Abstract
The present study was conducted to evaluate the ameliorative efficacy of Spirulina platensis (SP) on reproductive dysfunctions induced by cadmium chloride (CdCl2) in male rats. Rats (n = 40) were divided into five groups (eight rats/each). Group 1: served as control without any treatment. Group 2: Rats were administered SP (150 mg/kg body weight (BW)) in drinking water for 10 days. Group3: Rats were subcutaneously injected with CdCl2 (2 mg/kg BW) daily for 10 days. Group 4: Rats were co-treated with both CdCl2 (2 mg/kg BW) and SP (150 mg/kg BW) daily for 10 days (SP prophylactic group). Group5: Rats received CdCl2 for 10 days followed by administration of SP alone in drinking water daily for another 30 days with the same mentioned routes and doses (SP treatment group). From our findings, the administration of SP alone or co-administration with Cd significantly attenuated the harmful effects of Cd, suggesting its beneficial role in improving spermatogenesis and steroidogenesis after Cd exposure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The adverse health effects related to the reproductive dysfunction and male infertility have become a source of greatest concern worldwide. About half of the male infertility cases in humans of unknown origin suggest the involvement of physiological disorders or due to occupational or environmental exposures as many of these factors have been implicated to determine suboptimal quality of semen and to reduce reproductive hormone production (Patra et al. 2011).
Cadmium (Cd) is a toxic metal present in the environment either naturally or as a contaminant arising from different sources including agricultural and industrial ones (Järup and Äkesson 2009). Acute and chronic Cd toxicity have been reported to result in a variety of biochemical and physiological disorders due to extensive injury to different body organs both in humans and laboratory animals (Gaurav et al. 2010). Studies have demonstrated that the testis is highly sensitive to toxicity with cadmium (Liu et al. 2009), manifested by decline in male fertility parameters, such as decreased count of spermatozoa and poor semen quality (Wafaa et al. 2012; Ekhoye et al. 2013). The adverse impacts of Cd on male fertility come through its capability to induce degenerative changes in components of reproductive system (testes, epididymis, and seminal vesicles as well as Leydig cells), promoting an inhibition of steroidogenesis and generation of reactive oxygen species that lead to cell necrosis and apoptosis (Rennolds et al. 2012; Nair et al. 2013).
Currently, spirulina, a microscopic multi-cellular filamentous blue green algae (Cyanobacterium) known as a “super food” is gaining the attention of medical scientists due to the ability in protecting the body physiological system against oxidative damage as well as nutraceutical and source of potential pharmaceuticals. Therefore, its long history of safe use makes it a unique blue green algae (Sheshadri and Umesh 1992). It has been used as a nutritional supplement for human and animal consumption as it is rich in proteins, lipids, carbohydrates, sterols, and some essential elements such as zinc, magnesium, and selenium (Babadzhanov et al. 2004). Spirulina platensis (Arthrospira platensis) is a natural source of vitamins, macronutrients and micronutrients like amino acids, gamma linolenic acid, carotenoids, especially β-carotene, α-linolenic acid, phycocyanin and phycocyanobil in chlorophyll, and xanthophyll phytopigments (Upasani and Balaraman 2003; Gong et al. 2005; Bermejo et al. 2008). The administration of Arthrospira produced a significant protective effects against oxidative damage caused by Cd in liver and kidney of rats (Jeyaprakash and Chinnaswamy 2005), nephrotoxicity of mercury in mice (Sharma et al. 2007) as well as a protective role in the oxidative stress in nervous system of offspring from rats exposed to fluoride (Banjia et al. 2013) and enhance the spermatogenesis and steroidogenesis in diabetic rats (Nah et al. 2012). However, role of SP in ameliorating the reproductive dysfunctions induced by Cd is not fully established. Therefore, the present study aimed to determine whether SP could attenuate Cd-induced testicular damage, by investigating the possible antagonistic actions of SP on several aspects of testicular dysfunctions, biochemical and pathological abnormalities in male rat testes induced by cadmium chloride (CdCl2), as well as the expressions of some steroidogenic genes in testicular tissues.
Material and methods
Animals
Healthy adult male Sprague–Dawley rats (n = 40 and average body weight of 180–200 g) were used in this study. They were obtained from the Laboratory Animal Housing Unit, Research, Institute, Dokki, Cairo, Egypt. The animals were clinically healthy and kept under hygienic conditions in stainless steel cages with hard wood shavings as bedding. They were maintained on basal ration, given water ad libitum and exposed to 12 h light-darkness cycle for 2 weeks of acclimatization before use.
Tested compounds
CdCl2 was procured from El-Faraana Co. for Trading, Egypt, in the form of white powder. Spirulina platensis (SP) was procured from Free Trade Egypt Co., Behira, Egypt.
Experimental design
The 40 experimental rats were assigned into five equal groups each containing eight rats (with nearly equal body weight (BW)). Group 1 was kept as control and received no treatments. Group 2 was administered SP (150 mg/kg BW) in drinking water using water bottle for 10 days. The body weights of rats were measured every other day, and the amount of SP in drinking water (150 ml) was newly adjusted to 150 mg/kg BW. Group 3 was given CdCl2 (2 mg/kg BW) by subcutaneous injection (S/C) daily for 10 days. Group 4: Rats were co-treated with both CdCl2 (2 mg/kg BW) and SP (150 mg/kg BW) daily for 10 days (SP prophylactic group). Group 5: Rats were received CdCl2 for 10 days and then the cadmium administration was stopped and the same rats continued to receive SP alone in drinking water daily for another 30 days at the same mentioned routes and doses (SP treatment group).
