilized and put in storage at – 70 °C.
The optical densities (O.D.) of PBP extract were analyzed at 562, 620, and 652 nm absorbance by spectrophotometry. All spectrophotometry analyses were made using a Shimadzu BioSpec-mini DNA/RNA/Protein UV-visible Spectrophotometer. The equations shown in the Appendix were used to determine the concentration of C-PC, allophycocyanin (APC), and phycoerythrin (PER), respectively, (Boussiba and Richmond 1979).
The purity of CPC and APC in the extract was evaluated with a ratio of A620/A280 for C-PC and A652/A280 for APC. Data are shown in Table 1.
Experimental design
A total of 70 male CD-1 mice strain were obtained from the breeding colony of the Autonomous University of Hidalgo State (UAEH). Mice aging 2–6 weeks and weighing 35–45 g were randomly allocated into seven groups (n = 10, for each group): group 1 received daily oral saline solution (SS) for 10 days and another intraperitoneal (IP) dose of SS on the tenth day; group 2 was given the same SS oral treatment and an IP dose of CdCl2 at 2mg/kg BW on the tenth day; group 3 was orally administered the PBP extract at 200 mg/kg BW for 10 days and an IP dose of SS on tenth day; group 4 was orally administered the PBP extract at 50 mg/kg BW for 10 days and a IP dose of CdCl2 at 2mg/kg BW on the tenth day; groups 5 and 6 received the same treatment as group 4 but with a PBP dose of 100 and 200 mg/kg BW, respectively; group 7 was administrated with the same treatment as group 4 but with Sp at 300 mg/kg BW. Cd was administrated as CdCl2 diluted in saline solution (all used reagents were Sigma-Aldrich analytical purity unless stated otherwise). The PBP and Sp were diluted in saline solution. All procedures and handling of the animals were in accordance with the Mexican Official Regulation (NOM ZOO–062-200-1999) entitled “Especificaciones técnicas para la producción, cuidado y uso de los animales de laboratorio.”
Sample collection
On the 11th day of the treatment, blood was obtained by retro-orbital puncture, and sacrifice was conducted by cervical dislocation. Testes were resected, and one of them was immediately fixated on modified Davidson solution (22% formaldehyde, 33% ethylic alcohol, 11% glacier acetic acid and 34% water; Latendresse et al. 2002) for histology, as it is highly recommended for this kind of tissue. Sperm was extracted by pressure, pulled from the epididymis and deferent tubes, and suspended in M-16 medium, which had been incubated for 30 min at 37 °C with 5% CO2-(Albert and Roussel 1983). The non-fixated testes were washed with saline solution and then processed with 0.5 mL saline in an ultrasonic processor (Gex130pb). Blood samples were collected without anticoagulant and centrifuged at 5000 rpm for 10 min; the separated serum was separated and acquired. All separated samples were stored at – 70 °C until further testing.
Testosterone ELISA
The separated serum was used to measure free testosterone (Cayman Testosterone ELISA kit item.582701), following all the manufacturer’s instructions. The sensitivity of the assay was approximately 6 pg/mL, and the level expressed in pg/mL. The plate was read at 415 nm on an absorbance microplate reader (BioTek, ELx800).
Sperm quality
Sperm samples were further used to determine motility, concentration, and viability according to the World Health Organization (WHO) manual for sperm examination and processing (WHO 2010). In brief, sperm motility was determined by optical microscopy, which counted the sperms on 10 aleatory ranges; the result was expressed in percentage of progressive mobile sperm. Sperm concentration was counted manually on a hemocytometer and expressed in units of 106 /mL. Viability was determined by eosin-nigrosin staining on a sample smear: from 200 sperms, only those without staining were considered viable; thus, the result was expressed as percentage. Within the same smeared and stained samples, morphology test was performed based on Wyrobek and Bruce’s (1975) criteria; abnormalities on central piece, head, and tail were reported.
Acrosomal reaction (AR)
AR was performed on samples containing sperm concentration of 5 × 106/mL. Each sample was incubated on albumin-rich M-16 medium for 60 min for spontaneous reaction and with 20 mmol GABA for induced reaction. The reaction was stopped with paraformaldehyde 10%, rinsed three times with ammonia solution, centrifuged between rinses, and smeared and stained with 0.22% Coomassie Blue. Finally, after counting 200 sperms on each smear on an optic microscope (Carl Zeiss), the obtained result was expressed in percentage of positive AR (Larson and Miller 1999; Piña-Guzmán et al. 2009).
Testes histopathology
The fixated testes were transferred to a 10% formalin solution after 24 h of fixation. Thereafter, they were dehydrated on ascending alcohol solutions (70–100%), cleared in xylene, included in paraffin, and sliced on a microtome (American Optical) at 5 μm. Samples were stained with hematoxylin and eosin and examined on an optical microscope at 100× and 400× (Latendresse et al. 2002).
Antioxidant enzyme activity
The following tests were performed with both processed testes and sperm sample, at 37 °C:
Catalase activity (Cat)
Cat was assessed by the Aebi (1984) method, determined by degradation of H2O2 (20 mMol) with the sample diluted in 1 mL of sucrose buffer (pH7), and read at 240 nm. The used extinction coefficient was 0.036 mM/cm, and the activity was expressed as μmol of consumed H2O2 per minute by protein mg or 5 × 106/mL sperm sample.
Superoxide dismutase activity (SOD)
SOD was analyzed by the McCord and Fridovich (1969) method and performed with a Ransod kit (Crumlinm Country Antrim, RU). Briefly, xanthine and xanthine oxidase were used to generate superoxide radical that reacts with INT to form a reddish dye of formazan, which is inhibited by the activity of the sample SOD when converting superoxide into oxygen, determined at 505 nm. The absorbance was measured at 30 s and 3 min to determine the inhibition of the reaction and expressed as U SOD per minute by protein mg or 5 × 106/mL sperm sample.
