Abstract
Water-based or flexographic inks in paper and plastic industries are more environmentally favourable than organic solvent-based inks. However, their use also creates new challenges because they remain dissolved in water and alter the recycling process. Conventional deinking technologies such as flotation processes do not effectively remove them. Adsorption, coagulation/flocculation, biological and membrane processes are either expensive or have negative health impacts, making the development of alternative methods necessary. Cellulose nanofibers (CNF) are biodegradable, and their structural and mechanical properties are useful for wastewater treatment. TEMPO-oxidised CNF have been evaluated for the decolourisation of wastewaters that contained copper phthalocyanine blue, carbon black and diarlyide yellow pigments. CNF in combination with a cationic polyacrylamide (cPAM) has also been tested. Jar-test methodology was used to evaluate the efficiency of the different treatments and cationic/anionic demand, turbidity and ink concentration in waters were measured. Results show that dual-component system for ink removal has a high potential as an alternative bio-based adsorbent for the removal of water-based inks. In addition, experiments varying CNF and cPAM concentrations were performed to optimise the ink-removal process. Ink concentration reductions of 100%, 87.5% and 83.3% were achieved for copper phthalocyanine blue, carbon black and diarlyide yellow pigments, respectively. Flocculation studies carried out show the decolourisation mechanism during the dual-component treatment of wastewaters containing water-based inks.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recycling printing plastic and paper products from industrial waste streams is gaining importance owing to the demand for raw materials, environmental concerns, solid waste considerations and circular economy objectives. For environmental and health reasons, the use of traditional organic ink solvents has gradually been replaced by water and non-volatile products. Nevertheless, water-based inks can also become an ecological and recycling problem because they remain dissolved in the process water. Although ink manufacturers have made efforts to produce deinkability and recyclability inks, new deinking strategies are still necessary to remove water-based ink from wastewaters.
In the last years, several studies have been conducted for inks removal using different treatments. Adsorption is the most frequent process employed for the treatment of coloured effluents, using activated carbon (Wu et al. 2001a; Yang and Al-Duri 2001; Noonpui et al. 2010), resins (Karcher et al. 2002; Greluk and Hubicki 2013), agricultural and wood wastes (Annadurai et al. 2002; Robinson et al. 2002; Noonpui et al. 2010; Adegoke and Bello 2015), microorganisms (Fu and Viraraghavan 2003; Aksu et al. 2008) or bio-polymers (Wu et al. 2001b; Roussy et al. 2005; Raval et al. 2016). However, this process usually comprises a simple transfer of pollutant from a dispersed phase to a concentrated one. The necessity to control the discharge of these loaded material and the use of expensive materials sometimes makes the process not viable. Photochemical and catalytic degradation processes also remove inks (Arslan and Balcioglu 2001; Genc 2004), but these oxidation processes may cause pollution hazards since, in some cases, the products generated during the oxidation are more hazardous for the environment than the original contaminants. Coagulation/flocculation are shown to be simpler and more cost-effective, but the use of metal compounds such us aluminium, copper or bromide (Songsiri et al. 2002; Gecol et al. 2003; Kacha et al. 2003; Kim et al. 2004; Fernandez and Hodgson 2013) is controversial due to the possible impact on cancer or Alzheimer diseases.
Therefore, wastewater treatments are still important for printing and plastic industries due to the straighten regulations (Noonpui et al. 2010) and, new bio-technologies are needed for water-based ink removal.