Sample collection and preparation
At the end of the experiment, the animals were sacrificed followed by collection of blood without anticoagulant, then centrifuged at 3000 rpm for 10 min for separation of serum which was stored at −20 °C for hormonal analysis (determination of testosterone (TES), leutinizing hormone (LH), and estradiol (EST) levels). After dissection, the testicles were removed, trimmed off the attached tissues, grossly examined, and weighed. Tissue samples of testes were rapidly removed from depicted rats, quickly kept in liquid nitrogen until RNA extraction for determination of 3β-hydroxysteroid dehydrogenase type 6 (3β-HSD6), 17β-hydroxysteroid dehydrogenase type 3 (17β-HSD3) and Nr5A1 (=steroidogenic factor 1 (SF1)) expression level using a semi-quantitative real-time RT-PCR. Tails of epididymes were excised immediately for semen evaluation. Other samples from testes, seminal vesicles, and prostate glands of both control and treated animals were fixed in 10 % neutral buffered formalin for histopathological examination.
Reproductive organ weights
Testes were excised, and the epididymes were carefully removed from them. Then, testes weights were recorded; similarly, the seminal vesicles were excised from prostates, and their weights were taken.
Semen evaluation (epididymal spermatozoal examination)
The cauda epididymis of one testis was excised and received in a sterilized petri dish containing warm normal saline at 37 °C, and then, it was macerated by sterilized scissors to obtain the epidydimal contents in a suspension that was handled as the semen (Wales 1971). The percent of motile spermatozoa was microscopically estimated at 400× magnification (sperm motility) as described by Slott et al. (1991), and sperm cell concentration per milliliter of semen was performed according to the method of Robb et al. (1978); the count was repeated five times for each sample to minimize the error. Sperm abnormalities were recorded using the method of Filler (1993). To assess the incidence of abnormalities in head, neck/mid-piece, and tail, at least 500 spermatozoa were observed per animal.
Hormonal assays
Serum hormonal TES, LH, and EST levels were determined using an enzyme-linked immunosorbent assay (ELISA) with commercial kits, following manufacturer’s instructions. Serum TES and estradiol were evaluated using Oxis International, Inc., USA, Catalog Nos. 11150 and 11110 kits, respectively. The sensitivity of assays were 0.05 ng/ml and 10 pg/ml, respectively, and the level expressed respectively as ng/ml and pg/ml. Serum LH was quantified using BioCheck, Inc., USA, Catalog No. BC-1031 kit; the sensitivity of the assay was 1 mIU/ml.
Analysis of gene expressions by real-time RT-PCR in testicular tissues
To evaluate the effects of cadmium chloride on the steroidogenesis in Leydig cells, testicular messenger RNA (mRNA) levels of the steroidogenic pathway genes 17β-HSD3, 3β-HSD6, and NR5A1 were analyzed by semi-quantitative real-time RT-PCR according to Meadus (2003). Briefly, total RNA was isolated from testes samples using Qiagen RNA extraction kits, (Cat. No. 74104). The amount of extracted RNA was quantified and qualified using NanoDrop® ND-1000 Spectrophotometer, NanoDrop Technologies, Wilmington, Delaware, USA. The purity of RNA was checked and ranged between 1.8 and 2.1, demonstrating the high quality of the RNA. The mRNA was stored at −20 °C before RT-PCR. RNA was reversed for production of complementary DNA (cDNA) using QIAGEN Long Range 2 Step RT-PCR Kit, (Cat. No.205920). One microliter of total cDNA was mixed with 12.5 μl of 2× SYBR® Green PCR mix with ROX from Bio-Rad, 5.5 μl of autoclaved water, and 0.5 μl (10 pmol/μl) of each forward and reverse primer for the measured genes. The housekeeping gene β-actin was used as a constitutive control for normalization. Primer sequences of rat 3β-HSD6, 17β-HSD3, Nr5A1, and β-actin were obtained from the published sequences of Nah et al. (2012). The primer design was optimized for RT-PCR with EugeneTM version 2.2 (Daniben Systems, Cincinnati, OH, USA) (Table 1).
Histopathological investigation
Tissue specimens from testis, seminal vesicles, and prostate glands of both control and treated animals were fixed in 10 % neutral buffered formalin, dehydrated in gradual ethanol (70–100 %), cleared in xylene, and embedded in paraffin. Five micron thick paraffin sections were prepared and then routinely stained with hematoxylin and eosin (H and E) dyes according to Bancroft and Stevens (1996).
Statistical analysis
Data were statistically analyzed using the software SPSS/PC+ (2001) for obtaining mean data and standard error. Data were analyzed using one-way ANOVA to determine the statistical significance of differences among experimental groups.
Results
Effects of CdCl2, SP powder, and their co-exposure on body weight
Results presented in Table 2 show that CdCl2 administration significantly decreased (p < 0.05) the BW of exposed rats during the entire experimental period compared with the control and other treatments. The prophylactic use of SP (Cd + SP) or administration of SP alone could maintain the BW of rats similar to control value. However, using of SP as treatment (Cd then SP) could enhance the BW change of animals but still lower than control group.
Effects of CdCl2, SP powder, and their co-exposure on relative weights of reproductive organs
With regards to relative testicular weight, CdCl2-administered animals gained less testicular weight than the controls (p < 0.05; Table 3). However, co-administrated groups of SP either as prophylactic or as treatment could normalize the CdCl2 reducing effect, and their testicular weights were maintained at the level of the control. The relative weights of both seminal vesicle and prostate gland do not significantly changed (p < 0.05) in all experimental groups than control.
Seminal picture
The counts of epididymal sperm and motility were notably decreased in CdCl2-administered rats. The administration of SP alone could numerically increase the motility of the sperms (93.33 ± 1.66) and significantly (p < 0.05) increase their counts (74 ± 1.28) than control group (89.00 ± 2.88 and 69 ± 0.66, respectively). In addition, the co-administration of SP with CdCl2 (prophylactic) largely counteracted the unfavorable actions of CdCl2 and was more effective in increasing the motility % (83.33 ± 1.66) and sperm count (61 ± 0.88) to be comparable to those of control rats than CdCl2 then SP (treatment group) which showed a motility % of 76.66 ± 3.33 where the sperm count of this group was (59.33 ± 0.22).