Glutathione peroxidase activity (GpX)
GpX was measured by the Paglia and Valentine (1967) method, using a Ransel RS504 Kit (Crumlin, Country Antrim, RU), where GpX catalyzes the oxidation of glutathione by the cumene hydroperoxide. In the presence of glutathione reductase, the NADPH converts to NADP+, and this can be read at 340nm. The difference of absorbance was measured after 1 and 2 min; the activity was proportional to the difference in measurements. The difference was expressed in mU GpX per minute by protein mg or 5 × 106/mL sperm sample.
Oxidative stress
Lipid peroxidation
Lipid peroxidation was determined on testes and sperm samples by means of malondialdehyde (MDA), using the thiobarbituric acid reactive substances (TBARS; Tsikas 2017). Samples were added to 1.0 mL of reactive mixture containing 0.375% of TBA and 15% of trichloroacetic acid (TCA) in 0.20 N HCl. After incubating for 30 min in boiling water, samples were cooled and centrifuged at 1000 rpm for 10 min at 4 °C. The absorbance of the supernatant was measured at 532 nm, and the used extinction coefficient was 156,000 M-1cm-1.
Assay of advanced oxidation protein products (AOPP)
AOPP was performed on testes samples based on the method described by Witko-Sarsat et al. (1996). It was determined by the transformation of iodine to diatomic iodine and followed the change of the O.D. at 340 nm. Ten microliters of sample was added to a mixture containing 990 μL of phosphate buffer 10 mMol (pH7.4), 50 μL of KI, and 100 μL of glacier acetic acid. Using chloramine T curve as pattern, the results were reported in μM of chloramine T. Protein content of the testes samples was determined by the Bradford (1976) method.
Reactive oxygen species (ROS)
ROS were evaluated by cellular ROS assay kit (ab113851) (Abcam plc). It uses 5,6-chloromethyl diacetate-2,7-dichlorofluorescein and acetic ester to produce the DCFDA/H2DCFDA reaction. The sperm samples were treated with 10 μL of DCF and incubated at 37 °C for 30 min in complete darkness. The fluorescence was determined at 493 nm for excitation and 521 nm for emission with a PerkinElmer fluorescence spectrophotometer LS-55.
Statistics
Statistical analysis
Shapiro-Wilk normality tests were performed for all variables. Upon normal distribution, the one-way analysis of variance (ANOVA) test was performed, followed by Tukey as a post hoc test. Data were expressed in mean + SEM. The p value of less than 0.05 (p < 0.05) was considered of statistical significance. All analyses were performed on SigmaPlot11.0 software (Systat Software, 2005). Graphs were created using GraphPad Prism 6 (GraphPad Software, 2012).
Testes image analysis
All images were analyzed using ImageJ 1.52 software (Wayne Rasband National Institutes of Health, USA). The cellular populations in the testes were defined manually; after this, the percentage of cells against the field was determined. Thereafter, using a known scale, a comparison was performed to obtain the occupied area in mm2. Once these parametric data were obtained, all variables were used in the described statistical analysis.
Results
Weight
No significant changes were found among body weights throughout the 10 days of treatments (p 0.64). Relative weight of the testes was significantly higher (p 0.002) in the groups treated with PBPs at 50 and 200 mg/kg (0.425 ± 0.0369 g, 0.424 ± 0.0306 g) alongside Sp at 300 mg/kg plus Cd (0.407 ± 0.0153 g) than in the group treated with PBPs (0.299 ± 0.0200 g; Fig. 1A). To verify that this increase in the weight of the testes was due to vascular alterations and possibly due to edema caused by Cd, the results of the total protein test found by the Bradford method are shown (Fig. 1B). Relative weights of the seminal vesicles were not significantly different among groups (p 0.554).
Oxidative stress
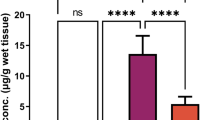
MDA in the sperm samples showed higher levels (p 0.003) in the groups treated with Cd (2221.314 ± 268.862 nM MDA), PBPs at 50 and 100 mg/kg and Cd (1914.183 ± 164.913nM MDA, 1902.805 ± 114.579nM MDA) than in the control group (1077.324 ± 226.953nM MDA). Furthermore, all Cd-treated samples including PBP had MDA levels close to the Cd-treated group (Fig. 2A). Unlike sperm samples for the same test performed on testicular tissue, the MDA production in all groups was lower (p < 0.001) than in the control group (5.591 ± 0.885nM MDA/mg; Fig. 2B). Regarding the production of ROS in sperm sample, interestingly, control and Cd groups showed similar ROS production measurement (p < 0.001), and the highest concentrations were found in the group treated only with PBPs (0.767 ± 0.133) and PBPs at 50 mg/kg and Cd (0.728 ± 0.107), while the lowest concentrations were found in the groups treated with PBPs at 200 mg/kg and Cd (0.148 ± 0.0144) and with Sp and Cd (0.124 ± 0.0259; Fig. 2C). The obtained concentrations of AOPP on the testes where significantly higher (p 0.001) in the control groups (51.531±3.553 μM/L) and the group treated with PBP at 50 mg/kg and Cd (49.526 ± 4.4 μM/L), and with Sp and Cd (55.564 ± 3.487 μM/L) than in the group treated only with Cd (30.614 ± 5.039 μM/L), as it had the lowest concentration of AOPP, followed by the group treated only with PBPs (35.763±3.103 μM/L; Fig. 2D).
Effect of PBPs and Sp against Cd on oxidative stress markers A MDA in sperm (p 0.003), a versus controls; B) MDA in testicular tissue (p<0.001), a versus all treated groups; C ROS by DCF fluorescence in sperm (p<0.001), c versus PBP, d versus 50 + Cd and D AOPP in testicular tissue (p0.001), b versus Cd, c versus PBP (Mean + SEM. ANOVA, Tukey) (p < 0.05; n = 10 replicates)
Antioxidant enzyme activity
SOD activity in sperm showed a lower value in the group treated with Sp and Cd (3.564 ± 0.1U SOD/min) towards control group (4.084 ± 0.0459 U SOD/min); however, none of the other groups showed statistically significant differences (p 0.023; Fig. 3A). SOD activity of testicular tissue also showed no significant differences among treated groups (p 0.093; Fig. 3B).
