Abstract
Modified graphene oxide with 5,10,15,20-tetrakis-(4-hexyloxyphenyl)-porphyrin and palladium (II) (signified by GO-CPTMS@Pd-TKHPP) prepared as a novel antifouling polyether sulfone (PES) blended nanofiller membrane. The membrane efficiency has been analyzed such as pure water flux (PWF), hydrophilicity, and antifouling features. By increasing of modified graphene oxide percentage from 0 to 0.1 wt.% in the polymer matrix, the PWF was incremented from 14.35 to 37.33 kg/m2·h at 4 bar. The membrane flux recovery ratio (FRR) has been investigated by applying powdered milk solution; the FRR results indicated that the 0.1 wt.%-modified graphene oxide membrane showed a positive effect on fouling behavior with Rir and FRR value 8.24% and 91.76%, respectively. The nanofiltration membrane performance was assessed applying the Direct Red 16 dye rejection. It was demonstrated that the optimal membranes (0.1 wt.%-modified graphene oxide) had notable dye removal (99.58% rejection). The results are also verified by measuring the scanning electron microscopy (SEM), water contact angle (WCA), and atomic microscopy analysis (AFM).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The most important environmental issue with colored wastewater is the removal of colors from the sewage (Elimelech & Phillip 2011). Industrial colored wastewater produced from textiles, paper, plastics, leather, food, and cosmetics must be treated before wastewater drains into the environment (to recover paint) to prevent health hazards, and degradation of the ecosystem is separated and decomposed (Deka et al. 2014, Moradi et al. 2020). Membrane filtration as a very competitive candidate for water treatment technology has been considered for decades due to less energy efficiency, low cost-effectiveness, and stability compared to biological methods (Asadi et al. 2016, Gholami et al. 2020), adsorption (Balea et al. 2017, Balea et al. 2019), and photocatalytic degradation (Zangeneh et al. 2018, Zangeneh et al. 2019c). Because of this necessity, the application of the membrane processes among the modern methods for separation is of high interest for scientists (Ang et al. 2015, You et al. 2012). Polymeric membranes are very popular with regard to their excellent formation and their unique physicochemical properties in water refinery (Warsinger et al. 2018). The high flux permeation, acceptable solid elimination, and low clogging are integral requirements for the high efficiency of membrane filtration. Despite the hydrophobic nature of polymers, it leads to precipitation of foulant materials (pathogens, NOMs, proteins, polysaccharides, micropollutants, and color) on the surface of the membrane, which subsequently diminishes flux permeation (Hairom et al. 2014, Manda et al. 2014). The membrane’s chemistry and morphology are affected by organic deposits that have destructive effects and are also an important barrier to the further development of membrane applications. Due to extensive research and efforts, one of the accepted solutions for membrane resistance to clogging for water treatment is to change and improve the membrane’s hydrophilicity. The very good dispersion of nanofiller material in the entire membrane matrix, that so-called mix matrix membrane (MMM), in recent years, has brought a new concept for membrane fabrication with high flux, high rejection, and high fouling resistance (Mukherjee et al. 2019). Organic and inorganic nanoparticles due to having specific functional points such as high hydrophilicity, high specific surface area, and good correction can be used as appropriate filler to make nanocomposite membranes. The excerpted of nanomaterials that used in this field are metal–organic framework (MOF)(Emam et al. 2019), polycitrate alumoxane (PC-A)(Pirsaheb et al. 2019), SiO2 nanoparticles (Ding et al. 2019, Zangeneh et al. 2019a), ZnO nanoparticle (Modi & Bellare 2019), zeolite (Mahmodi et al. 2020a, Mahmodi et al. 2020b), graphene oxide (GO)(Januário et al. 2020, Song et al. 2020, Yuan et al. 2020), and graphitic carbon nitride (g-C3N4) (Li et al. 2019).
Graphene oxide-based membranes (GO), which are made from two dimensions of GO nanostructures and polymer, are considered as promising candidates for water purification and desalination beyond traditional porous polymer membranes (Fathizadeh et al. 2017, Hegab & Zou 2015). Considering the high adaptability, high mechanical strength, high surface hydrophilic nature, and 2-D interrelation structure for the separation of ions as well as displacement of molecules, graphene oxide-based membranes have exhibited excellent performance in relation to high flux and impressive antifouling (Huang et al. 2013, Koltonow & Huang 2016, Papageorgiou et al. 2015). Because of agglomeration and the electrostatic repulsion between nanosheets graphene oxide in aqueous solutions, the water stability of the unpolished graphene oxide is weak and tends to break down normally. Therefore, researchers have provided many modifications to solve this problem (Chen & Yan 2010, Hua et al. 2015, Wang et al. 2011).