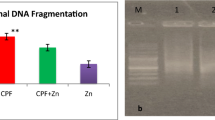
The S/C injection of CdCl2 to rats resulted in a significant increase (p < 0.05) in the incidence of abnormal spermatozoa (17.50 ± 0.29) compared to control (6.91 ± 0.33). The prophylactic use of SP significantly decreased (p < 0.05) the % of sperm abnormalities (8.42 ± 0.29) than CdCl2 group and gave results near to control values; the CdCl2 then SP (treatment) group could also decrease the % of abnormal spermatozoa (12.04 ± 0.31) than CdCl2 group but still significantly higher than control. However, the % of abnormal spermatozoa in rats administered SP alone (6.08 ± 0.12) was similar to those in control group (Fig. 1). As illustrated in Fig. 2, the primary pathologic spermatozoa were detached, broken, and abnormal head shape as well as detached tail, while the secondary abnormalities were coiled, curved, and looped tail and cytoplasmic droplets.
Hormonal analysis
The effects of administration of CdCl2 and SP on hormonal levels of treated rats are represented in Table 4. The results showed a significant decrease in serum level of TES in the CdCl2 group compared to control. The administration of SP alone showed a significant increase in serum TES level compared to control. On the other hand, CdCl2 + SP group (prophylactic) and CdCl2 then SP treatment group showed non-significant differences in TES level than control. The serum level of LH hormone did not significantly differ among all the experimental groups. Additionally, EST level showed non-significant differences among treated groups expect in the CdCl2 then SP group where the EST level was significantly decreased than control groups.
Expressions of steroidogenic genes in testicular tissues
In CdCl2-treated rat testes, steroidogenic genes’ mRNA expressions were significantly downregulated compared to the control. SP intake significantly upregulated 3β-HSD6 and 17β-HSD3 to be better than control group. However, administration of SP with CdCl2 (prophylactic group) significantly increased 3β-HSD6 mRNA and 17β-HSD3 levels to be similar to control values. The rats administered SP following CdCl2 (treatment group) showed a significant improvement in the expression of these two genes but still lower than prophylactic group and control group values (Fig. 3). Administration of SP alone significantly upregulated the expression of the Nr5A1 mRNA; however, there were no significant differences in its level among the all other treatment groups compared to control.
Expressions of steroidogenic genes in rat testes. Real-time RT-PCR analysis of steroidogenic genes (Nr5A1, 3β-HSD6, and 17β-HSD3). β-actin was used as an internal control. Data expressed as mean ± SE (n = 3 replicates). Columns carrying different superscripts are significantly different (one-way ANOVA) (p ≤ 0.05)
Histopathological findings
Testes
The testes of control or SP-received rats showed uniform seminiferous tubules with complete spermatogenesis and interstitial connective tissue. The tubular epithelium was highly intact and contained Sertoli cells resting on the basement membrane, together with spermatocytes and spermatogonia. Round and elongated spermatids were embedded in or associated with the Sertoli cells at different stages of the spermatogenic cycle (Fig. 4a 1). While, the testes of cadmium-received group showed testicular degeneration that was represented by severe vacuolation (Fig. 4a 2), desquamation, and necrosis (Fig. 4a 3) in the germinal epithelium of the seminiferous tubules. These tubules were almost devoid of spermatids and spermatozoa. Few leukocytes of neutrophils were infiltrated the tubular structures and the interstitium. Regarding to the interstitium, there was severe congestion of the interstitial blood vessels, edema, and hemorrhage (Fig. 4a 4). Cadmium and spirulina group (treatment) revealed moderate vacuolation in the testicular epithelium with slight improvement of spermatogenesis (Fig. 4a 5). Spirulina and cadmium group (prophylactic) showed normal seminiferous tubules with complete spermatogenesis. Sometimes, slight vacuolation in the spermatocytes was rarely seen (Fig. 4a 6).
a 1–6 Testis of rats from control group shows uniform seminiferous tubules with complete spermatogenesis and interstitial Leydig cells (1); cadmium-received group shows testicular degeneration represented by vacuolated germinal cells with rare spermatogenesis (arrows) (2) and necrosis in seminiferous tubule (arrow) (3) besides severe congestion (arrow) and hemorrhage (arrowhead) (4); cadmium and spirulina group (treatment) shows moderate vacuolation in the testicular epithelium (arrows) (5), and spirulina and cadmium group (prophylactic) shows normal seminiferous tubules with slight vacuolation and complete spermatogenesis (arrows) (6). HE ×400. b 7–12 Seminal vesicle of rats from control group shows normal epithelial lining and seminal fluid (7); cadmium-received group shows severe vacuolations (arrows) (8), and hyperplasia in the lining epithelium (arrow) (9) besides edema, congestion (arrowhead), hemorrhage, and few round cell infiltrations (arrow) (10); cadmium and spirulina group (treatment) shows nuclear stratification (arrows) and rare seminal fluid (11), and spirulina and cadmium group (prophylactic) shows normal fluid and lining epithelium (arrows) (12). HE ×400. c 13–18 Prostate gland of rats from control group shows normal pattern and acini (13× 100); cadmium-received group shows severe dilation of some acini (arrowhead) with presence of corpora amylacea (C), and hemorrhage (arrow) (14× 100), inflammatory edema with few round cell infiltration (arrow) (15) and hyperplasia and nuclear stratification in epithelial lining (arrows) with no evidence of prostatic fluid (arrowhead) (16); cadmium and spirulina group (treatment) shows inactive lining epithelium (arrow) and rare prostatic fluid (arrowheads) (17), and spirulina and cadmium group (prophylactic) shows normal prostatic fluid and hyperplasia in the lining epithelium (arrows) (18). HE ×400
Seminal vesicle
The seminal vesicle of control or SP-received rats showed normal epithelial lining and seminal fluid in its lumen (Fig. 4b 7). Cadmium-received group showed severe vacuolations (Fig. 4b 8), and hyperplasia in the lining epithelium (Fig. 4b 9) besides edema, congestion, hemorrhage, and few round cell infiltrations (Fig. 4b 10). Cadmium and spirulina group (treatment) revealed some nuclear stratification and scanty seminal fluid in the lumen (Fig. 4b 11). Meanwhile, the spirulina and cadmium group (prophylactic) showed normal fluid and lining epithelium (Fig. 4b 12).