Effect of PBP and Sp against Cd in enzymes with antioxidant capacity A SOD in sperm (p 0.023)a versus controls; B SOD in testis (p0.093, no significant differences were found); C Cat in sperm (p0.011), a versus controls, g versus Sp + Cd, D) Cat in testis (p 0.009), b versus Cd, E GpX in sperm, there are no significant statistical differences although not the possibility that they exist due to the variance of the data is excluded and F Gpx in testis (p<0.001) c versus PBP; g versus Sp + Cd, (Mean+ SEM. ANOVA Tukey) (p < 0.05; n = 10 replicates)
Cat activity in sperm also showed close levels in control (0.0435 ± 0.0109 H2O2/minute), Cd-treated (0.0574 ± 0.00736 H2O2/minute), and PBP-treated groups (0.0489 ± 0.00493 H2O2/minute), while a higher (p < 0.001) activity was found in the groups treated with PBPs at 50 and 100 mg/kg and Cd (0.0518 ± 0.00922 H2O2/minute, 0.0876 ± 0.00988 H2O2/minute), and with Sp and Cd (0.0959 ± 0.0130 H2O2/minute), which presented the highest measured activity (Fig. 3C). Regarding Cat activity on testicular tissue, it was found that the control group had a mean activity (1.594 ± 0.359 H2O2/minute) and the highest activity corresponded to the Cd-treated group (2.543 ± 0.416 H2O2/minute) and it was only comparable to the group treated with PBPs at 200 mg/kg and Cd (1.033 ± 0.181 H2O2/minute), which had the lowest activity (p 0.009; Fig. 3D).
Regarding GpX activity in sperm samples, no significant differences were found among groups (p 0.073; Fig. 3E). The determination of GpX activity in testicular tissue showed a more stable behavior than in sperm. While the control group showed intermediate activity (38.553 ± 2.091 mU GpX/min), it slightly increased in Cd-treated group (49.488 ± 3.512 mU GpX/min), and it was the highest in the PBP-treated group (55.368 ± 1.416 mU GpX/min). Interestingly, the PBP- and Cd-treated groups showed a slight dose-dependent increase in GpX activity, and unlike all other groups, the Sp- and Cd (31.519 ± 2.861 mU GpX/min)-treated group showed a decrease of its activity (p < 0.001) compared to the ones with the highest activity (Fig. 3F).
Sperm quality
Differences (p < 0.001) in the percentages of progressive motility were observed as Cd treatment highly diminished the motility (14.5 ± 3.76%) when compared to motility of the control group (71 ± 3.145%); further, motility was found to be somewhat restored as the PBP concentration increased, meaning that the groups treated with PBP (56 ± 6.182 %), PBP at 200 mg/kg BW. and Cd (61 ± 5.26%) alongside Sp and Cd (57 ± 7%) were close to control group’s percentage of motility and statistically different from the groups treated with Cd (Fig. 4A). In terms of sperm concentration, no statistically significant differences were found (p = 0.579; Fig. 4B). Viability analysis, likewise motility, showed a significant drop in the percentage (p 0.001) of the Cd-treated group (14 ± 2.854%) when compared against control group (30.5 ± 2.478%), and it was somewhat restored by PBP administration, except in the group treated with PBP at 100 mg/kg BW and Cd (16.5 ± 1.195%), in which a low percentage of viability was found (Fig. 4C). With respect to the analysis of morphological alterations, no differences were found among groups, as the most common alterations included head detachment and curled tails.
Effect of PBP and SP against Cd on sperm quality parameters A progressive motility (p < 0.001), a versus control and 200 + Cd, c against Fb and Sp + Cd; B concentration (p0.579, no significant differences were found) and C viability (p 0.001), a versus control (Mean + SEM .ANOVA Tukey) (p < 0.05; n = 10 replicates)
Acrosomal reaction
The control groups (8.063 ± 0.704%; 15.75 ± 0.856%) and groups treated only with PBPs (12.813 ± 1.221%; 15.313 ± 0.59%) showed the highest percentages of sperm with a positive spontaneous and induced AR, and the group treated with Cd (6 ± 0.543%; 8.063±0.608%) showed a low percentage of both, also a dose-dependent protective factor of PBP was found, as the PBP at 200 mg/kg BW and Cd (11.875 ± 0.736%; 13.25 ± 0.627%) as well as Sp and Cd (8.938 ± 1.159%; 11.125 ± 0.925%)-treated groups showed a measurement more similar to the PBP treated one (12.813± 1.221%; 15.313± 0.59%). Meanwhile, groups treated with PBP at 50 (3.813 ± 0.326%; 4.875± 0.479%) and 100 mg/kg BW and Cd (5.875 ± 0.73%; 7.063± 0.601%) showed a percentage even lower than the one treated with Cd, however, these groups were statistically close (p < 0.001; Fig. 5A). Interestingly, only the control group showed a bigger difference of 8.688 ± 1.709% between the spontaneous and induced AR (p < 0.001; Fig. 5B).
Effect of FBPs and Sp against Cd on A the percentage of spermatozoa that spontaneously and induced AR in 75 minutes. (p <0.001) b against Cd; c against. PBP; d against. 50 + Cd; e against 100 + Cd; g against Sp + Cd. B The difference in percentage of spontaneous versus induced AR (p 0.001) a against all groups (Mean + SEM. ANOVA Tukey) (p < 0.05; n = 10 replicates)
Serum testosterone levels
Testosterone levels were found to be conserved only in the control (2268.203 ± 642.249 pg/mL), PBP-treated (2018.171± 341.341 pg/mL), and, surprisingly, the Sp- and Cd-treated groups (2018.171 ± 341.341 pg/ml), as the Cd (333.654 ± 75.946 pg/mL) and all PBP and Cd-treated groups had diminished testosterone levels ( p< 0.001; Fig. 6).