Today, researchers have shown that the hydrophilicity of graphene oxide with hydrophilic agents helps to solve the problem of decomposition and clogging of GO and GO-based membranes, which strongly suggest that this strategy will help to reduce the contamination of pollutant by modified membranes (Cote et al. 2010, Hu et al. 2016, Lee et al. 2004). In cases of reducing the membrane fouling and degradation of nanosheets by combining triethanolamine (TEOA) with modified titanate nanowires (TNWs) and graphene oxide, Liu and his colleagues have made photocatalytic membranes that were able to solve membranes and graphene oxide problems. The results (flux: 42 L/m2·h; dye rejection: 95%) show that the modified membrane, due to its efficient modifications, has a high degree of antifouling and hydrophilicity that was used to remove Congo Red dye (Liu et al. 2017b). Huang et al., in 2014, developed a combination of graphene oxide and metal–organic framework (ZIF-8) to improve the membrane’s performance for better separation of gasses, which in bicontinuous ZIF-8@GO-based membrane increased hydrogen permselectivity relative to the unmodified state due to the presence of graphene oxide (Huang et al. 2014). In the next work that was introduced in 2017, Ayyaru and Ahn donated hydrophilicity and antifouling properties to the PVDF nanocomposite ultrafiltration membranes by inserting sulfonated graphene oxide (SGO). According to the results, due to the improvement of the graphene oxide by the −SO3H agent, the water permeation flux increased 146.6% and flux recovery ratio (88.7%) compared to the unmodified graphene oxide (water flux: 53.3%; FRR: 75%), indicating that the problem of graphene oxide and the membrane have also been resolved (Ayyaru & Ahn 2017). In 2018, Abdi and coworkers presented a hybrid combination (metformin/GO/Fe3O4) that was successfully introduced into the polymeric membrane for the removal of colored materials and heavy metals. The results of this combination were high hydrophilicity, PWF and antifouling effect, which has been applied to remove direct red-16 and copper ions with 99% and 92% removal, respectively (Abdi et al. 2018). The graphene oxide framework built by use of 1,4-cyclohyxanediamine (CDA) and p-phenylenediamine(pPDA) was introduced by Qian and et al. in 2018 and exposed in the field of modified graphene oxide. The synthesized composition was used in the alumina membrane, which showed great results, that can be mentioned to high flux (20.1 kg/m2·h) and high ionic rejection (99.9%) (Qian et al. 2018).
According to our studies, no publication has been found on the use of GO modified by CPTMS@Pd-TKHPP to improve nanofiltration membrane for colored effluent separation. In this study, with the aim of increasing membrane hydrophilicity and decreasing the water contact angle, the hydrophilic functional groups were added amine, aromatic rings −COOH, −OH, =O, and Pd metal. The addition of non-bounded lone pairs and double bonds as well as hydrophilic functional groups can create an aqueous layer on the surface of the membrane that helps to prevent the formation of a foulant layer on the membrane surface. It was also expected that the addition of aromatic rings during the nanoparticle modification would increase membrane porosity which can improve the membrane pure water flux by itself. As a notice, this modification can be used to construct a novel membrane by the phase inversion method. In the current study, the membrane modified with GO-CPTMS@Pd-TKHPP was firstly synthesized and characterized. In order to identify membrane characteristics, water contact angle, SEM, AFM, and antifouling tests are used. It should be mentioned that the optimal membranes have been used to reject dye from wastewater.
Materials and methods
Materials
The polyvinyl pyrrolidone (PVP) (Mw = 25,000 g/mol) was obtained (Merck Co., Germany). Dimethylacetamide (DMAc) and PES (Mw = 58,000 g/mol) were pouched (BASF Co., Germany). In all experiments, distilled water has been used. 4-Hydroxy benzaldehyde and 1-bromohexane were prepared from Sigma Aldrich, USA. Graphite powder, 3-(choloropropyl)-trimethoxysilane palladium (II) chloride, dimethylformamide (DMF), and ethanol were prepared from Merck, Germany. All the chemical reagents used in our experiment were used without further purification.
Modified graphene oxide nanocomposites preparation
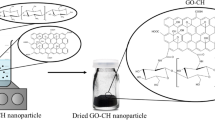
The GO-CPTMS@Pd-TKHPP (Fig. 1) was synthesized by the following methods.
According to the usual way, graphene oxide (GO) was prepared based on modified hummers method from graphite powder (Zaaba et al. 2017); then graphene oxide-3-(choloropropyl)-trimethoxysilane (GO-CPTMS) was synthesized by adding CPTMS to GO in a solution of toluene; then the mixture was stirred and refluxed for 24 h at 110°C; the solid was filtered and dried; after this, 4. 5,10,15,20-tetrakis-(4-hydroxyphenyl)-porphyrin (THPP) was prepared; synthesis of THPP was achieved according to the following procedure. Pyrrole was leisurely added under stirring to a solution of 4-hydroxy benzaldehyde in refluxing propionic acid. Refluxing was continued for 2 h; after this step, the reaction mixture was washed several times and cooled slowly, filtered, and dried; the dark purple crystalline product was precipitated. Then, THPP was added to a solution of GO-CPTMS in refluxing dioxane, and in the presentation of Na2CO3 and KI, a black powder of GO-CPTMS@THPP was obtained. Next 3-(choloropropyl)-trimethoxysilan 5,10,15,20-tetrakis-(4-(hexyloxy) phenyl)-porphyrin GO-CPTMS@Pd-TKHPP was obtained by adding 1-bromohexane to a solution of DMF and K2CO3 (4:4) in a flask and GO-CPTMS@THPP; then, the obtained mixture was refluxed up for 24 h, then dried, and the gray powder was obtained. Finally, GO-CPTMS@Pd-TKHPP was obtained by adding PdCl2 to GO-CPTMS@Pd-TKHPP in the presence of ethanol solvent and reflux condition for nearly 24 h (Bahrami & Kamrani 2018).