Prostate glands
The prostate glands of control or spirulina-received rats revealed normal pattern and acini (Fig. 4c 13). Cadmium-received group showed severe dilation of some acini with presence of corpora amylacea (Fig. 4c 14), inflammatory edema with few round cell infiltrations (Fig. 4c 15), and hyperplasia and nuclear stratification in epithelial lining with no evidence of prostatic fluid (Fig. 4c 16). These epithelia were enlarged with vacuolated cytoplasm and large vesicular nuclei. Cadmium and SP group (treatment) revealed inactive lining epithelium and rare prostatic fluid (Fig. 4c 17). However, the SP and Cd group (prophylactic) showed normal prostatic fluid and hyperplasia in the lining epithelium (Fig. 4c 18).
Discussion
This study was designed to investigate the beneficial effects of SP intake against the reproductive disorders induced by Cd in male rats. In the present work, it was found noticeable that subcutaneous administration of CdCl2 in a daily dose of 2 mg/kg BW for 10 days to male rats revealed clear signs of toxicity such as a significant decrease in BW gain, testicular weight, sperm cell concentration, motility percent with concurrent increase in the percentage of sperm cell abnormalities accompanied with declined concentration of testosterone, and a significant downregulation of mRNA expressions of steroidogenic enzyme genes in testes of treated rats compared to control and SP co-administered groups.
Subcutaneous administration of cadmium chloride evoked several histopathological changes in the testes represented by testicular degeneration, desquamation, and necrosis in the germinal epithelium of the seminiferous tubules with the absence of spermatids and spermatozoa. Hyperplasia in the lining epithelium of seminal vesicle and prostate gland were also noticed indicating impaired spermatogenesis and steroidogenesis after Cd exposure.
The decreased BW following Cd intoxication is in agreement with the results of Gaurav et al. (2010) who reported that Cd reduced the BW, and this may be due to the ability of Cd in the induction of lipid peroxidation and consequently cell damage. Interestingly, the significantly decreased BW gain of Cd-administered rats was highly improved by prophylactic use of SP (CdCl2 + SP) or administration of SP alone that could maintain the body gain of rats similar to control value. However, using of SP as treatment (CdCl2 and then SP) could enhance the gain of animals. The ameliorative effects of SP on the BW gain could be also attributed to the supplementation of body by essential nutrients, like proteins, biochelating vitamins, and amino acids (Mazo et al. 2004; Sharma et al. 2007). In addition, other constituents like β-carotene (Seshadri et al. 1991), SOD enzyme (Henrikson 1989), selenium, magnesium, zinc and manganese, α-lipoic acid, riboflavin, and some phytopigments such as xanthophyll, phycocyanin, and chlorophyll also have proven beneficial effects (Reddy et al. 2000; Careri et al. 2001; Chamorro et al. 2002).
Our results concerning the effect of CdCl2 on testicular weight, structure, and functions as well as semen quality (sperm count, motility, and morphology) are in total agreement with those obtained by Wafaa et al. (2012) and Ekhoye et al. (2013). The decreased number of spermatozoa caused by CdCl2 was also reported by Oteiza et al. (1999), Tbeileh et al. (2007), and Mudathir et al. (2008). Similarly, Hew et al. (1993) stated that CdCl2 (1 mg/kg) as single I/P injection in rats resulted in the inability of sperms to release from epithelium of seminefrous tubules suggesting that Cd starts to exert its harmful effect on spermatogenesis in its early stage so decrease sperm production and sperm production efficiency.
Decreased sperm counts and relative testicular weight following Cd administration obtained in this study were associated with a significant decline in the serum testosterone level, and these findings run parallel with the results of Biswas et al. (2001), Santos et al. (2006), and Thompson and Bannigan (2008). Sperm production decrease in rat testes could be attributed to the decreased levels of testosterone which are very important to promote testicular growth. Besides, Cd exposure can interfere with hypothalamic-pituitary-testicular axis (Lafuente et al. 2000) and disrupt the initial steps of gamete production that are hormone-mediated and dependent (Gunnarsson et al. 2003).
The present results also ameliorated the testicular weight loss and the decreased sperm count after Cd treatment to the alterations in testicular morphology as represented by testicular degeneration, severe vacuolation, desquamation, and necrosis in the germinal epithelium of the seminiferous tubules which were almost devoid of spermatids and spermatozoa. These findings were also supported by Rekha et al. (2011), while Yang et al. (2006) reported return of the weight loss of testes to the adverse effects induced by Cd on germ cell count and the percent of elongated spermatids because the weight of testes depends largely on undifferentiated spermatogenic cell mass. In the same context, the testicular weight loss, disturbances in blood–testis barrier, necrotic changes, edema as well as reduction of germ cells have been found as results of cadmium-induced testicular injury (Siu et al. 2009).
Regarding the sperm motility, our data showed that Cd-administered rats exhibited a significant decrease in sperm motility. Similar result was obtained by Oliveira et al. (2006). The decreased sperm motility in Cd-treated rats could be attributed to the ability of Cd to bind with calmodulin instead of calcium inhibiting its role in sperm motility (Schlingmann et al. 2007). El-Missiry and Shalaby (2000) related the decrease in sperm concentration and motility % and the increase in the abnormal sperm following Cd administration to the ability of Cd to induce lipid peroxidation and necrotic and apoptotic changes in testicular tissue of rats due to changes in the levels of circulating androgen. CdCl2 could induce testicular toxicity by a significant increase in hydroxyl free radical formation, generation of ROS, reduction of GSH content, and oxidative damage to macromolecules (Manna et al. 2008; Tremellen 2008; Liu et al. 2009). Peroxidative damage to the plasma membrane as a result of oxidative stress can interfere with functions of sperms as spermatozoa that contain high amounts of polyunsaturated fatty acids (PUFAs) increased its susceptibility to Cd-induced oxidative stress (Agrawal and Saleh 2002; Vernet et al. 2004). Another possible explanation for the impact of CdCl2 on semen quality was that Cd could inhibit the activity of alkaline phosphatase enzyme (ALP) by competing with zinc that is essentially required for this enzyme and disrupt the zinc- and calcium-dependent cellular actions (Martin et al. 2007). These alterations observed in testicular structure and functions are highly correlated to the inhibition of spermatogenesis observed in this study which represented by downregulation of mRNA expression of enzyme genes (Nr5A1 mRNA, 3β-HSD6, and 17β-HSD3) besides defects in reproductive parameters and testicular degeneration.