Recent advances in nanoscale science suggest that many of the current water treatment problems might be solved or greatly ameliorate by using cellulose nanofibers (CNF) (Carpenter et al. 2015). The main advantages of CNF are their high potential availability, renewable nature, biodegradability, non-abrasive properties, high specific strength and safer handling (Besbes et al. 2011). CNF can be produced from different cellulose sources, when virgin fibres are the primary raw material (Taipale et al. 2010; Gonzalez et al. 2012; Liimatainen et al. 2013), using mechanical treatments or a combination of chemical, enzymatic and mechanical treatments. Chemical and enzymatic pre-treatments reduce the energy of the mechanical process to obtain CNF from 100 kWh/kg, for unmodified cellulose preparations, to 1–2 kWh/kg, depending on the extent of the pre-treatment (Isogai 2009; Siro and Plackett 2010) and allow to obtain high CNF quality. One of the most widely used chemical pre-treatment is TEMPO (2,2,6,6-tetramethyl-1-piperidinyloxy)-mediated oxidation (Nechyporchuk et al. 2016). As a result, the cellulose fibre structure can easily be disintegrated to individual nanofibrils due to repulsive forces among the ionised carboxylates (Saito et al. 2007; Isogai et al. 2011). However, some recent studies show that the market prices of the chemicals used in the oxidation process, mainly the TEMPO catalyst, increase the CNF production cost. Thus, the cost to achieve a 75% increase in breaking length by the addition of TEMPO-oxidised CNF was around 4 €/kg of paper. Therefore, further studies should be carried out to obtain CNF at lower cost (Delgado-Aguilar et al. 2015).
Moreover, the high aspect ratio, nanometre width and the high negative charge may impart CNF a high bridging ability, thus constituting a pseudo-microparticle system with cationic polymers that retains small particles, such us fillers, by inducing flocculation among them (Jin et al. 2014).
However, very few studies on the use of CNF as a water chemical have been published. Most of them focussed on functionalising the CNF surface to increase the binding efficiency of pollutants (Yang et al. 2014) and oils (Korhonen et al. 2011; Zhang et al. 2014). TEMPO-oxidised cellulose nanofibers have been used for the adsorption of various metals from aqueous solutions, most efficiently with lead, calcium and silver (Saito and Isogai 2005; Carpenter et al. 2015). From the literature, no reports were found on the use of CNF for water-based ink removal.
Therefore, an alternative method for flexographic inks removal based on TEMPO-oxidised cellulose nanofibers alone or in combination with cPAM has been developed and validated with three of the most commonly flexographic inks with a different core chemical structure (copper phthalocyanine blue, carbon black and diarlyide yellow pigments) used in printing paper and plastic products. The efficiency of the treatments has been studied using the Jar-test methodology by measuring cationic/anionic demand, turbidity and ink concentration in the treated water. Several experiments varying CNF and cPAM concentrations were performed to optimise the ink-removal process.
Experimental
Materials
Synthetic water-based ink solutions were prepared using copper phthalocyanine blue, carbon black and diarlyide yellow pigments. Their maximum wavelength (λ max) were 615, 500 and 440 nm, respectively.
CNF were obtained from never dried refined Eucalyptus globulus bleached kraft pulp, manufactured by Torraspapel, S.A. (Spain). CNF were obtained by TEMPO-mediated oxidation, by using 5 mmol of NaClO/g of dry pulp. The oxidation conditions were those described by Saito et al. (Saito et al. 2007). Once the pulp was oxidised, a filtration cleaning process was performed using distilled water to reach a pH value around 7. Finally, six steps of homogenisation at 600 bar were applied in a laboratory homogeniser PANDA PLUS 2000 manufactured by GEA Niro Soavy (Parma, Italy). CNF were characterised by determining nanofibrillation yield, carboxylated groups, cationic demand (CD), transmittance (T) and polymerisation degree (PD) according to Balea et al. (2016). Table 1 lists the properties of the synthetic water-based ink solutions and the CNF used in this study.
Linear cationic polyacrylamide (cPAM) polymer was kindly supplied by AQUA+TECH Switzerland. cPAM has a charge density of 1.3 × 10−3 eq/g and a molecular weight of 13 MDa.
Methods
Jar-test methodology
The experiments to assess the efficiency of the different treatments were carried out using a Jar-test methodology. A sample volume of 200 mL of the synthetic water-based ink solution was used. The additive was added to the sample and stirred for 30 min at 200 rpm followed by slow stirring at 40 rpm for 10 min. Then the sample was left 60 min to settle (Arsad and Ngadi 2014). This method was repeated when another additive was added. Finally, the supernatant was collected. Cationic/anionic demand, absorbance and turbidity were measured by duplicate. The average error between replicates was always under 5%. Ink concentration was calculated from the calibration ink curves plotting absorbance as a function of various concentrations of each synthetic water-based ink solutions.