Preparation of modified mixed matrix PES-GO membrane
The unmodified and modified membranes were carried out via the phase inversion method. The membrane solution composition for all cases has been listed in Table 1. In order to form the homogenous solution, first, a proper amount of modified GO was added into DMAc and sonicated (DT 102H Bandelin ultrasonic (Germany)) for 30 min. Then, PVP and PES were added to obtain the solution. After this step, the casting solution was kept on a continuous stirrer overnight at room temperature. Finally, in order to increase the amount of homogeneity, dope solutions were sonicated again (20 min) to make sure all air bubbles had been removed and impressive dispersing was done. Finally, by using a self-made knife, the solutions were casted on glassy plates (150-μm thickness) and immediately (without evaporation) moved to distilled water (nonsolvent bath at RT). After forming the polymeric membrane, they were moved to fresh distilled water (24 h). This will remove water-soluble components from the membrane matrix. Finally, the obtained membranes were warped between paper for drying (24 h) (Zinadini et al. 2014).
Characterization of modified GO membranes
The membrane morphology was considered by applying a scanning electron microscope (SEM)(Philips-XL30, the Netherlands) (20 kV acceleration voltage); first, membranes were cut and cleaned with filter paper to clean probable contaminants. Then, they were immersed into liquid nitrogen for 50–70 s. After that, for membrane drying, the frozen membranes were breaking and kept at RT (room temperature). Afterward, the dried membranes were coated by Au ion in order to produce electron conductivity. As the final step, SEM images were captured (20 kV in high vacuum condition).
The surface roughness for the prepared membrane was analyzed by applying atomic force microscopy (AFM) (Nanosurf® Mobile S (Switzerland)); this device was equipped with an optical probe microscope. Small square samples were prepared (2*2 cm) and fixed on a holder, and the surface was scanned (4.4 μm *4.4 μm). The results reflected as (Sa) mean roughness, the root of square data (Sq), and the average difference between the highest peak and the lowest valley (Sz) has been achieved with quantitative analysis.
A recent method to analyze the hydrophilicity of membranes is water contact angle (WCA) (G10, KRUSS, Germany) measurement. The propensity of small distilled water droplets to extend on the membrane surface is known as membrane hydrophilicity. For WCA measurement, in all cases, 2 μl of deionized water has been used. For reducing experimental errors, all examinations were repeated in 4 random sites and the mean valve was reported.
Membrane performance
Pure water flux and antifouling experiments
Membrane performance, pure water flux (PWF), and antifouling attributes of modified NF membranes with GO were examined in a dead-end setup (Fig. 2a) (150 ml and 12.56 cm2). As trance membrane pressure (TMP) nitrogen cylinder, the cell was equipped to force the feed pass through the membrane. In order to concentrate polarization reduction, constant simulation was applied for the cell (400 rpm). In order to earn a stable state, for the first 30 min of the experiment, the TMP was compressed at 5 bar and then it was reduced to 4 bar (Gholami et al. 2017). Based on Eq. (1), the PWF was calculated as
where Δt is the time of permeation, A (m2) is touching surface, and M is the weight of permeation.
Milk powder solution (8000 ppm), to consider the antifouling properties were tested as proper foulant (after PWF test). The milk powder permeation (JP (kg/m2·h)) was tested based on water permeation during the analysis (4 bar, 90 min). After that test, the fouled membrane was cleaned with deionized water (15 min immersed in water without any backwash), then the pure water flux was tested again with distilled water. Based on the following equation, the FRR can be calculated as
As a matter of fact, higher FRR for membranes indicates the distinct antifouling ability of the nanofiltration membranes.
Following the results in order to consider antifouling behavior, during filtration resistance fouling ratio was evaluated in detail, irreversible fouling (Rir), reversible fouling (Rr), and total fouling (Rt) were investigated according to the following equations:
Dye removal
As dye-containing azo groups, direct red-16 was chosen. In this case, to consider membrane efficiency, the filtration was operated in a dead-end setup (120 min at 4 bar); each run was tested by 150 ml of feed. After this step, in order to evaluate long-term filtration and industrial simulation, a cross-flow setup (Fig. 2b) was applied (driving force 4 bar, flow rate 300 L/h at 420 min). In all experiments to propose feed simulation, 50 mg/L of direct red-16 was chosen, which is in the range of typical textile wastewater industries. As a result, the flux recovery ratio and flux were reported based on Eqs. (1) and (2). By using UV-Vis spectrophotometer (520 nm for direct red-16 and 665 nm for methylene blue) (JENWAY 6320D), according to Eq. (6), the rejection was calculated as
where Cf is feed concentration and Cp is permeated concentration of dye (mg/L).
Results and discussion
Modified graphene oxide nanocomposites characterization
The prosperous synthesis of the GO-CPTMS is confirmed by the FT-IR spectra (Fig. 3c). The peak turning up at 810 cm−1 comes back to Si–O–Si, the symmetric vibration. The peak at 1107 cm−1 is relevant to asymmetrical Si–O–Si stretching. About GO-CPTMS@TKHPP (Fig. 3e), it recognized that the peak of the N–H bending and stretching frequencies are located at ~3300 cm−1 and ~960 cm−1 and shown free base porphyrins. When the PdCl2 was interred into the porphyrin ring, the N–H peak vibration of free base porphyrins vanished and features of the functional groups of Pd–N bond shaped at ~1009 cm−1(Fig. 3f), that analyzed the configuration of a metal–ligand bond (Bahrami & Kamrani 2018).