With regards to relative testicular weight, co-administrated groups of SP either as prophylactic or as treatment could normalize the Cd-reducing effect on testicular weight. Moreover, administration of SP alone could numerically increase the motility of the sperms and significantly increase their counts than control group. In addition, the co-administration of SP with CdCl2 (prophylactic) increased the motility % and sperm count to be comparable to those of control rats than CdCl2 then SP (treatment) group. The prophylactic use of SP significantly decreased the % of sperm abnormalities than CdCl2 group and gave results near to control values; the CdCl2 then SP (treatment) group could also decrease the % of abnormal spermatozoa than CdCl2 group but still significantly higher than control. This suggested the improving effects of SP on the quality of sperm parameters and fertility in male rats.
There was a significant decrease in serum level of TES in the CdCl2 group compared to control. While, administration of SP alone showed a significant increase in serum TES level compared to control. On the other hand, CdCl2 + SP group (prophylactic) and CdCl2 then SP group showed non-significant differences in TES level than control group. The serum level of LH and EST hormone did not significantly differ among the all experimental groups. Administration of SP alone caused a prominent increase in testosterone concentration associated with an elevation in epididymal sperm counts and their motility indicating beneficial effects of SP intake on spermatogenesis. In addition, the testicular NR5A1, 3β-HSD6 mRNA, and 17β-HSD3 mRNA levels in the Cd + SP group were significantly higher than the Cd group. This suggests that SP intake can effectively recover Cd-induced deregulation of gene expression in early (NR5A1) and final (3β-HSD6 mRNA and 17β-HSD3) step in the steroidogenic pathway.
Concurrent treatment with SP significantly attenuated the alteration of reproductive system induced by CdCl2 may be attributed to the presence of vitamins C and E; these antioxidant vitamins could protect the cells from the dangerous effect of free radicals as reported by Mathew et al. (1995) where vitamin E could inhibit the peroxidation of cell membrane lipids by trapping lipid peroxyl (LOO−) and many other radicals to help in counteracting the oxidative damage and keeping the levels of GSH and ascorbic acid in damaged tissues caused by different xenobiotic as well as Cd toxicity (Rana et al. 1996; Patil and Rao 1999). Moreover, SP also contains selenium that is involved in the formation of selenium containing enzymes glutathione peroxidase and protein besides some other compounds like selenocystien, selenoglutathione, and selenodimethylselenide which are known to counteract the toxic effects of heavy metals (Simsek et al. 2009). In addition, to the presence of brilliant blue polypeptide phycocyanin and allophycocyanin which are important constituents of phycobilisomes (Bhat and Madyastha 2001; Riss et al. 2007), phycocyanin significantly inhibited peroxyl radical-induced lipid peroxidation in rat liver microsomes (Bhat and Madyastha 2000). Phycocyanin compound of Spirulina may reduce the lipid peroxidation and has the ability to chelate metals including free iron (Premkumar et al. 2003). Chlorophyll and its derivatives in spirulina scavenge free radicals (Ferruzzi et al. 2002). Moreover, Spirulina was reported to enhance the activity of ALP enzyme (Sharma et al. 2007) and contain high amount of zinc (Jeyaprakash and Chinnaswamy 2005) which is required for this enzyme to enhance its role in improving semen quality.
In the present study, the S/C administration of Cd chloride to male albino rats evoked several histopathologic changes on testis, prostate gland, and seminal vesicle. The adverse effects of cadmium on histological structure of reproductive tissues have been reported even after administration of single doses where acute Cd injection caused some necrotic and apoptotic changes in the seminefrous tubular epithelium as well as edema, congestion, hemorrhage of testes besides diffuse necrosis of spermatozoa with impaired spermatogenesis as reported by Messaodi et al. (2010) and El-shahat et al. (2009). On the same context, Adamkovičová et al. (2010) also reported that I/P injection of a single dose of Cd (2 mg/kg body weight) to adult rats showed necrosis of seminiferous tubular epithelium, disruption of blood–testis barrier leading to edema and ischemia. Similar histological alterations were observed after administration of Cd in single dose in testes of mice (Massányi et al. 2008) and rabbits (Toman and Massányi 2002; Nemoto et al. 2009).
These effects of CdCl2 on testicular tissue and fertility could be resulted as a consequence of induction of inflammatory reactions and increased expressions of TNF α and NO in testicular tissue (Gurl et al. 2007). Increased production of NO leads to more cellular damage as it could react with super oxide anion to generate peroxy nitrite radical and lead to depletion of intracellular GSH consequently increasing the susceptibility to oxidative disorders (Clancy and Abramson 1995), and this could explain the deficient spermatogenesis and testicular degeneration observed in the present study. Moreover, the alteration in testosterone levels induced by cadmium chloride may be the cause of the significant histopathological changes observed in testes. This result agrees with Tbeileh et al. (2007). Another mechanism explaining the testicular tissue damage caused by cadmium chloride is the decreased expression or lack of metallothionein (MT-1 and MT-2) genes after Cd administration (Waisberg et al. 2003).