Testicular histopathology
Testes treated with saline solution did not show abnormalities (Fig. 7A). Conversely testes treated with Cd showed alterations at the vascular level with outflow of blood content and edema in the interstice, with destruction and alterations in the morphology of Leydig cells. Within the seminiferous tubules, there was destruction of the architecture of the tubule with alterations in the nuclei and cytoplasm of germinal cell populations from spermatogonia to spermatid. Migration of Sertoli cells towards the center of the tubule, with absence of sperm, and increased lumen of the seminiferous tubule due to the spaces left by cells that entered apoptosis/necrosis that resembled vacuolation (Fig. 7B). For testes treated only with PBPs (200 mg / kg BW), although they generally had an anatomy similar to that of the control group, there was a significant decrease in the presence of sperm from the lumen of the seminiferous tubule (Fig. 7C). Testes of the groups treated with different doses of PBPs and Cd showed variations in the alterations previously described in the Cd group. Moreover, there was an evident decrease in the degrees of severity depending on the increase in the dose of administered PBPs, so that the group treated with PBPs at 200 mg/kg showed, in most cases, a seminiferous tubule with characteristics very similar to the control group. However, vascular and Leydig cell abnormalities showed similar patterns in all cases (Fig. 7D–F). Finally, the group treated with Sp (300 mg/kg) and Cd showed great variability in its alterations. The most common was the decrease in some cell populations of the seminiferous tubule and in some cases with a considerable amount of areas of necrosis/apoptosis. However, despite having severe vascular alterations, unlike the groups treated with PBPs, a greater presence of Leydig cells with normal characteristics was found (Fig. 7G).
Micrograph at 100× of a mouse testis treated with A saline, B Cd (a, peritubular zone with areas of bleeding, edema, inflammatory infiltrates, and abnormal Sertoli cells; b, vacuolated germinal cells with traces of apoptosis), C phycobiliproteins for 10 days (a, tubule with no morphological sperm on the lumini), D phycobiliproteins 50 mg/kg of weight for 10 days and Cd, E phycobiliproteins 100 mg/kg of weight for 10 days and Cd, F phycobiliproteins 200 mg/kg of weight for 10 days and Cd, and G Spirulina 300 mg/kg of weight for 10 days and Cd (D to G; a, peritubular zone with areas of bleeding, edema, inflammatory infiltrates, and abnormal Sertoli cells; b, vacuolated germinal cells with traces of apoptosis in different degrees of damage)
An increase in interstitial space was expected due to the possible presence of edema; however, no significant differences were found in this regard among the groups. Moreover, no significant difference was found in the caliber and space occupied by the blood vessels, despite the abundant presence of extravasation of blood material in some cases, probably because these findings were more sporadic (in each group treated with Cd up to two or three analyzed testes). Regarding the average size of the seminiferous tubule, the same findings were observed. The only evident difference at this level was the integrity of Leydig cells, since only the control group had the majority of this population with normal morphology, while the remaining groups showed morphological alterations, including the group treated only with PBPs where in many cases there was a decreased presence of Leydig cells. All the Cd-treated groups’ Leydig cells showed severe morphological alterations, which were present neither in the control groups nor in those treated with PBPs (Table 2). In the seminiferous tubule, no significant differences were found in the space and/or percentage of Sertoli cells, so that these only migrated in some seminiferous tubules from the groups treated with Cd. However, the rest of the cell populations did show a decrease in space and percentage of space occupied within the tubule when compared to the control groups, mainly in the specific case of the spermatocyte population. Deriving from the increase in lumen within the seminiferous tubule, this last parameter showed that it differed from the control group and groups treated with PBPs at 200 mg/kg and Cd against the Cd-treated one (Table 3).
Discussion
During this experiment, Cd was administered at 2 mg/kg and was well-tolerated, as low mortality was found in all groups. The most frequent manifestations after the administration included generalized pain and lack in appetence. However, since the sacrifice was made 24 h after this administration, no considerable decrease in weight was detected, unlike other works with similar administration schedules (Ji et al. 2013). This same phenomenon was observed with administration of lower doses of Cd for longer periods (Haouem and El Hani 2013). Only in high-dose regimens, a significant fluctuation in body weight was reported. Sp is known for its chronic use and has the ability to stabilize the variation in weight (Finamore et al. 2017). However, PBPs are known not only for retaining this ability but also for decreasing the production of certain triglycerides at liver level and altering the cholesterol balance with mixed results (Riss et al. 2007).
In the case of the testes, weight might have increased due to the acute inflammation caused by the administration of Cd. Some testicles were observed enlarged and hardened at touch, with a reddish coloration and with injuries of apparent hematic origin. Therefore, it was corroborated that a large part of the functional and endocrine alterations are caused because the main mechanism of Cd damage is by direct effect on the endothelium, causing the outflow of hematic and inflammatory material (Bertin and Averbeck 2006). Within the seminiferous tubule, Cd led to cellular alterations and rendered their activity dysfunctional, causing areas of apoptosis, which affected all developmental stages of spermatogenesis. Further an early drop in serum testosterone levels was also observed, which indicated a reproductive function decline (Parizek 1957). These results are consistent with the observations made in the histological sections, since this decrease in testosterone is attributable to Leydig cell dysfunction and manifests up to 24 h after Cd challenge (Wang et al. 2017). It is striking that the Sp seems to protect against this testosterone drop, perhaps by containing hormonal precursors such as phytohormones or such, which are recently being reported on other algae and plants like Cynara scolymus L.(Górka and Wieczorek 2017; Mohammed et al. 2019).