In all cases, the preservation of the samples, the analyses and the measurements were performed according to the Standard Methods for the Examination of Water and Wastewater (APHA-AWWA-WEF, 2005).
FBRM methodology
The evolution of the flocculation processes was monitored in real time by using a focused beam reflectance measurement probe (FBRM) M500L supplied by Mettler Toledo (Columbus, USA). This device provides a chord length distribution of the suspended solids and the aggregates in the pulp suspension. A laser diode generates a laser beam, which is focused in a point at the end of the probe, just near the external surface of the sapphire window, which is at the end of the probe. The focal point moves at a high translational speed (2 m/s) in a circular path. When particles intercept the focal point path, light is reflected back to the detector, which receives the light pulses, the chord length of the each detected particle is calculated from the time duration of the light pulse and the translational speed of the focal point. Thousands of particles can be detected each second. Therefore, thousands of chord lengths can be measured and recorded per second, producing a histogram in which the number of detected counts is sorted into 90 chord length bins over an interval from 0.5to 1000 μm (Blanco et al. 2002).
In a typical flocculation trial, the FBRM probe was placed in the ink solution stirred at 200 rpm and 2.4 g of CNF hydrogel was added and dispersed at 200 rpm during 30 min. After that, stirring was reduced at 40 rpm and, after 10 min, cPAM was added. This caused the flocculation of the particles in suspension, which is monitored on real time by means of the chord length distribution. Trials were also carried out without ink, and with the inverse addition order. In the last case, the FBRM probe was placed in the ink solution stirred at 200 rpm and cPAM was added to the solution. After 30 min, 2.4 g of CNF hydrogel was added and dispersed at 200 rpm during 30 min. After that, stirring was reduced at 40 rpm during 10 min.
Results and discussion
Selection of the water-based ink removal system
By Jar-test methodology, single and dual-component systems were examined for water-based ink removal using the same dose for each additive (0.01 wt.%) (Fig. 1). Although CNF have shown to be efficient in water treatment technologies (Carpenter et al. 2015), this was not the case for the removal of water-based inks when CNF were used as a single-component system. However, the turbidity and ink removal results obtained by adding the negatively charge nanofibers alone suggest that a weak interaction was induced (Liu et al. 2003). On the other hand, the addition of cPAM only reduced water turbidity by 18.5% and ink concentration by 68.2% in yellow ink. The cPAM-based treatment was the most suitable for yellow ink removal (164 mg ink removal/g of treatment) but was significantly less effective for blue and black ink particles (3.74 and 1.65 mg ink removal/g of treatment). This can be due to the chemical structure of the inks. The open structure of yellow ink and the high electronic density of the oxygen atoms in the extremes of the molecule enhance the interaction with the cationic charges of cPAM. Printing paper and plastic products use a four-colour process (cyan-magenta-yellow-black) or multi-colour ink printing; therefore, the most suitable ink removal process must be efficient for different colour inks.
Surprisingly, the addition order of the additives in the dual-component system highly affected the ink removal behaviour. The addition of cPAM before CNF was less effective than the addition of cPAM after the nanofibers. CNF + cPAM was the best treatment and it has shown to highly reduce ink concentration: 94% for blue, 87.5% for black and 83.3%, for yellow inks. In addition, water turbidity removal was around 60% for blue and black inks and nearly 90% for yellow ink. Therefore, CNF + cPAM was the most efficient treatment to remove blue and black ink particles (21.13 and 8.82 mg ink removal/g of treatment).
When cPAM followed by CNF treatment was used, blue and black inks reduced turbidity by 28.3% and 18.2%, respectively. However, higher turbidity and ink concentration removals were observed in yellow ink samples with cPAM + CNF treatment compared with the other water-based ink solutions. Addition of CNF after cPAM to the yellow ink solution increased the ink-removal process by 41.1% in turbidity (49.4% total turbidity reduction) and 52.7% in ink concentration (84.94% total ink concentration reduction) compared with the single-component cPAM system.