Regarding the effect of oral administration of Cd on the histopathological findings in the prostate gland, the results showed severe dilation of some acini, presence of corpora amylacea, inflammatory edema, and hyperplasia of epithelial lining with no evidence of prostatic fluid. Similar results were reported by Alvarez et al. (2004) after oral Cd administration for 3 months suggesting the lack of the functionality of prostate gland and a decreased secretory capacity that accompanied with redox imbalance (Ramirez et al. 2002). Where, Cd has been reported to induce prostate TBARS as a result of lipid peroxidation that affect membrane integrity (Spatz 1992).
The pathological lesions induced by CdCl2 in our study were remarkably reduced by the administration of SP as prophylactic and treatment suggesting that SP confers histological protection, and this came in harmony and support the earlier observation of the present investigation where administration of SP with CdCl2 cause more significant improvement in the testicular structure and function. The role of SP in modulating the pathological alterations induced by metallic toxicity may be returned to the presence of β-carotene that is known to act as powerful antioxidant. The antioxidant mechanism of β-carotene has been suggested to be singlet oxygen quenching, free radical scavenging and chain breaking during lipid peroxidation (Krinsky and Deneke 1982; Gerster 1993). Luxia et al. (1996) reported that β-carotene of spirulina may decrease cell and macromolecule damage, particularly DNA, and help the repair and regeneration of damaged cells. Moreover, β-carotene reported to protect against oxidative damage of P450 systems in Leydig cells (Hanukoglu 2006).
Conclusion
From our findings, it is evident that administrating S. platensis alone could improve the parameters related to male fertility in rats. Moreover, concomitant exposure to S. platensis either as prophylactic or as treatment in Cd-intoxicated rats significantly ameliorated the Cd-induced alterations in the structure and functions of male reproductive system components and enhanced the semen quality parameters and fertility of rats. The role of Spirulina in reversing the toxic effects of cadmium may be due to the presence of several active constituents acting as free radical scavenging enzyme systems and provides protection against Cd-induced testicular damages.
Abbreviations
- CdCl2 :
-
Cadmium chloride
- SP:
-
Spirulina platensis powder
- TES:
-
Testosterone
- LH:
-
Leutinizing hormone
- EST:
-
Estradiol
- 17β-HSD3:
-
17β-Hydroxysteroid dehydrogenase type 3
- 3β-HSD6:
-
3β-Hydroxysteroid dehydrogenase type 6
- NR5A1:
-
Steroidogenic factor 1 (SF1)
References
Adamkovičová M, Toman R, Cabaj M (2010) Diazinon and cadmium acute testicular toxicity in rats examined by histopathological and morphometrical methods. Slovak J Anim Sci 43(3):134–140
Agrawal M, Saleh RA (2002) Role of oxidants in male infertility: rational, significance, and treatment. Urol Clin N Am 29(4):817–827
Alvarez SM, Gomez NN, Scardapane L, Zirulnik F, Martinez D, Gimenez MS (2004) Morphological changes and oxidative stress in rat prostate exposed to a non-carcinogenic dose of cadmium. Toxicol Lett 153(3):365–376
Babadzhanov AS, Abdusamatova N, Yusupova FM, Faizullaeva N, Mezhlumyan GL, Malikova MKH (2004) Chemical composition of spirulina platensis cultivated in Uzbekistan. Chem Nat Compd 40(3):276–279
Bancroft JD, Stevens A (1996) Theory and practice of histological techniques, 4th edn. Churchill Livingstone, New York
Banjia D, Otilia JF, Banjia N, Gouri P, Annamalaib AR (2013) Investigation on the role of Spirulina platensis in ameliorating behavioural changes, thyroid dysfunction and oxidative stress in offspring of pregnant rats exposed to fluoride. Food Chem 140(1–2):321–331
Bermejo P, Pinero E, Villar AM (2008) Iron-chelating ability and antioxidant properties of phycocyanin isolated from a protean extract of Spirulina platensis. Food Chem 110(2):436–445
Bhat VB, Madyastha KM (2001) Scavenging of peroxynitrite by phycocyanin and phycocyanobilin from Spirulina platensis: Protection against oxidative damage to DNA. Biochem Biophys Res Commun 285(2):262–266
Bhat VB, Madyastha KM (2000) C-phycocyanin a potent peroxyl radical scavenger in vivo and in vitro. Biochem Biophys Res Commun 275(1):20–25
Biswas NM, Sengupta R, Chatopadhyay GR, Choudhury A, Sarkar M (2001) Effect of ethanol on cadmium-induced testicular toxicity in male rats. Reprod Toxicol 15:699–704
Careri M, Furlattini L, Mangia A, Musc M, Anklman E, Theobald A, Von Holst C (2001) Supercritical fluid extraction for liquid chromatographic determination of carotenoids in spirulina algae: a chemometric approach. J Chromatogr 912:61–71
Chamorro G, Salazar M, Araújo KG (2002) Update on the pharmacology of Spirulina (Arthrospira), an unconventional food. Arch Latinoam Nutr 52(3):232–240
Clancy RM, Abramson SB (1995) Nitric oxide: a novel mediator of inflammation. Proc Soc Exp Biol Med 210(2):93–101
Ekhoye EI, Nwangwa EK, Aloamaka CP (2013) Changes in some testicular biometric parameters and testicular function in cadmium chloride administered Wistar rats. Br J Med Med Res 3(4):2031–2041