Interestingly, although MDA levels in testes were expected to be higher in the Cd-treated group, the highest level was found in the control group. This is probably because the presence of free Cd in tissue interfered with the reaction, thereby altering the measurement of TBARS/MDA. The same test performed on the spermatozoa showed a pattern according to expectations: the group treated with Cd showed a higher reaction rate and the rest of the groups showed a decrease in correlation to a higher concentration of PBPs and Sp (Mahmoudi et al. 2018; Obembe and Raji 2018), resembling the control and PBP groups. However, in the test of AOPP in testicular tissue, a similar result was obtained to that of TBARS/MDA test on the same tissue. These results apparently suggest that Cd does not produce oxidative stress directly in testicular tissue, since PBPs alone decrease the presence of ROS in the testis, and the groups treated with the combination of PBPs or Sp and Cd show levels close to those of the control group (Matović et al. 2011). This same phenomenon was manifested in the determination of ROS by DCF in sperm, where Cd by itself does not influence the production of intracellular hydrogen peroxide, while PBPs do have the ability to alter the redox balance. This was especially reflected in the groups with different concentrations of PBPs with Cd, where a decrease in DCF with fluorescent activity was observed as the concentration of PBPs increased. The same behavior was observed in group treated with Sp, where a high concentration of PBPs probably acted to decrease the levels of hydrogen peroxide in this group (Fernández-Rojas et al. 2014; Sandbichler and Höckner 2016; Wu et al. 2016).
In this regard, using different experimental schemes, many authors have reported that Cd does raise ROS production by either raising the Cd dose or combining it with a substance that promotes oxidative stress on its own (Pandya et al. 2012; Abarikwu et al. 2013). Other authors also agree with this argument. Thus, it is assumed that although Cd by itself does not produce a large amount of ROS, it could amplify their production under different experimental conditions; this further proves indirect ROS production mechanism, as in this work, Cd showed both direct damage of the testicular tissue and indirect damage by producing oxidative stress. This was reflected by the low sperm quality, as sperm is highly sensitive to ROS, particularly with regard to motility, viability, and AR (Li et al. 2016; Sandbichler and Höckner 2016).
Regarding SOD activity, it appears that neither Cd nor PBPs play any role in the production of the super oxide radical or in the proper functioning of the enzyme, although Sp may slightly affect its activity in the case of sperm’s SOD (Matović et al. 2011).
GpX activity is conditioned by several factors—one is the presence of its reduced glutathione substrates and the other is the presence of hydrogen peroxide. It was previously mentioned that Cd does not directly increase the presence of ROS as hydrogen peroxide, so the increase in the GpX activity in the group treated with Cd could be due to the decrease in the presence of reduced glutathione, which is known to get reduced in the presence of Cd (Ochi et al. 1987). In the case of the groups treated with PBPs, it was expected that they would have some modification in their GpX activity, as PBPs alters oxide reduction balance (Abarikwu et al. 2013). Finally, in the case of Sp, it is probable that a component was responsible for the decrease in activity and that it was found mainly in the testicle where GpX presented greater variability in its activity, most likely due to the SOD available on the Sp (Castro-García et al. 2018).
In the context of Cat activity, a dose-dependent modification of PBP and Sp activity was also observed. However, unlike GpX, it was seen more clearly that Cd alone increases Cat activity. This is probably because Cat is the main enzyme that compensates for the oxidative imbalance produced by Cd in the testis, and was modified by the activity of PBPs (Ige et al. 2012). However, at spermatic level, the modification of the activity of this enzyme seems to have been conditioned by the higher concentrations of PBPs in the presence of Cd, since only PBP and CD-treated groups showed increased activity, probably due to the imbalance in the production of hydrogen peroxide produced by the combination of Cd and PBPs (Pandya et al. 2012; Yang et al. 2016).
In the case of the aqueous extract of PBPs, it is important to discuss that it acted negatively at two crucial points: first instance was during the transition from spermatid to sperm, where some cell populations showed greater differences in size within the seminiferous tubule. This can be explained by the imbalance in the redox state, whether due to an increase or decrease in free radicals that intervene in cell differentiation, which is particularly crucial for this transition to occur. It particularly appears to have interfered with Sertoli cell function. Second instance was during AR where we observed that it even had a similar behavioral trend to that of the groups treated with Cd. These points in common are characterized by the use of lysosomes and peroxisomes, which in the first case carried out the function to resorb the cytoplasm and in the second carried out the AR. This confirms that PBPs have a great capacity for cell penetration and free radical scavenging as reported for in vitro assays. However, this also raises the question that in what cases PBP is beneficial (Sharma and Agarwal 1996; Sanocka and Kurpisz 2004; Hernández-Lepe et al. 2015; Medina et al. 2017; Koh et al. 2018; Kim et al. 2019).
Conclusion
PBPs demonstrate a strong antioxidant activity as they show protective properties against Cd-induced oxidative toxicity on testes and sperm, improving some reproductive functions. However, due to its high anti-oxidative and cell penetration capabilities, it can interfere with functions that require free radicals such as cytoplasmic resorption and AR.
It was shown that the complete Sp has other components that intervene in other toxic mechanisms of Cd and that also have a protective effect against it; these are not explored in this work.