Some references related to retention systems in papermaking industry combine a cationic polymer of high molecular weight (cPAM or cationic starch) with an anionic microparticle (bentonite or colloidal silica). Recent results reported by Jin et al. (2014) shown that cPAM followed by TEMPO-oxidised CNF constitute a pseudo-microparticle retention system of kaolin clay particles, where turbidity was reduced more than 65% when 0.01 wt.% CNF was added after 0.02 wt.% cPAM. However, for water-based inks removal the optimum treatment was the dual-component system CNF + cPAM. Flocculation studies have been carried out to better understanding the mechanism decolourisation process. The flocculation results are shown later.
According to the removal parameters studied, blue and black ink solutions had similar responses to each treatment. Yellow ink solutions had higher ink and turbidity removal compared with the other water-based ink solutions, regardless of the treatment applied. This is due to the ability of the yellow ink molecules to interact to cPAM, as shown by the high removal efficiency of yellow ink when cPAM was used (Fig. 1).
Another important parameter to consider after treatments is the charge of the supernatant fraction expressed by CD (Fig. 1 ). Surface charge of the fibres, conformational behaviour of the cationic polymer on the surfaces and the number of extended parts of the cationic polymer all affect the adsorption of charge microparticles presented in the suspension (Asselman and Garnier 2000) including bentonite, colloidal silica, kaolin clay or submicron ink particles, the latter one being the focus of this study. Inks solutions are anionic with a CD of 10.9, 9.3 and 57 μeq/L, for blue, black and yellow inks, respectively (Table 1). CNF are anionic and after a single-component CNF system the supernatant fraction had anionic charge with a CD up to 100 μeq/L. On the other hand, the cationic nature of the cPAM produced a cationic supernatant after cPAM treatment whose values were aligned with the charge nature of the water-based inks.
After both dual-component systems, supernatant had cationic charge due to part of cPAM that remained in the supernatant fraction. However, the addition of the cPAM after CNF reduced the cationic charge (absolute value) of the supernatant by 32% (blue ink), 64% (black ink) and 84% (yellow ink), compared with the cPAM added first. As expected, cPAM had better interaction with inks when negatively charged cellulose is present in the solution.
Optimisation of the dual-treatment CNF-cPAM
Once the best ink removal system was selected, the effect of cPAM dose added after 0.01 wt.% CNF was studied (Fig. 2). Higher water turbidity removal was achieved with the increase of cPAM addition and the cPAM doses were 0.01, 0.0075 and 0.01 wt.% for yellow, blue and black inks, respectively. Dual-component system of CNF (0.01 wt.%) and cPAM highly reduced the water turbidity: by 89.3%, 95.2% and 63.6% for yellow, blue and black inks, respectively (Fig. 2a). Ink concentration removal also increased with cPAM addition. The cPAM doses to achieve the highest ink removal was the same as for turbidity reduction. (Fig. 2b). Similar ink removal behaviour was observed at lower cPAM doses (below 0.005 wt.%) for the three water ink-based solutions. A cPAM dose of 0.01 wt.% highly reduced the yellow ink particles, 100.5 mg ink removal/g of treatment, and higher cPAM doses were less efficient for yellow ink removal. For blue and black inks, the highest ink removal efficiency was 26.6 and 8.8 mg ink removal/g of treatment using 0.0075 and 0.01 wt.% cPAM, respectively.
A high reduction in the CD is usually necessary to destabilise and remove the contaminants from the water (Latour et al. 2015). In this research, cPAM addition after CNF neutralised the anionic groups of the CNF decreasing the CD of the supernatant fraction (Fig. 2c). The neutralisation capacity of the dual-component system is mainly governed by the charge density of the products, which vary depending on the CNF and cPAM doses (Latour et al. 2015). The isoelectric point of the different ink wastewaters took place at approximately same cPAM doses (0.0075–0.01 wt.%) for all ink solutions. All inks achieved the maximum turbidity and ink removal at charge neutralisation conditions.
Colour changes of blue ink after dual treatment of 0.01 wt.% CNF and different doses of cPAM are shown in Fig. 3. At optimal cPAM dose (0.0075 wt.%), the flocs were clearly formed and they retained all the ink particles and, thus, transparent supernatant fractions were obtained. However, lower and higher cPAM doses than the optimum one had a negative effect on flocs appearance, suggesting that a weaker flocculation was induced, resulting in smaller, less denser and weaker flocs.