In Fig. 4, the EDX analysis of nanosheets (GO-CPTMS@Pd-TKHPP) displayed the attendance of the anticipation elements in the nanosheets of GO, such as silicon, oxygen, palladium, nitrogen, and carbon. As can been seen, the element distribution of the Si-Pd/GO is approximately 1.43 (Zhang et al. 2017).
XRD pattern of GO-CPTMS@Pd-TKHPP as nanostructure exhibits peaks at 2θ = 40.4, 46.8, 68 and 74.1° which correspond to (111), (200), (220), and (331) crystalline planes of Pd, respectively, illustrated that Pd element does not exist in the form of Pd(II) and it was shown Pd (0)(Fig. 5) (Fareghi-Alamdari et al. 2016, Zolfigol et al. 2013).
The stratification structure of GO-CPTMS@Pd-TKHPP was also perceived from transmission electron microscopy (TEM) and scanning electron microscopy (SEM). The scanning electron microscopy (SEM) illustration of GO-CPTMS@Pd-TKHPP (Fig. 6a) shows the ingredient scale, morphology, and superficies sameness. Transmission electron microscopy (TEM) image of the GO-CPTMS@Pd-TKHPP indicated which Pd(II)-TKHPP was seated on the surface of graphene oxide (Fig. 6b)(Bahrami & Kamrani 2018).
Morphology analysis
In order to consider the effect of GO-CPTMS@Pd-TKHPP, concentration on the structure and morphology of the membrane and SEM images are shown in Fig. 7. As shown in the picture, asymmetric structure with a uniform dispersion of nanoparticles is visible and can be easily recognized as finger-like pores with the thin top layer and thick bottom layer due to fast immigration of hydrophilic NPs in the phase inversion step. The identical building of the membranes implies that the impact of nanoparticles on morphology was low. So, this result shows that it was not an important influence on the formation of mold membrane. Small differences in the structure of membranes at low concentrations were observed that described the increase in porosity and consequently increased the radius of pores. In different concentrations of nanoparticles, 0.1 wt.% provided the minimum thickness of the top layer in the membrane. As seen in Fig. 8, by adding 0.1 wt.% modified graphene oxide nanocompound, the porosity was increased, and a further increase in the amounts of the nanosheets to more than 0.1 wt.% caused the reduction of pore radius (Vatanpour et al. 2011).
The prepared membranes pure water flux and hydrophilicity
Water contact angle measurement can be used to identify the hydrophilicity surface of prepared membranes. As illustrated in Table 2, by adding nanofiller to the membrane matrix, a logical trend on WCA reduction can be observed. It should be noted that, due to presenting a hydrophilic functional group on the GO, the membrane hydrophilicity was increased (membrane contact angle was decreased), causing an increase in membrane permeability due to hydrogen bonding between water and membrane surface.
Flux against the time of the membranes with different concentrations of modified graphene oxide nanosheets. The filtration process includes three phases (water flux, milk powder filtration, and second water flux) after washing fouled membranes (M1 = unfilled, M2 = 0.1 wt.%, M3 = 0.5 wt.%, and M4 = 1.0 wt.%)
Flux transmission depends on many factors, that hydrophilicity is one of the most important cases. The hydrophilic functional groups of the GO-CPTMS@Pd-TKHPP on the membrane surface caused a change in the pure water flux (PWF) that established hydrogen bonds between water molecules and membrane surface (Table 2). Adding the nanoparticles up to 0.1 wt.% led to an increase in PWF (M2). By adding nanoparticles to the membrane matrix, an increment is notable for M2. In higher loadings (M3, M4), the accumulation effect that caused PWF reduction might be due to porosity occupation (Fig. 8). It should be noted that the powdered milk solution rejection was more than 98%, showing the interrelation between flux increment and defects or cracks in the membrane due to a poor bond between the modified graphene oxide and PES.
Fouling behavior of the prepared membranes
Figure 9 shows the antifouling performance of the prepared membrane in three-step filtration. The results of 0.1 wt.% of modified graphene oxide (M2) demonstrated the highest permeability compared to the other concentrations. As can be obtained (Fig. 9), the difference in flux, in the first step and third step, observed between the modified and unmodified membranes is caused by the presence of hydrophilic function group on the GO surface, which has been donated to have a hydrophilic effect on the PES membrane.
FRR is one of the important parameters for antifouling assessment. The FRR diagram was displayed in Table 3. The greatest FRR for membrane has been achieved for membrane embedded with 0.1 wt.% (M1) of modified graphene oxide. Antifouling performance of embedded mixed matrix membranes could be related to hydrophilicity for modified membranes, although this effect has an optimal ratio (M2) in higher additives due to agglomeration effect on the acceptable trend (FRR reduction) is notable. That was induced by remaining functional groups of GO-CPTMS@Pd-TKHPP on the membrane surface. Creating a hydration layer on the membrane surface due to the presence of hydrophilic nanosheets and hydrogen bonding can inhibit the formation of a foulant layer on the membrane surface. The results demonstrated that the addition of the modified graphene oxide nanosheets (ingenious design with effective groups) in the PES membrane was useful to improve membrane permeability (Ng et al. 2013).
According to the deposition phenomenon, resistance parameters as irreversible fouling resistance (Rir) and reversible fouling resistance (Rr) were obtained, as shown in Fig. 10. Irreversible fouling resistance of membrane embedded with 0.1 wt.% nanoparticles has reached the lowest value, but Rr has also increased with increasing concentration. The trapped residual foulants into the membrane pores and valley surface are responsible for irreversible aggregation, which noticeably can be removed by the washing process (immersing in water). The M2 membrane (embedded with 0.1 wt.% modified graphene oxide) indicated the highest FRR (91.76%).