Abbreviations
- AOPP:
-
Assay of advanced oxidation products
- APC:
-
Allophycocyanin
- AR:
-
Acrosomal reaction
- Cat:
-
Catalase
- Cd:
-
Cadmium
- C-PC:
-
C-Phycocyanin
- ELISA:
-
Enzyme-linked immunosorbent assay
- GABA:
-
Gamma-aminobutyric acid
- GpX:
-
Glutathione peroxidase
- GSH:
-
Dehydrogenated glutathione
- MDA:
-
Malondialdehyde
- MT:
-
Metallothionein
- PER:
-
Phycoerythrin
- PBP:
-
Phycobiliproteins
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- Sp:
-
Spirulina
- TBARS:
-
Thiobarbituric acid reactive substances
- TCA:
-
Trichloroacetic acid
References
Abarikwu SO, Iserhienrhien BO, Badejo TA (2013)Rutin- and selenium-attenuated cadmium-induced testicular pathophysiology in rats. Hum Exp Toxicol 32:395–406. https://doi.org/10.1177/0960327112472995
Abdel-Daim MM, Abuzead SMM, Halawa SM (2013) Protective role of Spirulina platensis against acute deltamethrin-induced toxicity in rats. PLoS One 8:e72991. https://doi.org/10.1371/JOURNAL.PONE.0072991
Abdel-Daim M, El-Bialy BE, Rahman HGA et al (2016) Antagonistic effects of Spirulina platensis against sub-acute deltamethrin toxicity in mice: biochemical and histopathological studies. Biomed Pharmacother 77:79–85. https://doi.org/10.1016/J.BIOPHA.2015.12.003
Abdel-Daim MM, Dawood MAO, Elbadawy M, Aleya L, Alkahtani S (2020) Spirulina platensis reduced oxidative damage induced by chlorpyrifos toxicity in Nile tilapia (Oreochromis niloticus). Anim 10:473. https://doi.org/10.3390/ANI10030473
Adamkovicova M, Toman R, Martiniakova M, Omelka R, Babosova R, Krajcovicova V, Grosskopf B, Massanyi P (2016) Sperm motility and morphology changes in rats exposed to cadmium and diazinon. Reprod Biol Endocrinol 14:42. https://doi.org/10.1186/s12958-016-0177-6
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126. https://doi.org/10.1016/s0076-6879(84)05016-3
Albert M, Roussel C (1983) Changes from puberty to adulthood in the concentration, motility and morphology of mouse epididymal spermatozoa. Int J Androl 6:446–460. https://doi.org/10.1111/j.1365-2605.1983.tb00559.x
Andrews GK (2000) Regulation of metallothionein gene expression by oxidative stress and metal ions. Biochem. Pharmacol. 59:95–104
Asmuss M, Mullenders LH, Eker A, Hartwig A (2000) Differential effects of toxic metal compounds on the activities of Fpg and XPA, two zinc finger proteins involved in DNA repair. Carcinogenesis 21:2097–2104. https://doi.org/10.1093/carcin/21.11.2097
Bartosiewicz M, Penn S, Buckpitt A (2001) Applications of gene arrays in environmental toxicology: fingerprints of gene regulation associated with cadmium chloride, benzo(a)pyrene, and trichloroethylene. Environ Health Perspect 109:71–74. https://doi.org/10.1289/ehp.0110971
Benoff S, Hauser R, Marmar JL, Hurley IR, Napolitano B, Centola GM (2009) Cadmium concentrations in blood and seminal plasma: correlations with sperm number and motility in three male populations (infertility patients, artificial insemination donors, and unselected volunteers). Mol Med 15:248–262. https://doi.org/10.2119/molmed.2008.00104
Bertin G, Averbeck D (2006) Cadmium: cellular effects, modifications of biomolecules, modulation of DNA repair and genotoxic consequences (a review). Biochimie 88:1549–1559. https://doi.org/10.1016/j.biochi.2006.10.001
Bin-Jumah MN, AL-Huqail AA, Abdelnaeim N et al (2021) Potential protective effects of Spirulina platensis on liver, kidney, and brain acrylamide toxicity in rats. Environ Sci Pollut Res 28:26653–26663. https://doi.org/10.1007/S11356-021-12422-X
Boussiba S, Richmond AE (1979) Isolation and characterization of phycocyanins from the blue-green alga Spirulina platensis. Arch Microbiol 120:155–159. https://doi.org/10.1007/BF00409102
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Castro-García SZ, Chamorro-Cevallos G, Quevedo-Corona L, McCarty MF, Bobadilla-Lugo RA (2018) Beneficial effects of phycobiliproteins from Spirulina maxima in a preeclampsia model. Life Sci 211:17–24. https://doi.org/10.1016/j.lfs.2018.09.011
Chamorro-Cevallos G (2016) Methods for Extraction, Isolation and Purification of C-phycocyanin: 50 years of research in review. Int J Food Nutr Sci 3:1–10. https://doi.org/10.15436/2377-0619.16.946
Chamorro-Cevallos G, Garduño-Siciliano L, Martínez-Galero E, Mojica-Villegas A, Pages N, Gutiérrez-Salmeán G (2014) The protective effect of dietary Arthrospira (Spirulina) maxima against mutagenicity induced by benzo[alpha]pyrene in Mice. J Med Food 17:527–534. https://doi.org/10.1089/JMF.2013.0109
Fernández-Rojas B, Hernández-Juárez J, Pedraza-Chaverri J (2014) Nutraceutical properties of phycocyanin. J Funct Foods 11:375–392. https://doi.org/10.1016/J.JFF.2014.10.011
Finamore A, Palmery M, Bensehaila S, Peluso I (2017) Antioxidant, immunomodulating, and microbial-modulating activities of the sustainable and ecofriendly spirulina. Oxid Med Cell Longev 2017:3247528–3247514. https://doi.org/10.1155/2017/3247528
Genchi G, Sinicropi MS, Lauria G, Carocci A, Catalano A (2020) The effects of cadmium toxicity. Int J Environ Res Public Health 17
Górka B, Wieczorek PP (2017) Simultaneous determination of nine phytohormones in seaweed and algae extracts by HPLC-PDA. J Chromatogr B Anal Technol Biomed Life Sci 1057:32–39. https://doi.org/10.1016/j.jchromb.2017.04.048
Gutiérrez-Salmeán G, Fabila-Castillo L, Chamorro-Cevallos G (2015) Aspectos nutricionales y toxicológicos de spirulina (arthrospira). Nutr Hosp 32:34–40. https://doi.org/10.3305/nh.2015.32.1.9001