For the optimal cPAM doses, the effect of CNF dose on turbidity and ink concentration removal was studied. Doses of CNF lower and higher that the studied one were considered (0.005 wt.% and 0.02 wt.%). Highest turbidity and ink concentration removal were achieved at 0.01 wt.% CNF. A higher CNF dose (0.02 wt.%) reduced the ink concentration and water turbidity removal for all the inks studied. Such low levels of CNF (0.01 wt.%) will be promising in an industrial situation due to the high energy that is necessary to produce CNF. Maximum removal rates were also aligned with the CD results closed to the isoelectric point.
Therefore, ink concentration and turbidity removal had a clear relationship with flocculation behaviour and cationic charge.
Water-based ink solution decolourisation mechanism
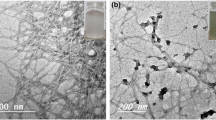
Flocculation studies have be carried out to better understanding the water-based ink solution decolourisation mechanism. Figures 4, 5 and 6 show the chord length distributions obtained before and after flocculation of CNF suspension and the effects of adding CNF to the inks before or after flocculation. The dimensions of CNF, cPAM and inks are quite low. However, the aggregates are detected. CNF interacts with cPAM, as it was expected (Figs. 4, 5 and 6a). The interaction of inks with cPAM, if it took place, it does not cause the formation of detectable aggregates. However, when CNF were added the suspension flocculated as shown by Figs. 4, 5 and 6b. Despite of it, the removal of blue or black inks was very low, which indicates that only a portion of ink was trapped inside the flocs. However, when CNF was allowed to interact with inks before the cPAM addition, removal of inks increased notably, although flocculation did not lead to such high number of flocs, especially in the case of blue ink (Fig. 4c). In the case of black ink the formed flocs were notably larger as shown by the chord length distributions (Fig. 5b, c). This confirms that the blue and black inks do not able to interact with cPAM. This can be due to their compact structure (Table 1) with many steric impediments for the cPAM chains to reach the active groups. The obtained results show that the inks must interact to CNF before their flocculation to be efficiently removed by the flocculation induced by cPAM. This interaction is not electrostatic, as both CNF and inks are anionic, but it is a physical adsorption, as proved by the fact that if the stirring rate used is too high during the interaction CNF-ink the removal efficiency decreases. This is possible by van der Waals forces due to the high molecular weight of the inks and its affinity to cellulose (as it is expected for flexographic inks). In the case of the yellow ink, the removal efficiency was similar due to the ability of this ink to interact with cPAM.
Conclusions
Water-based inks cannot be fully deinked by the traditional methods currently applied in recycled paper and plastic production. In this study, we demonstrate the feasible of flexographic inks removal using TEMPO-oxidised CNF hydrogels through its combination with a cPAM. The dual-component system based on CNF followed by cPAM was the best ink removal treatment, but the optimum treatment achieved depend on the type of ink. The treatments for higher ink reductions for each water-based ink solution studied are:
-
Blue ink (copper phthalocyanine blue)—0.01 wt.% CNF + 0.0075 wt.% cPAM, with 100% of ink removal and 95% of turbidity removal and 26.56 mg ink removed/g treatment.
-
Black ink (carbon black)—0.01 wt.% CNF + 0.01 wt.% cPAM, with 87,5% of ink removal and 63.6% of turbidity removal and 8.82 mg ink removed/g treatment.
-
Yellow ink (yellow 12)—0.01 wt.% CNF + 0.01 wt.% cPAM, with 83.3% of ink removal and 89.3% of turbidity removal and 100.5 mg ink removed/g treatment.
Flocculation trials show that first the ink is adsorbed onto the CNF, without increased of size particles and after the CNF, with the ink adsorbed, flocculates due to cPAM addition, removing the water-based inks.