AFM technique was used for measuring the membranes’ surface roughness. As a matter of fact, lower roughness replies to stronger antifouling property. In addition, foulants may be trapped in the valleys of the membrane with unusual surfaces, resulting in clogging of the valleys. The AFM and its associated results are presented in Fig. 11 and Table 4. The average roughness (Sa) of the bare nanofiltration membrane changed from 21.251 to 8.003 nm for the modified membranes with 0.1 wt.% of GO-CPTMS@Pd-TKHPP and then enhanced to 9.707 nm for the modified graphene oxide 1 wt.%. In the lower concentration of modified graphene oxide, because of less electrostatic interactions between the modified graphene oxide, they are tidied in membrane symmetrically, resulting in a smooth membrane surface. But due to the increment in agglomeration and pore size of modified graphene oxide, membrane surface roughness was enhanced at a higher concentration of modified graphene oxide (Zangeneh et al. 2019a).
Dye removal efficiency
Rejection and performance
The dye rejection results are indicated in Fig. 12, that described the membrane capability for direct red 16 and methylene blue dye removal from simulated feed (50 mg/L) after 60-min filtration as a function of the modified graphene oxide percentage in the casting solution. The dye removal efficiency for the modified membrane (M2) was achieved at 99.58% and 97.98% for direct red 16 and methylene blue, respectively. Although the removal percentage for M1 is lower (87.5% and 83.29%, respectively). This is caused by the presence of hydrophilic nanofiller in the membrane structures (Table 2) as an excellent refining agent (Wang et al. 2011). This high removal percentage of colored materials can be attributed to two factors: (i) chemical interactions (non-covalent bonds and π-π stacking) and (ii) electrostatic repulsion between color and membrane surface. In other words, because the direct red 16 and surface of the membrane both have a negative charge, they repel each other (given the existence dispersion of negative groups at the matrix of the dye molecule, the direct red-16 indicates a negative nature at the neutral pH), as well as non-covalent bonds (hydrogen bond) and π-π stacking between the agents of direct red 16 (–SO3, –NH2, –OH, and aromatic rings) and distributed GO-CPTMS@Pd-TKHPP on the membrane surface, prevent passing of dye through the membrane and for these reasons that the removal efficiency was more than 99% (Sabbatini et al. 2010, Zhang et al. 2019, Zhou et al. 2019). Although, as shown in Fig. 12 for methylene blue (aromatic rings –N and –S), the removal percentage has decreased which caused the positive nature of the methylene blue and low molecular weight in methylene blue than direct red 16 (methylene blue and direct red 16 molecular weight: 319.85 g/mol and 637.55 g/mol, respectively), so it is possible methylene blue to pass through the membrane and results show the dye removal reduction for methylene blue than direct red 16 (Fig. 12). It should be noted that the permeation flux of dye solutions was slightly lower than the pure water flux due to the adsorption of dye molecules on the membrane surface and the concentration polarization. According to the initial concentrations of direct red 16 (50 mg/L), the excellent rejection was obtained 99.58%, but for precise evaluation of the dye rejection at a high concentration (200 mg/L), the dye rejection was also investigated that achieved 97.29% (Bouazizi et al. 2017, Liu et al. 2017a); also, in Table 6 comparison, the performance of dye removal is provided (Table 5).
Long-term behavior
The results of long-term performance during the treatment of colored wastewater by the cross-flow system are displayed in Table 6 and Fig. 13. From the results, due to the high cross-flow velocity (CFV) and hence the increase of the Reynolds number (create turbulent flow due to Re > 4000) so that the cross-flow setup permeation showed enhancement in comparison with dead-end setup due to polarization concentration reduction (Bagheri et al. 2019).
According to Fig. 13 and Table 5, slump less flux in membrane permeability was observed in the prolonged dye removal process. This phenomenon can be justified by a reduction in the concentration polarization behind the membrane during the examination process. The drop of the membrane flux at the M1 (31.58% reduction) is much higher than that of the M2 (2.33% reduction), which can be attributed to the membrane modification by the hydrophilic nanoparticle and the donation of the antifouling property for the M2 membrane. According to the results presented, it can be obtained that the embedding of GO-CPTMS@Pd-TKHPP hydrophilic nanocomposite in the membrane matrix has improved the performance of the color removal, flux enhancement, and antifouling property (Zangeneh et al. 2019b).