Guzmán-Gómez O, García-Rodríguez RV, Quevedo-Corona L, Pérez-Pastén-Borja R, Rivero-Ramírez N, Ríos-Castro E, Pérez-Gutiérrez S, Pérez-Ramos J, Chamorro-Cevallos G (2018) Amelioration of ethanol-induced gastric ulcers in rats pretreated with phycobiliproteins of Arthrospira (Spirulina) Maxima. Nutrients 10. https://doi.org/10.3390/nu10060763
Haouem S, El Hani A (2013) Effect of cadmium on lipid peroxidation and on some antioxidants in the liver, kidneys and testes of rats given diet containing cadmium-polluted radish bulbs. J Toxicol Pathol 26:359–364. https://doi.org/10.1293/tox.2013-0025
Hartwig A, Asmuss M, Ehleben I, Herzer U, Kostelac D, Pelzer A, Schwerdtle T, Bürkle A (2002) Interference by toxic metal ions with DNA repair processes and cell cycle control: Molecular mechanisms. Environ Health Perspect 110:797–799. https://doi.org/10.1289/ehp.02110s5797
Hernández-Lepe MA, Wall-Medrano A, Juárez-Oropeza MA et al (2015) Spirulina y su efecto hipolipemiante y antioxidante en humanos: Una revisión sistemática. Nutr Hosp 32
Ige S, Olaleye S, Akhigbe R, Akanbi TA, Oyekunle OA, Udoh UAS (2012) Testicular toxicity and sperm quality following cadmium exposure in rats: Ameliorative potentials of Allium cepa. J Hum Reprod Sci 5:37–42. https://doi.org/10.4103/0974-1208.97798
Ji Y-L, Wang H, Zhang C, Zhang Y, Zhao M, Chen YH, Xu DX (2013)N-acetylcysteine protects against cadmium-induced germ cell apoptosis by inhibiting endoplasmic reticulum stress in testes. Asian J Androl 15:290–296. https://doi.org/10.1038/aja.2012.129
Kim Y-R, Do J-M, Kim KH, Stoica AR, Jo SW, Kim UK, Yoon HS (2019)C-phycocyanin from Limnothrix Species KNUA002 Alleviates Cisplatin-Induced Ototoxicity by Blocking the Mitochondrial Apoptotic Pathway in Auditory Cells. Mar Drugs 17:235. https://doi.org/10.3390/md17040235
Koh EJ, Kim KJ, Choi J et al (2018) Spirulina maxima extract prevents cell death through BDNF activation against amyloid beta 1-42 (Aβ 1-42 ) induced neurotoxicity in PC12 cells. Neurosci Lett 673. https://doi.org/10.1016/j.neulet.2018.02.057
Larson JL, Miller DJ (1999) Simple histochemical stain for acrosomes on sperm from several species. Mol Reprod Dev 52:445–449. https://doi.org/10.1002/(SICI)1098-2795(199904)52:4<445::AID-MRD14>3.0.CO;2-6
Latendresse JR, Warbrittion AR, Jonassen H, Creasy DM (2002) Fixation of testes and eyes using a modified Davidson’s fluid: comparison with Bouin’s fluid and conventional Davidson’s fluid. Toxicol Pathol 30:524–533. https://doi.org/10.1080/01926230290105721
Li YJ, Han Z, Ge L et al (2016)C-phycocyanin protects against low fertility by inhibiting reactive oxygen species in aging mice. Oncotarget. https://doi.org/10.18632/oncotarget.8165
Liu Q, Huang Y, Zhang R et al (2016) Medical application of Spirulina platensis derived C-phycocyanin. Evidence-based Complement Altern Med:2016
Mahmoudi R, Azizi A, Abedini S et al (2018) Green tea improves rat sperm quality and reduced cadmium chloride damage effect in spermatogenesis cycle. J Med Life 11:371–380. https://doi.org/10.25122/jml-2018-0005
Matović V, Buha A, Bulat Z, Đukić-Ćosić D (2011) Cadmium toxicity revisited: focus on oxidative stress induction and interactions with zinc and magnesium. Arch Ind Hyg Toxicol 62:65–76. https://doi.org/10.2478/10004-1254-62-2011-2075
McCord JM, Fridovich I (1969) Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem 244:6049–6055
Medina MF, Arrieta MC, Villafañe MN, Klyver SMR, Odstrcil IMA, González ME (2017) Early signs of toxicity in testes and sperm of rats exposed to low cadmium doses. Toxicol Ind Health 33:576–587. https://doi.org/10.1177/0748233716689524
Mohammed ET, Radi AM, Aleya L, Abdel-Daim MM (2019) Cynara scolymus leaves extract alleviates nandrolone decanoate-induced alterations in testicular function and sperm quality in albino rats. Environ Sci Pollut Res 27:5009–5017. https://doi.org/10.1007/S11356-019-07302-4
Mojica-Villegas MA, Izquierdo-Vega JA,Chamorro-Cevallos G, et. al. (2014) Protective effect of resveratrol on biomarkers of oxidative stress induced by iron/ascorbate in mouse spermatozoa. Nutrients. https://doi.org/10.3390/nu6020489, 6, 489, 503
Monsefi M, Alaee S, Moradshahi A, Rohani L (2009)Cadmium-induced infertility in male mice. Environ Toxicol 25. https://doi.org/10.1002/tox.20468
Obembe OO, Raji Y (2018) Effects of aqueous extract of Moringa oleifera seed on cadmium-induced reproductive toxicity in male Wistar rats. Afr Health Sci 18:653–663. https://doi.org/10.4314/ahs.v18i3.23
Ochi T, Takahashi K, Ohsawa M (1987) Indirect evidence for the induction of a prooxidant state by cadmium chloride in cultured mammalian cells and a possible mechanism for the induction. Mutat Res Fundam Mol Mech Mutagen 180:257–266. https://doi.org/10.1016/0027-5107(87)90222-3
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70:158–169
Pandya C, Pillai P, Nampoothiri LP, Bhatt N, Gupta S, Gupta S (2012) Effect of lead and cadmium co-exposure on testicular steroid metabolism and antioxidant system of adult male rats. Andrologia 44:813–822. https://doi.org/10.1111/j.1439-0272.2010.01137.x
Parizek J (1957) The destructive effect of cadmium ion on testicular tissue and its prevention by zinc. J Endocrinol 15:56–63. https://doi.org/10.1677/joe.0.0150056
Piña-Guzmán B, Sánchez-Gutiérrez M, Marchetti F, Hernández-Ochoa I, Solís-Heredia MJ, Quintanilla-Vega B (2009)Methyl-parathion decreases sperm function and fertilization capacity after targeting spermatocytes and maturing spermatozoa. Toxicol Appl Pharmacol 238:141–149. https://doi.org/10.1016/J.TAAP.2009.05.008