References
Adegoke KA, Bello OS (2015) Dye sequestration using agricultural wastes as adsorbents. Water Resour Ind 12:8–24
Aksu Z, Tatli AI, Tunc O (2008) A comparative adsorption/biosorption study of acid blue 161: effect of temperature on equilibrium and kinetic parameter. Chem Eng J 142:23–39
Annadurai G, Juang RS, Lee DJ (2002) Use of cellulose-based wastes for adsorption of dyes from aqueous solutions. Journal of Hazardous Materials 92:263–274
Arsad NJ, Ngadi N (2014) Chitosan-grafted Nanocellulose derived from empty fruit bunch for ethyl orange removal. In: Ahmed I (ed) Process and advanced materials engineering. Applied mechanics and materials. Trans Tech Publications Ltd, Stafa-Zurich, pp 784–787
Arslan I, Balcioglu IA (2001) Advanced oxidation of raw and biotreated textile industry wastewater with O-3, H2O2/UV-C and their sequential application. J Chem Technol Biotechnol 76:53–60
Asselman T, Garnier G (2000) Dynamics of polymer-induced hetero-flocculation of wood fibres and fines. Colloid Surf. A-Physicochem. Eng. Asp 174:297–306
Balea A, Merayo N, Fuente E, Delgado-Aguilar M, Mutje P, Blanco A, Negro C (2016) Valorization of corn stalk by the production of cellulose nanofibers to improve recycled paper properties. Bioresources 11:3416–3431
Besbes I, Alila S, Boufi S (2011) Nanofibrillated cellulose from TEMPO-oxidized eucalyptus fibres: effect of the carboxyl content. Carbohydrate Polymers 84:975–983
Blanco A, De la Fuente E, Negro C, Monte MC, Tijero J (2002) Focused beam reflectant measurement as a tool to measure flocculation. TAPPI J 1:14–20
Carpenter AW, de Lannoy CF, Wiesner MR (2015) Cellulose nanomaterials in water treatment technologies. Environ Sci Technol 49:5277–5287
Delgado-Aguilar M, Gonzalez I, Tarres Q, Alcala M, Pelach MA, Mutje P (2015) Approaching a low-cost production of cellulose nanofibers for papermaking applications. Bioresources 10:5345–5355
Fernandez EO, Hodgson KT (2013) Deinking flexographic-printed papers: destabilization of flexographic ink dispersions with copper compounds. TAPPI J 12:29–35
Fu YZ, Viraraghavan T (2003) Column studies for biosorption of dyes from aqueous solutions on immobilised Aspergillus niger fungal biomass. Water SA 29:465–472
Gecol H, Scamehorn JF, Christian SD, Riddell FE (2003) Use of surfactants to remove solvent-based inks from plastic films. Colloid Polym Sci 281:1172–1177
Genc N (2004) Photocatalytic oxidation of a reactive azo dye and evaluation of the biodegradability of photocatalytically treated and untreated dye. Water SA 30:399–405
Gonzalez I, Boufi S, Pelach MA, Alcala M, Vilaseca F, Mutje P (2012) Nanofibrillated cellulose as paper additive in eucalyptus pulps. BioResources 7:5167–5180
Greluk M, Hubicki Z (2013) Evaluation of polystyrene anion exchange resin for removal of reactive dyes from aqueous solutions. Chem Eng Res Des 91:1343–1351
Isogai A (2009) Individualization of nano-sized plant cellulose fibrils achieved by direct surface carboxylation using TEMPO catalyst. Biomacromolecules 10:1992–1996
Isogai A, Saito T, Fukuzumi H (2011) TEMPO-oxidized cellulose nanofibers. Nanoscale 3:71–85
Jin LQ, Wei YW, Xu QH, Yao WR, Cheng ZL (2014) Cellulose nanofibers prepared from TEMPO-oxidation of Kraft pulp and its flocculation effect on kaolin clay. J Appl Polym Sci 131:8
Kacha S, Derriche Z, Elmaleh S (2003) Equilibrium and kinetics of color removal from dye solutions with bentonite and polyaluminum hydroxide. Water Environ Res 75:15–20
Karcher S, Kornmuller A, Jekel M (2002) Anion exchange resins for removal of reactive dyes from textile wastewaters. Water Res 36:4717–4724
Kim TH, Park C, Shin EB, Kim S (2004) Decolorization of disperse and reactive dye solutions using ferric chloride. Desalination 161:49–58