El-Missiry MA, Shalaby F (2000) Role of beta-carotene in ameliorating the cadmium-induced oxidative stress in rat brain and testis. Biochem Mol Toxicol 14(5):238–243
El-shahat AE, Gabr A, Meki A, Mehena E (2009) Altered testicular morphology and oxidative stress induced by cadmium in experimental rats and protective effect of simultaneous green tea extract. Int J Morphol 27(3):757–764
Ferruzzi MG, Böhm V, Courtney PD, Schwartz SJ (2002) Antioxidant and antimutagenic activity of dietary chlorophyll derivatives determined by radical scavenging and bacterial reverse mutagenesis assays. J Food Sci 67(7):2589–2595
Filler R (1993) Method of epididymal sperm morphology. Cited in method of toxicology, Vol 3 Part A, Male Reproductive Toxicology. Academic press Limited, London, pp 334–343
Gaurav D, Shabad P, Dua KK (2010) Protective effect of Spirulina platensis on cadmium induced renaltoxicity in wistar rats. Arch Appl Sci Res 2(1):390–397
Gerster H (1993) Anticarcinogenic effect of common carotenoids. Int J Vitam Nutr Res 63(3):93–121
Gong R, Ding Y, Liu H, Chen Q, Liu Z (2005) Lead biosorption and desorption by intact and pretreated Spirulina maxima biomass. Chemosphere 58(1):125–130
Gunnarsson D, Nordberg G, Lundgren P, Selstam G (2003) Cadmium-induced decrement of the LH receptor expression and c-AMP levels in the testis of rats. Toxicology 183(1–3):57–63
Gurl E, Caner M, Bayraktar L, Dogruman H, Demirci H (2007) Effects of artichoke extract supplementation on gonads of cadmium-treated rats. Biol Trace Elem Res 119(1):51–59
Hanukoglu I (2006) Antioxidant protective mechanisms against reactive oxygen species (ROS) generated by mitochondrial P450 systems in steroidogenic cells. Drug Metabol Rev 38(1–2):171–196
Henrikson R (1989) Earth food spirulina. Cited from Recolina Limited. Ronore Enterprises Inc, Launa Beach, pp 27–65
Hew KW, Ericson WA, Welsh MJ (1993) A single low cadmium dose causes failure of spermiation in the rat. Toxicol Appl Pharmacol 121:15–21
Järup L, Äkesson A (2009) Current status of cadmium as an environmental health problem. Toxicol Appl Pharmacol 238(3):201–208
Jeyaprakash K, Chinnaswamy P (2005) Effect of Spirulina and Liv.52 on cadmium induced toxicity in albino rats. Indian J Exp Biol 43(9):773–781
Krinsky NI, Deneke SM (1982) Interaction of oxygen and oxyradicals with carotenoids. J Nat Cancer Inst 69(1):205–210
Lafuente A, Marquez N, Perez-Lorenzo M, Pazo D, Esquifino AI (2000) Pubertal and postpubertal cadmium exposure differentially affects the hypothalamic-pituitary-testicular axis function in the rat. Food Chem Toxicol 38(10):913–923
Liu J, Qu W, Kadiiska MB (2009) Role of oxidative stress in cadmium toxicity and carcinogenesis. Toxicol Appl Pharmacol 238(4):209–214
Luxia AS, Monica S, Ornella C, Plizzala B, Laura R, Livia B, Anio M, Ennio P (1996) Effect of β-carotene on cell cycle progression of human fibroblasts. Mutagenesis 17(11):2395–2401
Manna P, Sinha M, Sil PC (2008) Cadmium induced testicular pathophysiology: prophylactic role of taurine. Reprod Toxicol 26(3–4):282–291
Martin LJ, Chen H, Liao X, Allayee H, Shih DM, Lee GS, Hovland DN Jr, Robbins WA, Carnes K, Hess RA, Lusis AJ, Collins MD (2007) FK506, a calcineurin inhibitor, prevents cadmium-induced testicular toxicity in mice. Toxicol Sci 100(2):474–485
Massányi P, Lukáč N, Kováčik J, Toman R, Stawarz R, Cigánková V, Ciaglo A (2008) Heavy metals effects on testicular structure in vivo: abstracts from the 8th international conference on risk factors of food chain, Krakow. Slovak J Anim Sci 41:208
Mathew B, Sankaranarayanan R, Nair PP, Varghese P, SomanthanT ABP, Amm NS, Nair MK (1995) Evaluation to chemoprevention of oral cancer with Spirulina fusiformis. Nutr Cancer 24(2):194–202
Mazo VK, Gmoshinskiı IV, Zilova IS (2004) Microalgae Spirulina in human nutrition. Vopr Pitan 73(1):45–53
Meadus WJ (2003) A semi-quantitative RT-PCR method to measure the in vivo effect of dietary conjugated linoleic acid on protein muscle PPAR gene expression. Biol Proced 5:20–28
Messaodi I, Banni M, Said K, Kerkeni A (2010) Evaluation of involvement of testicular metallothine in gene expression in the protective effect of zinc against cadmium-induced testicular pathophysiology in rats. Reprod Toxicol 29(3):339–345
Mudathir FO, Suru SM, Fafunso MA, Obioha UE, Faremi Y (2008) Protective roles of onion and garlic extracts on Cd-induced changes in sperm characteristic and testicular oxidative damage in rats. Food Chem Toxicol 46(12):3604–3611
Nah WH, Kyoo K, Hae SA, Mi JK, Hee-Gyoo K, Jin HJ, Myung CG (2012) Effect of Spirulina maxima on spermatogenesis and steroidogenesis in streptozotocin-induced type I diabetic male rats. Food Chem 134(1):173–179
Nair AR, DeGheselle O, Smeets K, Van Kerkhove E, Cuypers A (2013) Cadmium-induced pathologies: where is the oxidative balance lost (or not)? Int J Mol Sci 14(3):6116–6143
Nemoto K, Miyajima S, Hara S, Saigusa R, Yamada M, Shikama H, Yotsuya S, Skimoto M, Degawa M (2009) Decreased gene expression of testicular cell specific proteins in cadmium-induced acute testicular toxicity. J Health Sci 55(6):952–956
Oliveira H, Loureiro J, Filipe L, Santos C, Ramalho-Santos J, Sousa M, Maria de Lourdes P (2006) Flow cytometry evaluation of lead and cadmium effects on mouse spermatogenesis. Reprod Toxicol 22(3):529–535