Riss J, Décordé K, Sutra T, Delage M, Baccou J-C, Jouy N, Brune J-P, Oréal H, Cristol J-P, Rouanet J-M(2007) Phycobiliprotein C-phycocyanin from Spirulina platensis is powerfully responsible for reducing oxidative stress and NADPH oxidase expression induced by an atherogenic diet in hamsters. J Agric Food Chem 55:7962–7967. https://doi.org/10.1021/jf070529g
Romay C, Ledón N, González R (1998) Further studies on anti-inflammatory activity of phycocyanin in some animal models of inflammation. Inflamm Res 47:334–338. https://doi.org/10.1007/s000110050338
Romay C, González R, Ledón N et al (2003) C-phycocyanin: a biliprotein with antioxidant, anti-inflammatory and neuroprotective effects. Curr Protein Pept Sci 4:207–216
Saez F, Drevet JR (2019) Dietary cholesterol and lipid overload: impact on male fertility. Oxid Med Cell Longev 2019:1–11
Sandbichler AM, Höckner M (2016) Cadmium protection strategies--a hidden trade-off? Int J Mol Sci 17. https://doi.org/10.3390/ijms17010139
Sanocka D, Kurpisz M (2004) Reactive oxygen species and sperm cells. Reprod Biol Endocrinol 2:12. https://doi.org/10.1186/1477-7827-2-12
Sharma RK, Agarwal A (1996) Role of reactive oxygen species in male infertility. Urology 48:835–850
Thévenod F, Lee WK (2013) Cadmium and cellular signaling cascades: interactions between cell death and survival pathways. Arch. Toxicol. 87:1743–1786
Tsikas D (2017) Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: analytical and biological challenges. Anal Biochem 524:13–30. https://doi.org/10.1016/j.ab.2016.10.021
Vázquez-Sánchez J, Ramón-Gallegos E, Mojica-Villegas A, Madrigal-Bujaidar E, Pérez-Pastén-Borja R, Chamorro-Cevallos G (2009) Spirulina maxima and its protein extract protect against hydroxyurea-teratogenic insult in mice. Food Chem Toxicol 47:2785–2789. https://doi.org/10.1016/j.fct.2009.08.013
Waisberg M, Joseph P, Hale B, Beyersmann D (2003) Molecular and cellular mechanisms of cadmium carcinogenesis. Toxicology 192:95–117
Wang H-F, Chang M, Peng T-T, Yang Y, Li N, Luo T, Cheng YM, Zhou MZ, Zeng XH, Zheng LP (2017) Exposure to cadmium impairs sperm functions by reducing CatSper in mice. Cell Physiol Biochem 42:44–54. https://doi.org/10.1159/000477113
WHO (ed) (2010) WHO laboratory manual for the Examination and processing of human semen, 5th Edition. WHO
Witko-Sarsat V, Friedlander M, Capeillère-Blandin C, Nguyen-Khoa T, Nguyen AT, Zingraff J, Jungers P, Descamps-Latscha B (1996) Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int 49:1304–1313. https://doi.org/10.1038/ki.1996.186
Wu HL, Wang GH, Xiang WZ, Li T, He H (2016) Stability and Antioxidant activity of food-grade phycocyanin isolated from Spirulina platensis. Int J Food Prop 19. https://doi.org/10.1080/10942912.2015.1038564
Wyrobek AJ, Bruce WR (1975) Chemical induction of sperm abnormalities in mice. Proc Natl Acad Sci U S A 72:4425–4429
Yang S-H, Long M, Yu L-H, Li L, Li P, Zhang Y, Guo Y, Gao F, Liu MD, He JB (2016) Sulforaphane prevents testicular damage in kunming mice exposed to cadmium via activation of Nrf2/ARE signaling pathways. Int J Mol Sci 17:1703. https://doi.org/10.3390/ijms17101703
Yoshida H, Kong YY, Yoshida R, Elia AJ, Hakem A, Hakem R, Penninger JM, Mak TW (1998) Apaf1 is required for mitochondrial pathways of apoptosis and brain development. Cell 94:739–750. https://doi.org/10.1016/S0092-8674(00)81733-X
Zhao L l, Ru Y f, Liu M et al (2017) Reproductive effects of cadmium on sperm function and early embryonic development in vitro. PLoS ONE 12:e0186727. https://doi.org/10.1371/journal.pone.0186727
Acknowledgments
The authors regard their thanks to Jaramillo Ph.D. Paniagua PhD. Ramon PhD from ENCB for supporting with equipment to the realization this work.
Availability of data and materials
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Author information
Authors and Affiliations
Contributions
RIMG performed all experiments, analyzed all the data, and wrote the main manuscript (Main author PhD; student). GGS revised the manuscript and wrote introduction, MAMV designed and supervised all experiments, JMC animal manipulation support and consultant wrote the abstract, JBB supported most experiments and performed PBP extraction, and GCC main author. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This project was approved by IRB (CEI-ENCB), according to national normativity and policy, under the title “Efecto protector de las ficobiliproteínas de Spirulina (Arthrospira) maxima en dos modelos de toxicidad reproductiva inducida por cadmio en ratón.” Register number CEI-ENCB ZOO-025-2019.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Mohamed M. Abdel-Daim
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
- Cd toxic effects are non-limited to oxidative stress, as it exerts aggressively, especially damaging structural and proliferative tissue in the testicles.
- Sperm quality appears to be mainly affected to oxidative stress caused by Cd.
- PBPs and Sp show great antioxidant activity against Cd-induced testicular and sperm toxicity.
- PBPs show great antioxidant activity and cellular penetration as it also interferes with spermatogenesis and acrosomal reaction.
Appendix
Appendix
Formulae used to determine PBP concentration in Sp aqueous extract
Rights and permissions
About this article
Cite this article
Montaño-González, R.I., Gutiérrez-Salmeán, G., Mojica-Villegas, M.A. et al. Phycobiliproteins extract from Spirulina protects against single-dose cadmium-induced reproductive toxicity in male mice. Environ Sci Pollut Res 29, 17441–17455 (2022). https://doi.org/10.1007/s11356-021-16668-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-16668-3