Conclusion
In this work, a hydrophilic nanofiller (modified graphene oxide nanocomposite) was successfully used and blended with the bare membrane during the phase inversion to reclaim membrane flux, antifouling, and dye removal performance. The effect of modified graphene oxide in the casting solution was evaluated and chosen as the optimal membrane. The selected modified graphene oxide-PES membrane (0.1 wt.% of modified graphene oxide) exhibited better pure water flux 37.33 kg/m2·h and dye rejection of 99.58 % compared to the unmodified membranes. By embedding the modified graphene oxide, membrane hydrophilicity was significantly improved. Antifouling experiments showed that the addition of the modified graphene oxide in the casting solution led to an increment in flux recovery ratio from 58.65 to 91.76% and a decrement in irreversible fouling from 41.35 to 8.24 %. The result of long filtration in the cross-flow system revealed that the optimized membrane can be proposed for industrial wastewater treatment.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abdi G, Alizadeh A, Zinadini S, Moradi G (2018) Removal of dye and heavy metal ion using a novel synthetic polyethersulfone nanofiltration membrane modified by magnetic graphene oxide/metformin hybrid. J Membr Sci 552:326–335
Ang WL, Mohammad AW, Hilal N, Leo CP (2015) A review on the applicability of integrated/hybrid membrane processes in water treatment and desalination plants. Desalination 363:2–18
Asadi A, Zinatizadeh AA, Van Loosdrecht M (2016) High rate simultaneous nutrients removal in a single air lift bioreactor with continuous feed and intermittent discharge regime: process optimization and effect of feed characteristics. Chem Eng J 301:200–209
Ayyaru S, Ahn Y-H(2017) Application of sulfonic acid group functionalized graphene oxide to improve hydrophilicity, permeability, and antifouling of PVDF nanocomposite ultrafiltration membranes. J Membr Sci 525:210–219
Bagheri M, Akbari A, Mirbagheri SA (2019) Advanced control of membrane fouling in filtration systems using artificial intelligence and machine learning techniques: a critical review. Process Saf Environ Prot 123:229–252
Bahrami K, Kamrani SN (2018) Synthesis, characterization and application of graphene palladium porphyrin as a nanocatalyst for the coupling reactions such as: Suzuki-Miyaura and Mizoroki-Heck. Appl Organomet Chem 32:e4102
Balea A, Monte MC, de la Fuente E, Negro C, Blanco Á (2017) Application of cellulose nanofibers to remove water-based flexographic inks from wastewaters. Environ Sci Pollut Res 24:5049–5059
Balea A, Monte MC, Fuente E, Sanchez-Salvador JL, Blanco A, Negro C (2019) Cellulose nanofibers and chitosan to remove flexographic inks from wastewaters. Environmental Science: Water Research & Technology 5:1558–1567
Bouazizi A, Breida M, Achiou B, Ouammou M, Calvo JI, Aaddane A, Younssi SA (2017) Removal of dyes by a new nano–TiO2 ultrafiltration membrane deposited on low-cost support prepared from natural Moroccan bentonite. Appl Clay Sci 149:127–135
Chen J-L, Yan X-P(2010) A dehydration and stabilizer-free approach to production of stable water dispersions of graphene nanosheets. J Mater Chem 20:4328–4332
Cote LJ, Kim J, Zhang Z, Sun C, Huang J (2010) Tunable assembly of graphene oxide surfactant sheets: wrinkles, overlaps and impacts on thin film properties. Soft Matter 6:6096–6101
Deka JR, Liu C-L, Wang T-H, Chang W-C, Kao H-M(2014) Synthesis of highly phosphonic acid functionalized benzene-bridged periodic mesoporous organosilicas for use as efficient dye adsorbents. J Hazard Mater 278:539–550
Ding S, Zhang L, Li Y, L-a H (2019) Fabrication of a novel polyvinylidene fluoride membrane via binding SiO2 nanoparticles and a copper ferrocyanide layer onto a membrane surface for selective removal of cesium. J Hazard Mater 368:292–299
Elimelech M, Phillip WA (2011) The future of seawater desalination: energy, technology, and the environment. Science 333:712–717
Emam HE, Abdelhamid AE, Abdelhameed RM (2019) Refining of liquid fuel from N-containing compounds via using designed polysulfone@ metal organic framework composite film. J Clean Prod 218:347–356
Fareghi-Alamdari R, Golestanzadeh M, Bagheri O (2016)meso-Tetrakis [4-(methoxycarbonyl) phenyl] porphyrinatopalladium (II) supported on graphene oxide nanosheets (Pd (II)-TMCPP-GO): synthesis and catalytic activity. RSC Adv 6:108755–108767
Fathizadeh M, Xu WL, Zhou F, Yoon Y, Yu M (2017) Graphene oxide: a novel 2-dimensional material in membrane separation for water purification. Adv Mater Interfaces 4:1600918
Gholami F, Zinadini S, Zinatizadeh A, Noori E, Rafiee E (2017) Preparation and characterization of an antifouling polyethersulfone nanofiltration membrane blended with graphene oxide/Ag nanoparticles. Int J Eng Trans A Basics 30:1425–1433
Gholami F, Zinatizadeh A, Zinadini S, McKay T, Sibali L (2020) An innovative jet loop-airlift bioreactor for simultaneous removal of carbon and nitrogen from soft drink industrial wastewater: process performance and kinetic evaluation. Environ Technol Innov 19:100772