Korhonen JT, Kettunen M, Ras RHA, Ikkala O (2011) Hydrophobic Nanocellulose aerogels as floating, sustainable, reusable, and recyclable oil absorbents. ACS Appl Mater Interfaces 3:1813–1816
Latour I, Miranda R, Carceller R, Blanco A (2015) Efficiency of polyaluminum nitrate sulfate-polyamine hybrid coagulants for silica removal. Desalination and Water Treatment 57:17973–17984
Liimatainen H, Visanko M, Sirvio J, Hormi O, Niinimaki J (2013) Sulfonated cellulose nanofibrils obtained from wood pulp through regioselective oxidative bisulfite pre-treatment. Cellulose 20:741–749
Liu W, Ni Y, Xiao HJ (2003) Cationic montmorillonite: preparation and synergy with anionic polymer in filler flocculation. Pulp Pap Sci 29
Nechyporchuk O, Naceur Belgacem M, Bras J (2016) Production of cellulose nanofibrils: a review of recent advances. Ind Crop Prod 93:2–25
Noonpui S, Thiravetyan P, Nakbanpote W, Netpradit S (2010) Color removal from water-based ink wastewater by bagasse fly ash, sawdust fly ash and activated carbon. Chem Eng J 162:503–508
Raval NP, Shah PU, Ladha DG, Wadhwani PM, Shah NK (2016) Comparative study of chitin and chitosan beads for the adsorption of hazardous anionic azo dye Congo red from wastewater. Desalin Water Treat 57:9247–9262
Robinson T, Chandran B, Nigam P (2002) Removal of dyes from an artificial textile dye effluent by two agricultural waste residues, corncob and barley husk. Environ Int 28:29–33
Roussy J, Chastellan P, van Vooren M, Guibal E (2005) Treatment of ink-containing wastewater by coagulation/flocculation using biopolymers. Water SA 31:369–376
Saito T, Isogai A (2005) Ion-exchange behavior of carboxylate groups in fibrous cellulose oxidized by the TEMPO-mediated system. Carbohydr Polym 61:183–190
Saito T, Kimura S, Nishiyama Y, Isogai A (2007) Cellulose nanofibers prepared by TEMPO-mediated oxidation of native cellulose. Biomacromolecules 8:2485–2491
Siro I, Plackett D (2010) Microfibrillated cellulose and new nanocomposite materials: a review. Cellulose 17:459–494
Songsiri D, Min SS, Scamehorn JF, Osuwan S, Ellis JW (2002) Use of cationic surfactant to remove solvent-based ink from rigid high density polyethylene surfaces. Colloid surf. A-Physicochem. Eng. ASp 204:261–269
Taipale T, Osterberg M, Nykanen A, Ruokolainen J, Laine J (2010) Effect of microfibrillated cellulose and fines on the drainage of Kraft pulp suspension and paper strength. Cellulose 17:1005–1020
Wu FC, Tseng RL, Juang RS (2001a) Adsorption of dyes and phenols from water on the activated carbons prepared from corncob wastes. Environ Technol 22:205–213
Wu FC, Tseng RL, Juang RS (2001b) Enhanced abilities of highly swollen chitosan beads for color removal and tyrosinase immobilization. J Hazard Mater 81:167–177
Yang XY, Al-Duri B (2001) Application of branched pore diffusion model in the adsorption of reactive dyes on activated carbon. Chem Eng J 83:15–23
Yang R, Aubrecht KB, Ma HY, Wang R, Grubbs RB, Hsiao BS, Chu B (2014) Thiol-modified cellulose nanofibrous composite membranes for chromium (VI) and lead (II) adsorption. Polymer 55:1167–1176
Zhang Z, Sebe G, Rentsch D, Zimmermann T, Tingaut P (2014) Ultralightweight and flexible Silylated Nanocellulose sponges for the selective removal of oil from water. Chem Mat 26:2659–2668
Acknowledgements
The authors wish to thank the Economy and Competitiveness Ministry of Spain for the support of the projects with references CTQ2012-36868-C02-01 and CTQ2013-48090-C2-1-R.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Balea, A., Monte, M.C., de la Fuente, E. et al. Application of cellulose nanofibers to remove water-based flexographic inks from wastewaters. Environ Sci Pollut Res 24, 5049–5059 (2017). https://doi.org/10.1007/s11356-016-8257-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-8257-x