Oteiza PI, Adonaylo VN, Keen CL (1999) Cadmium-induced testes oxidative damage in rats can be influenced by dietary zinc intake. Toxicology 137(1):13–22
Patil GR, Rao MV (1999) Role of ascorbic acid on mercuric chloride toxicity in vital organs of mice. Indian J Environ Toxicol 9:53–55
Patra RC, Amiya KR, Swarup D (2011) Oxidative stress in lead and cadmium toxicity and its amelioration. Vet Med Int 2011:1–9
Premkumar K, Abraham SK, Santhiya ST, Ramesh A (2003) Protective effect of Spirulina on chemical-induced genotoxicity in mice. Fitoterapia 75(1):24–31
Ramirez D, Gonzalez R, Merino N, Rodriguez S, Ancheta O (2002) Inhibitory effects of Spirulinain zymozan-induced arthritis in mice. Mediat Inflamm 11(2):75–79
Rana SVS, Singh R, Verma S (1996) Protective effects of few antioxidants on liver function in rats treated with cadmium and mercury. Indian J Exp Biol 34(2):177–179
Reddy CM, Baht VB, Kiranmai G, Reddy MN, Reddanna P, Madyastha KM (2000) Selective inhibition of cyclooxygenase-2 by C-phcocyanin, a biliprotein from spirulina platensis. Biochem Biophys Res Commun 277(3):599–603
Rekha DK, Tripathi Y, Raghuveer CV, Sheila RP, Ramaswamy C, Priya K (2011) Role of vitamin c as an antioxidant in cadmium chloride Induced testicular damage. Int J App Biol Pharm Technol 2(3):484–488
Rennolds J, Malireddy S, Hassan F, Tridandapani S, Parinandi N, Boyaka PN, Cormet-Boyaka E (2012) Curcumin regulates airway epithelial cell cytokine responses to the pollutant cadmium. Biochem Biophys Res Commun 417(1):256–261
Riss J, Decorde K, Sutra T, Delage M, Baccou JC, Jouy N (2007) Phycobiliprotein C-phycocyanin from Spirulina platensis is powerfully responsible for reducing oxidative stress and NADPH oxidase expression induced by an atherogenic diet in hamsters. J Agric Food Chem 55(19):7962–7967
Robb EA, Amann R, Killian G (1978) Daily sperm production and epididymal reverse of pupertal and adult rats. J Reprod Fertil 54(1):103–107
Santos FW, Graca DL, Zeni G, Rocha JBT, Weis SN, Favero AM (2006) Sub-chronic administration of diphenyldiselenide potentiates cadmium-induced testicular damage in mice. Reprod Toxicol 22(3):546–550
Schlingmann K, Michaut MA, McElwee JL, Wolff CA, Travis AJ, Turner RM (2007) Calmodulin and CaMKII in the sperm principal piece: evidence for a motility related calcium/calmodulin pathway. J Androl 28(5):706–716
Seshadri CV, Umesh BV, Manoharan R (1991) β-Carotene studies in Spirulina. Bioresour Technol 38:111–113
Sharma MK, Ambika S, Ashok K, Madhu K (2007) Evaluation of protective efficacy of Spirulina fusiformis against mercury induced nephrotoxicity in Swiss albino mice. Food Chem Toxicol 45(6):879–887
Sheshadri CV, Umesh BU (1992) Spirulina, a nutritious food for the masses. Invent Intell 252–256
Simsek N, Karadeniz A, Kalkan Y, Keles ON, Unal B (2009) Spirulina platensis feeding inhibited the anemia- and leucopenia-induced lead and cadmium in rats. J Hazard Mater 164(2–3):1304–1309
Siu ER, Mruk DD, Porto CS, Chuen-Yan C (2009) Cadmium-induced testicular injury. Toxicol Appl Pharmacol 238(3):240–249
Slott V, Suarez J, Perrealt S (1991) Rat sperm motility analysis: methodologic considerations. Rep Toxicol 5(5):449–458
Spatz L (1992) Introduction. In: Spatz L, Bloom AD (eds) Biological consequences of oxidative stress. Oxford university press, New York, pp 3–22
Tbeileh N, El-Betieha A, Damni H, Khamas W (2007) Effects of long term exposure to cadmium chloride on fertility in adult male mice. Vet Res 1(2):40–48
Thompson J, Bannigan J (2008) Cadmium: toxic effects on the reproductive system and the embryo. Reprod Toxicol 25(3):304–315
Toman R, Massányi P (2002) Changes in the testis and epididymis of rabbits after an intraperitoneal and peroral administration of cadmium. Trace Elem Electrolytes 19(3):114–117
Tremellen K (2008) Oxidative stress and male infertility—a clinical perspective. Hum Reprod Update 14(3):243–258
Upasani CD, Balaraman R (2003) Protective effect of Spirulina on lead induced deleterious changes in the lipid peroxidation and endogenous antioxidants in rats. Phytother Res 17(4):330–334
Vernet P, Aitken RJ, Drevet JR (2004) Antioxidant stratigies in epididymis. Mol Cell Endocrinol 216(1–2):31–39
Wafaa AH, Akram ME, Noha AE (2012) Egyptian date palm pollen ameliorates testicular dysfunction induced by cadmium chloride in adult male rats. J Am Sci 8(4):659–669
Waisberg M, Joseph P, Hale B, Beyersmann D (2003) Molecular and cellular mechanisms of cadmium carcinogenesis. Toxicology 192(2–3):95–117
Wales RG (1971) Reproduction and breeding techniques for laboratory animals. Aust Vet J 47(11):552
Yang HS, Han DK, Kim JR, Sin JC (2006) Effect of α-tocopherol on cadmium induced toxicity in rat testis and spermatogenesis. J Korean Med Sci 21(3):445–451
Conflict of interest
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Farag, M.R., Abd EL-Aziz, R.M., Ali, H.A. et al. Evaluating the ameliorative efficacy of Spirulina platensis on spermatogenesis and steroidogenesis in cadmium-intoxicated rats. Environ Sci Pollut Res 23, 2454–2466 (2016). https://doi.org/10.1007/s11356-015-5314-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5314-9