Hairom NHH, Mohammad AW, Kadhum AAH (2014) Nanofiltration of hazardous Congo red dye: performance and flux decline analysis. Journal of Water Process Engineering 4:99–106
Hegab HM, Zou L (2015) Graphene oxide-assisted membranes: fabrication and potential applications in desalination and water purification. J Membr Sci 484:95–106
Hu M, Zheng S, Mi B (2016) Organic fouling of graphene oxide membranes and its implications for membrane fouling control in engineered osmosis. Environ Sci Technol 50:685–693
Hua Z, Tang Z, Bai X, Zhang J, Yu L, Cheng H (2015) Aggregation and resuspension of graphene oxide in simulated natural surface aquatic environments. Environ Pollut 205:161–169
Huang A, Liu Q, Wang N, Zhu Y, Jr C (2014) Bicontinuous zeolitic imidazolate framework ZIF-8@ GO membrane with enhanced hydrogen selectivity. J Am Chem Soc 136:14686–14689
Huang H, Song Z, Wei N, Shi L, Mao Y, Ying Y, Sun L, Xu Z, Peng X (2013) Ultrafast viscous water flow through nanostrand-channelled graphene oxide membranes. Nat Commun 4:2979
Januário EFD, Beluci NCL, Vidovix TB, Vieira MF, Bergamasco R, Vieira AMS (2020) Functionalization of membrane surface by layer-by-layerself-assembly method for dyes removal. Process Saf Environ Prot 134:140–148
Koltonow AR, Huang J (2016)Two-dimensional nanofluidics. Science 351:1395–1396
Lee N, Amy G, Croué J-P, Buisson H (2004) Identification and understanding of fouling in low-pressure membrane (MF/UF) filtration by natural organic matter (NOM). Water Res 38:4511–4523
Lessan F, Karimi M, Arami M (2016) Tailoring the hierarchical porous structure within polyethersulfone/cellulose nanosheets mixed matrix membrane to achieve efficient dye/salt mixture fractionation. J Polym Res 23:171
Li R, Ren Y, Zhao P, Wang J, Liu J, Zhang Y (2019) Graphitic carbon nitride (g-C3N4) nanosheets functionalized composite membrane with self-cleaning and antibacterial performance. J Hazard Mater 365:606–614
Liu C, Mao H, Zheng J, Zhang S (2017a) Tight ultrafiltration membrane: preparation and characterization of thermally resistant carboxylated cardo poly (arylene ether ketone) s (PAEK-COOH) tight ultrafiltration membrane for dye removal. J Membr Sci 530:1–10
Liu G, Han K, Ye H, Zhu C, Gao Y, Liu Y, Zhou Y (2017b) Graphene oxide/triethanolamine modified titanate nanowires as photocatalytic membrane for water treatment. Chem Eng J 320:74–80
Mahmodi G, Dangwal S, Zarrintaj P, Zhu M, Mao Y, Mcllroy DN, Saeb MR, Vatanpour V, Ramsey JD, Kim S-J(2020a) NaA zeolite-coated meshes with tunable hydrophilicity for oil-water separation. Sep Purif Technol 240:116630
Mahmodi G, Zarrintaj P, Taghizadeh A, Taghizadeh M, Manouchehri S, Dangwal S, Anil R, Ganjali MR, Ramsey JD, Kim S-J(2020b) From microporous to mesoporous mineral frameworks: an alliance between zeolite and chitosan. Carbohydr Res 107930:107930
Manda BK, Worrell E, Patel MK (2014) Innovative membrane filtration system for micropollutant removal from drinking water–prospective environmental LCA and its integration in business decisions. J Clean Prod 72:153–166
Modi A, Bellare J (2019) Efficient removal of dyes from water by high flux and superior antifouling polyethersulfone hollow fiber membranes modified with ZnO/cGO nanohybrid. Journal of Water Process Engineering 29:100783
Moradi G, Zinadini S, Rajabi L (2020) Development of high flux nanofiltration membrane using para-amino benzoate ferroxane nanoparticle for enhanced antifouling behavior and dye removal. Process Saf Environ Prot 144:65–78
Mukherjee R, Bhunia P, De S (2019) Nanofiltration range desalination by high flux graphene oxide impregnated ultrafiltration hollow fiber mixed matrix membrane. J Clean Prod 213:393–405
Ng LY, Mohammad AW, Leo CP, Hilal N (2013) Polymeric membranes incorporated with metal/metal oxide nanoparticles: a comprehensive review. Desalination 308:15–33
Panda SR, De S (2014) Preparation, characterization and performance of ZnCl2 incorporated polysulfone (PSF)/polyethylene glycol (PEG) blend low pressure nanofiltration membranes. Desalination 347:52–65
Papageorgiou DG, Kinloch IA, Young RJ (2015)Graphene/elastomer nanocomposites. Carbon 95:460–484
Pirsaheb M, Farahani MHDA, Zinadini S, Zinatizadeh AA, Rahimi M, Vatanpour V (2019) Fabrication of high-performance antibiofouling ultrafiltration membranes with potential application in membrane bioreactors (MBRs) comprising polyethersulfone (PES) and polycitrate-alumoxane (PC-A). Sep Purif Technol 211:618–627
Qian Y, Zhou C, Huang A (2018)Cross-linking modification with diamine monomers to enhance desalination performance of graphene oxide membranes. Carbon 136:28–37
Sabbatini P, Yrazu F, Rossi F, Thern G, Marajofsky A, de Cortalezzi MF (2010) Fabrication and characterization of iron oxide ceramic membranes for arsenic removal. Water Res 44:5702–5712
Safarpour M, Vatanpour V, Khataee A (2016) Preparation and characterization of graphene oxide/TiO2 blended PES nanofiltration membrane with improved antifouling and separation performance. Desalination 393:65–78
Song Y, Sun Y, Chen M, Huang P, Li T, Zhang X, Jiang K (2020) Efficient removal and fouling-resistant of anionic dyes by nanofiltration membrane with phosphorylated chitosan modified graphene oxide nanosheets incorporated selective layer. Journal of Water Process Engineering 34:101086
Vatanpour V, Madaeni SS, Moradian R, Zinadini S, Astinchap B (2011) Fabrication and characterization of novel antifouling nanofiltration membrane prepared from oxidized multiwalled carbon nanotube/polyethersulfone nanocomposite. J Membr Sci 375:284–294
Wang H-W, Hu Z-A, Chang Y-Q, Chen Y-L, Zhang Z-Y, Yang Y-Y, Wu H-Y(2011) Preparation of reduced graphene oxide/cobalt oxide composites and their enhanced capacitive behaviors by homogeneous incorporation of reduced graphene oxide sheets in cobalt oxide matrix. Mater Chem Phys 130:672–679
Wang Y, Zhu J, Dong G, Zhang Y, Guo N, Liu J (2015) Sulfonated halloysite nanotubes/polyethersulfone nanocomposite membrane for efficient dye purification. Sep Purif Technol 150:243–251
Warsinger DM, Chakraborty S, Tow EW, Plumlee MH, Bellona C, Loutatidou S, Karimi L, Mikelonis AM, Achilli A, Ghassemi A (2018) A review of polymeric membranes and processes for potable water reuse. Prog Polym Sci 81:209–237
You S-J, Semblante GU, Lu S-C, Damodar RA, Wei T-C(2012) Evaluation of the antifouling and photocatalytic properties of poly (vinylidene fluoride) plasma-grafted poly (acrylic acid) membrane with self-assembled TiO2. J Hazard Mater 237:10–19
Yuan X-T, Xu C-X, Geng H-Z, Ji Q, Wang L, He B, Jiang Y, Kong J, Li J (2020) Multifunctional PVDF/CNT/GO mixed matrix membranes for ultrafiltration and fouling detection. J Hazard Mater 384:120978
Zaaba N, Foo K, Hashim U, Tan S, Liu W-W, Voon C (2017) Synthesis of graphene oxide using modified hummers method: solvent influence. Procedia engineering 184:469–477
Zangeneh H, Zinatizadeh AA, Feyzi M, Zinadini S, Bahnemann DW (2018) Photomineralization of recalcitrant wastewaters by a novel magnetically recyclable boron doped-TiO2-SiO2 cobalt ferrite nanocomposite as a visible-driven heterogeneous photocatalyst. Journal of environmental chemical engineering 6:6370–6381
Zangeneh H, Zinatizadeh AA, Zinadini S, Feyzi M, Bahnemann DW (2019a) Preparation and characterization of a novel photocatalytic self-cleaning PES nanofiltration membrane by embedding a visible-driven photocatalyst boron doped-TiO2SiO2/CoFe2O4 nanoparticles. Sep Purif Technol 209:764–775
Zangeneh H, Zinatizadeh AA, Zinadini S, Feyzi M, Bahnemann DW (2019b) Preparation ultrafine L-methionine (C, N, S triple doped)-TiO2-ZnO nanoparticles and their photocatalytic performance for fouling alleviation in PES nanocomposite membrane. Compos Part B 176:107158
Zangeneh H, Zinatizadeh AA, Zinadini S, Feyzi M, Rafiee E, Bahnemann DW (2019c) A novel L-histidine (C, N) codoped-TiO2-CdS nanocomposite for efficient visible photo-degradation of recalcitrant compounds from wastewater. J Hazard Mater 369:384–397
Zhang J, Feng A, Bai J, Tan Z, Shao W, Yang Y, Hong W, Xiao Z (2017)One-pot synthesis of hierarchical flower-like Pd-Cu alloy support on graphene towards ethanol oxidation. Nanoscale Res Lett 12:521
Zhang X, Li H, Wang J, Peng D, Liu J, Zhang Y (2019)In-situ grown covalent organic framework nanosheets on graphene for membrane-baseddye/salt separation. J Membr Sci 581:321–330
Zhou M-Y, Zhang P, Fang L-F, Zhu B-K, Wang J-L, Chen J-H, Abdallah HM (2019) A positively charged tight UF membrane and its properties for removing trace metal cations via electrostatic repulsion mechanism. J Hazard Mater 373:168–175
Zhu J, Tian M, Zhang Y, Zhang H, Liu J (2015) Fabrication of a novel “loose” nanofiltration membrane by facile blending with Chitosan–Montmorillonite nanosheets for dyes purification. Chem Eng J 265:184–193
Zinadini S, Zinatizadeh AA, Rahimi M, Vatanpour V, Zangeneh H (2014) Preparation of a novel antifouling mixed matrix PES membrane by embedding graphene oxide nanoplates. J Membr Sci 453:292–301
Zolfigol MA, Khakyzadeh V, Moosavi-Zare AR, Rostami A, Zare A, Iranpoor N, Beyzavi MH, Luque R (2013) A highly stable and active magnetically separable Pd nanocatalyst in aqueous phase heterogeneously catalyzed couplings. Green Chem 15:2132–2140
Author information
Authors and Affiliations
Contributions
Foad Gholami: conceptualization, data curation, formal analysis, writing – original draft
Sirus Zinadini: funding acquisition, project administration
Soheila Nakhjiri Kamrani: investigation
Ali Akbar Zinatizadeh: supervision, writing – review and editing
Kiumars Bahrami: writing – review and editing, supervision
Corresponding author
Ethics declarations
Ethics approval
Not applicable
Consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Angeles Blanco
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gholami, F., Zinadini, S., Kamrani, S.N. et al. Color removal from wastewater using a synthetic high-performance antifouling GO-CPTMS@Pd-TKHPP/polyether sulfone nanofiltration membrane. Environ Sci Pollut Res 29, 20463–20478 (2022). https://doi.org/10.1007/s11356-021-16655-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-16655-8